Abstract
Objectives: Our clinical experience with low dose loxapine (5–15 mg/day) suggests promising efficacy and safety for irritability in autism spectrum disorders (ASD). We studied low dose loxapine prospectively in adolescents and adults with ASD and irritability. Additionally, we measured loxapine and metabolite concentrations, and brain-derived neurotrophic factor (BDNF) as a biomarker of neuromodulation.
Methods: We performed a 12 week open trial of add-on loxapine in subjects, ages 13–65 years, diagnosed with ASD, and Aberrant Behavior Checklist-Irritability (ABC-I) subscale scores >14. Loxapine was dosed flexibly up to 15 mg daily, starting with 5 mg on alternate days. From weeks 1 to 6, other psychoactive medications were tapered if possible; from weeks 6 to 12, all medication doses were held stable. The primary outcome was the Clinical Global Impressions-Improvement subscale (CGI-I), ratings of Much Improved or Very Much Improved. Secondary outcomes were the ABC-I, Repetitive Behavior Scale-Revised, and Schalock Quality of Life scale. Serum BDNF and loxapine and metabolite concentrations were assayed. BDNF rs6265 was genotyped.
Results: Sixteen subjects were enrolled; 12 completed all visits. Median age was 18 years (range 13–39). Median final loxapine dose was 7.5 mg/day (2.5–15). All 14 subjects (100%) with data at week 12 were rated as Much Improved on CGI-I at 12 weeks. Mean change on ABC-I at 12 weeks was −31%, p=0.01. Mean body mass index (BMI)-Z decreased between weeks 6 and 12, p=0.03. Side effects were minimal, and prolactin elevation occurred in only one subject. BDNF concentrations measured in 11 subjects increased significantly (p=0.04). Subjects with AG genotype for BDNF rs6265 required a lower dose of loxapine at study end, but had similar behavioral and BDNF concentration changes as the GG genotype.
Conclusions: Low dose loxapine shows promise as a repurposed drug for irritability in ASD. Loxapine effects on BDNF warrant further study.
Introduction
Irritability and aggression are major public health problems for adolescents with autism spectrum disorders (ASD), their families, and communities. Such problems result in physical injuries, sometimes including death, property destruction, and possible lifelong emotional trauma to siblings and parents. Lifetime medical costs are estimated at $3,200,000 per affected person (Ganz 2007). New medications to treat these problems are urgently needed in this rapidly growing population. ASD incidence has increased 50% since 2002, and now is identified in one in 54 boys and one in 88 children (Centers for Disease Control 2009). Atypical antipsychotic drugs currently remain the psychopharmacologic treatment of choice for these serious problems.
The multisite Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study in adults with schizophrenia (Lieberman et al. 2005) and the Treatment of Early-Onset Schizophrenia Spectrum Disorders (TEOSS) study in children and adolescents (McClellan et al. 2007) contributed to the current perspective that typical antipsychotics remain efficacious, cost much less, and have different adverse event profiles than atypical antipsychotics. The atypical antipsychotics risperidone and aripiprazole produce significantly more weight gain, metabolic syndrome, diabetes, stroke and heart disease than the classic antipsychotics, and carry black box warnings. However, risperidone and aripiprazole are both United States Food and Drug Administration (FDA) approved to treat irritability in individuals with ASD≤6 years of age.
Younger age is an identified risk factor for greater weight gain with atypical antipsychotics (Correll and Carlson 2006), rendering new drug development an even more urgent priority for children and adolescents. Common and serious risperidone side effects in individuals with developmental disabilities include marked appetite increase, weight gain, diabetes, and prolactin elevation (Hellings et al. 2001; Findling et al. 2003; Hellings et al. 2010, 2006; Correll et al. 2009). Aripiprazole may also cause significant associated weight gain in some younger individuals (Hellings et al. 2011; Marcus et al. 2011).
Loxapine is a medium potency, classic dibenzoxazepine antipsychotic drug developed in the 1980s to resemble clozapine, but absent agranulocytosis an adverse effect of the latter. Loxapine blocks dopamine D1, D2, and D4 receptors potently, and serotonin receptors less potently, thus partly resembling novel antipsychotics (Glazer 1999). Pharmacologically, loxapine may be somewhat “atypical” in low doses (Stahl 2002; Xu et al. 2003). This is confirmed by the positron emission tomography (PET) scan study findings of Kapur and coworkers (1997), that loxapine is an equipotent blocker of 5-HT2 and D2 receptors in vivo, in subjects with psychosis. Singh and colleagues (1996) found that after 6 and 12 weeks of loxapine treatment in 24 patients with schizophrenia, both lymphocyte 5HT-2A and D2-like platelet receptor binding were downregulated. Interestingly, however, the observed degree of improvement in psychotic symptoms did not correlate significantly with the receptor downregulation degree, reinforcing our interest in the possible brain-derived neurotrophic factor (BDNF) activity of loxapine. A study by Li et al. (2003) found that loxapine effects on dopamine and acetylcholine release in the medial prefrontal cortex and nucleus accumbens were comparable to those of atypical antipsychotics.
Low doses of loxapine have a different adverse event profile, also associated with less weight gain (Stahl 2002). Stahl also describes the use of conventional (classic) antipsychotics to “lead in” or “top up” atypical antipsychotics, which, importantly, may spare the metabolic adverse events of the latter. Whereas the maximum recommended loxapine dosage is 250 mg daily, our results suggest efficacy and tolerability in this population with low doses ranging from 5 to 15 mg daily. In animal studies, loxapine produced changes in cerebral subcortical inhibitory areas, with associated behavioral calming and suppression of aggression.
Based on our prior clinical experience suggesting rapid efficacy and minimal weight gain with loxapine treatment for irritability in adolescents and adults with ASD, we hypothesized that low dose loxapine, dosed at 5–15 mg/day is effective and tolerable for the treatment of irritability in ASD, with minimal or no associated weight gain. Following the findings of Brennand and coworkers (2011) discussed subsequently, that loxapine showed neurotrophic effects in pluripotent stem cells, we decided to measure BDNF change and BDNF gene polymorphisms as potential markers of neurotrophic effects. BDNF plays an important role in embryologic brain development and postnatal neurogenesis, synaptic plasticity, and neuronal survival, and has been implicated in the pathogenesis of neuropsychiatric and neurodegenerative disorders (Martinowich et al. 2007; Zuccato and Cattaneo 2009). The primary source of BDNF in peripheral circulation is believed to be the central nervous system (Krabbe et al. 2007). We therefore measured serum BDNF concentrations as a biomarker for the central neuromodulating effects of loxapine. In addition, the common single nucleotide polymorphisms (SNP) of BDNF rs6265 results in a Val66Met substitution within the amino-terminus of the prohormone domain of BDNF. The Met isoform appears to be associated with poor cognitive performance caused by decreased activity-dependent BDNF secretion (Egan et al. 2003). This SNP has been associated with autism (Nishimura et al. 2007) and may have a modifying effect on cortical anatomy in patients with autism (Raznahan et al. 2009). Reduced BDNF levels have been associated with various psychiatric and neurodegenerative disorders, including Alzheimer's disease, Parkinson's disease, and Huntington's disease (Zuccato and Cattaneo 2009), and BDNF haploinsufficiency has been associated with lower cognitive functioning and greater social impairments (Han et al. 2013). We therefore wished to examine the potential modifying effect of the BDNF rs6265 genotype on the response to loxapine in our cohort of patients with autism.
