Abstract
Background: Delays in diagnosis and treatment for breast cancer may contribute to excess deaths among African Americans. We examined racial differences in delays in diagnosis and surgical treatment for early-stage breast cancer and evaluated race-specific predictors associated with delay.
Methods: A retrospective cohort study was conducted among 634 African American and white women diagnosed with invasive breast cancer between 2005 and 2010 in New Jersey. Detailed medical-chart abstraction and patient interviews were undertaken. Time intervals were calculated from symptom recognition to diagnosis (diagnosis delay) and from diagnosis to first operation (surgical delay). Binomial regression models were used to examine racial differences in delay and factors associated with ≥2 months delay in the overall population and stratified by race. Reasons responsible for diagnosis delay were also examined by race.
Results: Compared to white women, African American women experienced significantly higher risk of ≥2 months delay in diagnosis and surgical treatment (adjusted relative risks=1.44 (1.12–1.86) and 3.08 (1.88–5.04), respectively). For the African Americans, predictors of diagnosis delay included mode of detection, insurance, and tumor size; for whites, mode of detection and tumor grade. Surgical delay was associated with operation type and education among African Americans but with operation type and tumor size for whites. Patient-related factors were commonly noted as reasons for diagnosis delay.
Conclusions: These findings emphasize the need to raise further awareness, especially among African American patients and their providers, of the importance of prompt evaluation and treatment of breast abnormalities. Research on effective ways to accomplish this is needed.
Introduction
For over two decades, African American (AA) women diagnosed with breast cancer (BC) have had higher mortality rates than white women, despite lower incidence.1,2 One possible reason for persistence of this racial disparity is delay in receipt of diagnosis and treatment, which can negatively impact patient outcomes. Delay of more than 2 months in treatment initiation has been associated with worse BC survival.3–5
Commonly used definition and analytic methods to examine racial disparity in delay leave crucial gaps in understanding race-associated differences. Time to diagnosis for BC encompasses the elapsed time from symptom onset to medical consultation, as well as the elapsed time from consultation to diagnosis. The former is often referred to as “patient delay”; the latter, “system delay.” In the majority of research conducted on racial disparities in diagnosis delay, population-based cancer registries or administrative databases, which do not capture date of symptom recognition, have been used.6–8 Therefore, mostly diagnosis delay has been examined, in part because the delay resulting from care seeking (patient delay) is excluded from its definition. This can result in substantial underestimation of the length of delay in general. In addition, this may bias racial comparison of delays because of higher rates of self-detection in minorities as compared to nonminorities, as well as racial/ethnic differences with respect to treatment-seeking behavior.9–13
Moreover, the frequently used analytic approach of multivariate adjustment of race in population-based studies fails to understand the basis of racial difference by minimizing race to a risk factor.14 To actively assess racial disparity in care, it is more appropriate to have unadjusted models linking race to health outcomes of interest and then to stratify data by race to explore the contributing factors of differences.15 Limited studies have examined race-specific differences in predictors of diagnosis and surgical delay in BC patients.16 Epidemiologic research in other diseases has found that stratifying analysis by race allows exploration of between-race and within-race differences in risk factors of interest.17–20
We utilized detailed medical-record and interview data on a large sample of AA and white women with early-stage BC who participated in the Breast Cancer Treatment Disparity Study (BCTDS). The study aims to examine whether racial differences exist in delay from symptom recognition to diagnosis and surgery and to determine the factors that predict delay for AAs and whites.
Materials and Methods
Study population
The BCTDS is a cohort study designed to achieve a comprehensive understanding of how the process and structure of care for BC patients vary by race. The study expands upon the Women's Circle of Health Study (WCHS), a multisite case-control study in New York City and New Jersey (NJ) designed to evaluate risk factors for early and aggressive BC in AA and white women.21,22 The BCTDS cohort included NJ cases from the WCHS meeting the following criteria: AA or white women newly diagnosed with stages I, II, and T3N1M0 BC during 2005–2010 and no history of cancer other than nonmelanoma skin cancer.
