Abstract
Duchenne muscular dystrophy (DMD) is an X-linked lethal muscle disease caused by dystrophin deficiency. Gene therapy has significantly improved the outcome of dystrophin-deficient mice. Yet, clinical translation has not resulted in the expected benefits in human patients. This translational gap is largely because of the insufficient modeling of DMD in mice. Specifically, mice lacking dystrophin show minimum dystrophic symptoms, and they do not respond to the gene therapy vector in the same way as human patients do. Further, the size of a mouse is hundredfolds smaller than a boy, making it impossible to scale-up gene therapy in a mouse model. None of these limitations exist in the canine DMD (cDMD) model. For this reason, cDMD dogs have been considered a highly valuable platform to test experimental DMD gene therapy. Over the last three decades, a variety of gene therapy approaches have been evaluated in cDMD dogs using a number of nonviral and viral vectors. These studies have provided critical insight for the development of an effective gene therapy protocol in human patients. This review discusses the history, current status, and future directions of the DMD gene therapy in the canine model.
Duchenne Muscular Dystrophy and the Canine Duchenne Muscular Dystrophy Model
Duchenne Muscular Dystrophy (DMD) is a fatal muscle disease caused by null mutations in the dystrophin gene, a 2.4 mb gene in the X-chromosome.1,2 DMD occurs in ∼1 in 5,000 male births.3 Affected boys show delayed motor skill development between ages 2 and 5. They lose ambulation in their early teens and die around age 20 because of cardiorespiratory failure (Table 1).4 The current standard of care includes steroids, palliative support, and symptom management.5,6 Unfortunately, these therapies cannot solve the fundamental problem of dystrophin deficiency in DMD. Gene therapy has the potential to bring back the missing protein and radically change the disease course.7
Table 1.
A Comparison of Canine and Human Duchenne Muscular Dystrophy
| Canine DMD | Human DMD | |
|---|---|---|
| General | ||
| Mutation type | Point mutations, deletions, insertions | Mainly deletions (∼60%) and duplications (∼10%) |
| Lifespan reduction | By 75% | By 75% |
| Disease course | Progressive and severe | Progressive and severe |
| Birth body weight | Same as a normal puppy | Same as a normal baby |
| Neonatal death | 15–30% | Rare |
| Onset of disease | Birth (weak milk sucking or death) to 3 months (activity reduction) | 2–5 years; patients cannot reach motor development milestones |
| Ambulation | Rarely lost | Wheelchair-bound by early teenage |
| Growth retardation | Common | Rare unless the patient is on steroids |
| Kyphosis | Yes | Yes |
| Muscle wasting | Yes | Yes |
| Limb muscle hypertrophy | Cranial sartorius | Calf muscle |
| Histopathology | ||
| Pathology at birth | Minimal | Minimal |
| Limb muscle fibrosis | Yes | Yes |
| Centronucleation | Limited | Limited |
| Limb muscle MRI | ||
| Abductor | N/A | Affected |
| Biceps femoris | Affected | Affected, prominent fat replacement |
| Cranial tibialis | N/A | Relatively spared |
| Gluteus | N/A | Affected |
| Gracilis | Affected | Often preserved |
| Sartorius | Hypertrophic | Relatively spared |
| Semitendinosus | Severely affected | Less affected |
| Heart | ||
| Abnormal ECG | Frequent | Frequent |
| Function reduction | Detectable by 6 months of age | Evident by 16 years of age |
| Death from heart failure | Seldom | More common than used to |
| Cognitive defect | ||
| Prevalence | N/A | One-third of patients |
| Correlation with gene mutation | N/A | Often involves dystrophin C-terminus |
| Correlation with muscle disease | N/A | No correlation |
| Gene therapy tested | ||
| Gene replacement and RNA repair | Full-length dystrophin plasmid, adenovirus minidystrophin, gutted adenovirus full-length dystrophin, AAV microdystrophin, AAV exon skipping, AON exon skipping | Full-length dystrophin plasmid, AAV microdystrophin, AON exon skipping |
| DNA repair | Only been tested in one GRMD dog | N/A |
| Dystrophin independent | N/A | AAV follistatin tested in BMD patients |
AAV, adeno-associated virus; AON, antisense oligonucleotide; BMD, Becker muscular dystrophy; DMD, Duchenne muscular dystrophy; GRMD, golden retriever muscular dystrophy; N/A, no information available.
Dystrophin is a 427 kDa subsarcolemmal protein. It has four major functional domains: the N-terminal, rod, cysteine-rich, and C-terminal domains (Fig. 1). The N-terminal domain binds to γ-actin. The rod domain constitutes of 24 spectrin-like repeats and four intervening hinges. Repeats 1–3 have been suggested to interact with the membrane lipid bilayer. Repeats 11–15 form the second actin-binding domain. Repeats 16 and 17 contain the neuronal nitric oxide synthase (nNOS)-binding motif. Repeats 20–23 interact with microtubule. Hinges are thought to provide flexibility to the dystrophin protein. The cysteine-rich domain and a part of hinge 4 bind to the transmembrane glycoprotein dystroglycan that interacts with laminin in the extracellular matrix (ECM). The C-terminal domain interacts with dystrobrevin and syntrophin. Through interaction with dystroglycan and actin/microtubule, dystrophin links the ECM with the cytoskeleton and provides mechanic stability to muscle cells during contraction. Dystrophin also mediates muscle signaling through its interaction with nNOS, syntrophin, and dystrobrevin. Transmembrane protein sarcoglycans and sarcospan further strengthen the structure connection between the cytoskeleton and the ECM. The dystrophin-associated glycoprotein complex (DGC) formed by dystrophin and its partners provides essential support for normal muscle structure and function (Fig. 1).
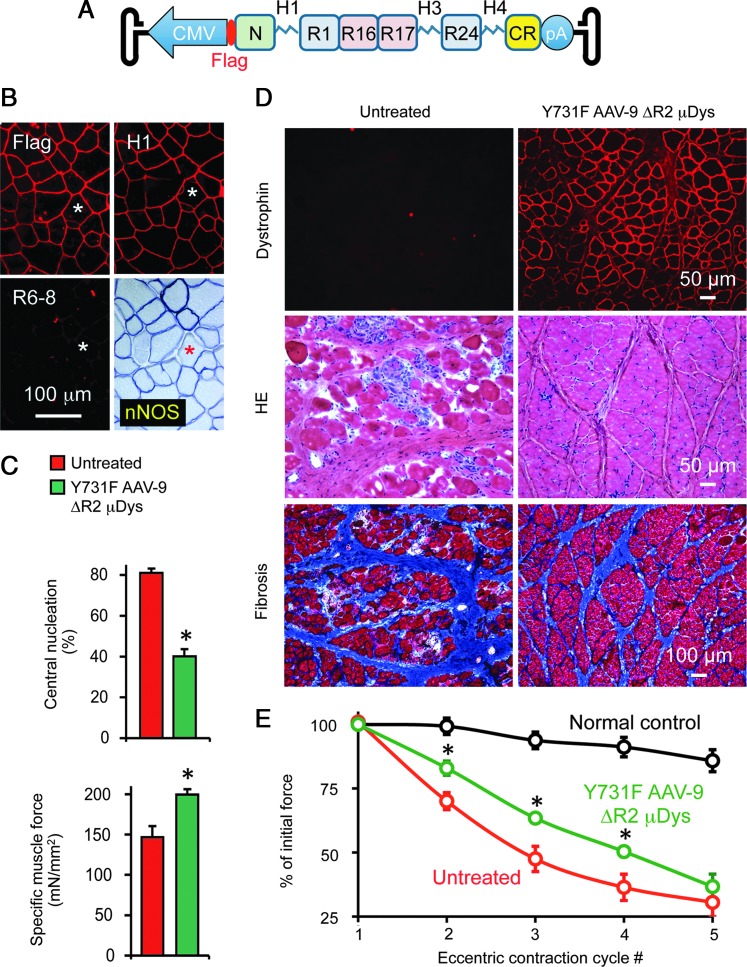
FIG. 1.
Dystrophin, minidystrophin, microdystrophin, and the DGC. Dystrophin and its associated proteins constitute the DGC. The DGC provides mechanical support and signaling function for muscle. Nitric oxide generated by nNOS dilates the vasculature during muscle contraction to meet metabolic needs of the muscle. Minidystrophins are about half the size of full-length dystrophin. The representative ΔH2-R15 minidystrophin protein contains all the known functional domain of the full-length protein (see reference 73). Microdystrophins are about one-third the size of full-length dystrophin. The representative ΔR2-15/ΔR18-19/ΔR20-23/ΔC microdystrophin protein restored sarcolemmal nNOS expression in mdx mice and improved muscle function in adult dystrophic dogs (see reference 40). C, dystrophin C-terminal domain; CR, dystrophin cysteine-rich domain; DGC, dystroglycan complex; ECM, extracellular matrix; H, hinge in the middle rode domain of dystrophin; N, dystrophin N-terminal domain; SG, sarcoglycan complex; SS, sarcospan. Numerical numbers refer to the number of spectrin-like repeats in the dystrophin rod domain. Note, the lipid-binding property of spectrin-like repeats 1–3 is not depicted.
