Abstract
Spontaneous canine models exist for several inherited retinal dystrophies. This review will summarize the models and indicate where they have been used in translational gene therapy trials. The RPE65 gene therapy trials to treat childhood blindness are a good example of how studies in dogs have contributed to therapy development. Outcomes in human clinical trials are compared and contrasted with the result of the preclinical dog trials.
Introduction
Development and testing of therapies in animal models is critical prior to commencing human clinical trials. Animal studies can provide proof of principle for the therapeutic approach, can be used to modify or refine the approach, and also provide information on the safety of the treatment. Studies utilizing spontaneously occurring canine models of inherited retinal dystrophies have been important in the translation of therapies from the laboratory to the clinic—most notably, gene therapy for the treatment of Leber congenital amaurosis (LCA) caused by RPE65 gene mutations. Several additional canine retinal dystrophy models are currently being used for preclinical therapeutic trials, some of which are currently, or soon will be, translated from these large-animal studies to human clinical trials.
Existing Canine Models
Retinal dystrophies encompass several groups of conditions, including LCA, retinitis pigmentosa (RP), cone–rod dystrophy, achromatopsia, and macular dystrophy. Dogs with spontaneous inherited retinal dystrophies that provide models within each of these categories have been identified (Table 1). The studies to identify the gene mutations underlying retinal dystrophies in dogs have served two purposes: first, the conditions result in vision loss in pet or working animals and identifying the causal mutations enables DNA testing, selective breeding, and eradication of the condition; second, and most relevant for this review, is that often mutations occur in the homologous gene in both dog and man and the resulting phenotype in both species is comparable. Establishing a research colony of affected animals allows detailed examination of the pathophysiological processes and the testing of therapies.
Table 1.
Dog Models of Inherited Retinal Degenerations with Identified Gene Mutations
| Condition | Gene | Breed | Reference to mutation | References to therapy |
|---|---|---|---|---|
| Leber congenital amaurosis models | ||||
| Congenital stationary night blindness/retinal dystrophy | Rpe65 | Briard | 14 | 16–20,22,24,41,43,45–47,94–100 |
| Autosomal recessive retinitis pigmentosa models | ||||
| Rod–cone dysplasia type 1 | Pde6b | Irish setter, red and white setter | 52 | 54 |
| Rod–cone dysplasia type 1a | Pde6b | Sloughi | 101 | |
| (Originally classified as a cone–rod dystrophy type 1) | Pde6b | American Staffordshire terrier | 102 | |
| Rod–cone dysplasia type 2 | C1orf36 | Collie | 103 | |
| Rod–cone dysplasia type 3 | Pde6a | Cardigan Welsh corgi | 53 | 56 |
| Early retinal degeneration | Stk38l | Norwegian elkhound | 104 | |
| Golden retriever PRA type 1 | Slc4A3 | Golden retriever | 105 | |
| Progressive retinal atrophy of Papillons type 1 | Cngb1 | Papillon | 106 | |
| Progressive retinal atrophy | Ccdc66 | Schapendoes | 113 | |
| Progressive retinal atrophy | Sag | Basenji | 107 | |
| Progressive rod–cone degeneration | Prcd | Multiple breeds | 108 | |
| Rod–cone degeneration type 4 | C2orf71 | Gordon setter | 109 | |
| Autosomal dominant retinitis pigmentosa models | ||||
| Dominant progressive retinal atrophy | Rhodopsin | Mastiff | 57 | 61 |
| X-linked retinitis pigmentosa models | ||||
| X-linked progressive retinal atrophy 1 | Rpgr | Siberian huskySamoyed | 65 | 66–68 |
| X-linked progressive retinal atrophy 2 | Rpgr | Mongrel | 65 | 66–68 |
| Cone–rod dystrophy models | ||||
| Cone–rod dystrophy 2 | Iqcb1 | Pit bull terrier | 102 | |
| Cone–rod dystrophy 3 | Adam9 | Glen of Imaal terrier | 110 | |
| Cone–rod dystrophy | Nphp4 | Standard wirehaired dachshund | 111 | |
| Cone–rod dystrophy | Rpgrip1 | Miniature longhaired dachshund | 69 | 74 |
| Achromatopsia models | ||||
| Achromatopsia | Cngb3 | Alaskan malamute | 77 | 79,81 |
| Achromatopsia | Cngb3 | German shorthaired pointer | 77 | 79,81 |
| Achromatopsia | Cnga3 | German shepherd dog | 112 | |
| Macular dystrophy models | ||||
| Canine multifocal retinopathy 1 | Best 1 | Mastiff and related breeds | 89 | 92 |
| Canine multifocal retinopathy 2 | Best 1 | Coton de Tulear | 89 | |
| Canine multifocal retinopathy 3 | Best 1 | Lapponian herder | 90 | 92 |
Those in bold have had reported gene therapy trials.
Dog eyes show many similarities to human eyes, for example, in size and morphology and also in density of photoreceptor distribution. The human retina has a region known as the macula, which has a high photoreceptor density and contains the fovea, a cone-only region, from which the inner retinal neurons are displaced to enable highest visual acuity. The macula and fovea are important for high visual acuity color vision. Laboratory rodents that are commonly used as models for retinal dystrophies lack a central retinal region of high photoreceptor density. One major advantage of the dog models is that there is an area centralis that has a higher photoreceptor density, particularly of cones, than the peripheral retina, making it analogous to the human macula.1 Although the photoreceptor packing density is not so high as in the human macula and dogs do not have a true fovea, the center of the area centralis in the dog has recently been shown to have a small foveal-like “bouquet” of cones.2
Gene Therapy for Leber Congenital Amaurosis
Leber congenital amaurosis (LCA) is a genetically heterogeneous condition that results in progressive vision loss starting in childhood. It is an uncommon condition, with incidence being reported to be between 3 in 100,000 and 1 in 81,000.3,4 Mutations of the retinal pigment epithelium (RPE) expressed gene, RPE65, account for about 6% of LCA cases.5 Gene therapy for LCA resulting from mutations in RPE65 (LCARPE65) highlights the use of a large-animal model for proof-of-principle studies. RPE65 is a retinoid isomerase forming a required part of the visual cycle that supplies and recycles light-sensitive retinoid (11-cis retinal) to the rod and cone photoreceptors.6,7 Although there may be a second source of retinoid for the cone photoreceptors,8 it is insufficient in the absence of the RPE visual cycle to allow for normal function.9
In the absence of normal RPE65 activity, the rod and cone photoreceptor opsins do not receive the retinoid 11-cis retinal, which they require to combine with the opsin protein to form the light-sensitive visual pigments. Lack of 11-cis retinal results in very reduced sensitivity and function of the photoreceptors such that affected patients require bright lighting levels for vision. There is thus a very early lack of visual function prior to an advanced loss of photoreceptors. This “disconnect” between loss of function and loss of photoreceptors provides a “window of opportunity” for therapeutic intervention aiming to establish visual cycle activity and thus supply the remaining photoreceptors with retinoid. This proved to be important for the gene augmentation therapeutic approach. The poorly functioning photoreceptors of LCARPE65 patients do degenerate and the retinal pigment epithelium that houses the RPE65 protein and plays a major role in the visual cycle accumulates retinyl esters because of the blockage of the visual cycle (for a review, see ref.10).