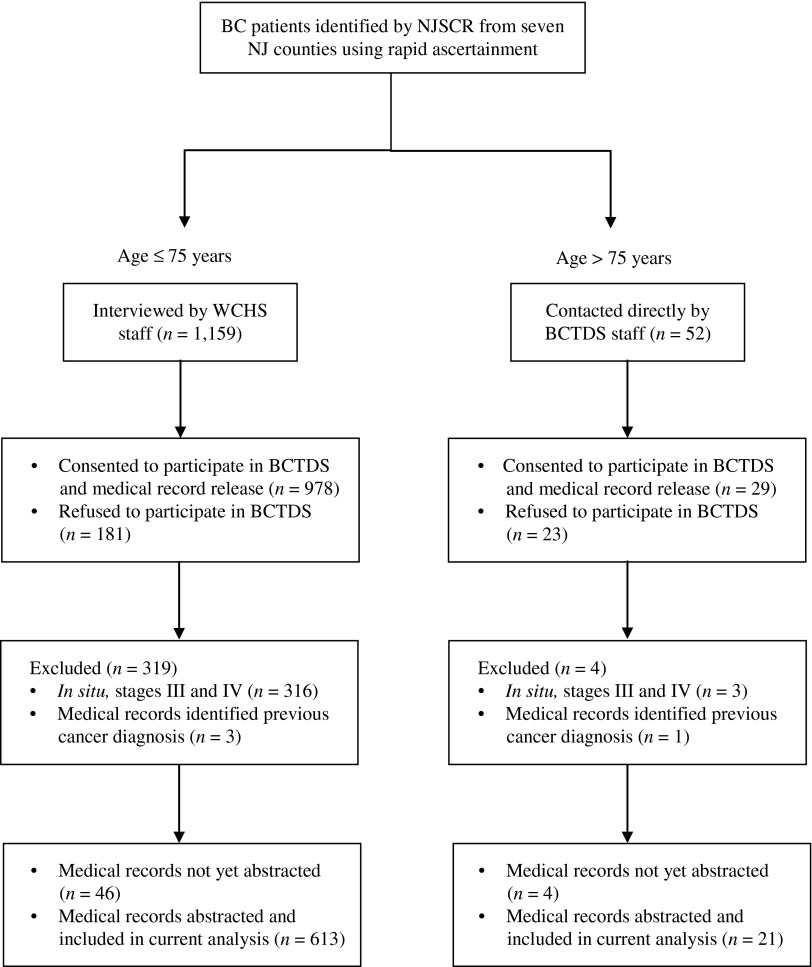
Cases in NJ were identified by rapid ascertainment by the New Jersey State Cancer Registry (NJSCR) staff from all the major hospitals in seven NJ counties: Bergen, Essex, Hudson, Mercer, Middlesex, Passaic, and Union. All eligible AA women ≤85 years of age were identified by the NJSCR and randomly frequency matched with white women by age (±5 years) and county of residence. The NJSCR obtained verbal consent from identified participants to be contacted by the research study staff. The WCHS was restricted to women ≤75 years of age, so the research staff contacted consenting patients in that age group to participate in an in-home interview for the WCHS. At the end of these home interviews, patients were asked for participation in the BCTDS. Patients >75 years old were not eligible to participate in the WCHS but were also identified by the NJSCR and invited to participate only in the BCTDS (Fig. 1). Written informed consents were obtained from all patients.
FIG. 1.
Subject-selection flow diagram. BC, breast cancer; BCTDS, Breast Cancer Treatment Disparity Study; NJSCR, New Jersey State Cancer Registry; WCHS, Women's Circle of Health Study.
Approximately 84.4% (978 of 1,159) of the cases ≤75 years old from WCHS (AA=80.9%; white=88.7%) and 55.8% (29/52) of the cases >75 years old (AA=60.0%; white=53.1%) consented to participate in the BCTDS and signed a medical-records release. In the ≤75-years group, those who refused vs. consented were more likely to be AAs (p<0.0001) and have poorly differentiated tumor grade (p=0.0273) but were not different in the stage distribution (p=0.696). On the other hand, among older women (i.e., >75 years old), only information on race was available, and no difference was noted by race between respondents and nonrespondents (p=0.627). The response rate was significantly lower among older patients; reasons for nonresponse included passive refusal (n=3), unable to be contacted (n=3), active refusal (n=15), and deceased before consenting (n=2). Consenting patients also provided names and addresses of their BC treatment providers and consented to participate in a follow-up telephone interview. Institutional review boards at all participating institutions approved the study.
Medical-records abstraction
Medical records were obtained from all healthcare providers identified by the patient, including primary physician; surgical, medical, and radiation oncologists; and relevant hospitals. Records of diagnostic information, pathology reports, operative notes, adjuvant treatment reports, and discharge summary from hospitalizations were obtained from 1 year prior through 1 year after the initial diagnosis. Trained abstractors reviewed medical records to capture the date and method of first symptom recognition (by patient, physician, or screening mammogram) and the dates and findings of preoperative investigations, especially the mammographic evaluation and the purpose of the mammogram (screening or diagnostic). Physician recommendations and the patient's compliance with them; the dates of physician visits, biopsy and all other procedures and treatments, along with missed appointments; and the reasons for delay were captured. Information was also abstracted on sociodemographic factors, family history of BC, comorbidities, tumor and receptor characteristics, and treatments received, along with the administering facility.
Information on race is recorded at several places in the medical charts, posing a logistical challenge to delete such information; therefore, abstractors were not blinded to patient race but were unaware of the study hypothesis. A standardized training was implemented for all abstractors to ensure the uniformity of information ascertainment, as well as to check for completeness and to prevent systematic differences in data abstraction between abstractors. At first, the training materials were distributed to all the abstractors for review. These materials included a study overview, information on procedures for abstraction, the abstraction form, and a procedure manual consisting of coding instructions. Both group and individual sessions were conducted with the abstractor by the principal investigator (PI) and the lead study coordinator. In these sessions, the abstraction form was reviewed item by item, along with the coding instructions in the manual. Familiarity with BC pathology and treatment terminology was established among the abstractors, who also demonstrated a medical-record abstraction supervised by the PI and the lead study coordinator. The first few charts abstracted (approximately 10) by each medical-record abstractor were also reviewed by the lead study coordinator, and discrepancies were discussed. Weekly consults with the PI were scheduled to address difficult cases and unresolved abstraction questions. If the PI was uncertain, surgical and medical oncologists were invited to the weekly meeting to resolve the uncertainty. The procedure manual was updated regularly as needed to reflect the changes recommended by the group, and abstractors were retrained on the changes. Following abstraction, data were entered into a Microsoft Access database version 2010 (Microsoft Corporation, Redmond, WA) that mirrored the layout of items in the abstraction form. During data entry, values were checked for errors and upon detection sent to the original abstractor with instructions to check the medical chart. This procedure allowed for an additional review of the abstracted data and served as a further training opportunity for abstractors to avoid repetition of similar errors.
Medical records were abstracted for a total of 634 (AA=304; white=334) patients, who comprised this study's sample for analysis.