More than 60 dystrophin-deficient animal models have been reported in the literature.8 These models have played a pivotal role in elucidating the biological function of dystrophin and pathogenic mechanisms of DMD. They are also essential for the establishment of the scientific premise for DMD gene therapy.9–11 The majority of the proof-of-principle gene therapy studies are conducted in the mdx mouse, a spontaneous dystrophin-deficient mouse strain with a nonsense mutation in the exon 23 of the dystrophin gene.12,13 Several gene therapy strategies have effectively ameliorated muscle pathology and enhanced muscle force in mdx mice. However, translation to patients has encountered great difficulties. A major reason for the delay in translation is the inherent limitations of the mdx model. For example, mdx mice show very mild clinical symptoms and they cannot accurately model the immune response to the gene therapy vector. The huge body size difference between a mouse and a boy also presents a significant scale-up challenge.
Concurrent with the discovery of the mdx mouse,14 a canine DMD (cDMD) model was established.15,16 This dystrophic dog is a golden retriever. Hence, it is called the golden retriever muscular dystrophy (GRMD) dog. The GRMD dog carries a point mutation (adenine to guanine transition) in the intron 6 of the dystrophin gene. This mutation disrupts normal splicing. As a consequence, exon 7 is excluded from the final messenger RNA. Connection of exons 6 and 8 introduces a frameshift mutation. Dystrophin translation is aborted in exon 8 because of the premature stop codon in the mutated transcript.15 Since then, dystrophin-deficient dogs have been described in many other breeds.15,17–33 The majority of these reports are descriptive case studies. Dystrophin mutations have been determined in some breeds. However, research colonies have only been established in a few breeds (GRMD, beagle with GRMD mutation, corgi with intron 13 insertion, Labrador retriever with intron 19 insertion, and Cavalier King Charles spaniel with exon 50 point mutation).8,34 DMD is a worldwide disease occurring in every race and every country. The genetic background of the patients is highly variable and complex. A pure breed cannot model this heterogeneity. To overcome this shortcoming, we have generated hybrid DMD dogs.35–40 These dogs carry the genetic information from several breeds and thus can better reflect the human condition.
In contrast to mdx mice, dystrophin-deficient dogs share many clinical features of human patients (Table 1). At birth, affected puppies are often weak and cannot compete with littermates for milk. As they reach 2–3 months of age (∼3 years of age in humans), they begin to show signs of limb muscle weakness such as frequent rests, difficulty in walking, and reduced activity. The condition continues to deteriorate. Conspicuous muscular dystrophy is seen around 6 months of age. Typical symptoms at this age include excessive salivation, stunted growth, muscle wasting, abnormal gait, joint contracture, dysphagia, and aspiration pneumonia. By 3 years of age (∼20 years of age in humans), affected dogs either die from cardiorespiratory complications or are euthanized because of poor health condition (Table 1). The cDMD model not only shares symptomatic similarity to human patients, but also has histological lesions resembling those of human patients. For example, limb muscle fibrosis is a common feature in DMD patients. This is observed in cDMD dogs but not in mdx mice. Centronucleation is not a prominent feature in patients because of poor muscle regeneration. This is reflected in cDMD dog muscle but not in mdx muscle.
Besides clinical manifestations and histology, the dog also has the advantage to simulate the immune response observed in DMD patients in gene therapy. Adeno-associated virus (AAV) is the most advanced viral vector for DMD gene therapy. However, AAV-mediated DMD gene therapy has been deterred by the cellular immune response.41,42 For example, muscle injection results in persistent AAV transduction in mdx mice. But nominal transduction is detected in DMD patients following direct injection.43 Similar to human patients, intramuscular injection also induces robust immune rejection in affected dogs (Table 2).44–46 For this reason, the canine model will be very useful to dissect the underlying mechanisms of the immune response and to develop creative strategies to evade immune surveillance.
Table 2.
Cellular Immune Response to Adeno-Associated Virus in the Canine Duchenne Muscular Dystrophy Model
| Serotype | Dog age | Route of delivery | CTL | Comments | References |
|---|---|---|---|---|---|
| AAV-1 | Young adult | Local limb muscle injection and limb perfusion | No | The vector does not express a protein. | Vulin et al. (2012)9 |
| AAV-2 | Not tested in cDMD dogs | Local limb muscle injectiona | Yesa | CTL to either capsid (Wang et al. 2007)44 or transgene (Yuasa et al. 2007)a,45 | Yuasa et al. (2007)a,45, Wang et al. (2007)a,44 |
| AAV-6 | Adult | Local limb muscle injection | Yes | CTL to capsid. CTL is reduced by immune suppression and elimination of the contaminating capsid gene. | Wang et al. (200744,124, 2014129), Shin et al. (2012)37 |
| AAV-6 | Young adult | Local injection to heart | No | The vector does not express a protein. | Bish et al. (2012)102, Barbash et al. (2013)101 |
| AAV-8 | Young adult | Local limb muscle injection and limb perfusion | Yes | Local injection resulted in at least 1 m expression. Intravascular delivery led to at least 2 m expression but there was a clear trend of expression reduction over time. | Ohshima et al. (2008)131 |
| AAV-8 | Young adult | Local limb muscle injection | No | Single-dog study. Expression lasted for at least 2 m. | Koo et al. (2011)133 |
| AAV-8 | Young adult | Limb perfusion | No | The vector does not express a protein. | Le Guiner et al. (2014)100 |
| AAV-9 | Neonatal | Intravenous injection | No? | Severe innate immune response. No CD4+ and CD8+ T cell infiltration. | Kornegay et al. (2010)84 |
| Y731F AAV-9 | Adult | Local limb muscle injection | Yes | CD4+ and CD8+ T cell infiltration despite transient immune suppression. But saturated expression was observed for at least 2 m. | Shin et al. (2013)40 |
Study performed in normal dog muscle.
cDMD, canine DMD; CTL, cytotoxic T lymphocyte.
Scale-up is a significant challenge in human gene therapy. There are issues related to vector purity, procedure safety, vector dose and dose regimen, host response, metabolic rate and body weight of the host, and so on. Large-scale vector production may amplify contaminations that are negligible in small-scale preparations.47 Infusion of trillions of viral particles to a dystrophic boy may lead to unexpected inflammatory and/or immune response and possibly fatal complications.48 A phenotypic large animal model (such as cDMD dogs) will be ideal to address these issues.
Collectively, given the biological and immunological similarities between dystrophic dogs and DMD patients, also given the advantage for scale-up, the cDMD model represents a highly valuable tool for the development and fine-tuning of gene therapy protocols before the human trial.
Current Status of DMD Gene Therapy in the Canine Model
Discovery of the dystrophin gene opens the door to correct DMD by gene therapy.49 The 2.4 mb full-length dystrophin gene contains 79 exons, and it produces a 11.5 kb cDNA. Since dystrophin deficiency underlies DMD pathogenesis, the majority of gene therapy approaches have been centered on the restoration of dystrophin expression. Currently, there are three distinctive classes of approaches, including gene replacement, gene repair, and dystrophin-independent gene therapy. All these approaches have been evaluated in the cDMD model.
Dystrophin replacement therapy in the cDMD model
Direct injection of a plasmid to muscle is perhaps the simplest method. However, it is very inefficient. Only a few dystrophin-positive cells (less than 1%) were observed after intramuscular injection of dystrophin plasmids to either newborn or adult GRMD dogs.50–52 Limited expression and immune cell infiltration were observed following electrotransfer of canine dystrophin plasmids to GRMD muscle.53,54
Adenovirus is the first viral vector used for delivering dystrophin to the canine muscle. Since the first-generation adenoviral vector has a packaging capacity of 8.2 kb,55 investigators used a 6.2 kb, minimized dystrophin gene called the Δ17–48 minigene.56 This minigene is isolated from a very mild patient who was ambulant at age 61.57 A large portion of the rod domain (from exon 17 to 48) is absent in this minidystrophin because of an in-frame deletion. Compared with plasmid injection, adenoviral delivery to neonatal GRMD puppies resulted in significantly much more efficient transduction.58,59 However, minidystrophin expression did not last long because of strong cellular immune responses to the adenoviral vector and human minidystrophin. Application of immune suppressive drug cyclosporine only moderately prolonged gene transfer.58 Gutted adenoviral vector has a carrying capacity up to 35 kb.60 It offers a great opportunity to deliver the full-length cDNA. Gilbert et al. generated a full-length human dystrophin gutted adenoviral vector and tested it in GRMD puppies. Unfortunately, only limit transduction was observed.61
AAV is a 4.7 kb single-stranded DNA virus. AAV-based gene replacement therapy has shown unprecedented clinical success in treating inherited diseases.62 However, there is a major limiting factor to use AAV in DMD gene therapy. The maximum packaging capacity of the AAV vector is 5 kb. This excludes the possibility of delivering the full-length dystrophin cDNA or even a truncated minidystrophin gene with the AAV vector.63 To overcome this hurdle, investigators engineered super small microdystrophin. The microgene carries only one-third of the dystrophin coding sequence (∼4 kb).64,65 Unlike the Δ17–48 minigene, there is no human precedent of a functional microgene. Although patients with super-large in-frame deletions have been identified and expression of micro-size dystrophin has been confirmed in some cases, unfortunately, these patients invariably displayed severe clinical disease.66–69 Since a spontaneous functional microgene does not exist, researchers have built a series of artificial microgenes based on our understanding on dystrophin. To determine the therapeutic potential of the microgene, various configurations of AAV microdystrophin vectors were injected in mdx mice. The majority of these rationally designed microgene vectors significantly protected mouse skeletal muscle and the heart.70–82 Surprisingly, when microdystrophin was initially tested in the cDMD model, it did not deliver therapeutic benefits.83 In one case, the phenotype of the treated dogs even became much worse.84
Over the last few years, several critical but previously unrecognized aspects of dystrophin biology are elucidated. Specifically, it is found that (1) minidystrophins with paired spectrin-like repeats are functionally superior to the ones with odd number repeats82; (2) hinge 2 negatively influences microdystrophin function85; (3) spectrin-like repeats 16 and 17 (R16/17) are required to anchor nNOS to the sarcolemma to prevent functional ischemia73,86; and (4) codon optimization can significantly improve microdystrophin function.87 To determine whether incorporation of these new developments can enhance the performance of the microgene in muscles of large mammals, we generated the ΔR2-15/ΔR18-19/ΔR20-23/ΔC microgene.73,88 This microgene has four spectrin-like repeats. Among which, two of the repeats are R16 and R17. We have also replaced hinge 2 with hinge 3 (Fig. 2). We delivered the codon-optimized canine version of this microgene to mdx mice and dystrophic dogs using tyrosine-engineered AAV-9.40 Systemic gene transfer restored sarcolemmal nNOS expression and enhanced muscle function in mdx mice. (Fig. 2B and C)40
FIG. 2.
