Abstract
Objective: The objective of this study was to determine the efficacy and safety of valproic acid versus risperidone in children, 3–7 years of age, with bipolar I disorder (BPD), during a mixed or manic episode.
Methods: Forty-six children with Diagnostic and Statistical Manual of Mental Disorders. 4th ed., Text Revision (DSM-IV-TR) diagnosis of bipolar disorder, manic, hypomanic, or mixed episode, were recruited over a 6 year period from two academic outpatient programs for a double-blinded, placebo-controlled trial in which subjects were randomized in a 2:2:1 ratio to risperidone solution, valproic acid, or placebo.
Results: After 6 weeks of treatment, the least-mean Young Mania Rating Scale (YMRS) total scores change, adjusted for baseline YMRS scores, from baseline by treatment group was: Valproic acid 10.0±2.46 (p=0.50); risperidone 18.82±1.55 (p=0.008); and placebo 4.29±3.56 (F=3.93, p=0.02). The mixed models for repeated measure (MMRM) analysis found a significant difference for risperidone-treated subjects versus placebo treated subjects (p=0.008) but not for valproic acid-treated subjects versus placebo-treated subjects (p=0.50). Treatment with risperidone over 6 weeks led to increased prolactin levels, liver functions, metabolic measures, and weight/body mass index (BMI). Treatment with valproic acid led to increases in weight/BMI and decreases in total red blood cells (RBC), hemoglobin, and hematocrit.
Conclusions: In this small sample of preschool children with BPD, risperidone demonstrated clear efficacy versus placebo, whereas valproic acid did not. The laboratory and weight findings suggest that younger children with BPD are more sensitive to the effects of both of these psychotropics, and that, therefore, frequent laboratory and weight monitoring are warranted.
Introduction
Bipolar disorder does present in preschool children; however, it is rare (Luby et al. 2009). In Kraepelin's classic 1929 text, Manic-Depressive Insanity and Paranoia, he commented about mania in children, “In rare cases the first beginnings can be traced back to even the tenth year….” (Kraepelin 1921). Dilsaver and Akiskal identified mania in 11/40 preschool children presenting for treatment at a community mental health clinic.(Dilsaver and Akiskal 2004). Scheffer and Niskala reported cases with positive treatment response in a clinical setting in a large group of preschoolers with mania (Scheffer and Niskala 2004). More recently, Luby and colleagues at Washington University identified the clinical characteristic of bipolar I disorder in 21 preschool subjects versus (depressed preschoolers Luby and Belden 2008). They found that compared with a group of preschoolers with major depressive disorder, the bipolar subjects were more severely depressed and had higher rates of comorbid disorders.
There have been few medication trials in this population. Biederman and colleagues at Massachusetts General Hospital conducted an 8 week, open-label trial of olanzapine versus risperidone in 30 children, 4–6 years of age, diagnosed with bipolar I, II, or not otherwise specified (NOS) disorder by Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV) criteria (American Psychiatric Association 1994). The mean dose of risperidone was 1.4+0.5 mg/day, and the mean dose of olanzapine was 6.3+2.3 mg/day. Both medications resulted in clinically significant reductions in Young Mania Rating Scale (YMRS) scores, with 69% of the risperidone group rated as “much or very much improved” versus 53% of the olanzapine-treated group, with no significant difference in the rate of response between these two medications. Mota-Castillo and colleagues reported the results of a small case series of seven bipolar children ages 25 months–7 years who had a positive response to valproic acid (VPA) (Mota-Castillo et al. 2001). Scheffer and Niskala also reported positive results with VPA in an open prospective trial with VPA, in preschoolers with bipolar disorder (BPD) (Scheffer and Niskala 2004).
Pavuluri et al. (2010) conducted a landmark, outpatient controlled trial with 66 children and adolescents (mean age 10±3 years) with mania. These subjects were randomly assigned to either risperidone (0.5–2 mg/day) or divalproex (60–120 μg/mL) in a double-blinded design for a 6 week period. They reported that the response rate on YMRS was 78% for risperidone and 45% for divalproex (p<0.01). This study also demonstrated that risperidone was associated with more rapid improvement and greater reduction in manic symptoms than divalproex.
The Stanley Medical Research Foundation funded study was designed to test the efficacy of risperidone versus VPA in children and adolescents with symptomatic bipolar I or II disorder during a mixed, manic, or hypomanic episode. The hypothesis of this study was that differential efficacy would be observed with the following predicted order of response: Risperidone>VPA>placebo.
Methods
This study was conducted in accordance with ethical principles as described in the Declaration of Helsinki, and all applicable local institutional review board regulations. The institutional review boards of each study site approved the protocols. Written informed assent was obtained from the patient and written informed consent was obtained from the patient's legally authorized representative before enrollment in the study. During the course of the study, an independent data safety monitoring board reviewed and interpreted safety data on a regular basis.