A spontaneous retinal dystrophy dog model, initially described as an autosomal recessive congenital stationary night blindness, had been recognized and studied for many years.11 The affected dogs were blind at lower lighting levels (but could see in brighter lights), had very reduced electroretinographic responses (electrical retinal responses to stimulation with flashes of light), and had reduced pupillary light reflexes and nystagmus (the presence of nystagmus is a common feature of early vision loss). Notably, there was a very slow loss of photoreceptors over many years, during which accumulations of vacuoles in the RPE became pronounced.12,13 Eventually, molecular genetic studies revealed the dog model to have a mutation in Rpe65. The affected dog breed was the briard (a French breed), and because of the initial phenotyping studies by a Swedish group, the model was often referred to as the Swedish briard. The gene mutation in the “Swedish” briard is a 4 bp deletion in the Rpe65 gene with a resultant frameshift and premature termination codon.14
Subsequent to the announcement of this finding at a research meeting (Association for Research in Vision and Ophthalmology [ARVO], 1998), briards in the United States with retinal dystrophy were also found to have the same mutation as the Swedish briard.15 Concurrent progress in gene transfer to the retina using adeno-associated viral (AAV) vectors provided a great opportunity to use the newly identified dog model for LCARPE65 for gene therapy proof-of-principle trials. Acland et al. announced at the ARVO meeting in 2001 the dramatic results of the gene therapy trials using the Rpe65-mutant briard dog.16 AAV vectors carrying the Rpe65 cDNA were introduced by subretinal injection and shown to transduce the RPE restoring the visual cycle and resulting in a remarkable improvement in retinal function as assessed by the electroretinogram (ERG) and also in vision as assessed by obstacle course vision testing.16
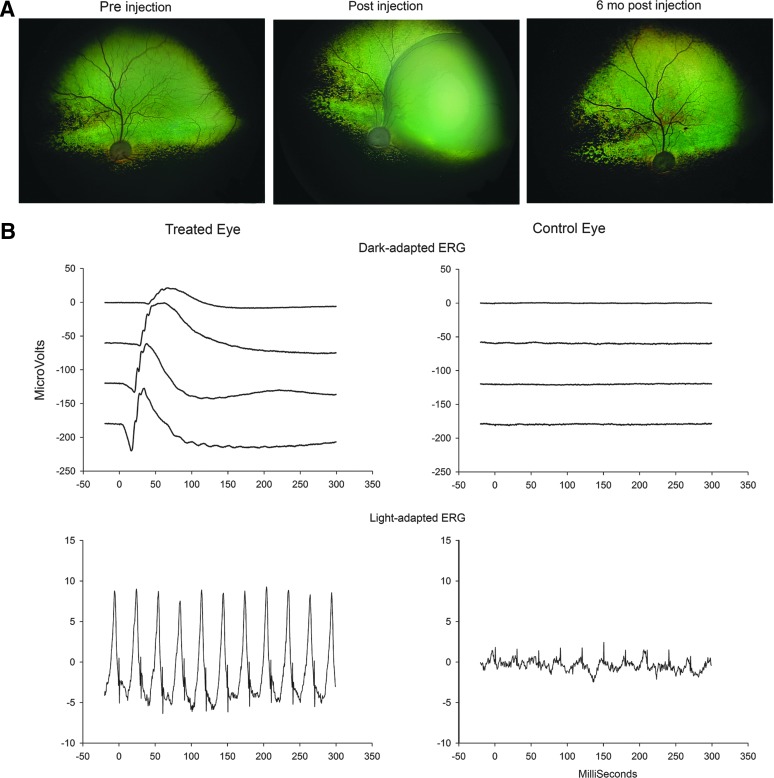
This and follow-on studies from the same and other groups further investigated the therapy and outcome. The therapy in the dog model led not only to a functional visual cycle that supplied 11-cis retinal to the photoreceptors providing an excellent improvement in photoreceptor function and sensitivity as assessed by ERG and vision testing, but also to the normalization of pupillary light reflexes, reduction in nystagmus, and increased visual evoked cortical activity.16–24 Furthermore, the rescue was long-lasting.17 (See Fig. 1 for results of AAV gene augmentation in a mutant dog.)
FIG. 1.
RPE65 gene therapy in a dog. (A) Fundus photographs of the treated eye of an Rpe65-mutant dog preinjection, immediately postinjection, and 6 months postinjection. Note the large region of retinal detachment resulting from the subretinal administration of the viral vector. (B) Electroretinographic outcome. Part of a dark-adapted intensity response series and 33 Hz light-adapted cone flicker response is shown from the treated eye and control eye of an Rpe65-mutant dog.43,47 AAV2/2hRPE65p.hRPE65 vector had been delivered by subretinal injection 4 months previously in the treated eye. The ERG tracings show that there is excellent restoration of both rod- and cone-mediated ERG responses. The magnitude of ERG amplitudes approaches those of normal dogs. (Dark-adapted intensities from top to bottom: 0.0016, 0.0099, 0.064, and 0.399 cdS/m2. Light-adapted 33 Hz cone flicker intensity 2.5 cdS/m2 superimposed on a rod suppressing 30 cd/m2 white-background light). ERG, electroretinogram.
The proof-of-principle gene therapy studies in the briard dog preceded gene therapy studies in Rpe65 mouse models.25–27 On the basis of the success of gene therapy in the dog, three separate groups started phase I dose escalation clinical trials (trials NCT00643747, NCT00516477, and NCT00481546; see Table 2). All three groups reported the initial results of the clinical trials in 2008, showing results from the first three patients treated in each trial.28–30 The initial reports indicated that there were no major adverse effects and that there was evidence of improved visual function in some of the patients.28–30 Subsequently, additional patients have been injected and more detailed analyses of the outcomes have been published.
Table 2.
Current Human Clinical Trials for RPE65 Gene Therapy from ClinicalTrials.gov
| ClinicalTrials.gov identifier | Date | Title | Phase | Sponsor | Locations |
|---|---|---|---|---|---|
| NCT00481546 | 5/31/2007 | Phase I Trial of Ocular Subretinal Injection of a Recombinant Adeno-Associated Virus (rAAV2-CBSB-hRPE65) Gene Vector to Patients with Retinal Disease Due to RPE65 Mutations (Clinical Trials of Gene Therapy for Leber Congenital Amaurosis) | I | University of Pennsylvania | Shands Children's Hospital, University of Florida, Gainesville, FL, Scheie Eye Institute, University of Pennsylvania, Philadelphia, PA |
| NCT00516477 | 8/13/2007 | A Phase 1 Safety Study in Subjects with Leber Congenital Amaurosis (LCA) Using Adeno-Associated Viral Vector to Deliver the Gene for Human RPE65 into the Retinal Pigment Epithelium (RPE) [AAV2-hRPE65v2-101] | I | Spark Therapeutics LLC | The Children's Hospital of Philadelphia, Philadelphia, PA |
| NCT00643747 | 3/20/2008 | An Open-label Dose Escalation Study of an Adeno-associated Virus Vector (AAV2/2-hRPE65p-hRPE65) for Gene Therapy of Severe Early-onset Retinal Degeneration | I/II | University College London | Moorfields Eye Hospital NHS Foundation Trust, London, UK |
| NCT00749957 | 9/8/2008 | A Multiple-Site, Phase 1/2, Safety and Efficacy Trial of a Recombinant Adeno-Associated Virus Vector Expressing RPE65 (rAAV2-CB-hRPE65) in Patients with Leber Congenital Amaurosis Type 2 | I/II | AGTC | University of Massachusetts Medical School, Worcester, MA. Casey Eye Institute, Oregon Health & Science University, Portland, OR |
| NCT00821340 | 1/12/2009 | Phase I Trial of Ocular Subretinal Injection of a Recombinant Adeno-Associated Virus (rAAV2-hRPE65) Gene Vector to Patients with Retinal Disease Due to RPE65 Mutations | I | Hadassah Medical Organization | Hadassah Medical Organization, Jerusalem, Israel |
| NCT00999609 | 10/21/2009 | A Safety and Efficacy Study in Subjects with Leber Congenital Amaurosis (LCA) Using Adeno-Associated Viral Vector to Deliver the Gene for Human RPE65 to the Retinal Pigment Epithelium (RPE) [AAV2-hRPE65v2-301] | III | Spark Therapeutics LLC | University of Iowa, Children's Hospital of Philadelphia, Philadelphia, PA |
| NCT01208389 | 9/22/2010 | A Follow-On Study to Evaluate the Safety of Re-Administration of Adeno-Associated Viral Vector Containing the Gene for Human RPE65 [AAV2-hRPE65v2] to the Contralateral Eye in Subjects with Leber Congenital Amaurosis (LCA) Previously Enrolled in a Phase 1 Study | I/II | Spark Therapeutics LLC | The Children's Hospital of Philadelphia, Philadelphia, PA |
| NCT01496040 | 11/24/2011 | Prospective Monocentric Open-Label Non-Randomized Uncontrolled Phase I/II Clinical Gene Therapy Protocol for the Treatment of Retinal Dystrophy Caused by Defects in RPE65 | I/II | Nantes University Hospital | CHU Nantes, Nantes, France |
The group based at University College London has treated 12 patients (NCT00643747); the group headed by The Children's Hospital of Philadelphia has reported on the outcome of 12 patients (NCT00516477, NCT00999609, and NCT01208389), and the group headed by University of Pennsylvania has reported on 15 patients (NCT00481546 and NCT00749957). Subsequently, clinical trials have started in Jerusalem, sponsored by Hadassah Medical Organization (NCT00821340),31 and in France, at Nantes University Hospital (NCT01496040). Although each group has used an AAV2 construct injected subretinally, the constructs differed as to which promoter was used and whether an intron or kozak sequence was included. Furthermore, the titers and volume injected varied between the trials. No significant immune responses were reported for any study.32,33
Some procedural-related complications did develop, for example, a macula hole in one patient,29 and as patients were analyzed it became apparent that causing a foveal detachment during subretinal administration of the vector could be deleterious and resulted in foveal thinning in some patients.34 Procedural changes have been implemented so that injections avoid causing foveal detachment, and at least one group has started injecting in two sites in the same eye and speculated that in the future three sites of injection may provide optimal retinal coverage with minimal risk of procedural complications.34 Assessment of outcome of therapy has consisted of a battery of different specialized tests designed to detect and quantify improvements in visual function over baseline. These included testing ability to perceive light (such as full-field sensitivity and microperimetry), visual acuity, ability to negotiate an obstacle course, investigation of eye movements and fixation, pupillometry, electrophysiological testing, and functional MRI (see ref.10 for a comprehensive review).
