Abstract
To address the growing need for corneal transplants two main approaches are being pursued: allogenic and synthetic materials. Allogenic tissue from human donors is currently the preferred choice; however, there is a worldwide shortage in donated corneal tissue. In addition, tissue rejection often limits the long-term success of this approach. Alternatively, synthetic homologs to donor corneal grafts are primarily considered temporary replacements until suitable donor tissue becomes available, as they result in a high incidence of graft failure. Tissue engineered cornea analogs would provide effective cornea tissue substitutes and alternatives to address the need to reduce animal testing of commercial products. Recent progress toward these needs is reviewed here, along with future perspectives.
Introduction
An estimated 10 million people worldwide suffer from corneal vision loss, and in the United States alone ∼40,000 corneal transplants are performed each year.1 The answer to the growing need for corneal transplants is limited to two main approaches: allogenic and synthetic materials. In the case of corneal diseases and blindness resulting from limbal stem cell deficiency (e.g., ocular burns), autologous limbal stem cell transplantation has been presented as effective therapeutic option for corneal regeneration to prevent neovascularization, chronic inflammation, and stromal scarring.2
To date, allogenic materials originating from human donors are the preferred choice in corneal graft replacement,3 but this approach suffers from a number of disadvantages. The major drawbacks are limited availability of quality-donor graft material, and occurrence of tissue rejection. Although corneas are the most transplanted tissues, eye bank programs cannot fulfill the demand for transplants; therefore, long waiting lists are still a limiting factor.4 Furthermore, over 10% of the transplant population rejects replacement cornea within the first year of implantation.5
Recently, traditional penetrating keratoplasty, where all five layers of the cornea are transplanted, has been effectively supplanted by partial lamellar keratoplasty, such as endothelial keratoplasty and deep anterior lamellar keratoplasty, which has significantly increased the rate of implant success.4 Specifically, endothelial keratoplasty selectively replaces only the endothelial layer, whereas in anterior lamellar keratoplasty only several layers of the stroma are transplanted. Large prospective studies have shown a 10-year graft survival rate for penetrating keratoplasty ranging from 89% to 35% depending on the initial pathological condition that lead to the transplant.6–8 Once the tissue rejection process begins, the transplanted corneal graft can be medically treated with topical corticosteroids such as dexamethasone at 0.1%, or prednisolone acetate at 1%, and if tissue rejection continues the corneal graft must be surgically removed and replaced.3 Rates for regraft rejection can be as high as 50%. The tissue rejection process has been described as painful for the patient and can lead to permanent blindness.5
In addition, there is an overwhelming need for transplantable cadaveric corneas in the developing world.9 In many regions around the world, widespread cadaveric donation for corneal transplantation is limited due to religious and cultural factors, lack of general education, and absence of eye banking facilities. Therefore, there is an enormous demand for an alternative to cadaveric corneal transplantation.
The need for alternative options to cadaveric corneas will continue to grow in importance as a result of increasing incidence of transmissible diseases (e.g., human immunodeficiency virus), aging of the population, and the popularity of refractive surgery, which renders corneas unusable for later transplantation. In the context of severe ocular surface pathologies, where the risk of transplant rejection is significantly higher, synthetic homologs, known as keratoprostheses, are chosen as option to donor corneal grafts for full-thickness corneal replacement. Keratoprostheses are used to replace corneas at high risk of immunological rejection or endothelial failure after penetrating keratoplasty and in eyes with limbal stem cell deficiency. Several keratoprostheses are in clinical use but the Boston type-1 keratoprosthesis is by far the most common. It is comprised of front and back plates, an optical polymethylmethacrylate section, and a titanium-locking ring mounted on a donor cornea, which serves to anchor the device in the host eye. Short-term visual recovery is good but long-term prognosis is limited by various complications, including glaucoma and endophthalmitis, requiring a high level of medical intervention, thus, reducing the long term use of these devices.4 Furthermore, osteo-odonto keratoprosthesis has shown good long-term anatomical survival rate and is currently the most common treatment in the case of end-stage inflammatory corneal disease, such as Stevens–Johnson syndrome and thermal and chemical burns.10
In addition, increased interest in reducing animal testing for pharmaceutical and cosmetic products drives the need for in vitro preclinical cornea tissue models. The need within the cosmetics industry to produce tissue analogs for animal testing models continues to grow to replace eye irritation and toxicity tests.11
In this review we provide insight into the human cornea with respect to the approaches being pursued to engineer the epithelium, stroma, endothelium, and full-thickness human cornea systems, and discuss the preclinical in vitro tissue models currently in clinical use.
