Abstract
Objectives: The purpose of this study was to describe rates and patterns of long- and short-acting alpha agonist use for behavioral problems in a primary care population following Food and Drug Administration (FDA) approval of the long-acting alpha agonists guanfacine and clonidine.
Methods: Children and adolescents 4–18 years of age, who received an alpha agonist prescription between 2009 and 2011, were identified from a sample of 45 United States primary care practices in two electronic health record-based research networks. Alpha agonist receipt was identified using National Drug Codes and medication names. The proportion of subjects receiving long- and short-acting prescriptions in each year was calculated and examined with respect to reported mental health diagnoses, and whether indications for use were on-label, had evidence from clinical trials, or had no trial evidence.
Results: In a cohort of 282,875 subjects, 27,671 (10%) received any psychotropic medication and only 4,227 subjects (1.5%) received at least one prescription for an alpha agonist, most commonly a short-acting formulation (83%). Only 20% of alpha agonist use was on-label (use of long-acting formulations for attention-deficit/hyperactivity disorder [ADHD]). Most subjects (68%) received alpha agonists for indications with evidence of efficacy from clinical trials but no FDA approval, primarily short-acting formulations for ADHD and autism; 12% received alpha agonists for diagnoses lacking randomized clinical trial evidence in children, including sleep disorders and anxiety, or for which there was no documented mental health diagnosis. Rates of long-acting alpha agonist use increased more than 20-fold from 0.2% to 4%, whereas rates of short-acting alpha agonist use grew only slightly between 2009 and 2011 from 10.6% to 11.3%.
Conclusions: Alpha agonist use was uncommon in this population, and most subjects received short-acting forms for conditions that were off-label, but with clinical trial evidence. The safety and efficacy of use for conditions, including sleep disorders and anxiety, lacking evidence from randomized trials, warrant further investigation.
Introduction
In September 2009 and October 2010, the United States Food and Drug Administration (FDA) approved long-acting forms of the α-2 adrenergic agonists, guanfacine and clonidine, respectively, as treatment for attention-deficit/hyperactivity disorder (ADHD) in children and adolescents (hereafter “children”) (Kapvay 2010; Intuniv 2011). Prior to these approvals, no form of alpha agonist had been approved for use in neurodevelopmental disorders among children. However, as recommended by American Academy of Child and Adolescent Psychiatry (AACAP) practice parameters (Pliszka 2007), short-acting alpha agonists or the clonidine patch have been used off-label for many years as a treatment for ADHD (Hirota et al. 2014). In addition, alpha agonists have been used off-label as a first-line pharmacological treatment for moderate or severe tics (Weisman et al. 2013), and have been frequently prescribed for insomnia symptoms in children with ADHD, anxiety, and mood disorders (Owens et al. 2010). Current AACAP parameters cautiously affirm the use of alpha agonists for tics (Murphy et al. 2013) and for insomnia with ADHD (Pliszka 2007), but not for other disorders.
Although there is evidence that alpha agonist use has increased over time (Rubin, et al. 2012; Fontanella et al. 2014), changes in prescribing patterns and rates of use of alpha agonists after FDA approval, both on- and off-label, have not been studied. In this study, we used electronic health record (EHR) data from a large sample of United States primary care pediatric practices to describe prescription patterns of alpha agonists from 2009 to 2011. These data include all children seen in a primary care setting, both children with contact with behavioral health specialists, including psychiatrists, as well as children treated exclusively in primary care.
Methods
Setting
This study was conducted within two EHR-based pediatric practice-based research networks: The Pediatric Research Consortium (PeRC) of The Children's Hospital of Philadelphia (CHOP), a two-state (Pennsylvania and New Jersey), hospital-owned, primary care network including 26 practices and >200,000 children, and the national Electronic Pediatric Research in Office Settings (ePROS) network, a subnetwork of the American Academy of Pediatrics (AAP) Pediatric Research in Office Settings (PROS) consisting, at the time of this study, of 19 practices (see Acknowledgments) using seven different EHR vendors and serving ∼90,000 children in 15 states (Fiks et al. 2012).
Design and sample selection
We conducted a retrospective review of EHRs from these practices to identify a cohort of children ages 4–18 years seen in office between January 1, 2009 and December 31, 2011. To focus on the use of alpha agonists for behavioral complaints, children with an EHR diagnosis of hypertension were excluded.