The results have been very encouraging, with patients having improved ability to perceive light, improved visual acuity in some instances, improved pupillary responses and visual cortex responses, and for some patients improved ability to negotiate obstacles.28–42 Close examination of the outcome for patients in one trial showed that several hours of dark adaptation were required to achieve maximum rod sensitivity in the treated area, whereas cones showed a normal speed of recovery. The markedly slow regeneration of rods was an indication of incomplete restoration of the visual cycle, and the possibility that a resistive barrier slowed the transport or diffusion of 11-cis retinal from the RPE to the photoreceptors was raised.40
Despite the safe and encouraging improvement in visual function, the degree of rescue achieved in human patients has been nowhere near that achieved in the preclinical Rpe65-mutant dog trials. With the exception of one patient with a transient improvement in the multifocal ERG,38 there was no change in ERG responses. In contrast, in dogs there is a remarkable improvement in the ERG, indicating restoration of function close to normal levels (Fig. 1). Vision in dogs after treatment appears very close to normal, while human patients remain visually impaired. So why is there such a difference in outcome between dog and human? One major reason is that human patients have a much faster relative decline in photoreceptor numbers compared with dogs and invariably already have a marked loss of photoreceptors when treated, whereas the dogs treated up to middle age have good preservation of photoreceptors that respond well to restoration of visual cycle function.41
Cideciyan et al. suggested that use of older Rpe65-mutant dogs is required to more accurately model the stage of degeneration present in human patients when they are treated.41,43 We found that robust rescue could still be achieved in middle-aged dogs, which is a reflection of the slow degeneration that occurs in the model.43 One of the authors (S.P.J.) has noted that a breeding line of Rpe65-mutant dogs maintained at Michigan State University develops an early photoreceptor degeneration in the center of the area centralis. This is a region of higher photoreceptor density and it is conceivable that this particular region of the dog retina more closely models the dynamics of photoreceptor loss that occurs in human patients.44 Other breeding lines have not been reported to develop this same early degeneration in the area centralis; this may reflect a difference in background genetics. Additional closer examination of the retinas of affected dogs showed that, although both rods and M/L cones are very slowly lost, the S-cones are rapidly lost in the dog model.45
The initial treatment protocols have been modified based on the experiences with the first patients, and now in a follow-on study (NCT01208389), the second eye of some patients has been treated successfully and with no adverse immune responses or reduction in the rescue previously achieved in the first treated eye.37 In preparation for human trials to treat the second eye of patients, preclinical studies in the Rpe65-mutant dogs had been performed. These showed that administration of vector to the second eye was safe, did not cause an adverse immune response, and allowed for transduction of the RPE and comparable rescue to that of the first treated eye.46,47
Cideciyan et al. investigated photoreceptor loss in the gene therapy-treated retinal areas of human patients and found that although the therapy did restore function to photoreceptors, it did not lead to their preservation in the long-term. They also investigated long-term preservation in Rpe65-mutant dogs and showed in older dogs in which photoreceptor loss was already established at the time of treatment that, although function was successfully restored, long-term photoreceptor preservation was not achieved.41 Clearly, a greater understanding of the reasons why photoreceptors die despite restoration of function and why human rod recovery kinetics remain delayed is needed. This may allow modifications to the treatment protocol to optimize outcomes by improving rod kinetics and enabling preservation of photoreceptors. Studies of older dogs with established retinal degeneration or of the more rapidly degenerating area centralis that is present in some lines may be valuable for these investigations.
Gene Therapy Using Canine Retinitis Pigmentosa Models
Retinitis pigmentosa (RP) is a genetically heterogeneous condition with a growing number of mapped loci and causal genes being identified for the recessive (35 loci and 32 genes), dominant (21 loci and 20 genes), and X-linked (5 loci and 2 genes) modes of inheritance (information on loci and genes for nonsyndromic RP from RetNet48 as of December 2014). Rod photoreceptors are initially affected, leading to night blindness and loss of peripheral vision. With progression, cones are also lost, often leading to blindness. With photoreceptor death, characteristic fundus “bone-spicule”-shaped pigment deposits develop, giving the condition its name. The dog equivalent of RP is known as progressive retinal atrophy (PRA). It occurs across many dog breeds, and an increasing number of the causal mutations have been identified (see Table 1). Colonies of some of the forms of PRA have been established, allowing for more detailed investigations of the phenotype, the disease mechanisms, and also the opportunity to test therapeutic interventions such as gene therapy.
Gene therapy reports in canine models of RP include those for recessive RP (Pde6a and Pde6b), for dominant RP (Opsin), and for X-linked RP (Rpgr). Translational studies for some of these are currently underway.
Gene therapy in dog models of autosomal recessive RP
There are currently publication and conference reports of two gene therapy studies using dog models of autosomal recessive RP. Both of these have mutations in rod phototransduction genes: the alpha and beta subunits of cyclic GMP (cGMP) phosphodiesterase (Pde6a and Pde6b).
PDE6A and PDE6B mutations are responsible for 3–4% and 4–5% of human autosomal recessive RP cases, respectively.49 The protein products (cGMP phosphodiesterase alpha and beta subunits: PDEα and PDEβ) combine as a heterotrimer with two inhibitory gamma subunits, and when the holoenzyme is activated the α/β active subunits hydrolyze cGMP. Reduced cGMP levels lead to closure of cyclic nucleotide gated channels and hyperpolarization of the rod photoreceptor. Dog models with null mutations in Pde6a and Pde6b exist and have very similar phenotypes.50,51 They both result in a stunting of photoreceptors' outer segments during retinal maturation with a relatively rapid loss of rod photoreceptors followed by a slower secondary loss of cone photoreceptors. Affected dogs are night blind with a lack of rod-mediated ERG responses.
The Pde6b model (known as rod–cone dysplasia type 1; rcd1) has a nonsense mutation that results in a lack of Pdeβ, an accumulation of cGMP in the affected rods, and death of the rod cells most probably by apoptotis.52 The Pde6a model (known as rod–cone dysplasia type 3; rcd3) has a 1 bp deletion with a resulting frameshift and premature stop codon and a similar phenotype to that of rcd1 dogs.53 One difference between the models is that in the absence of Pdeβ the alpha and gamma subunits of Pde can still be detected in the rod outer segment prior to degeneration; however, in the absence of Pdeα, the beta and gamma subunits appear not to transport to outer segments and are not detectable on Western blots.51
Gene therapy trials performed on eight Pde6b mutant dogs have been published. The dogs were treated at 20 days of age (because of the early onset of the photoreceptor degeneration); 4 received an AAV2/5 vector and 4 an AAV2/8 vector, the latter at a 10-fold higher titer, via subretinal injections. The canine Pde6b cDNA was delivered under control of a human rhodopsin kinase promoter in both vectors. The treated eyes were reported to have improved rod-mediated ERGs, and dim light vision testing of each eye in turn showed that when the treated eye was uncovered the dogs were able to negotiate obstacles, but when the untreated eye was uncovered they acted as if they were blind. Retinal morphology showed preservation of the photoreceptor layer in the treated regions, and rod outer segments expressing Pde6 and Gnat1 were detected in the treated areas on immunohistochemical (IHC) analysis.54
Pde6b mutant dogs have also been used for an alternative gene therapy strategy to restore vision. This is intended for use in patients once photoreceptors have been lost, and therefore gene augmentation to provide a normal copy of the mutated gene is no longer applicable. The approach is to transform inner retinal neurons, which remain following the loss of the photoreceptors, into light-sensitive cells. This is achieved by expressing a light-sensitive channel in the cell membrane. Either bipolar cells or ganglion cells can be targeted. A recent publication reported on a study that utilized a so-called optopharmacological approach. An AAV vector was used to transduce the target cells to express a light-gated ionotropic glutamate receptor (LiGluR). To enable the LiGluR to respond to light, a photoswitchable molecule to which the ligand for LiGluR (glutamate) is attached (photoswitchable tethered ligand maleimide–azobenzene–glutamate; MAG) is delivered by an intravitreal injection.