Anatomy and Functions of the Human Cornea
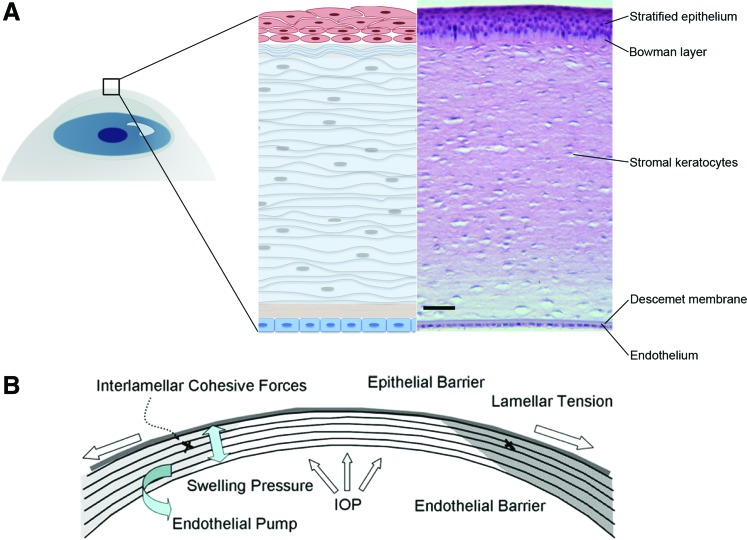
The human cornea is a transparent, avascular, connective tissue that primarily provides an optical interface with substantial refractive power. Additionally, the cornea provides protection for the eye, both mechanical and from infections, and transparency. The adult human cornea measures between 12 and 13 mm in the horizontal and vertical diameters,12 0.5 mm in thickness at the center and then progressively increases toward the sclera.13 The cornea is an organ comprised of three distinct cellular layers, corneal epithelium, stroma, and endothelium, separated by two acellular collagenous interfaces referred to as Bowman's layer and Descemet's membranes (Fig. 1A). The cornea is rich in collagens and contains a unique class of small leucine-rich proteoglycans, including keratan sulfate glycosaminoglycan. The corneal epithelium is a stratified, nonkeratinized squamous tissue with 4–6 cell layers comprising 10% of the overall corneal thickness (40–50 μm).14 The epithelium is one of the most highly innervated tissues in the body, and it is equipped with nociceptive nerve endings that terminate within the epithelial layers.15 The epithelium provides a biological barrier function, essential for corneal transparency, which regulates transfer of water and all soluble components into or out of the stroma. The corneal epithelium in conjunction with the tear film presents a smooth interface at the anterior surface of the eye allowing coherent refraction of light as it enters the cornea.16 In addition, the tear film functions as a reservoir for antibacterial and growth factors, crucial in the maintenance of epithelial homeostasis, proliferation, and repair. Bowman's membrane, (also termed the Anterior Limiting Lamina), is a 15 μm-thick acellular layer of amorphous condensed collagen immediately posterior to the epithelial basement membrane. Bowman's membrane may act as a molecular barrier and/or contribute to corneal shape.17 The corneal stroma makes up ∼90% of the overall cornea thickness and consists of layers of aligned collagen fibrils termed lamellae.18 Approximately 200 to 250 lamellae extend from limbus to limbus with collagen fibril orientation offset between adjoining layers. The lamellar collagen fibrils are heterotypic hybrids of type I and V, displaying remarkably small diameter (∼25 nm). Significant amounts of the fibril-associated collagens XII and XIV and nonfibrillar collagens type VI are also present. A high concentration of small leucine-rich proteoglycans, including decorin, lumican, keratocan, decorated with dermatan sulfate and keratan sulfate are present in the lamellae, credited with maintaining the interfibrillar spacing required for transparency, and contributing to regulation of corneal hydration.13,16 The regular lamellar layering becomes increasingly random in the anterior stroma where more oblique branching and interweaving is observed.19 Interlamellar branching is also more extensive in the corneal periphery than in the center.20 The interweaving of collagen bundles between neighboring lamellae provides an important structural foundation for