Telephone interview
To obtain further details on BC diagnosis and supplement the information abstracted from medical records, semistructured phone interviews were conducted with a subset of patients. The interviews were on average 30 minutes long, and a telephone-recording jack was used to tape record both sides of the conversation for transcription. The interview consisted of a brief structured interview followed by a longer semistructured interview. In the structured part, patients were asked how and when their BC was first found, the date of their first physician visit for their BC, and what treatments were recommended. Participants were also queried as a yes/no response on the presence of situational barriers, such as transportation issues, responsibility for daily caring of dependents (caring demand) and job-related demand when they were diagnosed with BC. In the semistructured component of the interview, patients were asked to elaborate on factors that influenced their choice of treatment, including the role of situational barriers. Only quantitative data collected in the structured part of the interview were used in the present analysis.
Female interviewers who received specialized training in BC biology, treatment, and interviewing techniques conducted the interviews. The training was developed and conducted by the PI, a medical anthropologist, and a national expert on qualitative methodologies. The training was focused on ethnographic interview types, with emphasis on semistructured interviews. The training interviewers also reviewed BC pathology and treatment, followed by a question-by-question review of the semistructured interview questionnaire. Prior to the training, all interviewers were provided with reading material to familiarize themselves with staging and treatment terminology. A procedure manual with detailed instructions was also provided to the interviewers for reference. Because qualitative studies require a much smaller sample size, we aimed to conduct interviews with at least 50% of patients. Patients were recruited consecutively, and a total of 369 interviews were completed (AA=143; whites=226).
Study outcomes
Diagnosis delay was defined as days from date of first symptom recognition to date of biopsy-proven diagnosis. The first symptom or abnormality was detected as either a sign or a symptom by the patient, on a clinical breast exam (CBE) conducted by the patient's physician, or by a screening mammogram. If the month and year were known but the exact date of the symptom recognition was not documented, the 15th of the month was used as a proxy. This approach has been used previously.23 The date of first abnormality detection was completely unavailable for 12 women, so 622 (AA=295; white=327) women were included in the analysis for diagnosis delay.
Surgical delay was defined as the interval in days from biopsy-proven diagnosis to first operation (breast-conserving surgery [BCS] or mastectomy). Examination of surgical delay excluded patients whose first operation was done without a diagnostic biopsy (n=1), if cancer was diagnosed with an excisional biopsy with no subsequent surgical treatment (n=22), who received neoadjuvant chemotherapy (n=35), or if the date of first operation was unavailable (n=1). After these exclusions, 575 (AA=271; white=304) women were examined for surgical delay.
Predictors of delay
We examined the impact of several patient, clinical, and health system factors in predicting delay for both AAs and whites. These factors included age at diagnosis; education; medical insurance; mode of detection; family history of BC; comorbidity burden; operation type; tumor size, grade, and lymph node status; and surgical-facility type (categorized according to the American College of Surgeons Commission on Cancer).24 Situational barriers, including transportation issues, caring demand, and job-related demand, were examined separately for patients who completed the telephone interviews.
Statistical analysis
Descriptive statistics by race were examined for sociodemographic, clinical, and tumor characteristics. Racial differences in study outcomes were examined using percentages of patients with delay in diagnosis and surgery of <2 months, 2 to <3 months, and ≥3 months. Unadjusted binomial regression models were used to compute the relative risk (RR) with 95% confidence interval (CI) comparing AAs vs. whites for delays at ≥2 and ≥3 months. Race-adjusted and race-stratified multivariable binomial regression models were utilized to examine independent predictors of ≥2 months delay. For both outcomes, the multivariable models were adjusted for the following predictors: age at diagnosis, medical insurance, family history of BC, comorbidity count, and tumor size, grade, and lymph node status. In addition, mode of detection was included for the diagnosis-delay model, and operation type and surgical facility were included for the surgical-delay model. A separate adjusted regression analysis including interviewed patients was conducted to examine situational barriers as predictors of each delay. Finally, we examined reasons for ≥2 months delay in diagnosis if available from medical records, overall as well as by race. All statistical tests were two-sided, and all analyses were performed using SAS software, version 9.2 (SAS Institute Inc., Cary, NC).
Results
A total of 304 AA and 330 white women were included in the study. Compared to whites, AAs were more likely to have less than a 4-year college degree, nonprivate medical insurance, and more comorbidities (Table 1). The telephone-interview participants were similar to the overall population (data not shown). Using both the medical-record and interview data, we identified information on first symptom recognition for all patients in the study. AA women were more likely to self-detect their cancer rather than have it detected through a screening mammogram or CBE by the physician. Situational barriers were distributed similarly between both races. AAs were more likely than whites to be diagnosed with larger (>2 cm), poorly differentiated, and triple-negative tumors (Table 2). No differences by race were seen for operation type, but AAs were more likely to receive surgery at a community facility.
Table 1.