When the MAG bound to the LiGluR is light activated, it undergoes a conformational change, making the glutamate accessible to the LiGluR, leading to activation of the channel and a change in cell membrane potential and thus neuronal signaling. Work in the retinal degeneration 1 (rd1) mouse that has a null mutation in Pde6b showed that this approach resulted in recordable light-induced responses in ganglion cells. Furthermore, it was shown that the expression of LiGluR coupled with intravitreous MAG was able to restore light-induced behavior in the photoreceptor-less mice. In a large-animal follow-on study, blind Pde6b-mutant dogs were used. An AAV construct was administered intravitreally to transduce ganglion cells with LiGuR. To show that the transduced ganglion cells had gained the ability to respond to light, a multielectrode array recording from retinal explants was performed. This showed that the transduced ganglion cells had a light-inducible response, but only in the presence of MAG.55 Further studies of this promising approach are expected to lead to human clinical trials.
The laboratory of one of the authors of this review (S.P.J.) reported on therapy with the rcd3 Pde6a mutant dog model using an AAV2/8 vector with a ubiquitous promoter (short CBA promoter) driving expression of the canine Pde6a cDNA. Treated eyes showed evidence of rod photoreceptor function on ERG, improved ability to negotiate obstacles in very dim light, and preservation of the outer nuclear layer of the retina in the injected area. Encouragingly, robust Pde6 expression was detected in the outer segments of the photoreceptors on IHC using an antibody that recognizes all subunits of Pde6. Despite the use of a ubiquitous promoter, the Pde6 protein was not detected in other parts of the retina. There was, however, evidence of some deleterious effects on therapy with photoreceptor rosette formation and associated patches of retinal thinning. This was similar to changes seen in the Rpgr dog model when a vector delivering a shortened cDNA was used (see below). The cause of the rosette formation requires further investigation and will be important to understand.56
Gene therapy in dog models of autosomal dominant RP
A dog model with an autosomal dominant phenotype was identified with a missense mutation (T4R) in rhodopsin.57 The mutation renders the affected retina sensitive to light damage because of instability of the mutant opsin when it is not conjugated to the chromophore 11-cis retinal to form rhodopsin.58,59 The effect of light exposure on progression of RP because of rhodopsin mutations needs careful consideration. In preparation for gene therapy experiments, development of a way of being able to visualize the retina during the subretinal injection process without causing light-induced damage was required. An operating microscope with an infrared viewing system was developed for this purpose.60 A study was reported that investigated whether AAV-mediated expression of mouse or human rhodopsin in the heterozygous mutant dog was safe and whether it provided protection against light damage.
Subretinal injection (conducted using the infrared microscope) of AAV2/5 delivering a human or mouse rhodopsin cDNA under control of the mouse opsin promoter was performed in affected dogs. A mild retinal thinning was noted in the region of the subretinal injection although expression of either mouse or human rhodopsin did not appear to be cytotoxic. However, when the dogs were exposed to a bright light known to cause retinal damage in the mutant but not normal dogs, there was no evidence of a protective effect from the expression of the transgene.61 Development of a therapy to reduce expression of the mutant allele may be required.62
Gene therapy in dog models of X-linked RP
Two dog models with RP GTPase regulator (Rpgr) mutations have been used in gene therapy trials. RPGR mutations result in X-linked retinal dystrophies with classifications including RP, cone–rod dystrophy, cone dystrophy, and macular degeneration. The splicing of RPGR is complex, with two groups of splice variants being expressed in the retina. One group is encoded by exons 1–13 plus 16–19 (Rpgrex1-19) and is highly expressed in developing photoreceptors.63 The second group of splice variants consists of exons 1–13 plus varying amounts of an alternatively splice C-terminal exon 14/15 (referred to as open reading frame 15 [ORF15]) (RpgrORF15) and appears in mature photoreceptors and is involved in trafficking of proteins through the photoreceptor cilium that connects the inner to outer segment. ORF15 is a common site for mutations in humans and is the site of the mutation in the two dog models. Despite both being caused by mutations in ORF15, the phenotypes are very different. The first, known as XLPRA1, has a milder rod–cone degeneration detectable at about 1 year of age.64–66 The second mutation (XLPRA2) causes a much more severe phenotype with photoreceptor outer segment disorganization from as early as 4 weeks of age in hemizygous males.64–66
Gene therapy in the XLPRA1 model was initially undertaken by administering a subretinal injection of AAV2/5 vector delivering a shortened version of canine RPGR exon 1-ORF15 cDNA (cshortRpgrex1-ORF15) under control of a mouse opsin (Mops) or human interstitial retinol binding protein (hIRBP) promoter. This resulted in patchy areas of retinal thinning associated with photoreceptor rosette formation.66,67 Changing the delivered transgene to the full-length human RPGRex1-ORF15 under the hIRBP or human G protein-coupled receptor kinase 1 (hGRK1) promoter was more successful. In two XLPRA1 dogs injected at 28 weeks of age (prior to retinal degeneration) in one eye with the AAV2/5-hIRBP-hRPGRex1-ORF15 vector, there was preservation of the photoreceptor layer thickness in the injected transgene-expressing area, prevention of the mislocalization of rod opsin and M/L cone opsin, and also a lack of Müller cell activation (opsin mislocalization and activation of Müller cells resulting in upregulation of glial fibrillary acidic protein expression are features of disease progression in the untreated retina).
Additionally, the bipolar cells in the treated areas did not retract their dendrites that remained associated with rod spherules and cone pedicles.66 Two XLPRA2 dogs were also treated by subretinal injection at 5 weeks of age. One received the construct with the hIRBP promoter and the second the same construct but with a hGRK promoter. There was preservation of outer retinal structure in both treated eyes, and opsin mislocalization was reversed in the eye injected with the hIRBP promoter and reduced in the eye treated with the hGRK promoter.66 Subsequent reports of additional early-stage (5 weeks), midstage (12 weeks; n=3), and late-stage (26 weeks; n=3) XLPRA2 dogs treated with the hIRBP promoted construct showed structural preservation, as well as electroretinographic functional preservation.67,68 These studies to investigate the degree of rescue at later-stage disease have important implications for future human therapy and are in apparent contrast to the RPE65 gene therapy study where once established photoreceptor loss continued despite restoration of function.41 Investigating the duration of rescue will also be important to see whether photoreceptor degeneration can preserve structure and function in the long-term.
Gene Therapy in a Canine Cone–Rod Dystrophy Model
Cone–rod dystrophies are a subset of photoreceptor dystrophies where cone photoreceptor dysfunction accompanies or precedes rod dysfunction (as opposed to RP where there is a rod-led dysfunction and/or degeneration). The cone–rod dystrophies show genetic heterogeneity, with one of the genes responsible being RP GTPase regulator-interacting protein 1 (RPGRIP1).