Outcome
The primary outcome measure was receipt of an alpha agonist prescription at any time during the study period (referred to as “use” for brevity). Alpha agonist use was identified from the 11 digit national drug code (NDC) specific to each drug, as well as by examining medication names. The medications included long-acting and short-acting forms of clonidine hydrochloride and guanfacine hydrochloride. We included any form of alpha agonist that appeared in the EHR, either because it was prescribed by the practice or abstracted into the EHR as part of medication reconciliation. We did not capture data on prescriptions from outside of primary care that were never entered into a child's primary care medical record.
Independent variables included patient sex, age, and mental health diagnoses. Mental health diagnoses were identified from encounters with codes from the International Classification of Diseases, Ninth Revision (ICD-9) classification (see footnote of Table 1).
Table 1.
Common Diagnosesa among Children Receiving Long- or Short-Acting Alpha Agonists
| Long-acting alpha agonist level of evidenceb (n=1065) | Short-acting alpha agonist level of evidenceb (n=3502) | |||
|---|---|---|---|---|
| Diagnosis | On-label | Off-label no randomized clinical trial evidence in pediatrics | Off-label evidence from clinical trials | Off-label no randomized clinical trial evidence in pediatrics |
| Attention-deficit/hyperactivity disorder (ADHD) | 863 (81.0%)e | N/A | 2392 (68.3%) | N/A |
| Tic disorderc | N/A | 26 (2.0%) | 161 (4.6%) | N/A |
| Conduct disorderd | N/A | 0 (0.0%) | 6 (0.2%) | N/A |
| Oppositional defiant disorderd | N/A | 4 (0.4%) | 27 (0.8%) | N/A |
| Autismd | N/A | 48 (4.5%) | 291 (8.3%) | N/A |
| Sleep disorderd | N/A | 10 (0.9%) | N/A | 127 (3.6%) |
| Anxietyd | N/A | 9 (0.8%) | N/A | 65 (1.9%) |
| Other diagnosis | N/A | 25 (2.3%) | N/A | 168 (4.8%) |
| No diagnosis | N/A | 80 (7.5%) | N/A | 265 (7.6%) |
340 subjects who received both long- and short-acting alpha agonists are counted in both columns.
The following diagnostic categories were included: Any mental health diagnosis (International Classification of Diseases, 9th Revision [ICD-9] codes 290-319.99), ADHD (314-314.99), autism (299-299.99), schizophrenia (295-295.99), bipolar disorder (296.00-296.10, 296.36-296.89), anxiety (300.00-300.29, 301.4), obsessive-compulsive disorder (300.3), conduct disorder (312.00, 312.89), depression (311, 296.20-296.35), oppositional defiant disorder (ODD) (313.81), sleep disorder (780.50-780.59, 327.0-327.8, 307.4, V69.4, V69.5), tic disorder (307.2-307.29, 333.3), and seizure disorder (345.00-345.99).
On-label indicates United States Food and Drug Administration (FDA) approval, which has only been obtained for long-acting alpha agonists for ADHD among subjects 6–17 years of age. Off-label with evidence from clinical trials indicates that review of the literature identified trials describing efficacy of alpha agonists for these indications, but FDA approval had not been obtained. Off-label with no trial evidence indicates that there were no published trials for these indications.
Subjects with comorbid ADHD and tics were counted in the ADHD group; these subjects did not have a diagnosis of ADHD.
These subjects did not have a diagnosis of ADHD or tic disorder.
Of these subjects, 848 (98%) were between the ages of 6 and 17, the age group for whom FDA approval was obtained.
Level of evidence for alpha agonist indication
We classified the diagnoses of children who received alpha agonists into three categories based on a review of clinical trial evidence in the literature and relevant treatment guidelines. First, “on-label” use was classified as use of a long-acting alpha agonist for ADHD, alone or with a stimulant medication, as this was the only FDA-approved indication in children at the time of this study (Kapvay 2010; Intuniv 2011) and is recommended by AAP guidelines (Wolraich et al. 2011). It should be noted that the AACAP practice parameters for ADHD, published in 2007, do not yet address long-acting alpha agonist prescribing but do recommend consideration of short-acting alpha agonists for children with ADHD who do not respond to stimulants or atomoxetine (Pliszka 2007).