Methods
We performed an exploratory, prospective 12 week open trial of add-on loxapine in consecutive outpatients meeting study inclusion criteria, ages 13–65 years, with ASDs including Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV) autistic disorder, pervasive developmental disorder-not otherwise specified, or Asperger's disorder, validated with the Autism Diagnostic Interview-Revised (ADI-R) (American Psychiatric Association 1994; Lord et al. 1994). Informed written consent, and assent when applicable, was first obtained from the subject themselves or their parents or guardians, following University of Kansas Medical Center Institutional Review Board (IRB) approval of the study. Each subject manifested irritability > 14 points on the Aberrant Behavior Checklist-Irritability (ABC-I) subscale (Aman et al. 1995). Loxapine was added and dosed flexibly up to 15 mg daily, starting with 5 mg on alternating but fixed days on a Monday, Wednesday, Friday, and Saturday, for the first 3 weeks, to minimize akathisia, as loxapine is available in 5 mg capsules as the lowest strength. This dosage was counted as 2.5 mg daily. Increase in medication > 5 mg daily was then either up to 10 mg daily or 5 mg daily plus 5 mg alternate days. Later this dosage was counted as 7.5 mg daily on averaging of doses.
From weeks 1 to 6, other psychoactive medications were tapered as tolerated, whereas from weeks 6 to 12 all medication doses were held stable. The primary outcome measure was the Clinical Global Impressions scale-Improvement (CGI-I) subscale (Guy 1976), with response defined as CGI-I of 2, Much Improved, or 1, Very Much Improved. Secondary outcome response measures rated by a parent, guardian, or caregiver, also unblinded, were the ABC-I, Repetitive Behavior Scale-Revised (RBS-R) (Lam and Aman 2007), and the Schalock Quality of Life (QOL) scale (Schalock et al. 1989). Movement side effects were rated on the Dyskinesia Identification User System (DISCUS) (Sprague and Kalachnik 1991) and antipsychotic side effects were rated on the Neuroleptic Side Effects Checklist (NLSEC) (Gualtieri et al. 1984).
At baseline and study end we collected morning, fasting blood for complete blood count and differential count, comprehensive metabolic panel, fasting lipids, hemoglobin A1c, and prolactin, in all subjects, and for BDNF in a subset of 11 subjects. An additional fasting, morning blood sample was collected at study end for a 12 hour post-loxapine dose study of loxapine and metabolite levels.
BDNF measurement
We measured serum BDNF concentrations as a biomarker for central neuromodulating effects of loxapine at baseline and at 12 weeks in 11 subjects (subject numbers 6 through 16). Morning, fasting venous blood was obtained in serum separator tubes, permitted to clot for 30 minutes and then centrifuged, and aliquots of serum were stored at at −80°C. At the conclusion of the study, baseline and follow-up samples from all subjects were analyzed simultaneously on a single plate to minimize interassay variability. Serum BDNF concentration was determined using a commercial BDNF ELISA kit (R&D Systems, Minneapolis, MN; sensitivity 20 pg/mL; intrassay and interassay variabilities of 3.8% and 7.6%, respectively). The average of duplicate assays for each sample was used for analyses, controlling for delta body mass index (BMI) and platelet count, because BDNF in peripheral circulation is stored in and released from platelets (Fujimura et al. 2002).
BDNF rs6265 genotyping
Genomic DNA was extracted from peripheral blood (n=10) or saliva (n=5) in 15 subjects using QIAamp DNA Mini kit (Qiagen, Valencia, CA). BDNF rs6265 was genotyped in duplicate using the Taqman SNP Genotyping Assay (Applied Biosystems, Foster City, CA).
Loxapine and metabolite levels
Loxapine and metabolite levels were measured in plasma samples obtained at final visit in 15 subjects (missing subject number 4). Blood was collected 12 hours after the last loxapine dose, in a K2-EDTA tube, and plasma was isolated by centrifugation. Isolated plasma was stored frozen within 30 minutes of collection, at −80°C. Samples were then analyzed as a batch for steady-state concentrations of loxapine, 7-hydroxyloxapine, 8-hydroxyloxapine, and amoxapine. These analytes were quantified using the Liquid chromatography–mass spectrometry (LC-MS)/MS method of Zimmer and coworkers (2000). Briefly, analytes were isolated from plasma by protein precipitation, separated by reverse-phase LC, and quantified using tandem MS. This method has been validated for human plasma samples containing 0.05–50 ng/mL of each analyte, demonstrating the sensitivity required for these clinical samples.
Statistical methods
Mixed models were used to evaluate the effect of loxapine over time on outcomes (Diggle et al. 2002). This type of model accounts for the within-subject correlation arising from repeated measurements, and accommodates intermittent missing data; thus all participants' data could be used even when visits were skipped. A compound symmetric variance–covariance structure was used to estimate error variance, and the Kenward–Roger adjustment to the degrees of freedom was used to control type I error (Kenward and Roger 1997). Mixed-effects logistic regression was used for the primary outcome, improvement on the CGI-I, and mixed-effects linear regression was used for all other outcomes. Week was treated as a categorical variable, and the F test of week was the main test of interest in all models. To account for multiple testing, p values for subscales were adjusted using the Bonferroni method (e.g., for the five subscales of ABC). Effect sizes were calculated by dividing the average estimated change from baseline to week 12 by the baseline standard deviation of the outcome.
Agreement between blinded and unblinded raters on the CGI was quantified using Cohen's κ. Changes in outcomes measured only at the baseline and final visit (blood pressure, pulse) were tested using paired t tests.
Because several subjects were still growing teenagers, changes in BMI were assessed using BMI-Z scores calculated by the LMS method (Cole 1990) to normalize for age and sex using standard United States growth charts (Kuczmarski et al. 2000). Change in adjusted serum BDNF concentrations was assessed by paired t test, and change in serum BDNF concentrations adjusted for change in BMI-Z and change in platelet count was assessed by repeated measured analysis of covariance (ANCOVA) with SPSS software (IBM, version 16).
Change in BMI-Z was assessed by Wilcoxon signed rank test. Hardy–Weinberg equilibrium of genotype distribution was assessed by χ2 test. Comparison of subjects by genotype for differences in clinical responses to loxapine were performed by Mann–Whitney U test.
Results
Sixteen subjects were enrolled; 12 completed all five study visits. Eleven were males and five were females. Median age was 18 years (range 13–39). Autistic disorder was diagnosed in 10 subjects, pervasive developmental disorder, not otherwise specified in 6 and Asperger disorder in none. Numbers of subjects with intellectual disability levels were: Mild, 5; moderate, 1; severe, 3; profound, 0; and unspecified, 7. Comorbid diagnoses were: Attention-deficit/hyperactivity disorder, 12; obsessive compulsive disorder, 8; intermittent explosive disorder, 13; Tourette disorder, 1; posttraumatic stress disorder, 1; and pica, 2.