A mutation was identified in the canine Rpgrip1 gene in dogs with a cone–rod dystrophy.69 Human RPGRIP1 mutations can cause recessive LCA or a recessive cone–rod dystrophy. It now appears that the situation is more complex in the canine model than a simple Rpgrip1 gene mutation causing the disease, with some studies discounting the mutation as disease causing and other implicating a necessary second locus on the same chromosome.70–72 The putative disease-causing mutation was an insert in exon 2 of the gene that would be predicted to result in a frame-shift and premature termination codon. However, the gene has complex splicing patterns in the retina and not all variants use the exon bearing the mutation.73 Despite the controversy as to the significance of the original Rgrip1 mutation, gene therapy has been performed on dogs derived from the original colony that was used to map the disease locus.74
A previous study using the dogs from the initial colony had shown an early loss of cone ERG responses and a slower and later loss of rod ERG responses.75 In the gene therapy trial, a vector construct of either AAV2/5 or AAV2/8, both with the canine Rpgrip1 cDNA driven by the human rhodopsin kinase promoter, was used. Five affected dogs were injected subretinally with the AAV2/5 vector and two dogs with the AAV2/8 construct. There was restoration of cone ERG responses in the treated eyes and a partial preservation of rod electroretinographic responses. Vision testing showed that treatment improved vision in both dim and bright conditions. The authors did note that, despite therapy preserving photoreceptors, advanced retinal thinning still developed.74 This suggests that further modifications of treatment may be needed for long-term success of the therapy.
Gene Therapy Using Canine Achromatopsia Models
Two canine achromatopsia models resulting from different mutations involving the cone cyclic-nucleotide gated channel beta subunit gene, Cngb3, have been used in gene therapy trials. Achromatopsia, also known as day blindness or rod monochromacy, is the result of loss of cone function. It is an uncommon condition affecting approximately 1 in 30,000 people.76 Affected patients have decreased visual acuity, a lack of color vision, photophobia, and nystagmus. They rely solely on rod photoreceptors for vision. Although there is genetic heterogeneity, mutations involving the subunits of the cone cyclic-nucleotide gated channel are most frequent, with those in CNGB3 being most prevalent. The dog achromatopsias present with very similar clinical signs to those of human patients. One of the models is the result of a large genomic deletion that includes the entire gene plus two neighboring genes.77,78
The mutation was identified in the Alaskan malamute and more recently in an apparently unrelated breed, the miniature Australian shepherd.77,78 The second canine model has a missense mutation in exon 6 of Cngb3 resulting in a functional null mutation. This was identified in the German shorthaired pointer breed of dog.77 Both dog models develop cones normally, and they express many normal cone markers and only have a very gradual loss of cones. During the period of retinal maturation detectable, but reduced amplitude, cone ERG responses can be detected, but these are rapidly lost.79 The very slow loss of cones provides a “window of opportunity” for gene therapy intervention, and both dog models have been used in proof-of-concept gene therapy studies. An AAV2/5 construct was used to deliver the human CNGB3 cDNA under control of promoters aimed to target cone photoreceptors. A promoter that effectively targeted dog M/L cones but not S cones had been identified and was successfully used to transduce the M/L cones in both models.80,81 A dog with successful treatment had restored daytime vision as assessed by ability to negotiate a maze82 and cone function was recordable by the ERG.
Subsequent to successful gene therapy in the dog, the Cngb3-mutant mouse was also rescued by gene therapy.83 The initial canine study, however, recognized that there were some dogs in which transgene expression occurred and yet detectable cone function did not result. It was noted that in dogs older than about 1 year of age the success rate was reduced despite the fact that the cones still appeared to be structurally normal. The reduced success rate in older dogs was addressed in a second study, where an intravitreal injection of ciliary neurotrophic factor (CNTF) was given 1 week before the viral vector construct (AAV2/5-PR2.1-hCNGB3) was delivered by subretinal injection. CNTF had already been shown in multiple animal models of inherited retinal degeneration to slow photoreceptor degeneration, and the results of phase I clinical trials in patients with advanced RP had already been reported.83,84
In both of the dog models, in animals treated at ages where gene therapy alone was not successful, the combined therapy resulted in restoration of cone function.78 Interestingly, the same study showed that intravitreal injection of CNTF alone resulted in small but detectable cone ERG responses, although these were recordable only for a short period of time. Intravitreal CNTF results in a temporary loss of photoreceptor outer segments (this has been termed “deconstruction”),78,85 and it appears that as the outer segments reform they act in a similar way to the outer segments in young animals. They have a transient period of small but detectable cone function (as seen in young untreated achromatopsia dogs) and with gene therapy can create functional cone CNG channels (again a feature of gene therapy in the younger dogs).79 The success of gene therapy in the dog achromatopsia model as well as the potential age limitations (a similar age limitation on rescue was also observed in the mouse83) provide useful information for the different groups currently gearing up to start human clinical trials. Currently, there are plans for clinical trials in the United States (Hauswirth, R24 EY022023, AGTC NCT01846052) and the United Kingdom (Ali, Medical Research Council).
Although not relevant for a review of canine retinal gene therapy trials, it is worth noting that gene therapy trials in sheep with spontaneous achromatopsia due to a mutation in the other cyclic-nucleotide channel subunit, Cnga3, have shown success.87 Clinical trials for CNGA3 achromatopsia are being planned in Israel87 and Germany.88
Gene Therapy with a Dog Macular Dystrophy Model
Best vitelliform macular dystrophy is a form of macular dystrophy that manifests as cystic like lesions in the macula consisting of large deposits of material in the subretinal space. With foveal involvement this can result in a serious loss of central vision. Mutations in the bestrophin 1 gene (BEST 1, also known as vitelliform macular dystrophy 2 [VMD2]) are responsible. The conditions resulting are often referred to as bestrophinopathies and there is some considerable phenotypic variation between patients. BEST 1 is expressed in the RPE and acts as an anion channel and also regulates intracellular calcium signaling. Mutations in canine Best1 result in a phenotype in dogs manifested as the development of multiple retinal elevations or blebs and is known as canine multifocal retinopathy (cmr). Three separate Best 1 mutations have been identified in dog breeds and the resulting conditions named cmr1, cmr2, and cmr3.89,90 These are predicted to have differing molecular disease-causing mechanisms and may model some of the range of phenotypes seen in affected human patients.
AAV vectors with transgene expression driven by the human VMD2 promoter have been developed and tested in wild-type dogs using either a reporter transgene (GFP) or the canine Best 1 cDNA. An AAV2/2 vector was found to be effective in targeting RPE.91 A construct using AAV2/1 was also tested, but when it was used to express the Best 1 transgene, it was found to result in expression in cones that led to their degeneration. The AAV2/2–VMD2 construct delivering either canine Best 1 or human BEST 1 cDNAs has been used to treat cmr-affected dogs. The therapy was reported to reverse lesions and to have no adverse effects.92,93 This potentially offers future translational opportunities to start clinical trials for the treatment of human bestrophinopathies.
Conclusions
Preclinical studies in dogs have been critical in bringing gene therapy to treat inherited retinal disease to the clinic. The advantages of the dog model include the similarity of eye size to that of human, enabling identical approaches to administer gene therapy, and the presence of a region of higher photoreceptor density, the area centralis. The spontaneous dog models often show a very similar disease phenotype to human patients, making them very useful for investigating disease mechanism and testing therapy. Although the laboratory mouse may be the workhorse for initial development of most treatments, this is not always the case (as with RPE65 and CNGB3 gene therapy trials) and the dog model can be used to make up for some of the limitations of the mouse models and allow refining of treatments prior to translation to clinical trials. Species differences do exist, meaning that extrapolation of successes between mouse, dog, and human should not be assumed. It seems likely that, with reports indicating that over 100 different breeds of dog are affected with retinal dystrophies, genetic screening within these breeds will further expand the existing “kennel” of canine retinal degeneration models that can be utilized for preclinical therapy development and refinement.