Y731F AAV-9-mediated ΔR2-15/ΔR18-19/ΔR20-23/ΔC microdystrophin expression ameliorated skeletal muscle disease in mdx mice and adult DMD dogs. (A) Schematic outline of the ΔR2-15/ΔR18-19/ΔR20-23/ΔC microgene (ΔR2 μDys) AAV vector. The microgene is driven by the ubiquitous CMV promoter. A flag tag is fused to the N-terminal end of the microgene for unequivocal determination of microgene expression. (B) Evaluation of the ΔR2 μDys expression in mdx mice. Representative serial muscle sections were stained for the Flag tag, hinge 1, repeats 6–8, and nNOS activity. *The same myofiber in serial sections. R6–8 is absent in ΔR2 μDys. (C) ΔR2 μDys therapy significantly reduced pathological central nucleation and enhanced specific muscle force in mdx mice. *p<0.05. (D) ΔR2 μDys therapy greatly improved overall histology, reduced muscle inflammation, and fibrosis in adult dystrophic dogs. The untreated and AAV-treated sides are the left and right sides of the extensor carpi ulnaris muscles of the same dog, respectively. (E) ΔR2 μDys therapy significantly preserved muscle force during eccentric contraction in affected dogs. *p<0.05. AAV, adeno-associated virus; DMD, Duchenne muscular dystrophy.
In six random-bred dystrophic dogs (10–28 months old), direct muscle injection resulted in saturated microdystrophin expression and dramatic histological improvement. Macrophage infiltration, fibrosis, and calcification were all greatly reduced (Fig. 2D).40 Importantly, treatment significantly protected the dystrophic muscle from eccentric contraction-induced force loss, a physiological hallmark of DMD (Fig. 2E).40 Our data suggest that microdystrophin may ameliorate muscular dystrophy in a large mammal, potentially in human patients. Our data also suggest that the ΔR2-15/ΔR18-19/ΔR20-23/ΔC microgene is an excellent candidate gene for treating DMD. Interestingly, soon after the publication of our study, Baroncelli et al. discovered a 3-year-old dog with a mild Becker muscular dystrophy (BMD) phenotype.29 On Western blot, the authors detected a micro-size dystrophin migrating at 130–140 kDa. Further investigations may reveal the location of the deletion in this BMD dog and provide critical insight to the design of next-generation microgene.
Dystrophin repair therapy in the cDMD model
The reading-frame rule explains the correlation between the mutation in the dystrophin gene and the clinical presentation in patients.9,90 Frame-shift mutation results in a complete loss of dystrophin and severe phenotype. However, patients with in-frame mutation often express a smaller but partially functional protein. These patients manifest a much milder clinical disease and are classified as BMD. The reading-frame rule suggests that lethal DMD can be converted to less severe BMD if an out-of-frame transcript can be converted to an in-frame transcript. Based on this theory, investigators have developed exon skipping. In this strategy, antisense oligonucleotides (AONs) are used to modulate the splicing machinery so that certain exons are excluded from the mRNA. The modified mRNA produces an internally deleted dystrophin protein similar to that observed in BMD patients.
Initial exon skipping studies were conducted using 2′-O-methylated phosphorothioate (2OMe-PS) and phosphorodiamidate morpholino oligomers (PMO). These AONs worked very well in cultured muscle cells obtained from different breeds of dystrophic dogs.19,91,92 Local injection also resulted in exon skipping in GRMD and beagle-background GRMD dogs.92,93 While a single AON seems sufficient for exon skipping in myoblasts,19,92 interestingly, a cocktail of AONs is required for efficient exon skipping in canine muscle in vivo.92 To test whole-body exon skipping, Yokota et al. delivered the PMO cocktail to beagle-background GRMD dogs by intravenous injection.92 Systemic therapy resulted in widespread dystrophin expression and clinical improvement. Short half-life and limited tissue penetration are the major limitations of 2OMe-PS and PMO. To overcome these obstacles, various modified AONs are developed.94 These modified AONs are conjugated with cell-penetrating peptides or polymers. Conjugation significantly enhances the tissue uptake of AONs in mdx mice. However, so far only the vivo-morpholino (PMO conjugated with the octa-guanidine dendrimer) has been tested in the dog model by local injection.95 As expected, the vivo-morpholino resulted in robust, much persistent exon skipping.95
An alternative approach to improve exon skipping is to deliver the AON with an AAV vector.96 In this approach, the U7 promoter is used to drive the expression of an AON that is fused to the U7 small nuclear RNA (snRNA).97,98 The snRNA allows efficient targeting of the AON to the spliceosome in the nucleus. AAV allows efficient tissue penetration and continuous production of the AON. Two different studies evaluated the AAV U7 approach for skeletal muscle gene therapy in GRMD dogs. Vulin et al. co-expressed two AONs (one for exon 6 skipping and the other for exon 8 skipping) in one vector using AAV-1 (AAV1-U7E6/8).99 Exclusion of exons 6 and 8 results in an in-frame Δ6–8 transcript). Investigators performed local injection and forelimb perfusion in six 5–15-month-old dogs and multimuscle injection in the hindlimb of four 3-week-old puppies. Efficient dystrophin restoration (20–80% positive myofibers) was observed up to 10 months after injection. Treatment reduced the number of calcified myofibers and improved parameters of magnetic resonance imaging (MRI). Further, specific muscle force was enhanced in treated puppies.99 Le Guiner et al. delivered the same set of AONs to fifteen 3–4-month-old juvenile GRMD dogs by forelimb perfusion.100 Instead of AAV-1, the authors used AAV-8 (AAV8-U7E6/8). At 2–3.5 months after injection, the authors observed high-level dystrophin expression (10–80% positive myofibers), reduced regeneration and fibrosis (but no change in inflammation and calcification), and improvement in MRI parameters. In dogs with ≥40% dystrophin-positive myofibers, treatment also prevented progressive force decline.100
Two independent groups examined AAV-6-mediated exon skipping in the heart of GRMD dogs using exactly the same U7E6/8 construct developed by Vulin et al.99,101,102 AAV-6 U7E6/8 was delivered by multiple transendocardial injection in young adult dogs (5–13 months old). Both groups achieved expected exon skipping and dystrophin restoration. Bish et al. followed 5 dogs for 13 months and observed a clear reduction of myocardial fibrosis and an improvement of the peak circumferential strain in cardiac MRI.102 Barbash et al. followed 5 treated dogs for 3 months and demonstrated the stabilization of left ventricular ejection fraction by cardiac MRI.101
Besides exon skipping, gene repair therapy can also be used to correct the mutation itself. Oligonucleotide-mediated gene correction and nuclease-based gene editing are two primary approaches. However, these DNA-level gene repair strategies are largely unexplored in the canine model. So far, only one study tried a chimeric RNA/DNA oligonucleotide in one 6-week-old GRMD puppy.103 The authors showed evidence of gene correction up to 1 year after therapy.
Dystrophin-independent gene therapy in the cDMD model
A number of cellular proteins have been shown to modify the dystrophic phenotype.104 These include utrophin, α7β1-integrin, myostatin, insulin-like growth factor-1, cytotoxic T cell GalNAc transferase, sarcoplasmic reticulum calcium ATPase (SERCA), peroxisome proliferator-activated receptor gamma coactivator 1α, osteopontin, and latent transforming growth factor-β (TGF-β) binding protein 4. Many of these have been confirmed in mouse studies by gene knock, transgenic overexpression, and AAV-mediated gene transfer. However, only two of the modifiers have been tested in the cDMD model.
Utrophin is a dystrophin homologous protein. It shares structural and functional similarity to dystrophin. A minimized utrophin has been developed based on the Δ17–48 minidystrophin gene. Cerletti et al. injected the miniutrophin adenoviral vector to 2-day-old GRMD puppies.105 In immune-suppressed animals, they achieved a 15% transduction efficiency and significant reduction of fibrosis at 2 months after treatment. The highly abbreviated microutrophin gene has also been generated recently.86,106 Therapeutic effect of microutrophin remains to be tested in affected dogs.