Median final loxapine dose was 8.4 mg/day (range 2.5–15 mg/day). See Table 1 for subject demographics, any medications that were tapered in the first 6 weeks, and any concomitant medications that were held stable during weeks 6–12. All subjects met criteria for response (Much Improved or Very Much Improved on CGI-I) at weeks 9 (for 12/12 subjects attending that visit) and 12 (for 14/14 subjects still in the study). Almost half of the subjects still in the study at week 6 (7/15; 46.7%) achieved response by week 6. Agreement between blinded and unblinded CGI ratings was moderate, with Cohen's κ=0.57. Because the blinded rater was unavailable for some visits, especially for the first six subjects, the unblinded ratings performed by the principal investigator were used for the analysis.
Table 1.
Subject Demographics, Loxapine Dosing, Weight, and Concomitant Medications
| Sub_ID | Age | Race | Gender | End-point Loxa-pine dose mg/day | Wt. base-line (kg) | Wt. wk 6 (kg) | Wt. end (kg) | Baseline medications tapered off (weeks 1–6) | Concomitant medications constant dose (weeks 1–12) |
|---|---|---|---|---|---|---|---|---|---|
| 1a | 17 | W | M | 7.5 | 83.7 | 83.8 | 83.4 | Escitalopram 20 mg daily | Clonidine 0.2 mg daily |
| 2a | 15 | AA | M | 7.5 | 68.0 | 68.9 | 66.3 | ||
| 3 | 26 | W | M | 15 | 139.0 | 139.0 | 137.6 | Oxcarbazepine | Ezetimibe+simvastatin |
| 4 | 24 | W | M | 2.5 | 94.6 | n/a | 94.4 | melatonin 3–6 mg h.s., krill oil, minocycline 100 mg daily | |
| 5 | 27 | W | F | 5 | 77.3 | 78.9 | 79.2 | Calcium, vitamin D, divalproex sodium125 mg (4 q.a.m.h.s.,3 q.noon) | |
| 6 | 39 | W | M | 10 | 75.7 | 77.2 | 77.2 | Olanzapine 2.5 mg t.i.d.b | |
| 7 | 22 | W | M | 5 | 71.0 | 68.3 | 64.4 | Mirtazapine 15 mg, aripiprazole 10 mg dailyb | Lamotrigine 100 b.i.d., amikacin 100 b.i.d. |
| 8a | 13 | W | M | 15 | 110.7 | 110.4 | 110.2 | Loratadine daily OTC, fluticasone inhaler TT puffs daily, doxycycline | |
| 9 | 33 | W | M | 7.5 | 104.8 | 103.9 | 97.5 | Risperidone 0.5 mL b.i.d.b | Escitalopram 15, lorazepam 1 mg a.m.,p.m., t.i.d. |
| 10 | 24 | W | F | 10 | 82.2 | 80.3 | 79.4 | Guanfacine1 mL t.i.d., divalproex sodium | Lamotrigine 100 q.a.m. |
| 11a | 16 | W | M | 10 | 71.7 | 71.2 | 71.2 | Ziprasidone 20 mg b.i.d.b | Atomoxetine 25 mg b.i.d. |
| 12a | 13 | AA | F | 5 | 60.3 | 60.1 | 63.2 | Dexmethylphenidate XR 20 mg, 10 mg noon, **quetiapine XR 150 | Dexmethylphenidate IR 5 mg q 4 p.m., lorazepam melatonin 1 mg h.s. |
| 13 | 26 | W | M | 10 | 70.8 | 73.0 | 74.8 | Mixed amphetamine salts XR 30 | Divalproex 1000 mg, trazodone100 h.s. |
| 14 | 17 | W | F | 5 | 64.0 | 60.8 | 60.8 | Olanzapine12.5 b.i.d.,b sertaline 25 mg | Naltrexone 50 daily, OCP |
| 15 | 19 | W | F | 10 | 119.7 | 122.5 | 121.6 | Ziprasidone 160 h.s.,blamotrigine 400 hs | Divalproex 1000 |
| 16a | 16 | W | M | 10 | 66.0 | 69.2 | 68.1 | Venlafaxine 50 daily | Sertraline 50 daily |
Adolescents, n=6.
Atypical antipsychotics tapered and stopped, n=7.
Secondary outcome measures are summarized in Table 2. Response to loxapine on CGI-Severity (CGI-S) was significant, with a severity decrease from a baseline mean of 5.8 to 4.1 at week 12 (mean improvement 29%, p<0.001, effect size Cohen's d=–3.1); see Figure 1. Response was also significant on the ABC-I; see Figure 2. Ratings decreased from a baseline mean of 26.5 to 18.2 at week 12, 31% improvement, p=0.01, effect size d= −1.1. Significant ABC improvements were also achieved on subscale II (Social Withdrawal, a core feature of autism) with p=0.006, subscale III (Stereotypy) with p=0.004, and subscale IV (Hyperactivity/Noncompliance), with p=0.04. Subscale V of the ABC (Inappropriate Speech) did not decrease significantly.
Table 2.
Outcomes on Secondary Measures at Baseline and Week 12; Results from Mixed Effect Regression Models
| na | Baseline (week 0) | End of study (week 12) | Percent change | Effect sizeb | |
|---|---|---|---|---|---|
| CGI-S | 16 | 5.8±0.15 | 4.1±0.16 | −29% | −3.1 |
| ABC | |||||
| I (Irritability) | 16 | 26.5±2.1 | 18.2±2.2 | −31% | −1.1 |
| II (Social Withdrawal) | 16 | 23.7±2.2 | 14.3±2.3 | −40% | −1.0 |
| III (Stereotypy) | 16 | 12.1±1.1 | 7.5±1.2 | −38% | −1.1 |
| IV (Hyperactivity) | 16 | 27.9±2.6 | 18.2±2.7 | −35% | −1.0 |
| V (Inappropriate Speech) | 16 | 6.4±1.0 | 4.7±1.0 | −27% | −0.5 |
| QOL | |||||
| I (Satisfaction) | 11 | 16.1±1.10 | 20.0±1.13 | 24% | 1.1 |
| II (Competence/Productivity) | 11 | 11.3±0.32 | 12.1±0.33 | 8% | 0.8 |
| III (Empowerment/Independence) | 11 | 15.4±0.72 | 18.2±0.73 | 18% | 1.0 |
| IV (Social Belonging) | 10 | 16.7±0.90 | 22.1±0.90 | 32% | 1.8 |
| RBS | |||||
| I (Stereotyped Behavior) | 11 | 5.7±0.92 | 2.2±0.95 | −62% | −0.9 |
| II (Self-Injury) | 11 | 6.8±1.25 | 4.5±1.28 | −34% | −0.6 |
| III (Compulsive Behavior) | 11 | 9.9±1.57 | 6.4±1.61 | −35% | −0.7 |
| IV (Ritualized Behavior) | 11 | 6.6±1.28 | 4.3±1.32 | −35% | −0.5 |
| V (Sameness Behavior) | 11 | 16.8±1.67 | 11.6±1.74 | −31% | −1.2 |
| VI (Restricted Behavior) | 11 | 4.6±0.91 | 3.9±0.94 | −16% | −0.2 |
All values are means±SEMs from mixed effects models.