Acknowledgments
S.M.P.-J. is the Myers-Dunlap Endowed Professor of Canine Health. A.M.K. acknowledges NIH/NEI Grants R01-06855, R01-EY19304, and R42-EY23123, Foundation Fighting Blindness, and Michigan State University Startup Funds.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Mowat FM, Petersen-Jones SM, Williamson H, et al. . Topographical characterization of cone photoreceptors and the area centralis of the canine retina. Mol Vis 2008;14:2518–2527 [PMC free article] [PubMed] [Google Scholar]
- 2.Beltran WA, Cideciyan AV, Guziewicz KE, et al. . Canine retina has a primate fovea-like bouquet of cone photoreceptors which is affected by inherited macular degenerations. PLoS One 2014;9:e90390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koenekoop RK. An overview of Leber congenital amaurosis: a model to understand human retinal development. Surv Ophthalmol 2004;49:379–398 [DOI] [PubMed] [Google Scholar]
- 4.Stone EM. Leber congenital amaurosis—a model for efficient genetic testing of heterogeneous disorders: LXIV Edward Jackson Memorial Lecture. Am J Ophthalmol 2007;144:791–811 [DOI] [PubMed] [Google Scholar]
- 5.Den Hollander AI, Roepman R, Koenekoop RK, et al. . Leber congenital amaurosis: genes, proteins and disease mechanisms. Prog Retin Eye Res 2008;27:391–419 [DOI] [PubMed] [Google Scholar]
- 6.Jin M, Li S, Moghrabi WN, et al. . Rpe65 is the retinoid isomerase in bovine retinal pigment epithelium. Cell 2005;122:449–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Redmond TM, Poliakov E, Yu S, et al. . Mutation of key residues of RPE65 abolishes its enzymatic role as isomerohydrolase in the visual cycle. Proc Natl Acad Sci USA 2005;102:13658–13663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang JS, Estevez ME, Cornwall MC, et al. . Intra-retinal visual cycle required for rapid and complete cone dark adaptation. Nat Neurosci 2009;12:295–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobson SG, Aleman TS, Cideciyan AV, et al. . Human cone photoreceptor dependence on RPE65 isomerase. Proc Natl Acad Sci USA 2007;104:15123–15128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cideciyan AV. Leber congenital amaurosis due to RPE65 mutations and its treatment with gene therapy. Prog Retin Eye Res 2010;29:398–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Narfstrom K, Wrigstad A, Nilsson SE. The briard dog: a new animal model of congenital stationary night blindness. Br J Ophthalmol 1989;73:750–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wrigstad A, Nilsson SE, Narfström K. Ultrastructural changes of the retina and the retinal pigment epithelium in briard dogs with hereditary congenital night blindness and partial day blindness. Exp Eye Res 1992;55:805–818 [DOI] [PubMed] [Google Scholar]
- 13.Wrigstad A, Narfström K, Nilsson SE. Slowly progressive changes of the retina and retinal pigment epithelium in briard dogs with hereditary retinal dystrophy. A morphological study. Doc Ophthalmol 1994;87:337–354 [DOI] [PubMed] [Google Scholar]
- 14.Veske A, Nilsson SE, Narfström K, et al. . Retinal dystrophy of Swedish briard/briard-beagle dogs is due to a 4-bp deletion in RPE65. Genomics 1999;57:57–61 [DOI] [PubMed] [Google Scholar]
- 15.Aguirre GD, Baldwin V, Pearce-Kelling S, et al. . Congenital stationary night blindness in the dog: common mutation in the RPE65 gene indicates founder effect. Mol Vis 1998;4:23. [PubMed] [Google Scholar]
- 16.Acland GM, Aguirre GD, Ray J, et al. . Gene therapy restores vision in a canine model of childhood blindness. Nat Genet 2001;28:92–95 [DOI] [PubMed] [Google Scholar]
- 17.Acland GM, Aguirre GD, Bennett J, et al. . Long-term restoration of rod and cone vision by single dose rAAV-mediated gene transfer to the retina in a canine model of childhood blindness. Mol Ther 2005;12:1072–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aguirre GK, Komaromy AM, Cideciyan AV, et al. . Canine and human visual cortex intact and responsive despite early retinal blindness from RPE65 mutation. PLoS Med 2007;4:e230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobson SG, Acland GM, Aguirre GD, et al. . Safety of recombinant adeno-associated virus type 2-RPE65 vector delivered by ocular subretinal injection. Mol Ther 2006;13:1074–1084 [DOI] [PubMed] [Google Scholar]
- 20.Narfstrom K, Katz ML, Bragadottir R, et al. . Functional and structural recovery of the retina after gene therapy in the RPE65 null mutation dog. Invest Ophthalmol Vis Sci 2003;44:1663–1672 [DOI] [PubMed] [Google Scholar]
- 21.Narfström K, Katz ML, Ford M, et al. . In vivo gene therapy in young and adult RPE65-/- dogs produces long-term visual improvement. J Hered 2003;94:31–37 [DOI] [PubMed] [Google Scholar]
- 22.Ford M, Bragadottir R, Rakoczy PE, et al. . Gene transfer in the RPE65 null mutation dog: relationship between construct volume, visual behavior and electroretinographic (ERG) results. Doc Ophthalmol 2003;107:79–86 [DOI] [PubMed] [Google Scholar]
- 23.Le Meur G, Stieger K, Smith AJ, et al. . Restoration of vision in RPE65-deficient briard dogs using an AAV serotype 4 vector that specifically targets the retinal pigmented epithelium. Gene Ther 2007;14:292–303 [DOI] [PubMed] [Google Scholar]
- 24.Jacobs JB, Dell'osso LF, Hertle RW, et al. . Eye movement recordings as an effectiveness indicator of gene therapy in RPE65-deficient canines: implications for the ocular motor system. Invest Ophthalmol Vis Sci 2006;47:2865–2875 [DOI] [PubMed] [Google Scholar]
- 25.Dejneka NS, Surace EM, Aleman TS, et al. . In utero gene therapy rescues vision in a murine model of congenital blindness. Mol Ther 2004;9:182–188 [DOI] [PubMed] [Google Scholar]
- 26.Pang JJ, Chang B, Kumar A, et al. . Gene therapy restores vision-dependent behavior as well as retinal structure and function in a mouse model of RPE65 Leber congenital amaurosis. Mol Ther 2006;13:565–572 [DOI] [PubMed] [Google Scholar]
- 27.Lai CM, Yu MJ, Brankov M, et al. . Recombinant adeno-associated virus type 2-mediated gene delivery into the Rpe65-/- knockout mouse eye results in limited rescue. Genet Vaccines Ther 2004;2:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bainbridge JW, Smith AJ, Barker SS, et al. . Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med 2008;358:2231–2239 [DOI] [PubMed] [Google Scholar]
- 29.Maguire AM, Simonelli F, Pierce EA, et al. . Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med 2008;358:2240–2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hauswirth WW, Aleman TS, Kaushal S, et al. . Treatment of Leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum Gene Ther 2008;19:979–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Banin E, Bandah-Rozenfeld D, Obolensky A, et al. . Molecular anthropology meets genetic medicine to treat blindness in the North African Jewish population: human gene therapy initiated in Israel. Hum Gene Ther 2010;21:1749–1757 [DOI] [PubMed] [Google Scholar]
- 32.Simonelli F, Maguire AM, Testa F, et al. . Gene therapy for Leber's congenital amaurosis is safe and effective through 1.5 years after vector administration. Mol Ther 2010;18:643–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cideciyan AV, Hauswirth WW, Aleman TS, et al. . Human RPE65 gene therapy for Leber congenital amaurosis: persistence of early visual improvements and safety at 1 year. Hum Gene Ther 2009;20:999–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacobson SG, Cideciyan AV, Ratnakaram R, et al. . Gene therapy for Leber congenital amaurosis caused by RPE65 mutations: safety and efficacy in 15 children and adults followed up to 3 years. Arch Ophthalmol 2012;130:9–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maguire AM, High KA, Auricchio A, et al. . Age-dependent effects of RPE65 gene therapy for Leber's congenital amaurosis: a phase 1 dose-escalation trial. Lancet 2009;374:1597–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ashtari M, Cyckowski LL, Monroe JF, et al. . The human visual cortex responds to gene therapy-mediated recovery of retinal function. J Clin Invest 2011;121:2160–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bennett J, Ashtari M, Wellman J, et al. . AAV2 gene therapy readministration in three adults with congenital blindness. Sci Transl Med 2012;4:120ra15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Testa F, Maguire AM, Rossi S, et al. . Three-year follow-up after unilateral subretinal delivery of adeno-associated virus in patients with Leber congenital amaurosis type 2. Ophthalmology 2013;120:1283–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Melillo P, Pecchia L, Testa F, et al. . Pupillometric analysis for assessment of gene therapy in Leber congenital amaurosis patients. Biomed Eng Online 2012;11:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cideciyan AV, Aleman TS, Boye SL, et al. . Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc Natl Acad Sci USA 2008;105:15112–15117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cideciyan AV, Jacobson SG, Beltran WA, et al. . Human retinal gene therapy for Leber congenital amaurosis shows advancing retinal degeneration despite enduring visual improvement. Proc Natl Acad Sci USA 2013;110:E517–E525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cideciyan AV, Hauswirth WW, Aleman TS, et al. . Vision 1 year after gene therapy for Leber's congenital amaurosis. N Engl J Med 2009;361:725–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Annear MJ, Mowat FM, Bartoe JT, et al. . Successful gene therapy in older Rpe65-deficient dogs following subretinal injection of an adeno-associated vector expressing RPE65. Hum Gene Ther 2013;24:883–893 [DOI] [PubMed] [Google Scholar]
- 44.Mowat F, Occelli L, Gervais K, et al. . Rapid photoreceptor degeneration in the area centralis and visual streak of Rpe65-deficient dogs: morphologic and histologic characterization. ARVO Meet Abstr 2013;54:1776 [Google Scholar]
- 45.Mowat FM, Breuwer AR, Bartoe JT, et al. . RPE65 gene therapy slows cone loss in Rpe65-deficient dogs. Gene Ther 2013;20:545–555 [DOI] [PubMed] [Google Scholar]
- 46.Amado D, Mingozzi F, Hui D, et al. . Safety and efficacy of subretinal readministration of a viral vector in large animals to treat congenital blindness. Sci Transl Med 2010;2:21ra16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Annear MJ, Bartoe JT, Barker SE, et al. . Gene therapy in the second eye of RPE65-deficient dogs improves retinal function. Gene Ther 2011;18:53–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.RetNet, www.sph.uth.tmc.edu/RetNet
- 49.Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet 2006;368:1795–1809 [DOI] [PubMed] [Google Scholar]
- 50.Aguirre GD, Rubin LF. Rod–cone dysplasia (progressive retinal atrophy) in Irish setters. J Am Vet Med Assoc 1975;166:157–164 [PubMed] [Google Scholar]
- 51.Tuntivanich N, Pittler SJ, Fischer AJ, et al. . Characterization of a canine model of autosomal recessive retinitis pigmentosa due to a PDE6a mutation. Invest Ophthalmol Vis Sci 2009;50:801–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suber ML, Pittler SJ, Quin N, et al. . Irish setter dogs affected with rod–cone dysplasia contain a nonsense mutation in the rod cGMP phosphodiesterase beta-subunit gene. Proc Natl Acad Sci USA 1993;90:3968–3972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Petersen-Jones SM, Entz DD, Sargan DR. cGMP phosphodiesterase-α mutation causes progressive retinal atrophy in the Cardigan Welsh corgi dog. Invest Ophthalmol Vis Sci 1999;40:1637–1644 [PubMed] [Google Scholar]
- 54.Petit L, Lheriteau E, Weber M, et al. . Restoration of vision in the pde6beta-deficient dog, a large animal model of rod–cone dystrophy. Mol Ther 2012;20:2019–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gaub BM, Berry MH, Holt AE, et al. . Restoration of visual function by expression of a light-gated mammalian ion channel in retinal ganglion cells or ON-bipolar cells. Proc Natl Acad Sci USA 2014;111:E5574–E5583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mowat FM, Bartoe JT, Bruewer A, et al. . Evaluation of rod photoreceptor function and preservation following retinal gene therapy in the PDE6a mutant dog. ARVO Meet Abstr 2012;53:1928 [Google Scholar]
- 57.Kijas JW, Cideciyan AV, Aleman TS, et al. . Naturally occurring rhodopsin mutation in the dog causes retinal dysfunction and degeneration mimicking human dominant retinitis pigmentosa. Proc Natl Acad Sci USA 2002;99:6328–6333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cideciyan AV, Jacobson SG, Aleman TS, et al. . In vivo dynamics of retinal injury and repair in the rhodopsin mutant dog model of human retinitis pigmentosa. Proc Natl Acad Sci USA 2005;102:5233–5238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu L, Jang GF, Jastrzebska B, et al. . A naturally occurring mutation of the opsin gene (T4R) in dogs affects glycosylation and stability of the G protein-coupled receptor. J Biol Chem 2004;279:53828–53839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Komaromy AM, Acland GM, Aguirre GD. Operating in the dark: a night-vision system for surgery in retinas susceptible to light damage. Arch Ophthalmol 2008;126:714–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iwabe S, Genini S, Sudharsan R, et al. . Assessment of AAV-mediated RHO augmentation in the canine T4R RHO model of autosomal dominant retinitis pigmentosa. Invest Ophthalmol Vis Sci 2014;55:3316 [Google Scholar]
- 62.Gorbatyuk MS, Aguirre GD, Acland GM, et al. . Molecular therapeutic approaches for treatment of autosomal dominant RP (ADRP) in a canine T4R rhodopsin model. Invest Ophthalmol Vis Sci 2003;44:2341 [Google Scholar]
- 63.Wright RN, Hong DH, Perkins B. Misexpression of the constitutive Rpgr(ex1-19) variant leads to severe photoreceptor degeneration. Invest Ophthalmol Vis Sci 2011;52:5189–5201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zeiss CJ, Acland GM, Aguirre GD. Retinal pathology of canine X-linked progressive retinal atrophy, the locus homologue of RP3. Invest Ophthalmol Vis Sci 1999;40:3292–3304 [PubMed] [Google Scholar]
- 65.Zhang Q, Acland GM, Wu WX, et al. . Different RPGR exon ORF15 mutations in Canids provide insights into photoreceptor cell degeneration. Hum Mol Genet 2002;11:993–1003 [DOI] [PubMed] [Google Scholar]
- 66.Beltran WA, Cideciyan AV, Lewin AS, et al. . Gene therapy rescues photoreceptor blindness in dogs and paves the way for treating human X-linked retinitis pigmentosa. Proc Natl Acad Sci USA 2012;109:2132–2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beltran WA, Cideciyan AV, Lewin AS, et al. . Gene augmentation for X-linked retinitis pigmentosa caused by mutations in RPGR. Cold Spring Harb Perspect Med 2014;pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Beltran WA, Cideciyan AV, Lewin AS, et al. . RPGR gene augmentation delivered at early, mid and late stage disease in a canine model of XLRP rescues photoreceptor structure and function. Invest Ophthalmol Vis Sci 2014;55:3321 [Google Scholar]
- 69.Mellersh CS, Boursnell ME, Pettitt L, et al. . Canine RPGRIP1 mutation establishes cone–rod dystrophy in miniature longhaired dachshunds as a homologue of human Leber congenital amaurosis. Genomics 2006;88:293–301 [DOI] [PubMed] [Google Scholar]
- 70.Miyadera K, Kato K, Aguirre-Hernandez J, et al. . Phenotypic variation and genotype-phenotype discordance in canine cone–rod dystrophy with an RPGRIP1 mutation. Mol Vis 2009;15:2287–2305 [PMC free article] [PubMed] [Google Scholar]
- 71.Kuznetsova T, Iwabe S, Boesze-Battaglia K, et al. . Exclusion of RPGRIP1 ins44 from primary causal association with early-onset cone–rod dystrophy in dogs. Invest Ophthalmol Vis Sci 2012;53:5486–5501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miyadera K, Kato K, Boursnell M, et al. . Genome-wide association study in RPGRIP1(-/-) dogs identifies a modifier locus that determines the onset of retinal degeneration. Mamm Genome 2012;23:212–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kuznetsova T, Zangerl B, Goldstein O, et al. . Structural organization and expression pattern of the canine RPGRIP1 isoforms in retinal tissue. Invest Ophthalmol Vis Sci 2011;52:2989–2998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lheriteau E, Petit L, Weber M, et al. . Successful gene therapy in the RPGRIP1-deficient dog: a large model of cone–rod dystrophy. Mol Ther 2014;22:265–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Turney C, Chong NH, Alexander RA, et al. . Pathological and electrophysiological features of a canine cone–rod dystrophy in the miniature longhaired dachshund. Invest Ophthalmol Vis Sci 2007;48:4240–4249 [DOI] [PubMed] [Google Scholar]