Study Subjects
Subjects were male or female outpatient subjects, 3.0–7 years, 11 months of age, with bipolar I disorder, mixed or manic episode, psychotic or nonpsychotic, according to Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision (DSM IV-TR) criteria, with a score≥20 (considered to be moderate severity) on the YMRS (Young et al. 1978; Fristad et al. 1992; American Psychiatric Association 2000) at the time of randomization. The DSM-IV-TR requires subjects to meet criterion A, including extreme and persistently elevated, expansive, or irritable mood lasting for at least 1 week, plus criterion B, manifested by three (or four, if the mood is irritable only) of seven symptoms during the period of mood disturbance. Also recorded was the onset of first episode, the number of episodes, the offset of last episode, and total duration of illness.
Subjects were excluded for: Clinically significant or unstable hepatic, renal, gastroenterological, respiratory, cardiovascular, endocrine, immunological, hematological, or other systemic medical conditions; neurological disorders including epilepsy, stroke, or severe head trauma; clinically significant laboratory abnormalities on complete blood count (CBC) with differential, electrolytes, blood urea nitrogen (BUN), creatinine, hepatic transaminases, urinalysis, thyroid indices (T3, total T4, tree T4, thyroid-stimulating hormone [TSH]) and electrocardiogram (ECG); mania caused by a general medical condition or substance-induced mania; mental retardation (intelligence quotient [IQ] <70); evidence of fetal alcohol syndrome or an alcohol-related neurodevelopmental disorder; or schizophrenia or other psychotic disorders (including schizophreniform disorder, schizoaffective disorder, delusional disorder, brief psychotic disorder, shared psychotic disorder, psychotic disorder caused by a general medical condition, substance-induced psychotic disorder, psychotic disorder not otherwise specified) as defined in the DSM-IV.
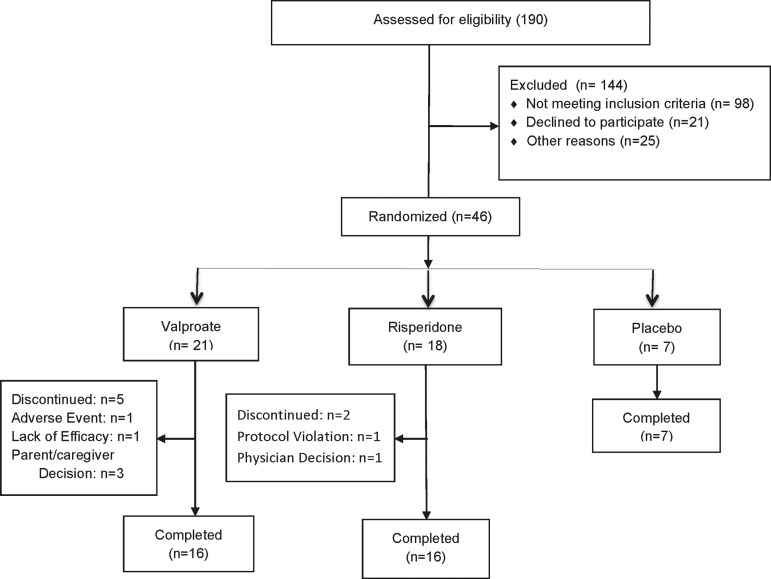
All diagnostic and rating evaluations were based on information obtained from the subject and other pertinent sources (e.g., parent, caregiver). Subjects were screened by telephone by a clinical nurse specialist using a screening form developed for previous pediatric bipolar medication trials, to determine if they qualified for the study according to study inclusion and exclusion criteria. A total of 180 potential subjects were screened. Patients with attention-deficit/ hyperactivity disorder (ADHD) were included if ADHD was comorbid with their BPD. The diagnosis of ADHD was made with reference to symptoms present during a euthymic period, as reported by parents. The telephone screening information was reviewed by a child psychiatrist, and if the patient seemed appropriate for the study, patient and guardian underwent a face-to-face, semistructured psychiatric interview by the same clinical nurse specialist with extensive training in the study instruments and well established inter-rater reliability (intraclass correlation coefficient [ICC] >0.9). The Washington University at St. Louis Kiddie-Schedule for Affective Disorders and Schizophrenia (K-SADS) (Geller et al. 2001) was used for subjects 6–7 years of age, and the Preschool Age Psychiatric Assessment (PAPA) (Angold and Costello 2000) was used for subjects 3–5 years of age. The Consolidated Standards of Reporting Trials (CONSORT) chart (Fig. 1) illustrates the flow of the subjects with a total of 46 (risperidone group, n=18; VPA group, n=21; placebo group, n=7) of the initial 180 potential subjects participating in the study.
FIG. 1.
Flow diagram of patients.
Subjects who met entry criteria were scheduled for a second diagnostic interview by an experienced child psychiatrist, 1–7 days later. Baseline assessment of the severity of the child's bipolar symptoms were made using the YMRS, the K-SADS Mania Rating Scale (Axelson et al. 2003); the Child Depression Rating Scale (CDRS-R) (Poznanski et al. 1984); and the Clinical Global Impressions (CGI) Severity and Improvement Scales, Bipolar Version (Spearing et al. 1997). The CGI-Bipolar Version (CGI-BP) includes separate ratings of a subject's overall psychiatric status and bipolar symptoms, and degree of change in each of these three domains. Socioeconomic status was determined by the Hollingshead four factor measure (Hollingshead 1975).