Myostatin is a TGF-β family muscle growth regulator.107 Myostatin inhibition has been shown to increase muscle size and reduce myopathy in mdx mice.108 Spontaneous mutation in the myostatin gene also leads to muscle hypertrophy in whippet dogs.109 To determine whether myostatin inhibition can ameliorate muscle disease in GRMD dogs, Bish et al. expressed a secreted dominant negative myostatin peptide in the liver of four 9–10-month-old GRMD dogs using AAV-8.110 Thirteen months after injection, they observed the expected increase in muscle mass. Furthermore, treatment reduced the serum creatine kinase level and muscle fibrosis. More recently, Cotten et al. crossed GRMD dogs with the myostatin-deficient whippets.111 The myostatin level was reduced in myostatin heterozygous GRMD dogs. Surprisingly, these dogs displayed a more severe phenotype. The discrepancy between Bish et al.'s study and Cotten et al.'s study remains to be explained. However, it should be pointed out that an increase in the myofiber size could be counterproductive in dystrophin-null muscle because the higher surface-to-volume ratio may result in higher sarcolemmal stress during contraction.
SERCA overexpression protects heart and muscle in rodent models of muscular dystrophy.112,116 AAV-mediated SERCA expression has also improved cardiac function in various canine models of heart failure.117,118 Based on these results, it is possible that AAV SERCA therapy may also reduce muscle disease in cDMD dogs.
Systemic AAV Delivery in Dogs
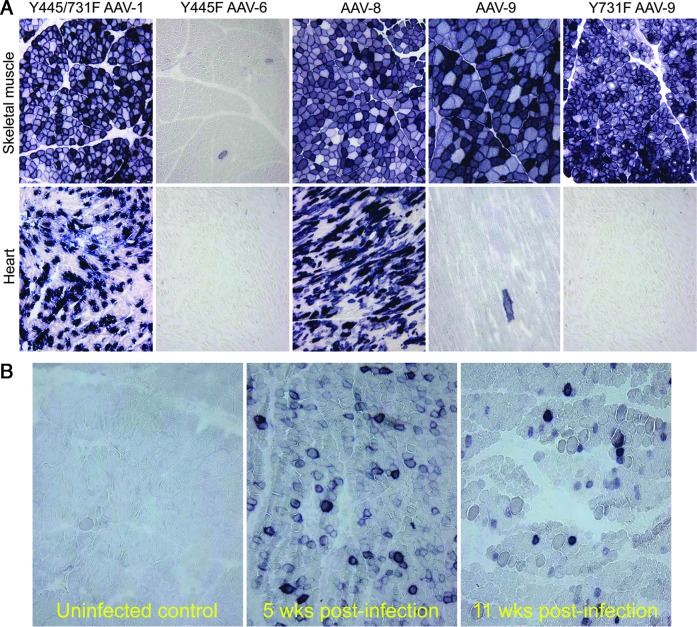
DMD affects all muscles in the body. Only whole-body gene therapy can truly change the outcome of the disease. Systemic gene delivery has been established in mice using AAV-1, 6, 8, and 9 since 2004.72,119,120 However, only a few studies have evaluated intravascular AAV delivery in dogs. We demonstrated the first successful systemic gene transfer in newborn dogs in 2008 using AAV-9 (Fig. 3).46 AAV-9 has been considered as a “cardiotropic” vector because of its efficient myocardial transduction in rodents.120,121 Unexpectedly, the dog heart was barely transduced by AAV-9.46 Over the last few years, we tested additional AAV serotypes and identified AAV-8 and Y445/731F AAV-1 as the preferred vectors for whole-body (heart and skeletal muscle) gene transfer in neonatal dogs (Fig. 3A).122,123 We also tested AAV-6. Interestingly, little muscle transduction was observed.123 This is in sharp contrast to what has been shown with direct AAV-6 injection in dog muscle.101,102,124 We believe that the difference is likely because of (1) the high-level preexisting AAV-6 neutralization antibody in the canine circulation,36,125 or (2) the presence of galectin 3 binding protein (G3BP) in dog serum.126 It has been shown that G3BP can cause AAV-6 particle aggregation and compromise transduction.126
FIG. 3.
Systemic AAV delivery in neonatal dogs. (A) Comparison of five different AAV variants in normal neonatal dogs. AAV-8 and tyrosine-engineered Y445/731F AAV-1 resulted in robust transduction in both skeletal muscle and the heart. AAV-9 and its variant Y731 AAV-9 effectively transduced only skeletal muscle. Y445F AAV-6 barely transduced striated muscle in neonatal dogs. (B) Evaluation of systemic AAV-9 gene transfer in neonatal affected dogs. An AAV-9 alkaline phosphatase reporter gene vector was injected to a 2-day-old affected puppy through the jugular vein. Efficient transduction was observed at 5 weeks postinfection. Transduction was still observed at the 11 weeks of age. However, expression appeared reduced.
Few studies have evaluated bodywide gene transfer in the cDMD model (Fig. 3B). Kornegay et al. delivered an AAV-9 microdystrophin vector to newborn GRMD puppies via the jugular vein.84 Injection resulted in widespread transduction. Unfortunately, these puppies also developed a fulminant inflammatory response and had to be euthanized. We recently explored systemic AAV-9 injection in adolescent dystrophic dogs.127 Gene transfer resulted in bodywide transduction in striated muscles without any untoward reaction.
Cellular Immune Response to AAV-Mediated Gene Transfer in the Canine Model
Host immune response is undoubtedly one of the greatest hurdles in gene therapy. It is influenced by the viral capsid, vector dose and purity, method of vector production and purification, delivery route, animal age and preexisting immunity, expression cassette (such as the promoter and microRNA142-3p-binding site), transgene product, regime of immune suppression, the species tested, and even the species origin of the transgene. Many studies have examined AAV immune reaction in normal and dystrophic dog muscle (Table 2). While it is generally thought that AAV can induce the cytotoxic T lymphocyte (CTL) response in canine muscle, there are important controversies that remain to be reconciled.
Vulin et al. found that intramuscular (3.5×1012 vg) or intravascular (1.4×1013 vg) injection of an AAV-1 vector that does not express a protein did not elicit the CTL response in GRMD dogs.99 However, Wang et al. detected a strong T cell response to AAV-1 capsid in normal dog muscle after injection of a much lower dose (5×1011 vg) of a canine factor IX vector.128
Two different groups reported cellular immune reaction to AAV-2 following direct muscle injection in normal dogs.44,45 Results of Yuasa et al. suggest that the immune response is against the transgene product (LacZ).45 But Wang et al. showed that the immune response is independent of the transgene product.44 Wang et al. observed robust mononuclear cell infiltration even with empty capsids.44
AAV-6 has been shown to induce capsid-specific T cell infiltration in skeletal muscle of normal and affected dogs following direction injection.44,124 A similar capsid-specific immune response was detected when AAV-6 was injected to the heart of nondystrophic dogs.117 In support of these observations, transient immune suppression significantly prolonged transgene expression.37,117,124 Further, elimination of the contaminating AAV-6 capsid gene reduced the immune response.129 Surprisingly, transendocardial injection of AAV-6 to normal or affected dog heart did not induce T cell reaction.101,102,130
Initial study with AAV-8 revealed transient expression and T cell infiltration in dog skeletal muscle irrespective of muscular dystrophy.131 Interestingly, vascular delivery seemed less immunogenic and resulted in somewhat longer expression.131 Since then, several groups have tested AAV-8 in normal132 and affected dogs.100,133,134 In contrast to the initial report by Ohshima et al., these later studies did not detect cellular immune reaction. Koo et al. expressed a canine-microdystrophin in a 9-week-old affected dog without immune suppression.133 Robust expression lasted for at least 2 months without the evidence of immune rejection. Three groups performed limb perfusion in normal,132 GRMD,100 and myotubularin myopathy dogs134 in the absence of immune suppressive drugs.100 Persistent transduction was observed up to 1 year (the longest time point) without any signs of the CTL response.
It is currently unclear why the observed immune responses to AAV-8 are different between Ohshima et al.'s study and other studies. It is possible that the use of the tissue-specific promoter and the species-specific transgene may have played a role. Ohshima et al. used a ubiquitous promoter, while Childers et al. and Koo et al. used the muscle-specific promoter. Ohshima et al. expressed LacZ and human microdystrophin, while Childers et al., Koo et al., and Qiao et al. expressed canine proteins. In the Le Guiner et al.'s study, no protein product was expressed. Regarding the species specificity of the transgene, an AAV-6 study also reached the same conclusion.124 Recently, several laboratories compared the immunity of AAV-8 to that of other AAV serotypes (AAV-1, 2, and 5 and rh32.33).124,131,135–140 Interestingly, all these studies suggest that AAV-8 is less immunogenic. Future mechanistic studies may reveal the molecular underpinning of the unique immune privilege demonstrated by AAV-8.