- 76.Michaelides M, Hunt DM, Moore AT. The cone dysfunction syndromes. Br J Ophthalmol 2004;88:291–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sidjanin DJ, Lowe JK, Mcelwee JL, et al. . Canine CNGB3 mutations establish cone degeneration as orthologous to the human achromatopsia locus ACHM3. Hum Mol Genet 2002;11:1823–1833 [DOI] [PubMed] [Google Scholar]
- 78.Yeh CY, Goldstein O, Kukekova AV, et al. . Genomic deletion of CNGB3 is identical by descent in multiple canine breeds and causes achromatopsia. BMC Genet 2013;14:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Komaromy AM, Rowlan JS, Corr AT, et al. . Transient photoreceptor deconstruction by CNTF enhances rAAV-mediated cone functional rescue in late stage CNGB3-achromatopsia. Mol Ther 2013;21:1131–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Komaromy AM, Alexander JJ, Cooper AE, et al. . Targeting gene expression to cones with human cone opsin promoters in recombinant AAV. Gene Ther 2008;15:1049–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Komaromy AM, Alexander JJ, Rowlan JS, et al. . Gene therapy rescues cone function in congenital achromatopsia. Hum Mol Genet 2010;19:2581–2593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Garcia MM, Ying GS, Cocores CA, et al. . Evaluation of a behavioral method for objective vision testing and identification of achromatopsia in dogs. Am J Vet Res 2010;71:97–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Carvalho LS, Xu J, Pearson RA, et al. . Long-term and age-dependent restoration of visual function in a mouse model of CNGB3-associated achromatopsia following gene therapy. Hum Mol Genet 2011;20:3161–3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tao W, Wen R, Goddard MB, et al. . Encapsulated cell-based delivery of CNTF reduces photoreceptor degeneration in animal models of retinitis pigmentosa. Invest Ophthalmol Vis Sci 2002;43:3292–3298 [PubMed] [Google Scholar]
- 85.Sieving PA, Caruso RC, Tao W, et al. . Ciliary neurotrophic factor (CNTF) for human retinal degeneration: phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proc Natl Acad Sci USA 2006;103:3896–3901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wen R, Song Y, Kjellstrom S, et al. . Regulation of rod phototransduction machinery by ciliary neurotrophic factor. J Neurosci 2006;26:13523–13530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Banin E, Obolensky A, Ejzenberg A, et al. . Gene therapy in a sheep model of CNGA3 achromatopsia. Invest Ophthalmol Vis Sci 2014;55:4566 [Google Scholar]
- 88.Zobor D, Stanzial F, Kellner U, et al. . Retinal structure and function in achromatopsia: the CNGA3 phenotype. Invest Ophthalmol Vis Sci 2014;55:346 [Google Scholar]
- 89.Guziewicz KE, Zangerl B, Lindauer SJ, et al. . Bestrophin gene mutations cause canine multifocal retinopathy: a novel animal model for best disease. Invest Ophthalmol Vis Sci 2007;48:1959–1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zangerl B, Wickstrom K, Slavik J, et al. . Assessment of canine BEST1 variations identifies new mutations and establishes an independent bestrophinopathy model (cmr3). Mol Vis 2010;16:2791–2804 [PMC free article] [PubMed] [Google Scholar]
- 91.Guziewicz KE, Zangerl B, Komaromy AM, et al. . Recombinant AAV-mediated BEST1 transfer to the retinal pigment epithelium: analysis of serotype-dependent retinal effects. PLoS One 2013;8:e75666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guziewicz K, Komaromy A, Iwabe S, et al. . Sustained therapeutic reversal of canine bestrophinopathy with gene therapy using recombinant AAV2. Invest Ophthalmol Vis Sci 2013;54:5965 [Google Scholar]
- 93.Guziewicz K, Beltran W, Cideciyan A, et al. . Gene therapy for bestrophinopathies. Acta Ophthalmol 2014;92 [Google Scholar]
- 94.Bennicelli J, Wright JF, Komaromy A, et al. . Reversal of blindness in animal models of Leber congenital amaurosis using optimized AAV2-mediated gene transfer. Mol Ther 2008;16:458–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jacobs JB, Dell'osso LF, Hertle RW, et al. . Gene therapy to abolish congenital nystagmus in RPE65-deficient canines. Invest Ophthalmol Vis Sci 2003;44:4249. [DOI] [PubMed] [Google Scholar]
- 96.Jacobs JB, Dell'osso LF, Wang ZI, et al. . Using the NAFX to measure the effectiveness over time of gene therapy in canine LCA. Invest Ophthalmol Vis Sci 2009;50:4685–4692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Narfström K, Bragadottir R, Redmond TM, et al. . Functional and structural evaluation after AAV.RPE65 gene transfer in the canine model of Leber's congenital amaurosis. Adv Exp Med Biol 2003;533:423–430 [DOI] [PubMed] [Google Scholar]
- 98.Narfström K, Seeliger M, Lai CM, et al. . Morphological aspects related to long-term functional improvement of the retina in the 4 years following rAAV-mediated gene transfer in the RPE65 null mutation dog. Adv Exp Med Biol 2008;613:139–146 [DOI] [PubMed] [Google Scholar]
- 99.Narfström K, Vaegan , Katz M, et al. . Assessment of structure and function over a 3-year period after gene transfer in RPE65-/- dogs. Doc Ophthalmol 2005;111:39–48 [DOI] [PubMed] [Google Scholar]
- 100.Petersen-Jones SM, Annear MJ, Bartoe JT, et al. . Gene augmentation trials using the Rpe65-deficient dog: contributions towards development and refinement of human clinical trials. Adv Exp Med Biol 2012;723:177–182 [DOI] [PubMed] [Google Scholar]
- 101.Dekomien G, Runte M, Godde R, et al. . Generalized progressive retinal atrophy of sloughi dogs is due to an 8-bp insertion in exon 21 of the PDE6B gene. Cytogenet Cell Genet 2000;90:261–267 [DOI] [PubMed] [Google Scholar]
- 102.Goldstein O, Mezey JG, Schweitzer PA, et al. . IQCB1 and PDE6B mutations cause similar early onset retinal degenerations in two closely related terrier dog breeds. Invest Ophthalmol Vis Sci 2013;54:7005–7019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kukekova AV, Goldstein O, Johnson JL, et al. . Canine RD3 mutation establishes rod–cone dysplasia type 2 (rcd2) as ortholog of human and murine rd3. Mamm Genome 2009;20:109–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Goldstein O, Kukekova AV, Aguirre GD, et al. . Exonic SINE insertion in STK38l causes canine early retinal degeneration (erd). Genomics 2010;96:362–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Downs LM, Wallin-Hakansson B, Boursnell M, et al. . A frameshift mutation in golden retriever dogs with progressive retinal atrophy endorses SLC4A3 as a candidate gene for human retinal degenerations. PLoS One 2011;6:e21452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Winkler PA, Ekenstedt KJ, Occelli LM, et al. . A large animal model for CNGB1 autosomal recessive retinitis pigmentosa. PLoS One 2013;8:e72229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Goldstein O, Jordan JA, Aguirre GD, et al. . A non-stop S-antigen gene mutation is associated with late onset hereditary retinal degeneration in dogs. Mol Vis 2013;19:1871–1884 [PMC free article] [PubMed] [Google Scholar]
- 108.Zangerl B, Goldstein O, Philp AR, et al. . Identical mutation in a novel retinal gene causes progressive rod–cone degeneration in dogs and retinitis pigmentosa in humans. Genomics 2006;88:551–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Downs LM, Bell JS, Freeman J, et al. . Late-onset progressive retinal atrophy in the Gordon and Irish setter breeds is associated with a frameshift mutation in C2orf71. Anim Genet 2012;44:169–177 [DOI] [PubMed] [Google Scholar]
- 110.Goldstein O, Mezey JG, Boyko AR, et al. . An ADAM9 mutation in canine cone–rod dystrophy 3 establishes homology with human cone–rod dystrophy 9. Mol Vis 2010;16:1549–1569 [PMC free article] [PubMed] [Google Scholar]
- 111.Wiik AC, Wade C, Biagi T, et al. . A deletion in nephronophthisis 4 (NPHP4) is associated with recessive cone–rod dystrophy in standard wire-haired dachshund. Genome Res 2008;18:1415–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dutrow EV, Tanaka N, Miyadera K, et al. . A missense mutation in canine CNGA3 eliminates retinal cone function: a novel model for achromatopsia. Invest Ophthalmol Vis Sci 2014;55:1641 [Google Scholar]
- 113.Dekomien G, Vollrath C, Petrasch- , Parwez E, et al. . Progressive retinal atrophy in schapendoes dogs: mutation of the newly identified ccdc66 gen. Neurogenetics 2010;11:163–174 [DOI] [PubMed] [Google Scholar]