Second, we defined a category of “off-label with evidence from clinical trials” for indications for which alpha agonists are not FDA-approved but have shown efficacy at reducing symptoms in clinical trials. This category included use of a short-acting alpha agonist for ADHD (with or without comorbid tics or aggression) (Chappell et al. 1995; Tourette's Syndrome Study Group 2002; Hazell and Stuart 2003; Palumbo et al. 2008), tic disorders (Cummings et al. 2002; Du et al. 2008), autism (Fankhauser et al. 1992; Handen et al. 2008), or aggression (defined as conduct disorder or oppositional defiant disorder [ODD]) (Kemph et al. 1993). Although the current AACAP guidelines for tics (Murphy et al. 2013) and autism (Volkmar et al. 2014) do include the potential use of alpha agonists, at the time of this study, there were no expert guidelines recommending the use of alpha agonists for conduct disorder or ODD. All other uses of alpha agonists (sleep problems, anxiety, depression, schizophrenia, bipolar, obsessive-compulsive disorder in children without ADHD, or in children with no mental health diagnosis) were categorized as “off-label with no randomized clinical trial evidence in children,” based on our review of Cochrane Database of Systematic Reviews (2014), the Agency for Healthcare Research and Quality (AHRQ) evidence-based reports (Volkmar et al. 2014), available pediatric psychotropic medication practice parameters (Steiner 1997; Greenhill et al. 2002; Birmaher et al. 2007; Connolly and Bernstein 2007; McClellan et al. 2007; Pliszka 2007; Steiner and Remsing 2007; American Academy of Child and Adolescent Psychiatry 2009; Murphy et al. 2013; Volkmar et al. 2014), and published pediatric sleep disorder guideline parameters (Morgenthaler et al. 2006).
Statistical analysis
Analyses were descriptive. First, we tabulated the number of children in the cohort receiving any psychotropic medication prescription and the proportion receiving at least one alpha agonist prescription during the study period, stratified by long- and short-acting alpha agonists. We then calculated the proportion of children receiving alpha agonist prescriptions who had a record in the EHR of any of the mental health diagnoses specified in Table 1, also stratified by long- and short-acting forms. We compared patterns of use among children with each of the three levels of evidence for alpha agonist use (on-label, off-label with evidence from clinical trials, off-label with no randomized clinical trial evidence). We then calculated the proportion of children receiving an alpha agonist each year who were classified at each evidence level to determine whether on-label use increased as a proportion of all alpha agonist use. Finally, as a secondary analysis and to investigate trends over time, we calculated proportion of children in the cohort receiving a psychotropic medication who received at least one long-acting or short-acting alpha agonist prescription in each year of the study (2009–2011).
All analyses were conducted in SAS software version 9.3 (SAS Institute Inc., Cary, NC) and Stata version 13.0 (StataCorp, College Station, TX). The Institutional Review Board (IRB) at the AAP approved this study, and the IRB at CHOP determined this study to be IRB exempt.
Results
Study population
The study cohort included 282,875 children (51% male, 44% ≥12 years of age), of whom 10% (27,671 children) received at least one prescription for a psychotropic medication during the study. Among all children, the proportion receiving at least one prescription for an alpha agonist during the study period was low (1.5%, 4227). Of those children prescribed an alpha agonist, 3162 (75%) received a short-acting alpha agonist only. In addition, 725 (17%) received a long-acting alpha agonist only and 340 (8%) received at least one prescription for both a long and short-acting alpha agonist. Compared with children not receiving alpha agonists, those on alpha agonists were more likely to be male (75%) and ≥12 years of age (59%).
Diagnoses of children on alpha agonists by level of evidence
Of the 1065 children who received a long-acting alpha agonist, 863 (81%) had an ADHD diagnosis (Table 1). Of those children, 98% were between the ages of 6 and 17, the FDA-approved age range for alpha agonists among children. Very few children received long-acting alpha agonists for other indications. Of the 3502 children who received short-acting alpha agonists, 68% had an ADHD diagnosis. The next largest categories were autism (8%) and tic disorders (5%), indications for which clinical trials have provided evidence but for which FDA approval has not been obtained; 332 children (8%) received an alpha agonist without a mental health diagnosis documented in the EHR.
Of all 4227 children receiving alpha agonists at any time during the study period, 863 (20%) received prescriptions that were on-label (long-acting for ADHD only), 2877 (68%) for indications that were off-label with evidence from clinical trials, and 487 (12%) for indications that were off-label, including anxiety, sleep disorders, or other diagnoses with no randomized clinical trial evidence in children or that had no diagnosis documented (Table 1).