Direct injection of AAV-9 evokes a strong cellular immune response in adult dog muscle.46 But this response is absent in neonatal dogs.46 We recently found that local delivery of a CMV-driving canine microgene also induced CD4+ and CD8+ T cell infiltration in adult DMD dogs despite the use of transient immune suppression.40 Nevertheless, we still observed robust expression for at least 2 months (the end of the study). So far, systemic AAV-9 injection has only been evaluated in newborn puppies. We achieved high-level persistent transduction in normal puppies.46,123 However, when the same technique was used in affected puppies, investigators observed a serious innate immune response that was so severe to require the termination of the study.84 The reason for this unexpected reaction is not clear. One possibility could be the use of a ubiquitous promoter and the human microgene.
Summary and Perspectives
Mdx mice and cDMD dogs were discovered simultaneously 30 years ago. However, the research use of the canine model has significantly lagged behind that of mdx mice. Using “mdx, gene therapy” and “GRMD, gene therapy” as key words, 620 and 27 records are retrieved in PubMed. That is to say that, for every 100 studies performed in mdx mice, there are only ∼4–5 studies performed using the canine model. Besides the high cost in maintaining these severely disabled cDMD dogs, the lack of a comprehensive and accurate characterization of the model also hinders the use of the cDMD model. There are several issues in this regard. First, a large-size population study on the natural history of the cDMD model remains to be conducted. Since most colonies only have a limited number of dogs, collaborative efforts from different laboratories will be needed to establish a solid baseline. Second, we need to develop standardized assays to reliably determine the outcomes of experimental interventions. This is especially important for cross-colony comparison. Numerous tools and protocols exist to study mouse muscle force. However, there aren't many options for dog muscle function evaluation. In fact, until recently, there is even no physiological assay to measure the contractility of a single dog muscle.38 Misinterpretation of the data has also been noticed because of insufficient understanding on dog-specific reagents (such as the antibody).141,142
In terms of gene therapy, we believe that the cDMD model will provide critical insights on issues that are very difficult to address or cannot be addressed in mdx mice, for example, the amount of the AAV vector needed to achieve bodywide transduction in a large mammal. Another important issue is the minimum level of dystrophin expression needed to protect muscle in a large mammal. Three recent AAV exon skipping studies have offered some clues. Vulin et al. found that 20–50% dystrophin-positive cells might be sufficient to improve muscle force.99 Le Guiner et al. reported that a correction of 33%, 35%, and 40% of myofibers might result in histopathology amelioration, MRI improvement, and muscle function preservation, respectively.100 Bish et al. showed that a level of 15–20% of normal dystrophin might offer some heart protection.102 This value is quite close to what has been observed in mdx mice.143,144 However, in the case microdystrophin, the level seems different between mice and dogs. Takeda and colleagues found that microdystrophin expression in 20% myofibers could not protect dog muscle although the same level expression improved muscle function in mdx mice.79,131 Besides answering these basic gene therapy questions, the cDMD model will also be essential to determine whether strategies that are shown to protect mouse muscle can or cannot treat muscular dystrophy in large mammals. New technologies that have (such as the use of the dual-AAV system to express a 6–8 kb minidystrophin gene)145–147 or have not (such as the use of nuclease to correct dystrophin gene mutation in vivo)148–150 been tested in mice may ultimately require corroboration in the canine model.
As we move forward from treating mdx mice to treating affected large mammals, great caution should be taken to not overinterpret the data. Results from canine studies may inform the design of the clinical trial, but they cannot fully predict what will happen in human patients because of many species-related differences. Nevertheless, a large therapeutic margin in the cDMD model may more likely translate to DMD patients.
Acknowledgments
DMD research in the Duan lab is supported by the National Institutes of Health (AR-49419, HL-91883), Department of Defense (MD130014), Muscular Dystrophy Association, Parent Project Muscular Dystrophy, Jesse's Journey—The Foundation for Gene and Cell Therapy, Hope for Javier, Kansas City Area Life Sciences Institute, and the University of Missouri.
Author Disclosure Statement
D.D. is a member of the scientific advisory board for Solid GT, a subsidiary of Solid Ventures.
References
- 1.Hoffman EP, Brown RH, Jr., Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 1987;51:919–928 [DOI] [PubMed] [Google Scholar]
- 2.Koenig M, Hoffman EP, Bertelson CJ, et al. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell 1987;50:509–517 [DOI] [PubMed] [Google Scholar]
- 3.Mendell JR, Lloyd-Puryear M. Report of MDA muscle disease symposium on newborn screening for Duchenne muscular dystrophy. Muscle Nerve 2013;48:21–26 [DOI] [PubMed] [Google Scholar]
- 4.Emery AEH, Muntoni F. Duchenne Muscular Dystrophy, 3rd ed. (Oxford University Press, New York, NY: ). 2003 [Google Scholar]
- 5.Kinnett K, Cripe LH. Transforming Duchenne care: meeting 25–26 June 2012, Ft. Lauderdale, Florida, USA. Neuromuscul Disord 2013;23:690–695 [DOI] [PubMed] [Google Scholar]
- 6.Bushby K, Finkel R, Birnkrant DJ, et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: implementation of multidisciplinary care. Lancet Neurol 2010;9:177–189 [DOI] [PubMed] [Google Scholar]
- 7.Rodino-Klapac LR, Chicoine LG, Kaspar BK, Mendell JR. Gene therapy for Duchenne muscular dystrophy: expectations and challenges. Arch Neurol 2007;64:1236–1241 [DOI] [PubMed] [Google Scholar]
- 8.McGreevy JW, Hakim CH, McIntosh MA, Duan D. Animal models of Duchenne muscular dystrophy: from basic mechanisms to gene therapy. Dis Model Mech 2015. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duan D. Duchenne muscular dystrophy gene therapy: lost in translation? Res Rep Biol 2011;2:31–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foster H, Popplewell L, Dickson G. Genetic therapeutic approaches for Duchenne muscular dystrophy. Hum Gene Ther 2012;23:676–687 [DOI] [PubMed] [Google Scholar]
- 11.Chamberlain JS. Gene therapy of muscular dystrophy. Hum Mol Genet 2002;11:2355–2362 [DOI] [PubMed] [Google Scholar]
- 12.Banks GB, Chamberlain JS. The value of mammalian models for Duchenne muscular dystrophy in developing therapeutic strategies. Curr Top Dev Biol 2008;84:431–453 [DOI] [PubMed] [Google Scholar]
- 13.Sicinski P, Geng Y, Ryder-Cook AS, et al. The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science 1989;244:1578–1580 [DOI] [PubMed] [Google Scholar]
- 14.Bulfield G, Siller WG, Wight PA, Moore KJ. X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc Natl Acad Sci USA 1984;81:1189–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooper BJ, Winand NJ, Stedman H, et al. The homologue of the Duchenne locus is defective in X-linked muscular dystrophy of dogs. Nature 1988;334:154–156 [DOI] [PubMed] [Google Scholar]
- 16.Valentine BA, Cooper BJ, Cummings JF, deLahunta A. Progressive muscular dystrophy in a golden retriever dog: light microscope and ultrastructural features at 4 and 8 months. Acta Neuropathol (Berl) 1986;71:301–310 [DOI] [PubMed] [Google Scholar]
- 17.Shimatsu Y, Yoshimura M, Yuasa K, et al. Major clinical and histopathological characteristics of canine X-linked muscular dystrophy in Japan, CXMDJ. Acta Myol 2005;24:145–154 [PubMed] [Google Scholar]
- 18.Valentine BA, Cooper BJ, de Lahunta A, et al. Canine X-linked muscular dystrophy—an animal model of Duchenne muscular dystrophy: clinical studies. J Neurol Sci 1988;88:69–81 [DOI] [PubMed] [Google Scholar]
- 19.Walmsley GL, Arechavala-Gomeza V, Fernandez-Fuente M, et al. A Duchenne muscular dystrophy gene hot spot mutation in dystrophin-deficient cavalier king charles spaniels is amenable to exon 51 skipping. PLoS One 2010;5:e8647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schatzberg SJ, Olby NJ, Breen M, et al. Molecular analysis of a spontaneous dystrophin ‘knockout’ dog. Neuromuscul Disord 1999;9:289–295 [DOI] [PubMed] [Google Scholar]
- 21.Kornegay JN, Tuler SM, Miller DM, Levesque DC. Muscular dystrophy in a litter of golden retriever dogs. Muscle Nerve 1988;11:1056–1064 [DOI] [PubMed] [Google Scholar]
- 22.Klarenbeek S, Gerritzen-Bruning MJ, Rozemuller AJ, van der Lugt JJ. Canine X-linked muscular dystrophy in a family of Grand Basset Griffon Vendeen dogs. J Comp Pathol 2007;137:249–252 [DOI] [PubMed] [Google Scholar]
- 23.Jones BR, Brennan S, Mooney CT, et al. Muscular dystrophy with truncated dystrophin in a family of Japanese Spitz dogs. J Neurol Sci 2004;217:143–149 [DOI] [PubMed] [Google Scholar]