shear (sliding) resistance and transfer of tensile loads between lamellae. The corneal stroma is populated by keratocytes, mesenchymal cells of neural crest origin. These cells are sandwiched between lamellae and maintain the matrix components of the lamellar connective tissue.21,22 The highly organized collagen lamellae provide mechanical support and biophysical properties required for transparency.23 These include the uniquely small diameter, regular spacing, and tight packing of collagen fibrils. At the posterior of the stromal layer is Descemet's membrane, a thickened basement membrane rich in type VIII collagen, which provides anchoring for the corneal endothelial layer.24 The endothelium provides a metabolic pump, removing water from the stroma and maintaining the correct level of stromal hydration responsible for corneal transparency (Fig. 1B).16,25
FIG. 1.
Human cornea structure and function. (A) Schematic and histological images of human cornea layers: the corneal stratified squamous epithelium with underlying the Bowman's layer, the stroma with keratocytes for the maintenance and production of extracellular matrix, the Descemet's membrane, and the single-layer endothelium. Scale bar 50 μm. (B) Collagen layers in the stroma provide mechanical tensile resistance, while a high concentration of small leucine-rich proteoglycans maintain the interfibrillar spacing required for transparency, and helping to regulate tissue hydration.10,13 Adapted from Tan et al.10 Color images available online at www.liebertpub.com/teb
The functions of the cornea dictate the three principal design requirements a corneal substitute or tissue model will need to satisfy: protection, transparency, and providing an optical interface with substantial refractive power. Particularly, the stroma is a highly organized, dense, avascular, and relatively acellular connective tissue. It consists of highly organized collagen fibrils, which provide lateral tensile strength.26 In addition, the epithelium represents the external surface of the ocular system, therefore protecting the stroma and the endothelium from chemical injuries and infections. In addition, the cornea functions as filters to protect the retina from damaging UV light.27 The transparency of the cornea is determined by to the collagen interfibrillar spacing, regulated by the hydrophilic stromal proteoglycans, which are ultimately regulated by the tissue state of hydration, through the endothelium pump.13
Current Tissue Engineering Approaches for Corneal Replacement
Tissue engineering principles have been applied in an effort to generate viable cornea tissue equivalents at different levels of complexity, starting from engineering of epithelium, stroma, and endothelium layers, to approaches to recreate the native innervation. These approaches have culminated in full-thickness cornea tissue equivalents, implemented in clinical settings, by using a plethora of biomaterial systems (Table 1) and various cell types (Table 2). In particular, the main approaches can be divided in purely cell-based, decellularized, synthetic-, and natural polymer-based constructs in combination with different cell types.
Table 1.