Number of subjects at baseline.
Cohen's d, defined as the average estimated change divided by the baseline standard deviation.
CGI-S, Clinical Global Impressions-Severity; ABC, Aberrant Behavior Checklist; QOL, quality of life; RBS, Repetitive Behavior Scale-Revised.
FIG. 1.
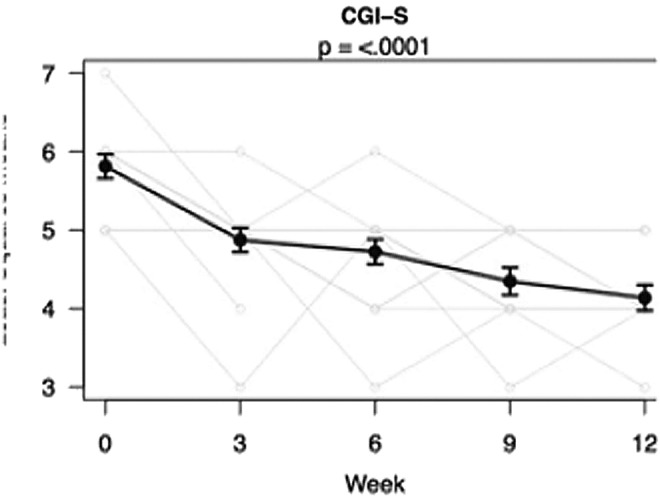
Change in Clinical Global Impressions-Severity (CGI-S) during the trial. Results are least squares means±standard errors from mixed models. Light gray lines are individual trajectories (with a small amount of noise added so that overlapping lines are visible).
FIG. 2.
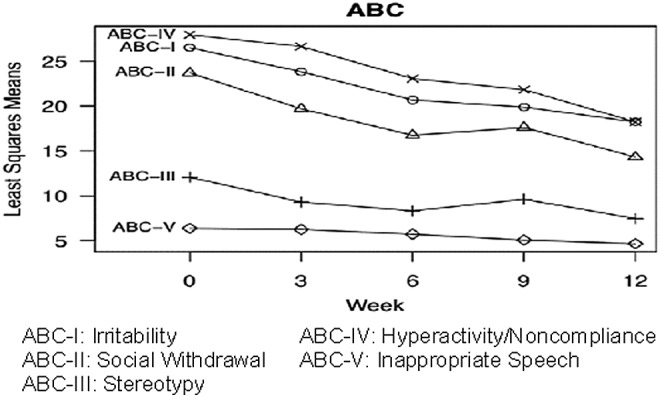
Change in Aberrant Behavior Checklist (ABC) subscales during the trial. Results are least squares means from mixed models. Subscales with significant changes over time are denoted with bolded text and an asterisk.
Outcomes on the RBS-R and QOL are shown in Figures 3 and 4. Subscale I. of the RBS-R (Stereotypic Behavior) changed from a baseline mean of 5.7 to 2.2 at the end, with −62% change, p=0.01. The remaining RBS-R subscales did not show significant changes. QOL subscale scores I (Satisfaction), III (Empowerment/Independence), and IV (Social Belonging/Community Integration) increased significantly, with p=0.03, 0.0002, and 0.0003, respectively.
FIG. 3.
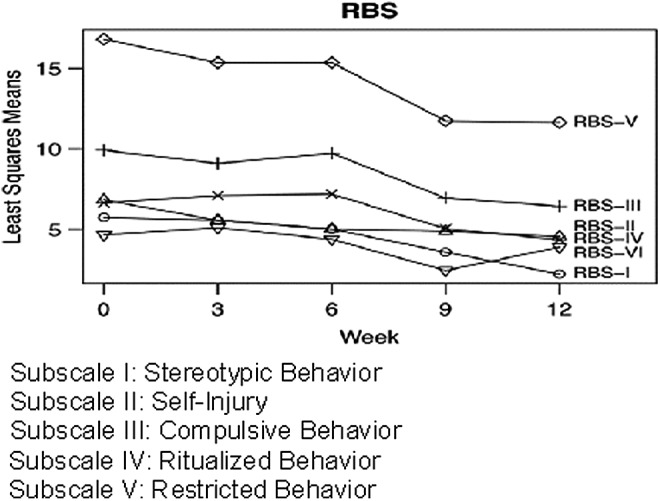
Change in Repetitive Behavior Scale-Revised (RBS-R) during the trial. Results are least squares means from mixed models. Subscales with significant changes over time are denoted with bolded text and an asterisk.
FIG. 4.
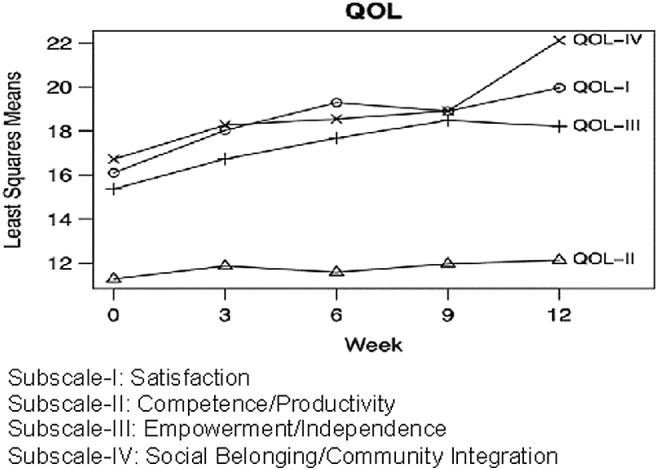
Change in quality of life (QOL) during the trial. Results are least squares means from mixed models. Subscales with significant changes over time are denoted with bolded text and an asterisk.
Of 16 subjects enrolled, 12 completed all visits. Subject 3 returned late for the final study visit because of family illness, but his outcomes data were still used. Subject 4 was lost to the study after week 3. Subject 14 was withdrawn at week 6 by her guardian after reported behavioral worsening.
Loxapine safety and adverse events
Changes in blood pressure and pulse were not significant (p>0.15 for all tests). Subject BMI-Z decreased significantly between weeks 6 and 12 (p=0.03); see Tables 1 and 3. Table 1 gives information regarding medications tapered in the first 6 weeks, during which 5 of 16 subjects had received atypical antipsychotics that were tapered and stopped. Change in BMI was not significant over the full study duration (baseline mean 28.5±7.0 kg/m2, end 28.2±7.2 kg/m2, p=0.3). Subject 9, a 33-year-old Caucasian male, with severe intellectual disability and ASD, initially weighing 104.8 kg, height 175.9 cm (BMI 33.9 kg/m2), is of note. After his taper off risperidone prior to week 6, he lost 5.7 kg over the next 6 weeks (final BMI 32.0 kg/m2). However, comparison of all seven subjects tapered off atypical antipsychotics versus the other nine subjects in the study showed no significant differences in change in BMI (p=0.45) or change in BMI-Z (p=0.17).