- 24.Bergman RL, Inzana KD, Monroe WE, et al. Dystrophin-deficient muscular dystrophy in a Labrador retriever. J Am Anim Hosp Assoc 2002;38:255–261 [DOI] [PubMed] [Google Scholar]
- 25.Paola JP, Podell M, Shelton GD. Muscular dystrophy in a miniature Schnauzer. Prog Vet Neurol 1993;4:14–18 [Google Scholar]
- 26.Wieczorek LA, Garosi LS, Shelton GD. Dystrophin-deficient muscular dystrophy in an old English sheepdog. Vet Rec 2006;158:270–273 [DOI] [PubMed] [Google Scholar]
- 27.Wetterman CA, Harkin KR, Cash WC, et al. Hypertorphic muscular dystrophy in a young dog. J Am Vet Med Assoc 2000;216:878–881 [DOI] [PubMed] [Google Scholar]
- 28.Baltzer WI, Calise DV, Levine JM, et al. Dystrophin-deficient muscular dystrophy in a Weimaraner. J Am Anim Hosp Assoc 2007;43:227–232 [DOI] [PubMed] [Google Scholar]
- 29.Baroncelli AB, Abellonio F, Pagano TB, et al. Muscular dystrophy in a dog resembling human Becker muscular dystrophy. J Comp Pathol 2014;150:429–433 [DOI] [PubMed] [Google Scholar]
- 30.Ito D, Kitagawa M, Jeffery N, et al. Dystrophin-deficient muscular dystrophy in an Alaskan malamute. Vet Rec 2011;169:127. [DOI] [PubMed] [Google Scholar]
- 31.Smith BF, Yue Y, Woods PR, et al. An intronic LINE-1 element insertion in the dystrophin gene aborts dystrophin expression and results in Duchenne-like muscular dystrophy in the corgi breed. Lab Invest 2011;91:216–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith BF, Kornegay JN, Duan D. Independent canine models of Duchenne muscular dystrophy due to intronic insertions of repetitive DNA. Mol Ther 2007;15:S51 [Google Scholar]
- 33.Beltran E, Shelton GD, Guo LT, et al. Dystrophin-deficient muscular dystrophy in a Norfolk terrier. J Small Anim Pract 2014. [Epub ahead of print]; doi: 10.1111/jsap.12292 [DOI] [PubMed] [Google Scholar]
- 34.Kornegay JN, Bogan JR, Bogan DJ, et al. Canine models of Duchenne muscular dystrophy and their use in therapeutic strategies. Mam Genome 2012;23:85–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fine DM, Shin JH, Yue Y, et al. Age-matched comparison reveals early electrocardiography and echocardiography changes in dystrophin-deficient dogs. Neuromuscul Disord 2011;21:453–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shin JH, Yue Y, Smith B, Duan D. Humoral immunity to AAV-6, 8, and 9 in normal and dystrophic dogs. Hum Gene Ther 2012;23:287–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shin JH, Yue Y, Srivastava A, et al. A simplified immune suppression scheme leads to persistent micro-dystrophin expression in Duchenne muscular dystrophy dogs. Hum Gene Ther 2012;23:202–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang HT, Shin JH, Hakim CH, et al. Dystrophin deficiency compromises force production of the extensor carpi ulnaris muscle in the canine model of Duchenne muscular dystrophy. PLoS One 2012;7:e44438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shin JH, Greer B, Hakim CH, et al. Quantitative phenotyping of Duchenne muscular dystrophy dogs by comprehensive gait analysis and overnight activity monitoring. PLoS One 2013;8:e59875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shin JH, Pan X, Hakim CH, et al. Microdystrophin ameliorates muscular dystrophy in the canine model of Duchenne muscular dystrophy. Mol Ther 2013;21:750–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Basner-Tschakarjan E, Mingozzi F. Cell-mediated immunity to AAV vectors, evolving concepts and potential solutions. Front Immunol 2014;5:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mendell JR, Rodino-Klapac L, Sahenk Z, et al. Gene therapy for muscular dystrophy: lessons learned and path forward. Neurosci Lett 2012;527:90–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mendell JR, Campbell K, Rodino-Klapac L, et al. Dystrophin immunity in Duchenne's muscular dystrophy. N Engl J Med 2010;363:1429–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Z, Allen JM, Riddell SR, et al. Immunity to adeno-associated virus-mediated gene transfer in a random-bred canine model of Duchenne muscular dystrophy. Hum Gene Ther 2007;18:18–26 [DOI] [PubMed] [Google Scholar]
- 45.Yuasa K, Yoshimura M, Urasawa N, et al. Injection of a recombinant AAV serotype 2 into canine skeletal muscles evokes strong immune responses against transgene products. Gene Ther 2007;14:1249–1260 [DOI] [PubMed] [Google Scholar]
- 46.Yue Y, Ghosh A, Long C, et al. A single intravenous injection of adeno-associated virus serotype-9 leads to whole body skeletal muscle transduction in dogs. Mol Ther 2008;16:1944–1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wright JF. Manufacturing and characterizing AAV-based vectors for use in clinical studies. Gene Ther 2008;15:840–848 [DOI] [PubMed] [Google Scholar]
- 48.Wilson JM. Progress in the commercial-scale production of adeno-associated viral vectors. Hum Gene Ther 2009;20:695. [DOI] [PubMed] [Google Scholar]
- 49.Kunkel LM. 2004 William Allan award address. cloning of the DMD gene. Am J Hum Genet 2005;76:205–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Howell JM, Fletcher S, O'Hara A, et al. Direct dystrophin and reporter gene transfer into dog muscle in vivo. Muscle Nerve 1998;21:159–165 [DOI] [PubMed] [Google Scholar]
- 51.Thioudellet C, Blot S, Squiban P, et al. Current protocol of a research phase I clinical trial of full-length dystrophin plasmid DNA in Duchenne/Becker muscular dystrophies. Part I: rationale. Neuromuscul Disord 2002;12:S49–S51 [DOI] [PubMed] [Google Scholar]
- 52.Duan D. Myodys, a full-length dystrophin plasmid vector for Duchenne and Becker muscular dystrophy gene therapy. Curr Opin Mol Ther 2008;10:86–94 [PubMed] [Google Scholar]
- 53.Pichavant C, Chapdelaine P, Cerri DG, Bizario JC, Tremblay JP. Electrotransfer of the full-length dog dystrophin into mouse and dystrophic dog muscles. Hum Gene Ther 2010;21:1591–1601 [DOI] [PubMed] [Google Scholar]
- 54.Pichavant C, Chapdelaine P, Cerri DG, et al. Expression of dog microdystrophin in mouse and dog muscles by gene therapy. Mol Ther 2010;18:1002–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Danthinne X, Imperiale MJ. Production of first generation adenovirus vectors: a review. Gene Ther 2000;7:1707–1714 [DOI] [PubMed] [Google Scholar]
- 56.Ragot T, Vincent N, Chafey P, et al. Efficient adenovirus-mediated transfer of a human minidystrophin gene to skeletal muscle of mdx mice. Nature 1993;361:647–650 [DOI] [PubMed] [Google Scholar]
- 57.England SB, Nicholson LV, Johnson MA, et al. Very mild muscular dystrophy associated with the deletion of 46% of dystrophin. Nature 1990;343:180–182 [DOI] [PubMed] [Google Scholar]
- 58.Howell JM, Lochmuller H, O'Hara A, et al. High-level dystrophin expression after adenovirus-mediated dystrophin minigene transfer to skeletal muscle of dystrophic dogs: prolongation of expression with immunosuppression. Hum Gene Ther 1998;9:629–634 [DOI] [PubMed] [Google Scholar]
- 59.O'Hara AJ, Howell JM, Taplin RH, et al. The spread of transgene expression at the site of gene construct injection. Muscle Nerve 2001;24:488–495 [DOI] [PubMed] [Google Scholar]
- 60.Parks RJ, Graham FL. A helper-dependent system for adenovirus vector production helps define a lower limit for efficient DNA packaging. J Virol 1997;71:3293–3298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gilbert R, Nalbantoglu J, Howell JM, et al. Dystrophin expression in muscle following gene transfer with a fully deleted (“gutted”) adenovirus is markedly improved by trans-acting adenoviral gene products. Hum Gene Ther 2001;12:1741–1755 [DOI] [PubMed] [Google Scholar]
- 62.Mingozzi F, High KA. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat Rev Genet 2011;12:341–355 [DOI] [PubMed] [Google Scholar]
- 63.Lai Y, Yue Y, Duan D. Evidence for the failure of adeno-associated virus serotype 5 to package a viral genome>or=8.2 kb. Mol Ther 2010;18:75–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scott J, Li S, Harper S, et al. Viral vectors for gene transfer of micro-, mini-, or full-length dystrophin. Neuromuscul Disord 2002;12:S23. [DOI] [PubMed] [Google Scholar]
- 65.Dickson G, Roberts M, Wells D, Fabb S. Recombinant micro-genes and dystrophin viral vectors. Neuromuscul Disord 2002;12:S40. [DOI] [PubMed] [Google Scholar]
- 66.Arikawa-Hirasawa E, Koga R, Tsukahara T, et al. A severe muscular dystrophy patient with an internally deleted very short (110 kD) dystrophin: presence of the binding site for dystrophin-associated glycoprotein (DAG) may not be enough for physiological function of dystrophin. Neuromuscul Disord 1995;5:429–438 [DOI] [PubMed] [Google Scholar]
- 67.Den Dunnen JT, Grootscholten PM, Bakker E, et al. Topography of the Duchenne muscular dystrophy (DMD) gene: FIGE and cDNA analysis of 194 cases reveals 115 deletions and 13 duplications. Am J Hum Genet 1989;45:835–847 [PMC free article] [PubMed] [Google Scholar]
- 68.Fanin M, Freda MP, Vitiello L, et al. Duchenne phenotype with in-frame deletion removing major portion of dystrophin rod: threshold effect for deletion size? Muscle Nerve 1996;19:1154–1160 [DOI] [PubMed] [Google Scholar]
- 69.Koenig M, Beggs AH, Moyer M, et al. The molecular basis for Duchenne versus Becker muscular dystrophy: correlation of severity with type of deletion. Am J Hum Genet 1989;45:498–506 [PMC free article] [PubMed] [Google Scholar]