Current Approaches for Tissue-Engineered Cornea Replacements
| Tissue layer | Biomaterial | Clinical status | Reference |
|---|---|---|---|
| Full-thickness cornea | Collagen-chitosan hydrogels | Animal model: pig | Rafat et al.78 |
| Full-thickness cornea | Dendrimer cross-linked collagen hydrogels | In vitro study | Duan and Sheardown,42 and Duan et al.81 |
| Full-thickness cornea | Poly(ethylene glycol)/poly(acrylic acid) hydrogels with peripheral skirt | In vitro study | Myung et al.86 |
| Full-thickness cornea | Fibrin-agarose hydrogels | In vitro study | Alaminos et al.80 |
| Full-thickness cornea | Cross-linked recombinant human collagen type III | Animal models | Griffith et al.79 |
| Phase I clinical trial: human | Li et al.,15 Liu et al.,77 and Fagerholm et al.86 | ||
| 4-year follow-up | Fagerholm et al.87 | ||
| Full-thickness cornea | Acellular porcine cornea | In vitro study | Luo et al.33 |
| Stroma | Surface patterned silk film | In vitro study | Lawrence et al.50 |
| Stroma | Magnetically aligned rat-tail type I | In vitro study | Torbet et al.52 |
| Collagen/proteoglycan hydrogels | Builles et al.57 | ||
| Stroma | Polyglycolic acid fibers | In vitro study | Hu et al.53 |
| Stroma | Gelatin hydrogels | In vitro study | Mimura et al.54 |
| Stroma | Bovine collagen film | In vitro study | Crabb et al.55 |
| Stroma | Poly(l,d lactic acid) | In vitro study | Wilson et al.56 |
| Stroma | Porous surface patterned silk film | In vitro study | Gil et al.65 and Wu et al.67 |
| Stroma | Poly(ester urethane) urea | In vitro study | Wu et al.58 |
| Epithelium | Thermoresponsive cell culture | In vitro study | Nishida et al.31 |
| Animal model: rabbit | Kobayashi et al.28 | ||
| Epithelium | Rat-tail type I collagen hydrogel | In vitro study | Mi et al.41 and Levis et al.29 |
| Epithelium | Chemically cross-linked collagen hydrogel | In vitro study | Duan and Sheardown42 |
| Epithelium | Patterned silk film | In vitro study | Lawrence et al.45 |
| Epithelium | Keratin film | In vitro study | Reichl et al.48 and Feng et al.49 |
| Endothelium | Decellularized human Descemets' membrane | In vitro study | Honda et al.69 |
| Endothelium | Gelatin hydrogel | In vitro study | Watanabe et al.70 |
| Endothelium | Hydroxyethyl chitosan, gelatin, and chondroitin sulfate | In vitro study | Liang et al.71 |
| Endothelium | Chitosan-poly(ethylene glycol) hydrogel films | In vitro study | Ozcelik et al.60 |
| Endothelium | Decellularized amniotic membrane | In vitro study | Fan et al.73 |
| Animal model: cat | |||
| Endothelium | Thermoresponsive cell culture | In vitro study | Teichmann et al.72 |
| Endothelium | Dense collagen hydrogel | In vitro study | Levis et al.74 |
Table 2.
Main Cell Types Used for Tissue-Engineered Cornea Replacements
| Cell | Reference |
|---|---|
| Primary animal-derived corneal epithelial cells | Myung et al.,85 Alaminos et al.,80 Nishida et al.,31 Kobayashi et al.,28 and Tegtmeyer et al.94 |
| Primary animal-derived corneal stromal cells | Myung et al.,85 Alaminos et al.,80 Hu et al.,53 Mimura et al.,54 and Tegtmeyer et al.94 |
| Primary animal-derived corneal endothelial cells | Alaminos et al.80 and Ozcelik et al.60 |
| Primary animal-derived dorsal root ganglion (DRG) cells | Li et al.15 |
| Immortalized human corneal epithelial cells | Li et al.,15 Griffith et al.,79 Lawrence et al.,45 and Kitazawa et al.97 |
| Immortalized human corneal stromal cells | Griffith et al.79 and Lawrence et al.50 |