Table 3.
Change of Body Mass Index (BMI) in N=15 Subjects for Weeks 1 to 6, N=14 Subjects for Weeks 6–12
| BMI | Mean | SD | p value |
|---|---|---|---|
| Baseline | 28.3 | ± 7.3 | —— |
| Week 6 | 28.6 | ± 7.5 | —— |
| Week 12 | 28.0 | ± 7.4 | —— |
| Change baseline to week 6 | 0.26 | ± 0.83 | 0.24 |
| Change week 6 to week 12 | −0.53 | ± 0.82 | 0.03 |
| Change baseline to week 12 | −0.27 | ± 1.1 | 0.36 |
No increases in metabolic indicators of fasting lipids, glucose, or Hb A1c were documented during the study. Subject 14, who received olanzapine at baseline and discontinued the study at week 6, had elevated triglycerides, cholesterol, and low density lipoprotein (LDL) at baseline, and elevated cholesterol and LDL at week 6. Subject 5 was missing baseline blood test results because of a laboratory error, and had elevated cholesterol and LDL at study end. The final visit lipid panel was also missing for Subjects 9 and 12. Prolactin increase occurred in Subject 6, from 7.2 ng/mL at baseline to 19.7 ng/mL at end. No significant changes were observed in white blood cell count, red blood cell count, platelet count, or hepatic or renal function.
Benzatropine was added for resting tremor in Subject 5, at 0.5 mg twice a day. Also, lorazepam 1 mg prn daily was added by a family practitioner for anxiety in Subject 12, together with melatonin 1 mg at bedtime for sleep. Movement side effects rated on the DISCUS scale scores were as follows: Subjects 1 and 11 manifested preexisting motor tics at baseline. Scores for Subject 1 decreased from 18 at baseline to 1 at end-point. Scores for Subject 11 were lower and varied from 2 at baseline to 3 at end. A mild tongue tremor was noted in Subject 12, an African American female 12 years of age, at weeks 6 and 12 (week 9 visit was missed). Her observations may have been confounded by her having been withdrawn from quetiapine extended release (XR) prior to week 6. Subject 13 manifested a mild tongue tremor only at week 9.
Qualitative side effect ratings on the NLSEC (Gualtieri et al. 1984) decreased on loxapine for all, except for four side effects. These were 1) loss of appetite, increased from one to three subjects; 2) muscle pains, increased from one to three subjects; and 3) urinary incontinence, increased from two to three subjects. For the fourth side effect, muscle stiffness, ratings remained the same, positive for three individuals at baseline and study end.
BDNF findings
For this group of 11 subjects, (who had available serum for BDNF measurement), numbers 6 through 16, 5 of whom were <18 years of age, BDNF concentration was significantly higher following loxapine treatment (see Fig. 5). This outcome held both when findings were unadjusted and adjusted for the appropriate covariates of delta platelets and delta BMI-Z calculations (Fujimura et al. 2002; Xu et al. 2003). Of the 15 subjects with DNA available for genotyping, 12 were homozygous for the major G allele of BDNF rs6265 and 3 were heterozygous for the minor A allele; this distribution was consistent with expected Hardy–Weinberg Equilibrium (χ2=0.19, p=0.67). BDNF rs6265 genotype was not associated with change in serum BDNF concentration, nor with any of the behavioral measures. However, genotype was associated with final dose of loxapine at study end among the 14 completers (mean±SD for GG vs. AG: 10.2±2.6 vs. 5.0±0 mg; median [range] for GG vs. AG: 10 [7.5–15] vs. 5 [5–5] mg; p=0.005).
FIG. 5.

Brain-derived neurotrophic factor (BDNF) changes.
Comparison of the seven subjects tapered off atypical antipsychotics versus the other four subjects in the study showed no significant difference in change in BDNF (p=0.32). Similarly, comparison of the three subjects tapered off antidepressants versus the other eight subjects in the study showed no significant difference in change in BDNF (p=0.50).
Loxapine and metabolite findings
Loxapine and its major metabolites were detected and quantified in plasma samples from 14 patients (Table 4). The mean loxapine concentration was 2.5 ng/mL, whereas the mean concentrations of the metabolites 7-hydroxyloxapine, 8-hydroxyloxapine, and amoxapine were 0.7, 3.4, and 0.7 ng/mL, respectively. There was significant interindividual variability in the concentrations of loxapine and its metabolites, and none of the analyte concentrations showed any apparent dose dependence.
Table 4.
Loxapine, Metabolite Levels, and Changes in Outcomes
| Percent change from baseline to week 12 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subject | Final loxapine dose (mg/day) | Loxapine (ng/mL) | 7-OH lox (ng/ml) | 8-Hydroxyloxapine (ng/mL) | Amoxapine (ng/mL) | CGI-S | ABC-I | ABC-II | ABC-III | ABC-IV |
| 1 | 7.5 | 1.8 | 0.3 | 1.2 | 0.6 | −33% | 28% | −16% | −6% | −19% |
| 2 | 7.5 | 1.7 | 0.6 | 1.7 | 0.8 | −20% | −25% | −6% | −45% | 0% |
| 3 | 15 | 1.9 | 0.7 | 4.3 | 0.2 | −50% | −86% | −65% | −100% | −86% |
| 4 (dropout) | 2.5 | |||||||||
| 5 | 5 | 3.4 | 0.9 | 2.1 | 0.7 | −33% | 0% | 0% | 0% | 0% |
| 6 | 10 | 3.9 | 1.7 | 7.9 | 1 | −17% | −31% | −29% | 8% | −29% |
| 7 | 5 | 0.9 | 1.2 | 3.8 | 0.2 | −20% | −50% | −39% | −54% | −27% |
| 8 | 15 | 3.2 | 1.2 | 3.8 | 1 | 0% | −21% | −58% | −27% | 0% |
| 9 | 7.5 | 3.3 | 0 | 2 | 0.5 | −20% | −40% | −48% | −39% | −77% |
| 10 | 10 | 1.2 | 0.3 | 2.2 | 0.3 | −17% | −7% | −20% | 0% | 0% |
| 11 | 10 | 0.9 | 0.5 | 1.6 | 0.6 | −33% | −68% | −64% | −57% | −74% |
| 12 | 5 | 3.6 | 0.3 | 2 | 1.6 | −33% | −9% | 0% | −13% | −3% |
| 13 | 10 | 5.1 | 0.6 | 6.6 | 0.8 | −33% | −18% | −86% | −100% | −40% |
| 14 (dropout) | 5 | |||||||||
| 15 | 10 | 1.9 | 0.7 | 4.6 | 0.2 | −43% | −22% | −20% | −25% | −28% |
| 16 | 10 | 2 | 1.3 | 3.3 | 0.7 | −33% | −50% | −38% | −47% | −25% |
| Mean: | 2.5 | 0.7 | 3.4 | 0.7 | ||||||
CGI-S, Clinical Global Impressions-Severity; ABC, Aberrant Behavior Checklist.