Study design
This was a 6 week, double-blind, randomized, placebo-controlled, outpatient study of monotherapy with risperidone, VPA, or placebo, conducted at two academic centers in the United States over a 60 month enrollment period. Subjects' guardians had to consent to the subjects' being washed out of their current medications at study entry. The washout period consisted of tapering any previous medications, including stimulants, over 1 week prior to study entry, except for those subjects taking aripiprazole or fluoxetine, who required a 4 week washout period. Other psychotropic medications required a 2 week washout. The study consisted of a 3–7 day screening period followed by a 6 week double-blind treatment period.
Active medication and placebo were administered in liquid form matched for taste and color. Medications were administered in a double-blinded manner on a twice-daily basis. Patients randomized to VPA were administered an initial dose of 10 mg/kg/day on a twice daily schedule beginning on day 0. VPA levels were adjusted to achieve a blood level of 80–100 μg/mL. An independent, unblinded study psychiatrist adjusted VPA doses to achieve a therapeutic level. The concurrent use of antipsychotic, antidepressant, and mood stabilizer/anticonvulsant medications other than the study drug was not allowed during study participation. The adjunctive use of oral chlorpromazine in low doses, 10–20 mg/day, two to three times a week, was allowed for sleep disturbance or agitation during the first 2 weeks of this trial.
Study outcomes
The a priori definition of response was a ≥50% improvement on the YMRS total score from baseline, or a CGI-Improvement score of 1 or 2 (“much” or “very much improved”). An initial target sample size of 60 patients per treatment group was selected to provide 80% power for an effect size of 0.46, treatment difference of 5.3, and pooled standard deviation of 11.6, assuming a two tailed type I error rate of 0.05. All statistical tests were two tailed, and p values of 0.05 were considered statistically significant. The study was funded for a 2 year period, and after 4 years of enrollment, the investigators decided to stop enrollment at 46 subjects after study funding ran out.
Subjects were assessed weekly for efficacy during the acute phase by one of the site principal investigators, who was blind to medication status and adverse events (AEs). An unblinded study coordinator performed the weekly side-effect ratings using the Side Effects Form for Children and Adolescents (SEFCA) (Klein and Slomkowski 1993), and coordinated dose increases with the unblinded medical monitor at each site. Subjects were rated weekly using the CGI-BP severity and improvement scales (Spearing et al. 1997), the YMRS, and the (CDRS-R).
Safety and tolerability assessments
Drug safety was assessed by monitoring of AEs, changes in vital signs, body weight, height, body mass index (BMI), and laboratory tests. Blood pressure and weight were recorded at each visit. Prolactin levels, glucose, and lipid levels were obtained at baseline and posttreatment. Blood samples were collected for assessment of hematology and clinical chemistry indices at screening, baseline and week 6. All laboratory tests were performed using validated procedures by a central laboratory certified by the College of American Pathologists and Clinical Laboratory Improvement Amendments. Vital signs were measured at all study visits. Physical examination and electrocardiograms were performed at the screening visit and week 6.
AEs were assessed with a checklist of items (SEFCA), each of which were rated on a scale of “not present” to “mild, does not interfere with functioning,” “moderate, some interference with functioning,” and “severe, functioning is significantly impaired because of side effects.” A significant AE was identified if it had not been present at baseline, but was reported during the course of the study, or if it had been present at baseline, but its severity was increased by at least 1 point on the scale during the study. The Barnes Akathisia Scale Abnormal Involuntary Movement Scale (Barnes 1989) and Simpson–Angus Scale (Simpson and Angus 1970) were used to assess for extrapyramidal symptoms.
Statistical analysis
Efficacy and safety analyses were conducted on the full analysis set which, following the intent-to-treat principle, included all randomized patients receiving at least one dose of the study drug. Changes from baseline of the primary efficacy measure were compared among groups using mixed models for repeated measure (MMRM), and treatment comparisons or secondary efficacy were analyzed using analysis of covariance (ANCOVA), imputing missing values based on last observation carried forward (LOCF). All analyses were on the intent-to-treat sample. All tests were two tailed and had an α level of 0.05, All analyses were performed using the SAS system (Version 9.2.; SAS Institute, Cary, NC).
Differences in demographics and clinical assessment variables at baseline among the three randomized groups were tested with an F test for continuous outcomes and χ2 for categorical outcomes. Differences in AEs were evaluated with Fisher's exact test. Treatment response defined as a >50% reduction in YMRS score was assessed with Fisher's exact test. Time to response was compared among groups using a Cox proportional hazards regression model. Logistic regression models were used to compute the odds of response/remission for the treatment groups. The logistic regression model provides an estimate of the odds of response/remission for risperidone versus placebo, VPA versus placebo, and risperidone versus VPA.
Results
Flow of subjects
The CONSORT diagram (Fig. 1) displays the flow of participants through the study. bjects were enrolled from September 2005 until September 2010. A total of 190 subjects were screened, and 144 (76%) were excluded: 98 subjects (69%) were excluded because they did not meet study inclusion criteria; 21 (14%) declined to participate, mostly because they did not want their child on medications; and 25 (17%) declined to participate because of transportation or other problems with making study regular appointments. Forty-six subjects were enrolled and 39 subjects completed the 6 weeks of this trial. The baseline characteristics of participants were similar among treatment groups and are summarized in Table 1. There were no significant differences among the groups on any of the demographic or clinical variables.