- 70.Yue Y, Li Z, Harper SQ, et al. Microdystrophin gene therapy of cardiomyopathy restores dystrophin-glycoprotein complex and improves sarcolemma integrity in the mdx mouse heart. Circulation 2003;108:1626–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gregorevic P, Allen JM, Minami E, et al. rAAV6-microdystrophin preserves muscle function and extends lifespan in severely dystrophic mice. Nat Med 2006;12:787–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gregorevic P, Blankinship MJ, Allen JM, et al. Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat Med 2004;10:828–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lai Y, Thomas GD, Yue Y, et al. Dystrophins carrying spectrin-like repeats 16 and 17 anchor nNOS to the sarcolemma and enhance exercise performance in a mouse model of muscular dystrophy. J Clin Invest 2009;119:624–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gregorevic P, Blankinship MJ, Allen JM, Chamberlain JS. Systemic microdystrophin gene delivery improves skeletal muscle structure and function in old dystrophic mdx mice. Mol Ther 2008;16:657–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bostick B, Yue Y, Lai Y, et al. Adeno-associated virus serotype-9 microdystrophin gene therapy ameliorates electrocardiographic abnormalities in mdx mice. Hum Gene Ther 2008;19:851–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bostick B, Shin J-H, Yue Y, Duan D. AAV-microdystrophin therapy improves cardiac performance in aged female mdx mice. Mol Ther 2011;19:1826–1832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu M, Yue Y, Harper SQ, et al. Adeno-associated virus-mediated microdystrophin expression protects young mdx muscle from contraction-induced injury. Mol Ther 2005;11:245–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yue Y, Liu M, Duan D. C-terminal truncated microdystrophin recruits dystrobrevin and syntrophin to the dystrophin-associated glycoprotein complex and reduces muscular dystrophy in symptomatic utrophin/dystrophin double knock-out mice. Mol Ther 2006;14:79–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yoshimura M, Sakamoto M, Ikemoto M, et al. AAV vector-mediated microdystrophin expression in a relatively small percentage of mdx myofibers improved the mdx phenotype. Mol Ther 2004;10:821–828 [DOI] [PubMed] [Google Scholar]
- 80.Fabb SA, Wells DJ, Serpente P, Dickson G. Adeno-associated virus vector gene transfer and sarcolemmal expression of a 144 kDa micro-dystrophin effectively restores the dystrophin-associated protein complex and inhibits myofibre degeneration in nude/mdx mice. Hum Mol Genet 2002;11:733–741 [DOI] [PubMed] [Google Scholar]
- 81.Wang B, Li J, Xiao X. Adeno-associated virus vector carrying human minidystrophin genes effectively ameliorates muscular dystrophy in mdx mouse model. Proc Natl Acad Sci USA 2000;97:13714–13719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Harper SQ, Hauser MA, DelloRusso C, et al. Modular flexibility of dystrophin: implications for gene therapy of Duchenne muscular dystrophy. Nat Med 2002;8:253–261 [DOI] [PubMed] [Google Scholar]
- 83.Sampaolesi M, Blot S, D'Antona G, et al. Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature 2006;444:574–579 [DOI] [PubMed] [Google Scholar]
- 84.Kornegay JN, Li J, Bogan JR, et al. Widespread muscle expression of an AAV9 human mini-dystrophin vector after intravenous injection in neonatal dystrophin-deficient dogs. Mol Ther 2010;18:1501–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Banks GB, Judge LM, Allen JM, Chamberlain JS. The polyproline site in hinge 2 influences the functional capacity of truncated dystrophins. PLoS Genet 2010;6:e1000958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lai Y, Zhao J, Yue Y, Duan D. alpha2 and alpha3 helices of dystrophin R16 and R17 frame a microdomain in the alpha1 helix of dystrophin R17 for neuronal NOS binding. Proc Natl Acad Sci USA 2013;110:525–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Foster H, Sharp PS, Athanasopoulos T, et al. Codon and mRNA sequence optimization of microdystrophin transgenes improves expression and physiological outcome in dystrophic mdx mice following AAV2/8 gene transfer. Mol Ther 2008;16:1825–1832 [DOI] [PubMed] [Google Scholar]
- 88.Li D, Yue Y, Lai Y, et al. Nitrosative stress elicited by nNOSmu delocalization inhibits muscle force in dystrophin-null mice. J Pathol 2011;223:88–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Monaco AP, Bertelson CJ, Liechti-Gallati S, et al. An explanation for the phenotypic differences between patients bearing partial deletions of the DMD locus. Genomics 1988;2:90–95 [DOI] [PubMed] [Google Scholar]
- 90.Beggs AH, Hoffman EP, Snyder JR, et al. Exploring the molecular basis for variability among patients with Becker muscular dystrophy: dystrophin gene and protein studies. Am J Hum Genet 1991;49:54–67 [PMC free article] [PubMed] [Google Scholar]
- 91.McClorey G, Moulton HM, Iversen PL, et al. Antisense oligonucleotide-induced exon skipping restores dystrophin expression in vitro in a canine model of DMD. Gene Ther 2006;13:1373–1381 [DOI] [PubMed] [Google Scholar]
- 92.Yokota T, Lu QL, Partridge T, et al. Efficacy of systemic morpholino exon-skipping in Duchenne dystrophy dogs. Ann Neurol 2009;65:667–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fletcher S, Ly T, Duff RM, et al. Cryptic splicing involving the splice site mutation in the canine model of Duchenne muscular dystrophy. Neuromuscul Disord 2001;11:239–243 [DOI] [PubMed] [Google Scholar]
- 94.Douglas AG, Wood MJ. Splicing therapy for neuromuscular disease. Mol Cell Neurosci 2013;56:169–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yokota T, Nakamura A, Nagata T, et al. Extensive and prolonged restoration of dystrophin expression with vivo-morpholino-mediated multiple exon skipping in dystrophic dogs. Nucleic Acid Ther 2012;22:306–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Goyenvalle A, Vulin A, Fougerousse F, et al. Rescue of dystrophic muscle through U7 snRNA-mediated exon skipping. Science 2004;306:1796–1799 [DOI] [PubMed] [Google Scholar]
- 97.Gorman L, Suter D, Emerick V, et al. Stable alteration of pre-mRNA splicing patterns by modified U7 small nuclear RNAs. Proc Natl Acad Sci USA 1998;95:4929–4934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Suter D, Tomasini R, Reber U, et al. Double-target antisense U7 snRNAs promote efficient skipping of an aberrant exon in three human beta-thalassemic mutations. Hum Mol Genet 1999;8:2415–2423 [DOI] [PubMed] [Google Scholar]
- 99.Vulin A, Barthelemy I, Goyenvalle A, et al. Muscle function recovery in golden retriever muscular dystrophy after AAV1-U7 exon skipping. Mol Ther 2012;20:2120–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Le Guiner C, Montus M, Servais L, et al. Forelimb treatment in a large cohort of dystrophic dogs supports delivery of a recombinant AAV for exon skipping in Duchenne patients. Mol Ther 2014;22:1923–1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Barbash IM, Cecchini S, Faranesh AZ, et al. MRI roadmap-guided transendocardial delivery of exon-skipping recombinant adeno-associated virus restores dystrophin expression in a canine model of Duchenne muscular dystrophy. Gene Ther 2013;20:274–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bish LT, Sleeper MM, Forbes SC, et al. Long-term restoration of cardiac dystrophin expression in golden retriever muscular dystrophy following rAAV6-mediated exon skipping. Mol Ther 2012;20:580–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bartlett RJ, Stockinger S, Denis MM, et al. In vivo targeted repair of a point mutation in the canine dystrophin gene by a chimeric RNA/DNA oligonucleotide. Nat Biotechnol 2000;18:615–622 [DOI] [PubMed] [Google Scholar]
- 104.Swaggart KA, McNally EM. Modifiers of heart and muscle function: where genetics meets physiology. Exp Physiol 2014;99:621–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cerletti M, Negri T, Cozzi F, et al. Dystrophic phenotype of canine X-linked muscular dystrophy is mitigated by adenovirus-mediated utrophin gene transfer. Gene Ther 2003;10:750–757 [DOI] [PubMed] [Google Scholar]
- 106.Odom GL, Gregorevic P, Allen JM, et al. Microutrophin delivery through rAAV6 increases lifespan and improves muscle function in dystrophic dystrophin/utrophin-deficient mice. Mol Ther 2008;16:1539–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lee SJ. Regulation of muscle mass by myostatin. Annu Rev Cell Dev Biol 2004;20:61–86 [DOI] [PubMed] [Google Scholar]
- 108.Bogdanovich S, Krag TO, Barton ER, et al. Functional improvement of dystrophic muscle by myostatin blockade. Nature 2002;420:418–421 [DOI] [PubMed] [Google Scholar]