Discussion
In this pilot study, the rate of response to loxapine was fairly typical, with 47% showing CGI-I ratings of Much Improved or Very Much Improved by week 6, and 100% showing these ratings by weeks 9 and 12. Akathisia was not observed as a problem. The response would likely have been more rapid if loxapine at 5 mg h.s. was added daily rather than for four times per week. Severity of symptoms rated on CGI-S decreased rapidly and significantly, with measurable improvement by week 3 and continuing until study end at week 12, as shown in Figure 1.
Global improvements were accompanied by significant improvement in several other secondary measure subscales, notably in Irritability, Hyperactivity/Noncompliance (greatest improvement), Social Withdrawal, and Stereotypic Behavior on the ABC. Inappropriate Speech did not change; the latter is limited by subjects' speech abilities; and additionally, the subscale consists of only four items. ABC total scores are not an appropriate use of the ABC (Aman 2012). Stereotypic Behavior also improved significantly on the RBS-R scale, confirming the ABC-Stereotypy subscale findings. RBS-R subscales measuring self-injury, and compulsive and restrictive behavior did not change significantly. QOL measures increased as irritability decreased. Treatment of these symptoms enables individuals to expand their social and community interactions, as evidenced by a significant increase in the Satisfaction, Empowerment, Independence, and Social Belonging/Community Integration subscales on the Schalock QOL scale.
The current study results will require validation in randomized, double-blind placebo-controlled trials, powered to show differences between loxapine and placebo. The recommended adult dose of loxapine for schizophrenia is 60–250 mg daily; however, loxapine appears to rapidly treat irritability in adolescents and adults with ASD in low doses of < 15 mg/day, and with positive effects on mood, irritability, and impulse control. Whereas extrapyramidal side effects and tardive dyskinesia are dose-related adverse events of typical antipsychotics including loxapine in conventional doses, the study doses were low in comparison, notably 5–15 mg/day. Only one published case report exists in the literature so far, describing successful loxapine treatment of a 10-year-old African American girl with autistic disorder and treatment-resistant aggression who showed response to a low dose of 15 mg/day (Reinblatt et al. 2006).
Mean subject BMI-Z decreased significantly between weeks 6 and 12 while all medication doses were held constant. This could be in part because psychoactive medications, including atypical antipsychotics, were tapered between baseline and week 6, which occurred in seven subjects, one of whom discontinued the study at week 6. Decreased appetite was a side effect noted on the NLSEC, increasing from two subjects at baseline to three at study end. Regardless of mechanism and explanation, this weight loss is a welcome observation insofar as so many patients tend to gain unacceptable weight with newer antipsychotics.
Overall, side effect rates were low, with only one subject requiring the addition of benzatropine to treat extrapyramidal side effects. Many NLSEC side effect items such as irritability and restlessness overlap with baseline psychopathology symptoms; these decreased from baseline to week 12. Observations are limited by lack of blinding, lack of placebo comparison group, and use of concomitant medications (although all drug doses were constant from weeks 6 to 12).
The subsample of 11 subjects showed a significant increase in BDNF levels, warranting further BDNF study in a larger, placebo-controlled trial. No difference in BDNF response was observed between youth < 18 years of age (n=5) and adults (n=11). In this small group, there were no significant relationships between BDNF changes and loxapine or loxapine metabolite levels or behavioral changes, but the sample size was underpowered for assessing these correlations; larger studies are warranted. Also, a study extension with long-term follow-up thereafter is required to address tardive dyskinesia concerns. Interestingly, subjects with AG genotype for BDNF rs6265 had similar behavioral and BDNF concentration changes in response to loxapine to those of subjects with GG genotype; however, they required half the dose of loxapine for these effects, suggesting a pharmacogenomic interaction between loxapine and rs6265 genotype. The presence of the minor A allele variant has been shown to decrease activity-dependent secretion of BDNF, and has been associated with poorer cognitive performance in typical subjects (Egan et al. 2003) and with differences in cortical anatomy in patients with autism (Raznahan et al. 2009). We hypothesize that loxapine may counteract the effect of the minor A allele, and, therefore, have a stronger effect in individuals who carry this genetic variation. Further studies are needed to determine if lower doses of loxapine are required to achieve similar clinical benefits among patients with heterozygosity for the minor allele of BDNF rs6265.
Recent evidence points toward the likelihood that antipsychotic drugs in general reduce brain matter volume, and antipsychotics may actually produce some of the brain abnormalities that have usually been attributed to schizophrenia (Moncrieff and Leo 2010). Loxapine, in contrast, may have neurotrophic effects, based on findings of a human pluripotent stem cell study by Brennand and coworkers (2011). In their study, four other comparison antipsychotics did not show neurotrophic effects, notably thioridazine, clozapine, olanzapine, and risperidone. Loxapine produced neural sprouting and improved neuronal connectivity. In the current study, we measured BDNF as a potential biomarker and mediator of brain health in association with loxapine treatment. BDNF modulates hippocampal plasticity and hippocampal-dependent memory in cell models, animals, and humans (Egan et al. 2003). Levels of serum BDNF may be lower in youth with ASDs and in those with intellectual disabilities, and the normal trajectory of BDNF increase with development may be less (Abdallah et al. 2013).
Limitations of our study include the small number of subjects, lack of a double-blind placebo control design, and the fact that raters of primary as well as of secondary measures were unblinded. Nevertheless, there was good correlation between primary ratings made by the blinded and unblinded raters for the CGI. In addition, in an add-on study design, with taper of some presenting medications in the first 6 weeks, improvement may have been partially the result of concomitant medications or taper of other medications that potentially worsened behavior. Similarly, observed BDNF level increases may have been, in part, caused by other concomitant medications such as antidepressants, which are known to increase BDNF. Although we observed no significant differences in change in BDNF between those with and without tapering of antidepressants or atypical antipsychotics, the number of subjects was underpowered to rule out such effects. Strengths of the present study include that it represents a first step toward identifying safer, effective treatment options in ASD, potentially also with more neuroptrophic benefits than some atypical as well as classic antipsychotics.
The lowest available loxapine dose is a 5 mg capsule, although Alexza Pharmaceuticals recently developed an oral spray formulation. The inhaled oral spray formulation was approved by the FDA for acute agitation in schizophrenia, but could pose administration problems for aggressive individuals with ASD, as well as recently identified bronchospasm risks. Lesem and coworkers (2011) published a multicenter, randomized, placebo-controlled study of inhaled loxapine for rapid acute treatment of agitation in individuals with schizophrenia, with positive findings. In a 2012 study, loxapine was effective for agitation during weaning from mechanical ventilation in 19 critical care patients, without adverse events after 150 mg administration via nasogastric tube, in up to two administrations, 90 minutes apart (Sztrymf et al. 2010).