Table 1.
Baseline Characteristics of Subjects by Treatment Group
| Valproic acid n=21 | Risperidone n=18 | Placebo n=7 | Statistic | |
|---|---|---|---|---|
| Age | ||||
| Mean (SD) | 6.03 (1.3) | 5.31 (1.3) | 5.19 (1.0) | F=1.96, p=0.15 |
| Ethnicity | ||||
| Caucasian | 16 (76) | 11 (61) | 5 (71) | χ2=12.97, p=0.23 |
| African American | 3 (14) | 2 (11) | 1 (14) | |
| Hispanic | 0 | 3 (17) | 0 | |
| Asian American | 0 | 0 | 1 (14) | |
| Native American | 1 (5) | 0 | 0 | |
| Other | 1 (5) | 2 (11) | 0 | |
| Gender | ||||
| Male | 13 (63) | 11 (61) | 5 (71) | χ2=0.25, p=0.88 |
| Female | 8 (38) | 7 (39) | 2 (29) | |
| Comorbid ADHD (%) | 34.8% | 37.0% | 15.2% | |
| Comorbid ODD (%) | 4.3% | 4.3% | 0% | |
| Comorbid GAD (%) | 10.9% | 8.7% | 6.5% | |
| YMRS | 29.76 (3.6) | 31.94 (4.4) | 30.57 (6.3) | F=1.18, p=0.32 |
| CDRS-R | 23.25 (3.8) | 22.88 (6.2) | 21.29 (2.1) | F=0.91, p=0.42 |
| F-GAS | 72.48 (13.2) | 70.22 (14.5) | 70.57 (12.2) | F=0.15, p=0.87 |
| C-GAS | 44.33 (4.9) | 44.83 (3.2) | 46.57 (3.6) | F=0.78, p=0.47 |
ADHD, attention-deficit/hyperactivity disorder; ODD, oppositional defiant disorder; GAD, generalized anxiety disorder; YMRS, Young Mania Rating Scale; CDRS-R, Child Depression Rating Scale; F-GAS, C-GAS, Children's Global Assessment Scale.
Demographic and clinical characteristics
As shown in Table 1, there were no significant differences among subjects assigned to risperidone, VPA, or placebo treatment on any demographic or clinical variable. The mean dose of risperidone at end-point was 0.5 mg/day (range 0.5–0.75 mg/day), and the mean dose of VPA was 300 mg/day. The mean level of VPA at study end-point was 81±24 μg/mL.
Primary outcomes
Means and standard deviations of all the outcome measures are reported by treatment in Table 2.
Table 2.
Efficacy Results at Study End-Point
| Study measure at end-point | Valproic acid mean (SE) n=21 | Risperidone mean (SE) n=18 | Placebo mean (SE) n=7 | |
|---|---|---|---|---|
| Least squares mean change in YMRS score from baseline | 10.0 (2.46) | 18.82 (1.55) | 4.29 (3.56) | F=3.93, p=0.02 |
| p=0.57 | p=0.001 | p=0.353 | ||
| Percent with Clinical Global Impressions Improvement Scale score of 1 or 2 | 50% | 88% | 0% | χ2=4.97 p=0.08 |
| p=0.008 | p=0.003 | |||
| Cohen's effect size (vs. placebo) | 1.66 | 3.58 | 0.56 | |
| Hazard ratio product-limit survival estimates (status=50% decrease in YMRS) | 1.95 (0.6–6.9) | 6.97 (1.9–25.9) | 0.51 (0.1–1.8) | |
| p=0.30 | p=0.004 | p=0.30 | ||
| Mean change CDRS-from baseline | 2.25 | 2.31 | 0.29 | F=0.44; p=0.64 |
| p=0.06 | p=0.16 | p=0.82 |
YMRS, Young Mania Rating Scale; CDRS, Child Depression Rating Scale.