| Immortalized human corneal endothelial cells | Griffith et al.79 |
| Primary human corneal epithelial cells | Duan and Sheardown,42 Duan et al.,81 Luo et al.,33 Mi et al.,41 Levis et al.,29 Duan and Sheardown42 Reichl et al.,96 and Van Goethem et al.95 |
| Primary human corneal fibroblast cells | Torbet et al.,52 Crabb et al.,55 Wilson et al.,56 Gil et al.,65 Lawrence et al.,50 and Reichl et al.96 |
| Primary human corneal endothelial cells | Honda et al.,69 Watanabe et al.,70 Liang et al.,71 Fan et al.,73 Teichmann et al.,72 and Reichl et al.96 |
| Primary human corneal stromal stem cells | Wu et al.58 and Wu et al.67 |
Corneal epithelium
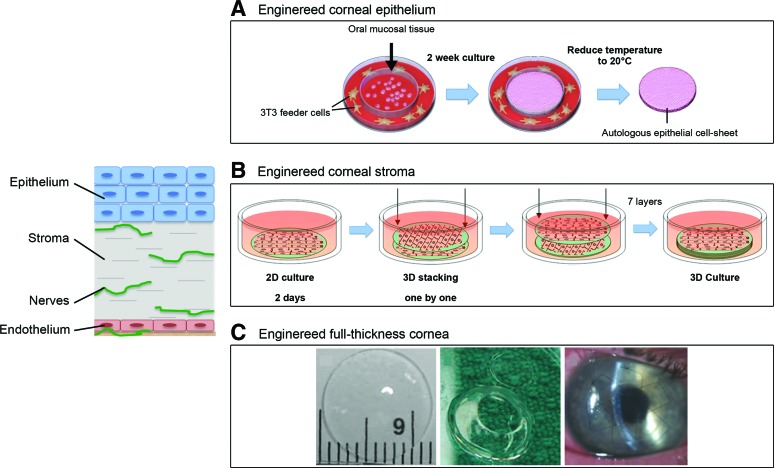
Epithelial and endothelial layers play pivotal roles in maintaining corneal deturgescence (relative state of hydration), and ultimately its transparency, through barrier and pump functions.13 In vitro the corneal epithelial layer has been successfully generated by cell sheet engineering, and it has been shown to promote in vitro growth of a functional stratified epithelium.28–30 In addition, engineered epithelial tissue sheets, prepared using thermoresponsive polymeric substrates, have been clinically evaluated using autologous oral mucosal epithelium (Fig. 2A). However, major drawbacks of this technique are the high variability and the extended time in culture required to generate adequate structures for transplantation.31 Similarly, human amniotic membranes have been extensively applied as substrates for corneal epithelial-derived in vitro expansion and for reconstruction of damaged cornea in several animal models (e.g., rat, rabbit, and goat).32–36 However, the high inter- and intra-tissue variability in morphological, chemical, and optical properties limit the use of the human amniotic membrane in clinical settings.37–39 Alternatively, human donor corneal stromal tissues have been proposed as substrates for human corneal epithelium growth, displaying, in vitro, features similar to the native limbal epithelium.40 The lack of corneal tissue donor availability significantly affects its clinical potential for corneal reconstruction. Among natural polymers, reconstituted type I collagen hydrogels can successfully encapsulate human corneal limbal epithelial cells, resulting in functional stratified epithelial layers.41 Furthermore, collagen hydrogels have been used to engineer corneal limbal crypts through microcontact printing to generate three-dimensional (3D) in vitro human limbal epithelial stem cell niche, which contributes to the maintenance of the corneal epithelium regeneration.29 Chemically cross-linked collagen hydrogels were also explored as corneal epithelium scaffold, due to the enhanced mechanical and optical properties in comparison to thermally driven collagen hydrogels.42 Owing to the optical properties, mechanical robustness, and its versatile processability,43 silk has been exploited as substrate for human epithelial cell growth and functional organization.44 Particularly, silk film topography significantly influenced initial human epithelial cell attachment, proliferation, and growth.45,46 Further studies have shown that silk surfaces can be optimized by contact guidance to direct collective epithelial cell migration and the direction of tissue epithelialization.47 Similarly, keratin-based substrates have been exploited for ocular surface reconstruction because of their good optical properties and ability to support epithelial cell growth in vitro.48,49
FIG. 2.