Overall trends in alpha agonist use
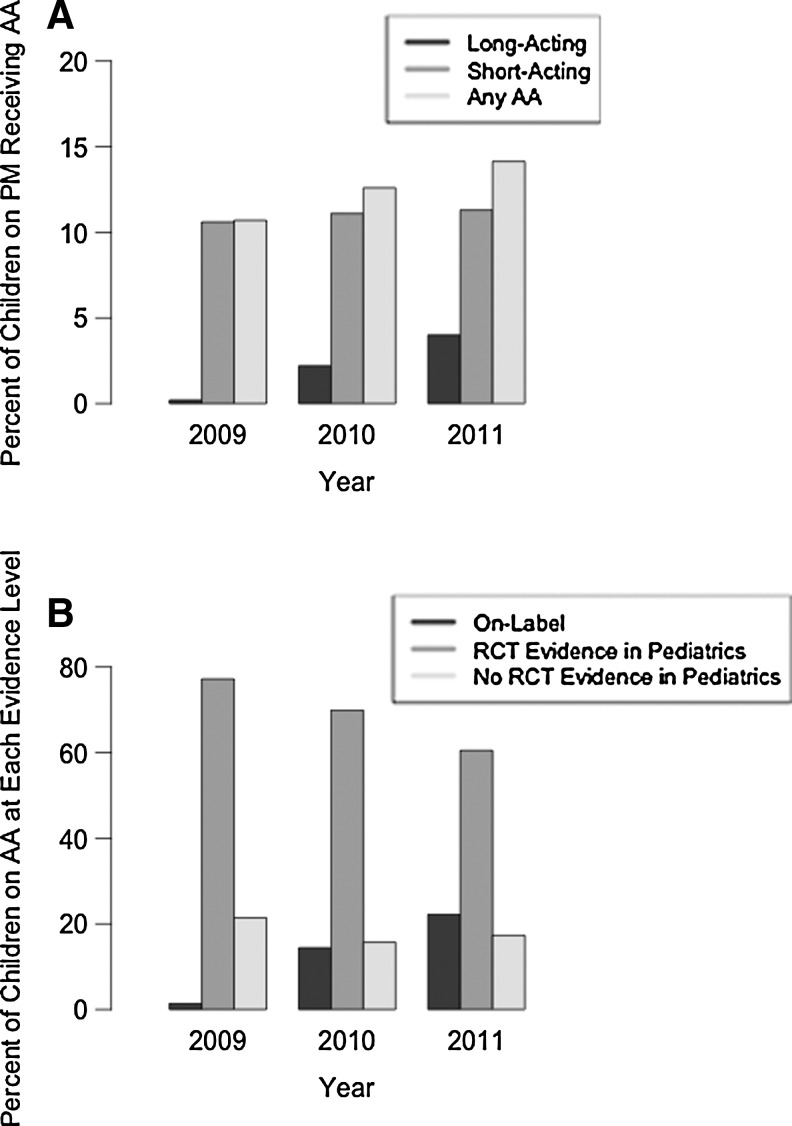
Among children receiving any psychotropic medication, overall rates of alpha agonist use increased from 11% in 2009 to 14% in 2011, largely driven by increases in long-acting alpha agonists, which increased more than 20-fold from 0.2% to 4% (Fig. 1A). The proportion of children receiving short-acting alpha agonists grew only slightly, from 10.6% to 11.3%. Following the FDA approval of long-acting alpha agonists in 2009, the proportion of alpha agonist use that was on-label increased over time, from 1% in 2009 to 22% in 2011 (Fig. 1B). No clear trend was observed for use without clinical trial evidence.
FIG. 1.
(A) Proportion of children receiving psychotropic medication who received alpha agonists in each study year (2009–2011). Note that the proportion of the entire cohort who received any psychotropic medication increased from 7.7% in 2009 to 8.4% in 2011. PM, psychotropic medication; AA, alpha agonist. (B) Change in the proportion of alpha agonist use at each level of evidence over time (2009–2011). AA, alpha agonist.
Discussion
In this study of children in primary care settings, we found limited prescribing of alpha agonist medications overall, with only 1.5% of children receiving any alpha agonist. A majority of children (68%) received alpha agonists for indications for which there was some clinical trial evidence but no FDA approval; 12% of children received alpha agonists for indications such as anxiety and sleep disorders for which there was no evidence from randomized clinical trials in children.