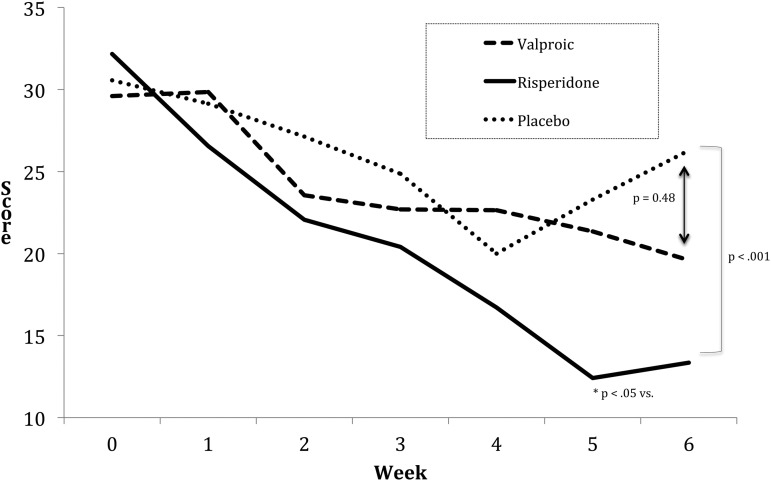
After 6 weeks of treatment, the least-mean YMRS total scores change, adjusted for baseline YMRS scores, from baseline by treatment group was: VPA 10.0±2.46 (p=0.50), risperidone 18.82±1.55 (p=0.008), and placebo 4.29±3.56 (F=3.93, p=0.02) (Fig. 2). The mixed model repeated measures (MMRM) analysis found a significant difference for risperidone-treated subjects versus placebo-treated subjects (p=0.008) but not for VPA-treated subjects versus placebo-treated subjects (p=0.50). There was also a significant difference between risperidone-treated subjects and VPA-treated subjects (p=0.004). Risperidone separated from placebo at week 4 (p=0.01) on the MMRM analysis. The CGI-BP Improvement Scale indicated improvement (“much” or “very much” improved) in 50% of VPA -treated subjects (p=0.001); 88% of risperidone-treated subjects (p=0.001); and 0% of placebo-treated subjects. Cohen's effect size for VPA-treated subjects was 1.66; for risperidone-treated subjects it was 3.58 and for placebo-treated subjects it was 0.56. A Cox survival analysis, with status equaling a 50% decline in YMRS from baseline, revealed that the hazard ratio (HR) reduction using the placebo group as a reference was: VPA-treated subjects 1.95 (0.6–6.9) (p=0.30) and risperidone-treated subjects 6.97 (1.6–7.9) (p=0.002). There were no significant changes in CDRS-R scores from baseline to end of treatment for any of the treatment groups (F=0.44; p=0.64) (Table 2).
FIG. 2.
Weekly Young Mania Rating Scale (YMRS) scores by treatment group.
Safety and tolerability
At study end-point, there were differences among groups for premature discontinuations. In the VPA-treated group, one subject discontinued at week 1 because of an AE (nausea); two discontinued at week 3 for increased “anger outbursts,” and three were discontinued by the caregiver because of the frequency of study visits. One subject who dropped out of the trial at week 3 because of increased anger outbursts had a VPA level of 103 μg/mL. A second subject dropped out at week 3, also because of increased anger outbursts, and had a VPA level of 104 μg/mL. A third subject dropped out at week 3 because of increased anger outbursts, and had a VPA level of 89 μg/mL.
Reasons for discontinuation in the risperidone group included: One for protocol violation (did not return after the first medication visit) and one by physician decision (agitation related to study blood draws). No subjects discontinued prematurely in the placebo-treated group.
There were no significant differences among the treatment groups in baseline to end-point changes on any ECG measures, thyroid function tests, electrolytes, BUN, or creatinine.
Repeated-measures ANOVA found significant differences from baseline to study end-point among the three treatment groups in measured weight gain (kg), BMI, albumin, insulin levels, and prolactin levels (Table 3). The risperidone- and VPA-treated groups had significant increases in weight and BMI, whereas the placebo-treated group did not.
Table 3.
Adverse Events
| Medication (n) | |||
|---|---|---|---|
| Adverse Event on SEFCA | Valproic Acid (21) | Risperidone (18) | Placebo (7) |
| Abdominal pain | 1 | 0 | 0 |
| Headache | 0 | 5 | 0 |
| Initial insomnia | 0 | 1 | 0 |
| Difficulty waking in the morning | 0 | 0 | 1 |
| Excitement | 2 | 0 | 0 |
| Irritability | 0 | 2 | 0 |
| Outburst of anger | 6 | 3 | 0 |
| Difficulty concentrating | 0 | 0 | 6 |
| Sadness | 3 | 4 | 2 |
| Nasal congestion | 1 | 0 | 0 |
| Enuresis | 0 | 2 | 0 |
| Total | 13 | 17 | 9 |
SEFCA, Side Effects Form for Children and Adolescents.
The VPA-treated group had statistically significant decreases from baseline to end-point in unconjugated bilirubin and albumin, total red blood cells (RBC), hemoglobin, and hematocrit. None of these changes were clinically significant.
The risperidone-treated group had statistically significant increases from baseline to end-point in unconjugated bilirubin, γ-glutamyl transferase (GGT), cholesterol, and prolactin levels. None of these changes were clinically significant. The mean prolactin level in the risperidone-treated group was 7.43±4.5 at baseline and 53.9±20 at study end-point. None of the subjects with elevated prolactin levels had developed any clinical symptoms related to this elevation.
Table 4 reports AEs that were commonly reported, which were not present at baseline but were reported during the course of the study or, which were present at baseline and whose severity was increased by at least a 1 point rating on the SEFCA during the study. The two groups treated with active medication reported more AEs. The majority of these AEs were mild, occurred in the 1st or 2nd week of treatment, and resolved over the course of the trial. This study was not powered to detect significant differences in AEs among the three treatment groups.
Table 4.