Characteristics of Study Participants, by Race
| African American | White | p-valuea | |
|---|---|---|---|
| Sociodemographics and clinical characteristics, % | n=304 | n=330 | |
| Age, years | 0.1487 | ||
| <55 | 46.4 | 52.1 | |
| ≥55 | 53.6 | 47.9 | |
| Education | <0.0001 | ||
| <4-year college | 63.2 | 46.0 | |
| ≥4-year college | 25.7 | 41.8 | |
| Unknown | 11.2 | 12.1 | |
| Health insurance | 0.0006 | ||
| Private | 61.8 | 75.5 | |
| Nonprivate | 31.9 | 19.1 | |
| Unknown | 6.3 | 5.5 | |
| Family history | 0.0487 | ||
| Yes | 36.8 | 44.5 | |
| No | 63.2 | 55.5 | |
| Comorbidity count | <0.0001 | ||
| 0 | 14.8 | 29.7 | |
| ≥1 | 85.2 | 70.3 | |
| Mode of detection | 0.0013 | ||
| Self-detected | 48.4 | 35.8 | |
| Clinical breast exam/mammogram | 51.6 | 64.2 | |
| Situational barriers,b% | n=143 | n=226 | |
| Caring demand | 0.2455 | ||
| Yes | 34.7 | 29.2 | |
| No | 64.6 | 70.8 | |
| Unknown | 0.7 | 0.0 | |
| Transportation issues | 0.9499 | ||
| Yes | 12.5 | 11.5 | |
| No | 85.4 | 87.2 | |
| Unknown | 2.1 | 1.3 | |
| Job-related demands | 0.2805 | ||
| Yes | 3.5 | 7.5 | |
| No | 69.2 | 66.8 | |
| Not employed | 27.3 | 25.7 |
p-values were generated from chi-square test of difference in proportions.
Patients who completed telephone interview.
Table 2.
Treatment and Tumor Characteristics of Study Participants, by Race
| Surgery and tumorcharacteristics, % | African American n=304 | White n=330 | p-valuea |
|---|---|---|---|
| Operation type | 0.7123 | ||
| Breast-conserving surgery | 56.9 | 59.1 | |
| Mastectomy | 42.4 | 40.6 | |
| No surgery | 0.7 | 0.3 | |
| Tumor size | <0.0001 | ||
| ≤1.0 cm | 29.6 | 42.4 | |
| >1.0 cm to ≤2.0 cm | 30.6 | 34.9 | |
| >2.0 cm | 39.8 | 22.7 | |
| Tumor grade | 0.0005 | ||
| Well/moderate | 52.0 | 67.0 | |
| Poor | 42.4 | 28.2 | |
| Unknown | 5.6 | 4.9 | |
| Lymph node | 0.3832 | ||
| Negative | 71.7 | 74.5 | |
| Positive | 26.6 | 24.8 | |
| Unknown | 1.6 | 0.6 | |
| ER and PR | <0.0001 | ||
| One positive | 16.1 | 9.1 | |
| Both positive | 55.6 | 76.1 | |
| Both negative | 27.3 | 14.8 | |
| Unknown | 1.0 | 0.0 | |
| Her2 | 0.1380 | ||
| Positive | 18.8 | 14.5 | |
| Negative | 76.6 | 82.7 | |
| Unknown | 4.6 | 2.7 | |
| Triple negative | 20.1 | 9.1 | <0.0001 |
| Surgical facility | 0.0964 | ||
| Community-based | 41.8 | 34.2 | |
| Teaching-based | 49.7 | 58.2 | |
| Other | 8.6 | 7.6 |
p-values were generated from chi-square test of difference in proportions.
ER, estrogen receptor; Her2, human epidermal growth factor receptor 2; PR, progesterone receptor.
As shown in Table 3, the median time to diagnosis was significantly longer for AAs than for whites (49 days vs. 36 days, p<0.001), with 75% of whites receiving diagnosis within 2 months from symptom recognition compared to 60% of AAs. Median time to first surgical treatment was 29 days for whites vs. 32 days for AAs (p=0.02), with 92% of whites compared to 80% of AAs receiving surgery within 2 months from diagnosis (p<0.001).
Table 3.
Distribution and Risk of Diagnosis and Surgical Delay Comparing African American with White Women
| African American | White | Unadjusted relative risk (95% CI) | |
|---|---|---|---|
| Diagnosis delay | n=295 | n=327 | |
| Median* | 49 days | 36 days | — |
| ≥2 months | 40.0% | 25.4% | 1.58 (1.25–1.99) |
| ≥3 months | 26.4% | 17.5% | 1.52 (1.12–2.05) |
| Surgical delay | n=271 | n=304 | |
| Median* | 32 days | 29 days | — |
| ≥2 months | 19.6% | 7.9% | 2.48 (1.57–3.90) |
| ≥3 months | 6.6% | 1.0% | 6.73 (2.00–22.60) |
p<0.05.
CI, confidence interval.
The unadjusted binomial regression models estimated that compared to whites, AAs were 52% more likely to experience ≥3 months and 58% more likely to experience ≥2 months delay in diagnosis (Table 3). Furthermore, AAs were almost 7 times and 2.5 times more likely than whites to experience ≥3 months and ≥2 months surgical delay, respectively.
Predictors of ≥2 months delay
In the adjusted analyses for the overall study population, AA women were 1.44 times and 3.08 times more likely than white women to experience diagnosis and surgical delay, respectively (Table 4). Furthermore, self-detection of cancer, as compared with CBE or screening mammogram (RR=1.73), was associated with significant increased risk of diagnosis delay. The risk of surgical delay was also significantly higher for <4-year compared to ≥4-year college education (RR=1.08) and for mastectomy compared to BCS (RR=2.45); it was lower for >1.0 cm compared to ≤1.0 cm tumors (RR=0.62). In the models adjusted for situational barriers, AAs were at increased risk for both diagnosis delay (RR=1.80) and surgical delay (RR=2.62) as compared to whites. In addition, job-related demands for those employed was associated with diagnosis delay (RR=1.67).
Table 4.