The AAP recently released a policy statement that “off-label” use does not indicate improper or contraindicated use, and that the purpose of off-label medication use is to benefit individual patients based on the judgment of their clinician (Frattarelli et al. 2014). However, much remains to be learned about medication use in conditions with little or no published clinical trial evidence. Our finding that ∼12% of alpha agonist prescriptions were for indications with no trial evidence, primarily sleep disorders and anxiety, suggests these are areas for which clinicians are actively seeking treatment options. Although there are some older, open trials examining the use of alpha agonists in children with posttraumatic stress disorder (Perry 1994), results from this study support increased research to document the benefits and risks of alpha agonist use in these disorders. Interestingly, close to 8% received an alpha agonist without a documented mental health diagnosis, raising additional concerns about documentation, and highlighting a continued need for a focus on medication reconciliation in primary care. This finding may reflect a lack of clinician documentation of an existing diagnosis, a lack of awareness by the primary pediatrician regarding a diagnosis made through the mental health system, or a child treated without receiving a specific diagnosis.
Not surprisingly, there was a substantial growth in the prescribing of long-acting forms of alpha agonists following FDA approval of the drugs clonidine and guanfacine, but limited growth in the prescribing of short-acting alpha agonists. Although long-acting alpha agonist use increased over time, the majority of alpha agonist prescriptions were for short-acting forms. The sustained predominance of short-acting forms over the course of the study period is consistent with prior research that found slow and inconsistent adoption of new psychotropic medications (Huskamp et al. 2013), but could also indicate a preference for focusing on symptoms confined to particular times of day (e.g., before bedtime). Regardless, in those disorders for which there is some evidence for use of a short-acting alpha agonist (e.g., autism), it is anticipated that further studies of the use of long-acting formulations will follow.
Growth in the use of long-acting formulations may reflect a number of factors. From a clinical perspective, continued growth in long-acting alpha-agonist prescribing is likely as clinicians look for medications to use either concurrently with, or instead of, stimulants, especially among families reluctant to use stimulants (Dosreis et al. 2003) or when problems persist after initiating stimulant medication (Wood et al. 2007). The increase in long-acting alpha agonist use in a broad sample of primary care practices after FDA approval may similarly reflect efforts to improve adherence. In ADHD, long-acting stimulant medication use has been associated with improved adherence in studies (Adler and Nierenberg 2010; Spencer et al. 2011); a caveat is that authors of those studies have received pharmaceutical industry support. Growth in use may also occur as long-acting alpha agonists appear on insurance company formularies. Advertising by the pharmaceutical industry may also affect these trends.
This study had several limitations. First, EHRs commonly lack medication end dates; as a result, we conservatively credited children for receiving alpha agonists only for new entries in the EHR. This method might understate the duration of use. Second, although medication reconciliation is a regular part of pediatric practice, medications prescribed by psychiatrists, neurologists, or developmental pediatricians may have been missed if those children failed to attend recommended health maintenance visits, physicians failed to document or were unaware of use of psychopharmacology prescribed by outside clinicians, and/or EHRs were not adequately integrated to provide a shared record of prescribed medications. Third, certain diagnoses treated with alpha agonists, such as sleep problems, may be inconsistently documented in EHR data. As a result, the actual use of alpha agonists for sleep problems may be greater than that observed. Fourth, given the breadth of the sample of practices included in this study, we lack data on formulary restrictions at different sites that may have shaped prescribing for newly licensed alpha agonists. Use is likely to grow as these medications are increasingly covered by insurance. Fifth, despite the diversity of practices included in ePROS and PeRC, it is possible that trends in certain settings may not reflect the broader changes in alpha agonist prescribing presented here. Sixth, a review of prescribing by different types of practices or providers was beyond the scope of this study and may be addressed in future work. Finally, this study focused on 3 years of data around the licensing of the long-acting alpha agonists. As such, we lack data on longitudinal trends in alpha agonist use among individual children that would be better appreciated through a longitudinal cohort study.