Laboratory and Weight Measures
| Valproic Acid | Risperidone | Placebo | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | End-point | Baseline | End-point | Baseline | End-point | Between-group F value | Normal values for age | |
| Bilirubin unconjugated mg/dL | 0.40±0.3 | 0.37±0.2a | 0.33±0.19 | 0.37±0.2a | 0.27±0.2 | 0.29±0.2a | F(2,44)=0.38, p=0.68 | <1 |
| Serum glutamic oxaloacetic transaminase(SGOT) units/L | 50.8±16 | 51.8±14.2 | 49±10 | 58.5±15.3 | 54.7±9.7 | 51.5±12 | F(2,44)=1.0, p=0.38 | 15–46 |
| Alanine aminotransferase (ALT/SGPT) units/L | 22.5±13 | 16.9±7.3 | 20.3±9 | 23.4±9.2 | 31.7±12 | 22.2±6 | F(2,44)=0.2.65, p=0.08 | 8–36 |
| γ-glutamyl transferase (GGT) U/L | 16.9+5 | 16.2+3 | 14.7+2.5 | 17.8+2.5a | 17.2+2 | 16.8+1.2 | F(2,44)=1.61, p=0.22 | 7–19 |
| Albumin g/dL | 4.5±0.2 | 4.1±0.2a | 4.5±0.3 | 4.5±0.2 | 4.5±0.1 | 4.5±0.2 | F(2,44)=12.46, p=0.001 | 3.5–5.5 |
| Total cholesterol mg/dL | 161±20 | 164.8±33a | 162.3±22 | 170.1±19a | 149.5±14 | 167±13a | F(2,44)=0.16, p=0.85 | 135–200 |
| Triglycerides mg/dL | 79.6±34 | 86.2±43 | 78.2±46 | 99.5±90 | 81.1±37 | 108.6±75 | F(2,44)=0.24, p=0.79 | <45 |
| Low density lipoproteins mg/dL | 92.3±22 | 93.83±31 | 95±17 | 98±19 | 87.2±15 | 97.3±13 | F(2,44)=0.15, p=0.89 | <190 |
| High density lipoproteins mg/dL | 53.6±11 | 53.7±13 | 51±9 | 55±7 | 46±10 | 48±9 | F(2,44)=0.1.14, p=0.33 | <110 |
| Red blood cell count mill/mm3 | 4.7±0.4 | 4.5±0.3a | 4.6±0.3 | 4.5±0.3 | 4.5±0.2 | 4.5±0.2 | F(2,44)=0.13, p=0.88 | 4.0–4.9 |
| Hemoglobin g/dL | 13.1±0.6 | 12.61±0.59a | 12.5±0.7 | 12.5±0.9 | 13±0.9 | 12.8±0.8 | F(2,44)=0.31, p=0.74 | 11.5–14.5 |
| Hematocrit % | 38.50±2.36 | 36.73±1.54a | 37.08±2.5 | 36.6±2.5 | 37.4±2.6 | 37.8±2.3 | F(2,44)=0.87, p=0.43 | 35–42 |
| Platelets×103/mm3 | 357+80 | 319+92 | 374.8+77 | 362.2+53 | 342+55 | 366+46 | F(2,44)=1.7, p=0.20 | 250–550 |
| Prolactin level ng/mL | 7.43±4.5 | 5.25±1.3 | 8.7±4.2 | 53.9±20a | 5.1±1.7 | 6.9±2.5 | F(2,44)=56, p=0.001 | 1.2–12.95 |
| Insulin level mU/L | 14.87±10 | 17.8±10 | 10±1.0 | 14±1.0 | 17.2±3.2 | 7.3±3.5a | F(2,44)=1.66, p=0.22 | 1.8–24.6 |
| Weight (kg) | 25.3±6.4 | 25.8±7.2a | 20.7±5.2 | 21.4±5.4a | 20.7±4.5 | 20.8±4.5 | F(2,44)=2.65, p=0.09 | |
| Body mass index | 17.4±2.2 | 19.0±3.4a | 16.8±3.0 | 17.0±2.1 | 16.2±0.9 | 16.3±1.0 | F(2,44)=03.39, p=0.04 | |
p<0.05 versus baseline within the valproate, risperidone, or placebo-treated groups.
Discussion
During this 6 week, double-blinded, placebo controlled trial, risperidone was efficacious on both the a priori, primary outcome variables: The mean changes from baseline on the YMRS and the CGI-Improvement Scales. Subjects treated with risperidone also demonstrated efficacy in the MMRM analysis, a large effect size and significantly better odds of survival than with placebo. The response rates on the CGI Improvement Scale observed for risperidone (88%) and VPA (50%) were similar to those reported in a recent meta-analysis by Liu et al. (2011) for VPA and risperidone in children and adolescents with BPD. On the whole, risperidone demonstrated a greater effect on mood and behavior than VPA. These results are similar to what Pavuluri et al. reported in an older sample of children and adolescents with BPD (Pavuluri et al. 2010) and what the Treatment of Early Age Mania (TEAM) trial reported in subjects 6–15 years of age with BPD I mania (Geller et al. 2012). Subjects treated with risperidone demonstrated a clinical response by weeks 2–3, whereas the VPA-treated subjects did not begin showing response until weeks 4–5.