Adjusted Relative Risk with 95% Confidence Interval for Predictors of Diagnosis and Surgical Delay ≥2 Months in the Overall Study Population
| Diagnosis delay | Surgical delay | |
|---|---|---|
| Panel Aa | n=622 | n=575 |
| Race | ||
| African American vs. white | 1.44 (1.12, 1.86)* | 3.08 (1.88–5.04)* |
| Age, years | ||
| ≥55 vs. <55 | 0.92 (0.71–1.20) | 0.75 (0.48–1.17) |
| Education | ||
| <4-year college vs. ≥4-year college | 0.98 (0.94–1.02) | 1.08 (1.02–1.13)* |
| Health insurance | ||
| Nonprivate vs. private | 1.22 (0.94–1.58) | 0.89 (0.56–1.43) |
| Mode of detection | ||
| Self-detected vs. clinical breast exam/mammogram | 1.73 (1.31–2.29)* | — |
| Family history | ||
| Yes vs. no | 0.90 (0.71–1.14) | 1.13 (0.74–1.72) |
| Comorbidity count | ||
| ≥1 vs. 0 | 1.11 (0.82–1.52) | 1.11 (0.64–1.94) |
| Tumor size | ||
| >1.0 cm vs. ≤1.0 cm | 0.77 (0.58–1.03) | 0.62 (0.40–0.96)* |
| Tumor grade | ||
| Poor vs. well/moderate | 0.86 (0.66–1.11) | 1.11 (0.70–1.78) |
| Lymph node | ||
| Positive vs. negative | 1.10 (0.85–1.43) | 0.91 (0.56–1.47) |
| Surgical facility | ||
| Community-based vs. teaching-based | 0.85 (0.56–1.29) | |
| Operation type | ||
| Mastectomy vs. breast-conserving surgery | — | 2.45 (1.53–3.94)* |
| Panel Ba | n=369 | n=339 |
| Race | ||
| African American vs. white | 1.80 (1.27–2.55)* | 2.62 (1.37–5.01)* |
| Caring demand | ||
| Yes vs. no | 0.82 (0.56–1.20) | 1.20 (0.63–2.26) |
| Transportation issues | ||
| Yes vs. no | 0.77 (0.41–1.42) | 1.41 (0.64–3.09) |
| If employed, job-related demands | ||
| Yes vs. no | 1.67 (1.05–2.66)* | 1.12 (0.38–3.28) |
Adjusted for all predictors within the panel.
p<0.05.
Table 5 shows results from race-stratified multivariable binomial regression models. Among white women, those who self-detected their cancer were 1.74 times more likely to experience diagnosis delay as compared to those whose cancer was detected by CBE or screening mammogram. Higher tumor grade was associated with more timely diagnosis (RR=0.51). Larger tumor size was associated with lower risk of surgical delay (RR=0.34), but white women who received mastectomy rather than BCS were more than 5 times likely to experience surgical delay (RR=5.35). Among AA women, those with nonprivate insurance and self-detected tumors were 1.4 and 1.81 times more likely to have diagnosis delay compared to those with private insurance and detection by CBE or screening mammogram, respectively. AA women with larger tumors were also less likely to delay diagnosis (RR=0.61). Education level below a 4-year college degree and receipt of mastectomy were associated with 1.10 and 1.96 times higher risk of surgical delay, respectively.
Table 5.
Adjusted Relative Risk with 95% Confidence Interval for Predictors of Diagnosis and Surgical Delay ≥2 Months, by Race
| African American | White | |
|---|---|---|
| Predictors of diagnosis delaya | n=295 | n=327 |
| Age, years | ||
| ≥55 vs. <55 | 0.90 (0.65–1.25) | 0.92 (0.60–1.41) |
| Education | ||
| <4-year college vs. ≥4-year college | 0.97 (0.92–1.03) | 1.00 (0.94–1.07) |
| Health insurance | ||
| Nonprivate vs. private | 1.40 (1.02–1.93)* | 1.11 (0.68–1.81) |
| Mode of detection | ||
| Self-detected vs. clinical breast exam/mammogram | 1.81 (1.24–2.65)* | 1.74 (1.13–2.68)* |
| Family history | ||
| Yes vs. no | 0.97 (0.72–1.31) | 0.81 (0.55–1.18) |
| Comorbidity count | ||
| ≥1 vs. 0 | 1.00 (0.67–1.51) | 1.20 (0.75–1.89) |
| Tumor size | ||
| >1.0 cm vs. ≤1.0 cm | 0.61 (0.40–0.91)* | 0.95 (0.61–1.46) |
| Tumor grade | ||
| Poor vs. well/moderate | 1.31 (0.82–1.56) | 0.51 (0.31–0.85)* |
| Lymph node | ||
| Positive vs. negative | 0.99 (0.72–1.38) | 1.24 (0.80–1.91) |
| Predictors of surgical delaya | n=271 | n=304 |
| Age, years | ||
| ≥55 vs. <55 | 0.69 (0.43–1.11) | 0.81 (0.27–2.39) |
| Education | ||
| <4-year college vs. ≥4-year college | 1.10 (1.04–1.16)* | 1.02 (0.88–1.19) |
| Health insurance | ||
| Nonprivate vs. private | 0.99 (0.60–1.63) | 0.60 (0.14–2.59) |
| Family history | ||
| Yes vs. no | 1.05 (0.64–1.73) | 2.06 (0.82–5.17) |
| Comorbidity count | ||
| ≥1 vs. 0 | 1.75 (0.74–4.11) | 0.87 (0.29–2.61) |
| Tumor size | ||
| >1.0 cm vs. ≤1.0 cm | 0.65 (0.38–1.10) | 0.34 (0.14–0.85)* |
| Tumor grade | ||
| Poor vs. well/moderate | 1.27 (0.75–2.16) | 0.77 (0.27–2.26) |
| Lymph node | ||
| Positive vs. negative | 0.84 (0.47–1.50) | 1.37 (0.50–3.75) |
| Surgical facility | ||
| Community-based vs. teaching-based | 0.91 (0.57–1.44) | 0.67 (0.26–1.77) |
| Operation type | ||
| Mastectomy vs. breast-conserving surgery | 1.96 (1.16–3.31)* | 5.35 (1.73–16.57)* |
Adjusted for all predictors within the panel.
p<0.05.