- 109.Mosher DS, Quignon P, Bustamante CD, et al. A mutation in the myostatin gene increases muscle mass and enhances racing performance in heterozygote dogs. PLoS Genet 2007;3:e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bish LT, Sleeper MM, Forbes SC, et al. Long-term systemic myostatin inhibition via liver-targeted gene transfer in golden retriever muscular dystrophy. Hum Gene Ther 2011;22:1499–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cotten SW, Kornegay JN, Bogan DJ, et al. Genetic myostatin decrease in the golden retriever muscular dystrophy model does not significantly affect the ubiquitin proteasome system despite enhancing the severity of disease. Am J Transl Res 2014;6:43–53 [PMC free article] [PubMed] [Google Scholar]
- 112.Shin JH, Bostick B, Yue Y, et al. SERCA2a gene transfer improves electrocardiographic performance in aged mdx mice. J Transl Med 2011;9:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ferretti R, Marques MJ, Pertille A, Santo Neto H. Sarcoplasmic-endoplasmic-reticulum Ca2+-ATPase and calsequestrin are overexpressed in spared intrinsic laryngeal muscles of dystrophin-deficient mdx mice. Muscle Nerve 2009;39:609–615 [DOI] [PubMed] [Google Scholar]
- 114.Goonasekera SA, Lam CK, Millay DP, et al. Mitigation of muscular dystrophy in mice by SERCA overexpression in skeletal muscle. J Clin Invest 2011;121:1044–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Morine KJ, Sleeper MM, Barton ER, Sweeney HL. Overexpression of SERCA1a in the mdx diaphragm reduces susceptibility to contraction-induced damage. Hum Gene Ther 2010;21:1735–1739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bouyon S, Roussel V, Fromes Y. SERCA2a gene therapy can improve symptomatic heart failure in delta-sarcoglycan-deficient animals. Hum Gene Ther 2014;25:694–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhu X, McTiernan CF, Rajagopalan N, et al. Immunosuppression decreases inflammation and increases AAV6-hSERCA2a-mediated SERCA2a expression. Hum Gene Ther 2012;23:722–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mi YF, Li XY, Tang LJ, et al. Improvement in cardiac function after sarcoplasmic reticulum Ca2+-ATPase gene transfer in a beagle heart failure model. Chin Med J 2009;122:1423–1428 [PubMed] [Google Scholar]
- 119.Wang Z, Zhu T, Qiao C, et al. Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nat Biotechnol 2005;23:321–328 [DOI] [PubMed] [Google Scholar]
- 120.Bostick B, Ghosh A, Yue Y, et al. Systemic AAV-9 transduction in mice is influenced by animal age but not by the route of administration. Gene Ther 2007;14:1605–1609 [DOI] [PubMed] [Google Scholar]
- 121.Pacak CA, Mah CS, Thattaliyath BD, et al. Recombinant adeno-associated virus serotype 9 leads to preferential cardiac transduction in vivo. Circ Res 2006;99:e3–9 [DOI] [PubMed] [Google Scholar]
- 122.Pan X, Yue Y, Zhang K, et al. Long-term robust myocardial transduction of the dog heart from a peripheral vein by adeno-associated virus serotype-8. Hum Gene Ther 2013;24:584–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hakim CH, Yue Y, Shin JH, et al. Systemic gene transfer reveals distinctive muscle transduction profile of tyrosine mutant AAV-1, −6, and −9 in neonatal dogs. Mol Ther Methods Clin Dev 2014;1:14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang Z, Kuhr CS, Allen JM, et al. Sustained AAV-mediated dystrophin expression in a canine model of Duchenne muscular dystrophy with a brief course of immunosuppression. Mol Ther 2007;15:1160–1166 [DOI] [PubMed] [Google Scholar]
- 125.Rapti K, Louis-Jeune V, Kohlbrenner E, et al. Neutralizing antibodies against AAV serotypes 1, 2, 6, and 9 in sera of commonly used animal models. Mol Ther 2011;20:73–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Denard J, Beley C, Kotin R, et al. Human galectin 3 binding protein interacts with recombinant adeno-associated virus type 6. J Virol 2012;86:6620–6631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yue Y, Pan X, Hakim CH, et al. Safe and bodywide muscle gene transfer in young adult Duchenne muscular dystrophy dogs. New Directions in Biology and Disease of Skeletal Muscle, 2014, Chicago, Illinois, June 29–July 22, 2014 [Google Scholar]
- 128.Wang Z, Storb R, Lee D, et al. Immune responses to AAV in canine muscle monitored by cellular assays and noninvasive imaging. Mol Ther 2010;18:617–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang Z, Halbert CL, Lee D, et al. Elimination of contaminating cap genes in AAV vector virions reduces immune responses and improves transgene expression in a canine gene therapy model. Gene Ther 2014;21:363–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bish LT, Sleeper MM, Brainard B, et al. Percutaneous transendocardial delivery of self-complementary adeno-associated virus 6 achieves global cardiac gene transfer in canines. Mol Ther 2008;16:1953–1959 [DOI] [PubMed] [Google Scholar]
- 131.Ohshima S, Shin JH, Yuasa K, et al. Transduction efficiency and immune response associated with the administration of AAV8 vector into dog skeletal muscle. Mol Ther 2009;17:73–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Qiao C, Li J, Zheng H, et al. Hydrodynamic limb vein injection of AAV8 canine myostatin propeptide gene in normal dogs enhances muscle growth. Hum Gene Ther 2009;20:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Koo T, Okada T, Athanasopoulos T, et al. Long-term functional adeno-associated virus-microdystrophin expression in the dystrophic CXMDj dog. J Gene Med 2011;13:497–506 [DOI] [PubMed] [Google Scholar]
- 134.Childers MK, Joubert R, Poulard K, et al. Gene therapy prolongs survival and restores function in murine and canine models of myotubular myopathy. Sci Transl Med 2014;6:220ra210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vandenberghe LH, Wang L, Somanathan S, et al. Heparin binding directs activation of T cells against adeno-associated virus serotype 2 capsid. Nat Med 2006;12:967–971 [DOI] [PubMed] [Google Scholar]
- 136.Lu Y, Song S. Distinct immune responses to transgene products from rAAV1 and rAAV8 vectors. Proc Natl Acad Sci USA 2009;106:17158–17162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang L, Figueredo J, Calcedo R, et al. Cross-presentation of adeno-associated virus serotype 2 capsids activates cytotoxic T cells but does not render hepatocytes effective cytolytic targets. Hum Gene Ther 2007;18:185–194 [DOI] [PubMed] [Google Scholar]
- 138.Xin KQ, Mizukami H, Urabe M, et al. Induction of robust immune responses against human immunodeficiency virus is supported by the inherent tropism of adeno-associated virus type 5 for dendritic cells. J Virol 2006;80:11899–11910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mays LE, Vandenberghe LH, Xiao R, et al. Adeno-associated virus capsid structure drives CD4-dependent CD8+ T cell response to vector encoded proteins. J Immunol 2009;182:6051–6060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Faust SM, Bell P, Cutler BJ, et al. CpG-depleted adeno-associated virus vectors evade immune detection. J Clin Invest 2013;123:2994–3001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rozkalne A, Adkin C, Meng J, et al. Mouse regenerating myofibers detected as false-positive donor myofibers with anti-human spectrin. Hum Gene Ther 2014;25:73–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kodippili K, Vince L, Shin JH, et al. Characterization of 65 epitope-specific dystrophin monoclonal antibodies in canine and murine models of Duchenne muscular dystrophy by immunostaining and western blot. PLoS One 2014;9:e88280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wells DJ, Wells KE, Asante EA, et al. Expression of human full-length and minidystrophin in transgenic mdx mice: implications for gene therapy of Duchenne muscular dystrophy. Hum Mol Genet 1995;4:1245–1250 [DOI] [PubMed] [Google Scholar]
- 144.Phelps SF, Hauser MA, Cole NM, et al. Expression of full-length and truncated dystrophin mini-genes in transgenic mdx mice. Hum Mol Genet 1995;4:1251–1258 [DOI] [PubMed] [Google Scholar]
- 145.Odom GL, Gregorevic P, Allen JM, Chamberlain JS. Gene therapy of mdx mice with large truncated dystrophins generated by recombination using rAAV6. Mol Ther 2011;19:36–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhang Y, Duan D. Novel mini-dystrophin gene dual adeno-associated virus vectors restore neuronal nitric oxide synthase expression at the sarcolemma. Hum Gene Ther 2012;23:98–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhang Y, Yue Y, Li L, et al. Dual AAV therapy ameliorates exercise-induced muscle injury and functional ischemia in murine models of Duchenne muscular dystrophy. Hum Mol Genet 2013;22:3720–3729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ousterout DG, Kabadi AM, Thakore PI, et al. Correction of dystrophin expression in cells from Duchenne muscular dystrophy patients through genomic excision of exon 51 by zinc finger nucleases. Mol Ther 2014. [Epub ahead of print]; doi: 10.1038/mt.2014.234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ousterout DG, Perez-Pinera P, Thakore PI, et al. Reading frame correction by targeted genome editing restores dystrophin expression in cells from Duchenne muscular dystrophy patients. Mol Ther 2013;21:1718–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Long C, McAnally JR, Shelton JM, et al. Prevention of muscular dystrophy in mice by CRISPR/Cas9-mediated editing of germline DNA. Science 2014;345:1184–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]