Although the clinical effect was significantly greater in the risperidone-treated group, there were also more endocrine and metabolic effects. The end-point prolactin levels were greater in the risperidone than in the VPA-treated group (p<0.05) (Table 3). There were no clinically meaningful AEs reported caused by the elevated prolactin levels in the risperidone group, but during the trial period of 6 weeks, clinical changes may yet occur. In adults, early peaks in serum prolactin (1st or 2nd week) are common with the majority of antipsychotics. This study replicates earlier findings (Pandina et al. 2006) that prolonged elevation of serum prolactin is common in youth treated with risperidone, and that close clinical monitoring is warranted
Treatment with VPA led to increases in total cholesterol and weight/BMI and decreases in serum albumin, total RBC, hemoglobin, and hematocrit. These metabolic and hematological changes have been noted in patients treated with VPA (Abaci et al. 2009; Bachmann et al. 2011), which suggests that these parameters should be monitored in young children treated with VPA for BPD.
The risperidone-treated group had elevated unconjugated bilirubin, GGT, and decreases in albumin and total protein. Insulin was statistically significantly elevated in the risperidone- treated group, but levels of insulin were only available in 22 subjects. Cholesterol was statistically elevated in the risperidone-treated group, and there was also a trend toward increases of triglycerides and low-density lipoproteins. Transient increases in hepatic function have been documented in other samples of children treated with risperidone (Erdogan et al. 2008). These are markers of general health, but more specifically reflect hepatic and metabolic function. All of these increases suggest that careful monitoring of hepatic and metabolic functions is warranted in younger children treated with risperidone; perhaps more frequently than is recommended in older children (Correll and Carlson 2006).
There appeared to be a relationship between valproic levels and increased mood lability. Six out of 21 (28%) of the VPA-treated subjects had increased mood lability that was documented as the AE “outbursts of anger.” In 3 of the 21 (15%) of VPA-treated subjects, higher plasma levels of VPA at week 3 (mean level of 98 μg/mL) led to symptomatic worsening and subsequent discontinuation during this trial in 3 subjects. This is an AE that several other investigators have observed with divalproex (Kowatch et al. 2000; Pavuluri et al. 2010), and suggests that some younger patients with BPD may develop more mood lability at higher plasma levels of VPA. This effect may by be mediated by a rise in serum ammonia levels (Carr and Shrewsbury 2007), which has been theorized to be caused by a VPA-linked carnitine deficiency (Raskind and El-Chaar 2000).
Clinical caveats
1. Risperidone is efficacious and works quickly in young patients with mania.
2. The mean dose of risperidone was 0.5 mg/day.
3. Treatment with VPA resulted in lower response rates, and took longer than treatment with risperidone.
4. The laboratory and weight findings in both treatment groups suggest that younger children with BPD are more sensitive to the effects of both of these psychotropics, and that, therefore, frequent laboratory and weight monitoring are warranted.
Limitations
This study was limited by the small sample size and short duration of treatment. The average VPA level of 81 μg/ml was relatively low, and may have limited the response rate to VPA.
Conclusions
In this small sample of preschool children with BPD, risperidone demonstrated clear efficacy versus placebo, whereas valproic acid did not. Treatment with risperidone over 6 weeks led to increased prolactin levels, liver functions, metabolic measures, and weight/BMI. Treatment with valproic acid led to increases in weight/BMI and decreases in total RBC, hemoglobin, and hematocrit. These findings suggest that younger children with BPD are more sensitive to the effects of both of these psychotropics, and that frequent laboratory monitoring is warranted.
Disclosures
Dr. Kowatch is a consultant and faculty for the Resource for Advancing Children's Health (REACH) Institute. He receives research support from the National Institute of Mental Health (NIMH). He is employed by Ohio State University and is an editor for Current Psychiatry. Drs. Scheffer, Monroe, Delgado, and Altaye, and Ms. Lagory disclosed no conflicts of interest.