The beneficial mood effects of loxapine observed may be clinically related to the loxapine N-demethylated metabolite which is a tricyclic antidepressant, marketed separately as Amoxapine®. Furthermore, loxapine's half-life is measured as 4 hours. However, in clinical experience, loxapine doses of 5 mg administered four times per week on a Monday, Wednesday, Friday, and Saturday frequently produced measurable improvement. The measured plasma concentrations of loxapine and of its metabolites are very low, and raise questions regarding the relationship between loxapine pharmacokinetics and pharmacodynamics. Larger, randomized controlled studies are needed to examine the possibility that peak loxapine concentrations exert a neurotrophic effect through induction of BDNF, with potential effects on neuronal numbers and architecture persisting beyond the time period in which therapeutic blood levels of loxapine were present.
Conclusions
Based on our findings, double-blind, placebo-controlled trials are warranted to study the efficacy, tolerability, and potential ease of use of loxapine in low doses for irritability in adolescents and adults with ASD. Loxapine may have atypical antipsychotic sparing properties that could be useful in ASD, and the role of BDNF in its mechanism of action warrants further study.
Clinical Significance
Loxapine could impact future public health cost reduction and law enforcement savings if treatment can be achieved with fewer metabolic side effects and more rapid behavioral control of irritability and aggression. Once-daily dosing of loxapine may rapidly reduce irritability in individuals who have responded only partially to atypical antipsychotics.
Our study is a first step in exploring loxapine's mechanism of action and possible neurotrophic effects via BDNF potentiation. Outcomes may relate to an important problem relevant to improvement in intellectual function and core features of autism, notably repetitive behaviors and social competence of individuals with intellectual and developmental disability (IDD), ASD, and severe irritability.
Disclosures
Dr. Hellings is an investigator for studies funded by Autism Speaks-Autism Treatment Network (ATN), Forest, the National Institute of Mental Health (NIMH), Shire, and Sunovion; has been an advisor to Abbott Laboratories; and a consultant to Ferring Pharmaceuticals. Dr. Aman has received research contracts, consulted with, or served on advisory boards of Biomarin Pharmaceuticals, Bristol-Myers Squibb, CogState, Confluence Pharmaceutica, Coronado Bioscience, Forest Research, Hoffman LaRoche, Johnson & Johnson, Novartis, Pfizer, Prophase LLC, and Supernus Pharmaceutica. Dr. Cain has a conflict of interest for Shire. At the time of the research, Dr. Han was funded by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (1ZIAHD008898). No other authors have conflicts of interest.
References
- Abdallah MW, Mortensen EL, Greaves-Lord K, Larsen N, Bonefeld-Jørgensen EC, Nørgaard-Pedersen B, Hougaard DM, Grove : Neonatal levels of neurotrophic factors and risk of autism spectrum disorders. Acta Psychiatr Scand 128:61–69, 2013 [DOI] [PubMed] [Google Scholar]
- Aman MG: Aberrant Behavior Checklist: Current identity and future developments. Clin Exp Pharmacol 2:e114, 2012 [Google Scholar]
- Aman MG, Burrow WH, Wolford PL: The Aberrant Behavior Checklist-Community: Factor validity and effect of subject variables for adults in group homes. Am J Ment Retard 100:283–192, 1995 [PubMed] [Google Scholar]
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC: American Psychiatric Association; 1994 [Google Scholar]
- Brennand KJ, Simone A, Jou J, Gelboin–Burkhart C, Tran N, Sangar S, Li Y, Mu Y, Chen G, Yu D, McCarthy S, Sebat J, Gage FH: Modeling schizophrenia using human induced pluripotent stem cells. Nature 473:221–225, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control: Prevalence of Autism Spectrum Disorders – Autism and Developmental Disabilities Monitoring Network, United States, 2006. MMWR Surveillance Summaries, 2009. Available at www.cdc.gov/mmwr/preview/mmwrhtml/ss5810a1.htm [PubMed]
- Cole TJ: The LMS method for constructing normalized growth standards. Eur J Clin Nutr 44:45–60, 1990 [PubMed] [Google Scholar]
- Correll CU, Carlson H: Endocrine and metabolic adverse effects of psychotropic medications in children and adolescents. J Am Acad Child Adol Psychiatry 45:771–791, 2006 [DOI] [PubMed] [Google Scholar]
- Correll CU, Manu P, Olshanskiy V, Napolitano B, Kane JM, Malhotra AK: Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA 302:1765–1773, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diggle P, Heagerty P, Liang KY, Zeger SL: Analysis of Longitudinal Data. Oxford, UK: Claredon Press, 2002 [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR: The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 112:257–269, 2003 [DOI] [PubMed] [Google Scholar]
- Findling RL, Kusumakar V, Daneman D, Moshang T, De Smedt G, Binder C: Prolactin levels during long-term risperidone treatment in children and adolescents. J Clin Psychiatry 64:1362–1369, 2003 [DOI] [PubMed] [Google Scholar]
- Fujimura H, Altar CA, Chen R, Nakamura T, Nakahashi T, Kambayashi J, Sun B, Tandon NN: Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation. Thromb Haemost 87:728–734, 2002 [PubMed] [Google Scholar]
- Ganz ML: The lifetime distribution of the incremental societal costs of autism. Arch Pediatr Adolesc Med 161:343–349, 2007 [DOI] [PubMed] [Google Scholar]
- Glazer WM: Does loxapine have “atypical” properties? Clinical evidence. J Clin Psychiatry 60 (Suppl 10):42–46, 1999 [PubMed] [Google Scholar]
- Gualtieri CT, Quade D, Hicks RE, Mayo JP, Schroeder SR: Tardive Dyskinesia And Other Clinical Consequences Of Neuroleptic Treatment In Children And Adolescents. Am J Psychiatry 141:20–23, 1984 [DOI] [PubMed] [Google Scholar]
- Guy W: ECDEU Assessment Manual for Psychopharmacology, revised ed. Washington, DC: United States Department of Health, Education, and Welfare; 1976 [Google Scholar]
- Han JC, Thurm A, Golden Williams C, Joseph LA, Zein WM, Brooks BP, Butman JA, Brady SM, Fuhr S, Hicks MD, Huey AE, Hanish AE, Danley KM: Association of brain-derived neurotrophic factor (BDNF) haploinsufficiency with lower adaptive behaviour and reduced cognitive functioning in WAGR/11p13 deletion syndrome. Cortex 49:2700–2710, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellings JA, Boehm D, Yeh H, Schroeder SR: Long-term clinical aripiprazole efficacy and weight changes in youth with developmental disabilities including autism spectrum disorders. J Ment Health Res Intellect Disabil 4:1–14, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellings JA, Schroeder SR, Cardona A: Long-term safety and adverse events of risperidone in children, adolescents and adults with pervasive developmental disorder. J Ment Health Res Intellect Disabil 3:1–13, 2010 [Google Scholar]