Nevertheless, this study highlights the advantages of studies employing EHR data from a large sample of United States primary care practices. Given the substantial increase in EHR use over the past decade (Hsiao and Hing 2014), these databases have become an increasingly important resource to study the usage patterns of psychotropic medications among children. In addition, the presence of blood pressure, growth, and laboratory and diagnostic test results in EHRs, an area for future research, further supports the use of the EHR to detect effectiveness, safety, and side effects of psychopharmacology. This work may be advanced as EHRs increasingly incorporate outcome data reported by children, parents, or teachers (Wu et al. 2010; Ahmed et al. 2012). In the context of these trends and as has been suggested by the National Institutes of Health (Lauer and Collins 2010), comparative effectiveness research will be warranted to better understand the response to, and side effects from, these treatments in larger numbers of children in actual practice settings.
Conclusions
In this large cohort of children receiving primary care, alpha agonist use was uncommon overall. A majority of alpha agonist use was with short-acting agents, and most children received short-acting forms for conditions that were off-label, but with clinical trial evidence. The safety and efficacy of off-label use in clinical practice, especially in the context of sleep and anxiety for which clinical trial evidence is lacking, warrant further investigation.
Clinical Significance
In this large sample of United States practices, most alpha agonist use was of short-acting forms that were off-label but with clinical trial evidence. However, many children received alpha agonists for indications with no clinical trial evidence, such as for anxiety or sleep disorders. Additional study is needed to document effectiveness and safety in clinical practice for these conditions.
Acknowledgments
We thank Andrew Suh from CHOP, and the providers, patients, and their families from PeRC and ePROS. The ePROS pediatric practices that participated in this study are listed by AAP Chapter. California-1: Shasta Community Health Center (Redding), practice of Mark M Simonian, MD (Clovis); Colorado: Fort Collins Youth Clinic PC (Fort Collins); Georgia: The Pediatric Center (Stone Mountain), Roswell Pediatrics (Cumming); Indiana: Jeffersonville Pediatrics (Jeffersonville); Kentucky: Union Pediatrics (Union); Maryland: Main Street Pediatrics (Towson); New Jersey: Delaware Valley Pediatric Associates PA (Lawrenceville), PASE Healthcare PC (Millburn); New York-2: East End Pediatrics (East Hampton); Missouri: Priority Care Pediatrics LLC (Kansas City); Ohio: Oxford Pediatrics and Adolescents (Oxford); Oklahoma: OUCP Sooner Pediatric Clinic (Oklahoma City); Oregon: Childhood Health Associates of Salem (Salem); Pennsylvania: Kressly Pediatrics (Warrington); Tennessee: Plateau Pediatrics (Crossville); South Carolina: AnMed Health Children's Health Center (Anderson).
Disclosures
Dr. Fiks is the co-principal investigator of an independent research grant from Pfizer for work in ADHD. The other authors have nothing to disclose.
References
- Cochrane Database of Systematic Reviews. Chichester: Wiley; 2014 [Google Scholar]
- Adler LD, Nierenberg AA: Review of medication adherence in children and adults with ADHD. Postgrad Med 122:184–191, 2010 [DOI] [PubMed] [Google Scholar]
- Ahmed S, Berzon RA, Revicki DA, Lenderking WR, Moinpour CM, Basch E, Reeve BB, Wu AW: The use of patient-reported outcomes (PRO) within comparative effectiveness research implications for clinical practice and health care policy. Med Care 50:1060–1070, 2012 [DOI] [PubMed] [Google Scholar]
- American Academy of Child and Adolescent Psychiatry: Practice parameter on the use of psychotropic medication in children and adolescents. J Am Acad Child Adolesc Psychiatry 48:961–973, 2009 [DOI] [PubMed] [Google Scholar]
- Birmaher B, Brent D, Bernet W, Bukstein O, Walter H, Benson RS, Chrisman A, Farchione T, Greenhill L, Hamilton J, Keable H, Kinlan J, Schoettle U, Stock S, Ptakowski KK, Medicus J: Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatry 46:1503–1526, 2007 [DOI] [PubMed] [Google Scholar]