References
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC: American Psychiatric Association; 1994 [Google Scholar]
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revsion. Washington, DC: American Psychiatric Association; 2000 [Google Scholar]
- Angold A, Costello EJ: The Child and Adolescent Psychiatric Assessment (CAPA). J Am Acad Child Adolesc Psychiatry 39:39–48, 2000 [DOI] [PubMed] [Google Scholar]
- Axelson D, Birmaher BJ, Brent D, Wassick S, Hoover C, Bridge J, Ryan N: A preliminary study of the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children mania rating scale for children and adolescents. J Child Adolesc Psychopharmacol. 13:463–470, 2003 [DOI] [PubMed] [Google Scholar]
- Bachmann T, Bertheussen KH, Svalheim S, Rauchenzauner M, Luef G, Gjerstad L, Tauboll E: Haematological side effects of antiepileptic drug treatment in patients with epilepsy. Acta Neurol Scand Suppl:23–27, 2011 [DOI] [PubMed] [Google Scholar]
- Barnes T: A rating scale for drug-induced akathisia. Br J Psychiatry 154:5, 1989 [DOI] [PubMed] [Google Scholar]
- Carr RB, Shrewsbury K: Hyperammonemia due to valproic acid in the psychiatric setting. Am J Psychiatry 164:1020–1027, 2007 [DOI] [PubMed] [Google Scholar]
- Correll CU, Carlson HE: Endocrine and metabolic adverse effects of psychotropic medications in children and adolescents. J Am Acad Child Adolesc Psychiatry 45:771–791, 2006 [DOI] [PubMed] [Google Scholar]
- Dilsaver SC, Akiskal HS: Preschool-onset mania: Incidence, phenomenology and family history. J Affect Disord. 82 Suppl 1:S35–43, 2004 [DOI] [PubMed] [Google Scholar]
- Erdogan A, Atasoy N, Akkurt H, Ozturk D, Karaahmet E, Yalug I, Yalug K, Ankarali H, Balcioglu I: Risperidone and liver function tests in children and adolescents: a short-term prospective study. Prog Neuropsychopharmacol Biol Psychiatry 32:849–857, 2008 [DOI] [PubMed] [Google Scholar]
- Fristad MA, Weller EB, Weller R. The mania rating scale: Can it be used in children?: A preliminary report. J Am Acad Child Adolesc Psychiatry. 31:252–257, 1992 [DOI] [PubMed] [Google Scholar]
- Geller B, Zimerman B, Williams M, et al. Reliability of the Washington University in St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS) mania and rapid cycling sections. J Am Acad Child Adolesc Psychiatry 40:450–455, 2001 [DOI] [PubMed] [Google Scholar]
- Geller B, Luby JL, Joshi P, Wagner KD, Emslie G, Walkup JT, Axelson DA, Bolhofner K, Robb A, Wolf DV, Riddle MA, Birmaher B, Nusrat N, Ryan ND, Vitiello B, Tillman R, Lavori P: A randomized controlled trial of risperidone, lithium, or divalproex sodium for initial treatment of bipolar I disorder, manic or mixed phase, in children and adolescents. Arch Gen Psychiatry 69:515–528, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RG, Slomkowski C: Treatment of psychiatric disorders in children and adolescents. Psychopharmacol Bull 29:525–535, 1993 [PubMed] [Google Scholar]
- Kowatch RA, Suppes T, Carmody TJ, Bucci JP, Hume JH, Kromelis M, Emslie GJ, Weinberg WA, Rush AJ: Effect size of lithium, divalproex sodium and carbamazepine in children and adolescents with bipolar disorder. J Amer Acad Child Adol Psychiatry 39:713–720, 2000 [DOI] [PubMed] [Google Scholar]
- Causes Kraepelin E. In: Manic-Depressive Insanity & Paranoia. Edinburgh: E & S Livingston, Ltd., 1921 [Google Scholar]
- Liu HY, Potter MP, Woodworth KY, Yorks DM, Petty CR, Wozniak JR, Faraone SV, Biederman J: Pharmacologic treatments for pediatric bipolar disorder: a review and meta-analysis. J Am Acad Child Adolesc Psychiatry 50:749–762, 2011 [DOI] [PubMed] [Google Scholar]
- Luby JL, Belden AC: Clinical characteristics of bipolar vs. unipolar depression in preschool children: an empirical investigation. J Clin Psychiatry 69:1960–1969, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby JL, Tandon M, Belden A. Preschool bipolar disorder. Child Adolesc Psychiatr Clin N Am 18:391–403, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota–Castillo M, Torruella A, Engels B, Perez J, Dedrick C, Gluckman M: Valproate in very young children: An open case series with a brief follow-up. J Affect Disord 67:193–197, 2001 [DOI] [PubMed] [Google Scholar]
- Pandina GJ, Aman MG, Findling RL: Risperidone in the management of disruptive behavior disorders. J Child Adolesc Psychopharmacol 16:379–392, 2006 [DOI] [PubMed] [Google Scholar]
- Pavuluri MN, Henry DB, Findling RL, Parnes S, Carbray JA, Mohammed T, Janicak PG, Sweeney JA: Double-blind randomized trial of risperidone versus divalproex in pediatric bipolar disorder. Bipolar Disord 12:593–605, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poznanski E, Freman L, Mokros H: Children's depression rating scale-revised. Psychopharmacol Bull 21:979–989, 1984 [Google Scholar]
- Raskind JY, El-Chaar GM: The role of carnitine supplementation during valproic acid therapy. Ann Pharmacother 34:630–638, 2000 [DOI] [PubMed] [Google Scholar]
- Scheffer R, Niskala J: The diagnosis and treatment of preschool mania. J Affect Disord 82:825–834, 2004 [DOI] [PubMed] [Google Scholar]
- Simpson GM, Angus JW: A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl 212:11–19, 1970 [DOI] [PubMed] [Google Scholar]
- Spearing MK, Post RM, Leverich GS, Brandt D, Nolen W: Modification of the Clinical Global Impressions (CGI) Scale for use in bipolar illness (BP): The CGI-BP. Psychiatry Res 73:159–171, 1997 [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: Reliability, validity and sensitivity. Br J Psychiatry 133:429–435, 1978 [DOI] [PubMed] [Google Scholar]