- Hellings JA, Zarcone JR, Crandall K, Wallace D, Schroeder SR: Weight gain in a controlled study of risperidone in children, adolescents and adults with mental retardation and autism. J Child Adolesc Psychopharmacol 11:229–38, 2001 [DOI] [PubMed] [Google Scholar]
- Hellings JA, Zarcone JR, Reese RM, Valdovinos MG, Marquis JG, Fleming KK, Schroeder SR: A crossover study of risperidone in children, adolescents and adults with mental retardation. J Autism Dev Disord 36:401– 411, 2006 [DOI] [PubMed] [Google Scholar]
- Hellings JA, Zarcone JR, Valdovinos MG, Reese RM, Gaughan E, Schroeder SR: Risperidone-induced prolactin elevation in a prospective study of children, adolescents, and adults with mental retardation and pervasive developmental disorders. J Child Adolesc Psychopharmacol 15: 885–892, 2005 [DOI] [PubMed] [Google Scholar]
- Kapur S, Zipursky R, Remington G, Jones C, McKay G, Houle S: PET evidence that loxapine is an equipotent blocker of 5-HT2 and D2 receptors: Implications for the therapeutics of schizophrenia. Am J Psychiatry 154:1525–1529, 1997 [DOI] [PubMed] [Google Scholar]
- Kenward MG, Roger JH: Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 53:983–997, 1997 [PubMed] [Google Scholar]
- Krabbe KS, Nielsen AR, Krogh–Madsen R, Plomgaard P, Rasmussen P, Erikstrup C, Fischer CP, Lindegaard B, Petersen AM, Taudorf S, Secher NH, Pilegaard H, Bruunsgaard H, Pedersen BK: Brain–derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia 50:431–438, 2007 [DOI] [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Grummer–Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL: CDC Growth Charts: United States. Advance Data from Vital and Health Statistics. No. 314 Hyattsville, MD: National Center for Health Statistics; 2000 [PubMed] [Google Scholar]
- Lam KS, Aman MG: The Repetitive Behavior Scale – Revised: Independent validation in individuals with autism spectrum disorders. J Autism Dev Disord 37:855–866, 2007 [DOI] [PubMed] [Google Scholar]
- Lesem MD, Tran–Johnson TK, Riesenberg RA, Feifel D, Allen MH, Fishman R, Spyker DA, Kehne JH, Cassella JV: Rapid acute treatment of agitation in individuals with schizophrenia: Multicentre, randomised, placebo-controlled study of inhaled loxapine. Br J Psychiatry 198:51–58, 2011 [DOI] [PubMed] [Google Scholar]
- Li Z, Ichikawa J, Meltzer H: A comparison of the effects of loxapine with ziprasidone and thioridazine on the release of dopamine and acetylcholine in the prefrontal cortex and nucleus accumbens. Psychopharmacology 167, 35 1–323, 2003 [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RSE, Davis SM, Davis CE, Lebowitz BD, Severe J, Hsiao JK: Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 353:1209–1223, 2005 [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A: Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 24:659–685, 1994 [DOI] [PubMed] [Google Scholar]
- Marcus RN, Owen R, Manos G, Mankoski R, Kamen L, McQuade RD, Carson WH, Findling RL: Safety and tolerability of aripiprazole for irritability in pediatric patients with autistic disorder: A 52-week, open-label, multicenter study. J Clin Psychiatry 72:1270–1276, 2011 [DOI] [PubMed] [Google Scholar]
- Martinowich K, Manji H, Lu B: New insights into BDNF function in depression and anxiety. Nat Neurosci 10:1089–1093, 2007 [DOI] [PubMed] [Google Scholar]
- McClellan J, Sikich L, Findling RL, Frazier JA, Vitiello B, Hlastala SA, Williams E, Ambler D, Hunt-Harrison T, Maloney AE, Ritz L, Anderson R, Hamer R, Lieberman JA: Treatment of early-onset schizophrenia spectrum disorders (TEOSS): rationale, design, and methods. J Am Acad Child Adolesc Psychiatry 46:969–978, 2007 [DOI] [PubMed] [Google Scholar]
- Moncrieff J, Leo J: A systematic review of the effects of antipsychotic drugs on brain volume. Psychol Med 40:1409–1422, 2010 [DOI] [PubMed] [Google Scholar]
- Nishimura K, Nakamura K, Anitha A, Yamada K, Tsujii M, Iwayama Y, Hattori E, Toyota T, Takei N, Miyachi T, Iwata Y, Suzuki K, Matsuzaki H, Kawai M, Sekine Y, Tsuchiya K, Sugihara G, Suda S, Ouchi Y, Sugiyama T, Yoshikawa T, Mori N: Genetic analyses of the brain-derived neurotrophic factor (BDNF) gene in autism. Biochem Biophys Res Commun 356:200–206, 2007 [DOI] [PubMed] [Google Scholar]
- Raznahan A, Toro R, Proitsi P, Powell J, Paus T, Bolton PF, Murphy DGM: A functional polymorphism of the brain derived neurotrophic factor gene and cortical anatomy in autism spectrum disorder. J Neurodev Disord 1:215–23, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinblatt SP, Abanilla PK, Jummani R, Coffey B: Loxapine treatment in an autistic child with aggressive behavior: therapeutic challenges. J Child Adolesc Psychopharmacol 16:639–643, 2006 [DOI] [PubMed] [Google Scholar]
- Schalock RL, Keith KD, Hoffman K, Karan OC: Quality of life: Its measurement and use. Ment Retard 27:25–31, 1989 [PubMed] [Google Scholar]
- Singh AN, Barlas C, Singh S, Franks P, Mishra RK: A neurochemical basis for the antipsychotic activity of loxapine: Interactions with dopamine D1, D2, D4 and serotonin 5-HT2 receptor subtypes. J Psychiatry Neurosci 21(1):29–35, 1996 [PMC free article] [PubMed] [Google Scholar]
- Sprague RL, Kalachnik JE: Reliability, validity, and a total score cut off for dyskinesia identification system: Condensed user rating scale (DISCUS) with mentally ill and mentally retarded populations. Psychopharmacol Bull 27:51–58, 1991 [PubMed] [Google Scholar]
- Stahl SM: Essential Psychopharmacology of Antipsychotics and Mood Stabilizers. New York: Cambridge University Press; 2002 [Google Scholar]
- Sztrymf B, Chevrel G, Bertrand F, Margetis D, Hurel D, Ricard JD, Dreyfuss D.Beneficial effects of loxapine on agitation and breathing patterns during weaning from mechanical ventilation. Crit Care 14:R86, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Goulding EH, Zang K, Cepoi D, Cone RD, Jones KR, Tecott LH, Reichardt LF: Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat Neurosci 6:736–742, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer D, Muschalek V, Muller C: Determination of metrifonate enantiomers in blood and brain samples using liquid chromatography on a chiral stationary phase coupled to tandem mass spectrometry. Rapid Commun Mass Spectrom 14:1425–1432, 2000 [DOI] [PubMed] [Google Scholar]
- Zuccato C, Cattaneo E: Brain-derived neurotrophic factor in neurodegenerative diseases. Nat Rev Neurol 5:311–322, 2009 [DOI] [PubMed] [Google Scholar]