- Chappell PB, Riddle MA, Scahill L, Lynch KA, Schultz R, Arnsten A, Leckman JF, Cohen DJ: Guanfacine treatment of comorbid attention-deficit hyperactivity disorder and Tourette's syndrome: Preliminary clinical experience. J Am Acad Child Adolesc Psychiatry 34:1140–1146, 1995 [DOI] [PubMed] [Google Scholar]
- Connolly SD, Bernstein GA: Practice parameter for the assessment and treatment of children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry 46:267–283, 2007 [DOI] [PubMed] [Google Scholar]
- Cummings DD, Singer HS, Krieger M, Miller TL, Mahone EM: Neuropsychiatric effects of guanfacine in children with mild tourette syndrome: A pilot study. Clin Neuropharmacol 25:325–332, 2002 [DOI] [PubMed] [Google Scholar]
- Dosreis S, Zito JM, Safer DJ, Soeken KL, Mitchell JW, Jr., Ellwood LC: Parental perceptions and satisfaction with stimulant medication for attention-deficit hyperactivity disorder. J Dev Behav Pediatr 24:155–162, 2003 [DOI] [PubMed] [Google Scholar]
- Du YS, Li HF, Vance A, Zhong YQ, Jiao FY, Wang HM, Wang MJ, Su LY, Yu DL, Ma SW, Wu JB: Randomized double-blind multicentre placebo-controlled clinical trial of the clonidine adhesive patch for the treatment of tic disorders. Aust N Z J Psychiatry 42:807–813, 2008 [DOI] [PubMed] [Google Scholar]
- Fankhauser MP, Karumanchi VC, German ML, Yates A, Karumanchi SD: A double-blind, placebo-controlled study of the efficacy of transdermal clonidine in autism. J Clin Psychiatry 53:77–82, 1992 [PubMed] [Google Scholar]
- Fiks AG, Grundmeier RW, Margolis B, Bell LM, Steffes J, Massey J, Wasserman RC: Comparative effectiveness research using the electronic medical record: An emerging area of investigation in pediatric primary care. J Pediatr 160:719–724, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanella CA, Hiance DL, Phillips GS, Bridge JA, Campo JV: Trends in psychotropic medication use for medicaid-enrolled preschool children. J Child Fam Stud 23:617–631, 2014 [Google Scholar]
- Frattarelli DA, Galinkin JL, Green TP, Johnson TD, Neville KA, Paul IM, Van Den Anker JN: Off-label use of drugs in children. Pediatrics 133:563–567, 2014 [DOI] [PubMed] [Google Scholar]
- Greenhill LL, Pliszka S, Dulcan MK, Bernet W, Arnold V, Beitchman J, Benson RS, Bukstein O, Kinlan J, McClellan J, Rue D, Shaw JA, Stock S: Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry 41:26s–49s, 2002 [DOI] [PubMed] [Google Scholar]
- Handen BL, Sahl R, Hardan AY: Guanfacine in children with autism and/or intellectual disabilities. J Dev Behav Pediatr 29:303–308, 2008 [DOI] [PubMed] [Google Scholar]
- Hazell PL, Stuart JE: A randomized controlled trial of clonidine added to psychostimulant medication for hyperactive and aggressive children. J Am Acad Child Adolesc Psychiatry 42:886–894, 2003 [DOI] [PubMed] [Google Scholar]
- Hirota T, Schwartz S, Correll CU: Alpha-2 agonists for attention-deficit/hyperactivity disorder in youth: a systematic review and meta-analysis of monotherapy and add-on trials to stimulant therapy. J Am Acad Child Adolesc Psychiatry 53:153–173, 2014 [DOI] [PubMed] [Google Scholar]
- Hsiao CJ, Hing E: Use and characteristics of electronic health record systems among office-based physician practices: United States, 2001–2013. NCHS Data Brief January:1–8, 2014 [PubMed] [Google Scholar]
- Huskamp HA, O'Malley AJ, Horvitz–Lennon M, Taub AL, Berndt ER, Donohue JM: How quickly do physicians adopt new drugs? The case of second-generation antipsychotics. Psychiatr Serv 64:324–330, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intuniv [package insert]: Wayne, PA: Shire Pharmaceuticals, Inc; 2011 [Google Scholar]
- Kapvay [package insert]: Atlanta: Shionogi Pharma, Inc.; 2010 [Google Scholar]
- Kemph JP, DeVane CL, Levin GM, Jarecke R, Miller RL: Treatment of aggressive children with clonidine: Results of an open pilot study. J Am Acad Child Adolesc Psychiatry 32:577–581, 1993 [DOI] [PubMed] [Google Scholar]