Of the 622 subjects examined for diagnosis delay, a total of 201 (32.3%) experienced ≥2 months diagnosis delay. Medical records for these patients were searched for reason(s) that could explain the delay. For more than half these patients (54.2%), no particular reason was identified or available in the records. Patient-related reasons were identified in 28.9% of cases; health system or provider related, in 16.9% of cases. Patient-related reasons included ignoring symptoms until they became worse, health-related or personal issues, insurance, and seeking a second opinion. Provider- and health-system-related reasons included dismissal of symptoms by provider, patient asked to follow up in 6 months, inconclusive diagnostic work-up requiring additional testing, and scheduling issues. No differences by race were noted for delay reasons.
Discussion
Among women with early-stage invasive BC in this study, AAs were significantly more likely than whites to experience diagnosis and surgical delays. Furthermore, variations were observed in both patient- and clinical-related factors that predicted delays between AA and white women.
Despite the possible negative impact of delayed diagnosis on BC survival, our study shows that AA women are continuing to experience significantly longer diagnosis delay as compared to their white counterparts in current practice, even after adjusting for important sociodemographic and clinical factors, as well as situational barriers. Diagnosis delay encompasses a time period that can be affected by a combination of patient-, provider-, and health-system-related factors.23,25 Particularly for AAs, who are more likely to be medically underserved, issues related to access to care are considered a major barrier to timely diagnosis. This factor was supported by our study, as nonprivate insurance was an independent predictor of diagnosis delay for AA women but not for white women or the overall population. In addition to socioeconomic factors, a variety of cultural and psychosocial factors have been related to diagnosis delay.26 But only a few studies have examined the impact of self-recognition of symptoms, primarily owing to difficulty in capturing this information.23,27,28 We found that patients who self-detected their cancer were more likely to experience diagnosis delay as compared to patients whose cancer was detected through CBE or screening mammography for both groups. The proportion of patients with ≥2 months diagnosis delay was higher among AA patients than in white patients, irrespective of the method of detection. This finding was true for both self-detected patients (47.1% and 34.5%, respectively) and for CBE/screening-mammogram-detected patients (33.8% and 20.4%, respectively). Patients who self-detect their lump are likely to have larger tumors and to have nonprivate insurance. Although none of the other clinical predictors were associated with diagnosis delay in the overall study population, the stratified analysis identified that longer delays were found among AA women with smaller tumors, whereas a lower-grade tumor was a factor of delay among white women. These findings likely reflect the priority given to women with tumors that appear large or aggressive.
Racial differences in treatment delay have been previously reported, with most studies showing longer delay for AAs in comparison to whites,5,6,23,29–32 although a few found no difference.8,33–35 Inability to differentiate the type and sequence of therapy, particularly neoadjuvant, radiation and surgical treatment sequence, was a limitation for many of the previous investigations. Although time is required for preoperative evaluation before the appropriate surgery is performed, it is shown that ≥2 months delay in initiating surgical treatment can negatively impact survival.3,5 Keeping the wait times between diagnosis and surgery below 60 days is considered an important measure of quality of care as well.36–38 Two recent studies specifically examined surgical delay and predictors associated with it; only one of the studies included race and reported significant differences.5,39 Similarly, AAs in our study were more likely than whites to experience surgical delay after accounting for differences in their sociodemographic and clinical characteristics. In the overall study population, lower education level, smaller tumor size, and mastectomy were additional predictors of surgical delay. Longer surgical delay for mastectomy compared to BCS can be attributed to additional time required for making treatment decisions, consultation for reconstructive surgery after mastectomy, and scheduling coordination between the breast and reconstructive surgeons. When examined separately, mastectomy was associated with a longer surgical delay compared to BCS in both groups, but the strength of association was much higher in whites than in AAs. Furthermore, smaller tumor size was identified as a predictor only among whites and lower educational attainment among AAs. This finding supports emerging evidence that a combination of both clinical and nonclinical factors play an important role throughout the continuum of BC care delivered to AA women.40
Our study has several limitations. The participation rate for older women was poorer than in younger women. Older women are generally at increased risk of delay, and their lower response rate may have underestimated delay estimates in our study. We were able to examine predictors only of 2-month delay and had limited statistical power to examine predictors of 3- month delay. Furthermore, the study was limited to NJ residents and may not be generalizable to women living elsewhere. Another potential limitation was the unavailability of precise dates for symptom recognition for several patients. For the 622 patients included in the analysis of diagnosis delay, 202 had the 15th day of the month captured for the date of symptom recognition. However, we cannot quantify how many of these dates were actual and how many were substituted. We believe that the missing information on day will be randomly distributed and nondifferential. It is not likely to create any systematic bias, especially since the abstractors were blinded to the study hypothesis. Nevertheless, we examined the distribution of the 202 patients with midmonth day by race. It was distributed approximately equally between whites (n=101) and AAs (n=101), so it is unlikely to bias race differences. On the other hand, the study was strengthened by its population-based design covering all major hospitals in a large area in NJ. We were able to define delay with more accuracy by measuring it from the time of first abnormality in contrast to examining it from the time of first health service contact, which may underestimate the delay period. We also had the advantage of examining several predictors, including sociodemographic, clinical, and treatment characteristics, from medical records and situational barriers from patient interviews.