Tissue-engineered approaches for corneal reconstruction. (A) Human epithelial cell sheet obtained from oral epithelial cells after removal of the cell sheet from the thermoresponsive poly(N-isopropylacrylamide)-pNIPAAM surface; (B) Assembly diagram for three-dimensional (3D) silk film corneal constructs seeded with human corneal fibroblasts. After 2 days, cell-seeded silk films were stacked one by one and three-dimensionally cultured. (C) Synthetically cross-linked collagen, molded into an implantable, full-thickness corneal substitute. Transparent samples were trephined to prepare a button for corneal implantation and then held in place by sutures in the recipient eye. Adapted from Kobayashi et al.,28 Griffith et al.,59 and Liu et al.77 Color images available online at www.liebertpub.com/teb
Corneal stroma
Reconstruction of corneal stroma is challenging due to its complex structure, mechanical strength and transparency. Therefore, engineering corneal stroma has been actively investigated by developing functional corneal stroma substrates through chemical, morphological, and mechanical cues.50–57 In particular, synthetic polymers have been explored for stromal corneal substrates because of their tunable mechanical properties. They have also been processed in micron- nano-sized fiber forms to direct stromal cell organization and differentiation in vitro, resulting in stratified collagen fibril lamellae in orthogonal orientations. A fibrous poly(ester urethane) urea substrate in combination with the use of human stromal stem cells, which are able to differentiate toward keratocyte lineages, showed promise for the in vitro engineering of the stroma via a two-dimensional (2D) approach, although lacking in adequate optical properties.58 The combination of synthetic polymers together with natural-derived materials provides enhanced biological responses and more amenable optical properties.59 As an example, hydrogels films prepared from chitosan blended with poly(ethylene glycol) and poly (l,d lactic acid) nanofibers in a composite with type I collagen hydrogel displayed improved mechanical, optical, and biological performances compared with synthetic fibers alone.56,60 Among the natural polymers, hydrogels from reconstituted type I collagen offer the advantage of encapsulating living cells and,61 particularly in the context of the human cornea, are widely used due to type I collagen dominant content in the corneal stroma. However, due to insufficient mechanical properties and the susceptibility to cell-mediated remodeling and degradation, chemical cross-linkers are commonly used with type I collagen hydrogels for corneal stromal replacements or regeneration.59,62,63 Alternatively, organized arrays of electrospun type I collagen nanofibers have been developed to provide spatial guidance to corneal fibroblasts, although reducing construct transparency.64 In an effort to combine mechanical, biological, and optical properties, corneal stromal substrates from silk films have been developed by optimizing topography, surface chemistry, porosity, and degradation profiles, demonstrating a capability to support corneal stromal cell differentiation in 2D and 3D film architectures (Fig. 2B).51,65–67 Mechanically robust, patterned, porous, and thin films of optically clear silk proteins, in combination with appropriate cell types, displayed great potential as functional cornea tissue equivalents to meet clinical needs. In addition, a dual-layer scaffold, comprised of a silk film and a silk porous membrane, has been used as substrate for human limbal epithelial and mesenchymal stromal cells, for the engineering of the corneal limbus.68
Corneal endothelium
Engineering of the endothelial layer has recently been explored mainly using natural polymer substrates, including type I collagen, gelatin, decellularized tissues, chitosan, and chondroitin sulfate, which showed evidence for the formation of a functional endothelium.60,69–74 Decellularized aminiotic membrane was clinically evaluated in combination with human corneal endothelial cells in a lamellar keratoplasty model, where the removal of the endothelium and part of the Descemet's membrane was performed and able to function as a corneal endothelium equivalent.73