- Lauer MS, Collins FS: Using science to improve the nation's health system NIH's commitment to comparative effectiveness research. JAMA 303:2182–2183, 2010 [DOI] [PubMed] [Google Scholar]
- McClellan J, Kowatch R, Findling RL: Practice parameter for the assessment and treatment of children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry 46:107–125, 2007 [DOI] [PubMed] [Google Scholar]
- Morgenthaler TI, Owens J, Alessi C, Boehlecke B, Brown TM, Coleman J, Jr., Friedman L, Kapur VK, Lee-Chiong T, Pancer J, Swick TJ, American Academy of Sleep Medicine: Practice parameters for behavioral treatment of bedtime problems and night wakings in infants and young children. Sleep 29:1277–1281, 2006 [PubMed] [Google Scholar]
- Murphy TK, Lewin AB, Storch EA, Stock S: Practice parameter for the assessment and treatment of children and adolescents with tic disorders. J Am Acad Child Adolesc Psychiatry 52:1341–1359, 2013 [DOI] [PubMed] [Google Scholar]
- Owens JA, Rosen CL, Mindell JA, Kirchner HL: Use of pharmacotherapy for insomnia in child psychiatry practice: A national survey. Sleep Med 11:692–700, 2010 [DOI] [PubMed] [Google Scholar]
- Palumbo DR, Sallee FR, Pelham WE, Jr., Bukstein OG, Daviss WB, McDermott MP: Clonidine for attention-deficit/hyperactivity disorder: I. Efficacy and tolerability outcomes. J Am Acad Child Adolesc Psychiatry 47:180–188, 2008 [DOI] [PubMed] [Google Scholar]
- Perry BD: Neurological sequelae of childhood trauma: PTSD in children. In: Catecholamine Function in Posttraumatic Stress Disorder: Emerging Concepts. Edited by Murburg M.M. Washington, DC: American Psychiatric Press, 223–255, 1994 [Google Scholar]
- Pliszka S: Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 46:894–921, 2007 [DOI] [PubMed] [Google Scholar]
- Rubin D, Matone M, Huang YS, dosReis S, Feudtner C, Localio R: Interstate variation in trends of psychotropic medication use among Medicaid-enrolled children in foster care. Child Youth Serv Rev 34:1492–1499, 2012 [Google Scholar]
- Spencer TJ, Mick E, Surman CB, Hammerness P, Doyle R, Aleardi M, Kotarski M, Williams CG, Biederman J: A randomized, single-blind, substitution study of OROS methylphenidate (Concerta) in ADHD adults receiving immediate release methylphenidate. J Atten Disord 15:286–294, 2011 [DOI] [PubMed] [Google Scholar]
- Steiner H: Practice parameters for the assessment and treatment of children and adolescents with conduct disorder. J Am Acad Child Adolesc Psychiatry 36:122s–139s, 1997 [DOI] [PubMed] [Google Scholar]
- Steiner H, Remsing L: Practice parameter for the assessment and treatment of children and adolescents with oppositional defiant disorder. J Am Acad Child Adolesc Psychiatry 46:126–141, 2007 [DOI] [PubMed] [Google Scholar]
- Tourette's Syndrome Study Group: Treatment of ADHD in children with tics: A randomized controlled trial. Neurology 58:527–536, 2002 [DOI] [PubMed] [Google Scholar]
- Volkmar F, Siegel M, Woodbury–Smith M, King B, McCracken J, State M: Practice parameter for the assessment and treatment of children and adolescents with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry 53:237–257, 2014 [DOI] [PubMed] [Google Scholar]
- Weisman H, Qureshi IA, Leckman JF, Scahill L, Bloch MH: Systematic review: Pharmacological treatment of tic disorders—efficacy of antipsychotic and alpha-2 adrenergic agonist agents. Neurosci Biobehav Rev 37:1162–1171, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolraich M, Brown L, Brown RT, DuPaul G, Earls M, Feldman HM, Ganiats TG, Kaplanek B, Meyer B, Perrin J, Pierce K, Reiff M, Stein MT, Visser S: ADHD: Clinical practice guideline for the diagnosis, evaluation, and treatment of attention–deficit/hyperactivity disorder in children and adolescents. Pediatrics 128:1007–1022, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JG, Crager JL, Delap CM, Heiskell KD: Beyond methylphenidate: Nonstimulant medications for youth with ADHD. J Atten Disord 11:341–350, 2007 [DOI] [PubMed] [Google Scholar]
- Wu AW, Snyder C, Clancy CM. and Steinwachs DM. Adding the patient perspective to comparative effectiveness research. Health Aff (Millwood) 29:1863–1871, 2010 [DOI] [PubMed] [Google Scholar]