This is one of the first few studies to examine differences in predictors of delay between and within races. The utilization of stratified analysis generated better understanding of how predictors of delay differ in each race. The findings of this study suggest that AAs experienced significantly longer diagnosis and surgical delays than did whites. Both patient and health system factors, such as education, self-recognition of symptoms, and insurance status, affected delay, especially in AA women. Interventions are needed to educate and motivate at-risk women and their providers about the importance of prompt assessment of breast symptoms, of adhering to screening guidelines, and of efficient scheduling of diagnostic procedures and initial definitive surgery.
Acknowledgments
This work was supported by grants from the American Cancer Society (RSGT-07-291-01-CPHPS), the Susan G. Komen Breast Cancer Foundation (POP131006), the National Cancer Institute (R01CA133264, R01 CA100598, P01 CA151135, K22 CA138563, P30CA072720, P30 CA016056), the US Army Medical Research and Material Command (DAMD-17-01-1-0334), the Breast Cancer Research Foundation, a gift from the Philip L. Hubbell family, and a gift from the Buckingham Foundation.
The study team is grateful for medical, surgical and radiation oncologists, and primary care physicians who understood the value of research and helped us obtain patients' medical records, without which the conduct of the study would have been impossible.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Howlader N, Noone AM, Krapcho M, et al. . SEER Cancer Statistics Review, 1975–2010. National Cancer Institute, Bethesda, MD, Available at: http://seer.cancer.gov/csr/1975_2010/ (based on November 2012. SEER data submission, posted to the SEER web site, April 2013; accessed on May16, 2014) [Google Scholar]
- 2.DeSantis C, Naishadham D, Jemal A. Cancer statistics for African Americans, 2013. CA Cancer J Clin 2013;63:151–166 [DOI] [PubMed] [Google Scholar]
- 3.McLaughlin JM, Anderson RT, Ferketich AK, Seiber EE, Balkrishnan R, Paskett ED. Effect on survival of longer intervals between confirmed diagnosis and treatment initiation among low-income women with breast cancer. J Clin Oncol 2012;30:4493–4500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richards MA, Westcombe AM, Love SB, Littlejohns P, Ramirez AJ. Influence of delay on survival in patients with breast cancer: Asystematic review. Lancet 1999;353:1119–1126 [DOI] [PubMed] [Google Scholar]
- 5.Smith EC, Ziogas A, Anton-Culver H. Delay in surgical treatment and survival after breast cancer diagnosis in young women by race/ethnicity. JAMA Surg 2013;148:516–523 [DOI] [PubMed] [Google Scholar]
- 6.Gorin SS, Heck JE, Cheng B, Smith SJ. Delays in breast cancer diagnosis and treatment by racial/ethnic group. Arch Intern Med 2006;166:2244–2252 [DOI] [PubMed] [Google Scholar]
- 7.Hoffman HJ, LaVerda NL, Levine PH, et al. . Having health insurance does not eliminate race/ethnicity-associated delays in breast cancer diagnosis in the District of Columbia. Cancer 2011;117:3824–3832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith ER, Adams SA, Das IP, Bottai M, Fulton J, Hebert JR. Breast cancer survival among economically disadvantaged women: The influences of delayed diagnosis and treatment on mortality. Cancer Epidemiol Biomarkers Prev 2008;17:2882–2890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coates RJ, Uhler RJ, Brogan DJ, et al. . Patterns and predictors of the breast cancer detection methods in women under 45 years of age (United States). Cancer Causes Control 2001;12:431–442 [DOI] [PubMed] [Google Scholar]
- 10.Thind A, Diamant A, Hoq L, Maly R. Method of detection of breast cancer in low-income women. J Womens Health (Larchmt) 2009;18:1807–1811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reifenstein K. Care-seeking behaviors of African American women with breast cancer symptoms. Res Nurs Health 2007;30:542–557 [DOI] [PubMed] [Google Scholar]
- 12.Blackman DJ, Masi CM. Racial and ethnic disparities in breast cancer mortality: Are we doing enough to address the root causes? J Clin Oncol 2006;24:2170–2178 [DOI] [PubMed] [Google Scholar]
- 13.Breen N, Yabroff KR, Meissner HI. What proportion of breast cancers are detected by mammography in the United States? Cancer Detecti Prev 2007;31:220–224 [DOI] [PubMed] [Google Scholar]
- 14.Jones CP. Invited commentary: “Race,” racism, and the practice of epidemiology. Am J Epidemiol 2001;154:299–304; discussion 305–306 [DOI] [PubMed] [Google Scholar]
- 15.Karter AJ. Race and ethnicity: Vital constructs for diabetes research. Diabetes Care 2003;26:2189–2193 [DOI] [PubMed] [Google Scholar]
- 16.McGee SA, Durham DD, Tse CK, Millikan RC. Determinants of breast cancer treatment delay differ for African American and White women. Cancer Epidemiol Biomarkers Prev 2013;22:1227–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilbert GH, Shah GR, Shelton BJ, Heft MW, Bradford EH, Jr., Chavers LS. Racial differences in predictors of dental care use. Health Serv Res 2002;37:1487–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu YW, Xing G, Fuentes-Afflick E, Danielson B, Smith LH, Gilbert WM. Racial, ethnic, and socioeconomic disparities in the prevalence of cerebral palsy. Pediatrics 2011;127:e674–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wightkin J, Magnus JH, Farley TA, Boris NW, Kotelchuck M. Psychosocial predictors of being an underweight infant differ by racial group: A prospective study of Louisiana WIC program participants. Matern Child Health J 2007;11:49–55 [DOI] [PubMed] [Google Scholar]