Corneal innervation
The cornea is one of the most innervated tissues in the human body, with nociceptive nerve protrusions, which end in the epithelial layer. The corneal nerves function as mechanical and thermal sensors, to maintain the overall cornea health. The progressive lack in innervation is at the base of the pathological conditions known as dry eye, resulting in a reduction in corneal sensitivity and ultimately in diffuse corneal ulcers. Despite the critical role of innervation in corneal functions, few attempts have been made to induce peripheral nerve proliferation within corneal tissue-engineered constructs. In vitro studies have shown that decoration of substrate with laminin-derived peptides promoted epithelial stratification and neuron growth.75 Furthermore, functional nerve regeneration was observed in a deep lamellar keratoplasty porcine model using cross-linked collagen substitutes, recovering preoperative nerve density at 1-year postsurgery.76
Full-thickness cornea equivalent
Direct in vivo implantation of corneal equivalent biomaterials without cells has been investigated to study the integration of implanted biomaterials with native corneal tissue.77,78 Efforts to mimic the three-layer structure of the cornea (epithelium, stroma, and endothelium) have been reported.42,79–81 Recent studies have been also focused on the use of decellularized biological material, as in the case of amniotic membrane and animal cornea. However, the results of an acellular porcine cornea in combination with amniotic epithelial cells in a rabbit lamellar keratoplasty resulted in degradation of the tissue-engineered cornea due to host rejection.33 Further efforts have been undertaken to develop in vitro corneal stroma equivalents, in combination with the engineering of the epithelial layer and promoting nerve ingrowth, which ultimately culminated in engineered full-thickness cornea for tissue replacement, based on type I collagen in combination with a variety of synthetic polymers (e.g., polyacrylamide, poly(ethylene glycol)).82–85 Biosynthetic corneas from cross-linked recombinant human collagen type III were implanted in an anterior partial keratoplasty surgery in human patients, to enhance endogenous tissue regeneration. The implants were stably integrated, innervated, and avascularized up to 2 years (Fig. 2C). However, the retaining sutures of the implants delayed epithelial closure and there was concurrency of tissue thinning and fibrosis.86 A 4-year follow-up showed the construct stably integrated without rejection, whereas future steps are focused on improving visual acuity based on materials with better shape retention.87
Cell sources
In an effort to generate an in vitro 3D cornea tissue model and to address the need of surgical replacements for partial and full keratoplasty, the most extensively investigated cell populations are epithelial and stromal cell lines for early investigations, while primary human epithelial and stromal cells are mainly implemented in in vitro 3D tissue models and preclinical approaches (Table 2). Furthermore, several studies report methods to isolate and utilize limbal stem cells for regenerating corneal epithelium, stromal stem cells, and mesenchymal stem cells for corneal repair.32,34,88–90 Human stromal stem cells have been used to repopulate mouse corneas and restore stromal thickness, fibril deficits, and transparency in cloudy corneas found in lumican−/− mice,91 and in natural and synthetic polymers-based in vitro systems.58,67 The cells were found to be stably integrated into the mouse cornea for >10 weeks. Importantly, the stem cells did not elicit an immune response, while under the same conditions corneal fibroblasts were immunogenic. The data suggest that a bioengineered cornea populated with such immune privileged cells could provide a viable supplement to corneal replacements and in vitro models. Therefore, human corneal stromal stem cells represent a promising avenue for clinical translational approaches.