- 20.Lipworth L, Mumma MT, Cavanaugh KL, et al. . Incidence and predictors of end stage renal disease among low-income blacks and whites. PloS One 2012;7:e48407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ambrosone CB, Ciupak GL, Bandera EV, et al. . Conducting molecular epidemiological research in the age of HIPAA: A multi-institutional case-control study of breast cancer in African-American and European-American women. J Oncol 2009;2009:871250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bandera EV, Chandran U, Zirpoli G, et al. . Body size in early life and breast cancer risk in African American and European American women. Cancer Causes Control 2013;24:2231–2243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gwyn K, Bondy ML, Cohen DS, et al. . Racial differences in diagnosis, treatment, and clinical delays in a population-based study of patients with newly diagnosed breast carcinoma. Cancer 2004;100:1595–1604 [DOI] [PubMed] [Google Scholar]
- 24.Bell M, Lommel T, Fischer JG, Lee JS, Reddy S, Johnson MA. Improved recognition of heart attack and stroke symptoms after a community-based intervention for older adults, Georgia, 2006–2007. Prev Chronic Dis 2009;6:A41. [PMC free article] [PubMed] [Google Scholar]
- 25.Chang SW, Kerlikowske K, Napoles-Springer A, Posner SF, Sickles EA, Perez-Stable EJ. Racial differences in timeliness of follow-up after abnormal screening mammography. Cancer 1996;78:1395–1402 [DOI] [PubMed] [Google Scholar]
- 26.Lannin DR, Mathews HF, Mitchell J, Swanson MS, Swanson FH, Edwards MS. Influence of socioeconomic and cultural factors on racial differences in late-stage presentation of breast cancer. JAMA 1998;279:1801–1807 [DOI] [PubMed] [Google Scholar]
- 27.Maly RC, Leake B, Mojica CM, Liu Y, Diamant AL, Thind A. What influences diagnostic delay in low-income women with breast cancer? J Womens Health (Larchmt) 2011;20:1017–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruddy KJ, Gelber S, Tamimi RM, et al. . Breast cancer presentation and diagnostic delays in young women. Cancer 2014;120:20–25 [DOI] [PubMed] [Google Scholar]
- 29.Fedewa SA, Edge SB, Stewart AK, Halpern MT, Marlow NM, Ward EM. Race and ethnicity are associated with delays in breast cancer treatment (2003–2006). J Health Care Poor Underserved 2011;22:128–141 [DOI] [PubMed] [Google Scholar]
- 30.Fedewa SA, Ward EM, Stewart AK, Edge SB. Delays in adjuvant chemotherapy treatment among patients with breast cancer are more likely in African American and Hispanic populations: A national cohort study 2004–2006. J Clin Oncol 2010;28:4135–4141 [DOI] [PubMed] [Google Scholar]
- 31.Caplan LS, May DS, Richardson LC. Time to diagnosis and treatment of breast cancer: Results from the National Breast and Cervical Cancer Early Detection Program, 1991–1995. Am J Public Health 2000;90:130–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gold HT, Do HT, Dick AW. Correlates and effect of suboptimal radiotherapy in women with ductal carcinoma in situ or early invasive breast cancer. Cancer 2008;113:3108–3115 [DOI] [PubMed] [Google Scholar]
- 33.Balasubramanian BA, Demissie K, Crabtree BF, Strickland PA, Pawlish K, Rhoads GG. Black Medicaid beneficiaries experience breast cancer treatment delays more frequently than whites. Ethn Dis 2012;22:288–294 [PubMed] [Google Scholar]
- 34.Williams DL, Tortu S, Thomson J. Factors associated with delays to diagnosis and treatment of breast cancer in women in a Louisiana urban safety net hospital. Women Health 2010;50:705–718 [DOI] [PubMed] [Google Scholar]
- 35.Hershman DL, Wang X, McBride R, Jacobson JS, Grann VR, Neugut AI. Delay of adjuvant chemotherapy initiation following breast cancer surgery among elderly women. Breast Cancer Res Treat 2006;99:313–321 [DOI] [PubMed] [Google Scholar]
- 36.McCahill LE, Privette A, James T, et al. . Quality measures for breast cancer surgery: Initial validation of feasibility and assessment of variation among surgeons. Arch Surge 2009;144:455–462; discussion 462–463 [DOI] [PubMed] [Google Scholar]
- 37.Gabel M, Hilton NE, Nathanson SD. Multidisciplinary breast cancer clinics. Do they work? Cancer 1997;79:2380–2384 [PubMed] [Google Scholar]
- 38.Richardson LC, Royalty J, Howe W, Helsel W, Kammerer W, Benard VB. Timeliness of breast cancer diagnosis and initiation of treatment in the National Breast and Cervical Cancer Early Detection Program, 1996–2005. Am J Public Health 2010;100:1769–1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wagner JL, Warneke CL, Mittendorf EA, et al. . Delays in primary surgical treatment are not associated with significant tumor size progression in breast cancer patients. Ann Surg 2011;254:119–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shelton RC, Clarke Hillyer G, Hershman DL, et al. . Interpersonal influences and attitudes about adjuvant therapy treatment decisions among non-metastatic breast cancer patients: An examination of differences by age and race/ethnicity in the BQUAL study. Breast Cancer Res Treat 2013;137:817–828 [DOI] [PMC free article] [PubMed] [Google Scholar]