Current Tissue Engineering Approaches for In Vitro Corneal Tissue Model
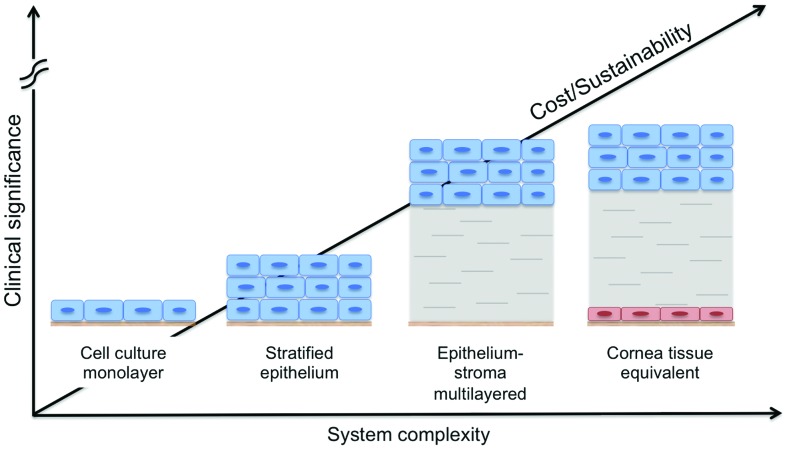
The need for in vitro preclinical cornea tissue models has increased in an effort to reduce animal testing for commercial products. In particular, the cosmetic and pharmaceutical industries encourage the development of viable tissue analogs for irritation and toxicity tests, driven by regulatory requirements. Analogous to the field of tissue-engineered skin, the Seventh Amendment of the European Union Cosmetics Directive requires that irritancy testing of cosmetic products needs to be carried out on in vitro models.92 Engineered tissue analogs may provide effective alternatives to supplant the need for animal testing. In particular, a tissue-engineered cornea could be used in place of the gold standard model for eye irritancy, the Draize Test, which is based on observations upon topical application of a substance in the rabbit eye.93 Furthermore, in vitro cornea models would also be beneficial for drug permeability studies, currently performed with bovine or porcine corneal explants, which have problems associated with reproducibility and species matching, similar to the Draize Test.94 In vitro cornea models range from simple monolayer systems to stratified cell cultures, to epithelium-stroma multilayered systems, and culminate in 3D full-thickness corneal equivalents (Fig. 3).
FIG. 3.
Schematic of cornea tissue models. In vitro cornea models range from simple monolayer systems to stratified cell cultures, to epithelium-stroma multilayered systems, and culminate in 3D full-thickness corneal equivalents, as function of system complexity, cost and culture sustainability, and clinical significance of each approach.92 Color images available online at www.liebertpub.com/teb
The use of a 3D tissue-engineered cornea in the context of in vitro tissue models has been explored to recapitulate the three layers of the cornea (i.e., epithelium, stroma, and endothelium) in combination with human cells for the study of complex physiological and pathophysiological processes in a highly controlled 3D environment (Table 3). The in vitro testing platforms developed up to date mainly comprise a corneal epithelial model, where stratified human epithelium was cultured on a porous permeable polycarbonate substrate and used to test the irritancy of 20 test compounds in comparison to the Draize Test.95 A simplified model based on the epithelium layer alone may be sufficient for acute surface irritancy testing, whereas a more complex tissue model is required to test drug irritancy, toxicity, and permeability through the entire cornea thickness. A three-layer model was developed based on a collagen gel, where corneal endothelial, stromal, and epithelial cells were used for permeability testing protocols. The model was initially proposed with bovine-derived cells and then improved with the use of human cells. Construct permeability was measured the same in engineered corneal construct and explanted human cornea control.96 However, construct material morphological and mechanical properties are far from native tissues. Furthermore, cornea tissue models for pathological chronic studies have mostly been implemented in 2D in vitro culture systems using immortalized corneal epithelial cells to study dry eye and gelatinous drop-like corneal dystrophy.97,98 The cornea in vitro disease model studies developed to date lack full recapitulation of the 3D multilayered native tissue structure, the interplay of the different cell types (i.e., epithelium, stroma, endothelium, and nerve), and sustained function, in case of chronic diseases.
Table 3.
Current Approaches and Clinical Status for Tissue-Engineered Cornea Tissue Models
| Tissue layer | Biomaterial | Clinical status | Reference |
|---|---|---|---|
| Full-thickness cornea | Collagen gel | In vitro study | Tegtmeyer et al.95 |
| Full-thickness cornea | Collagen gel | In vitro study | Reichl et al.97 |
| Epithelium | Permeable polycarbonate substrate | Commercial product | SkinEthic Laboratories |
| Van Goethem et al.96 | |||
| Epithelium | None | In vitro study | Kitazawa et al.98 |
| Epithelium | None | In vitro study | Liang et al.99 |
| Epithelium | Trans-well permeable membrane | Commercial product | Clonetics™ Human Corneal Epithelial Culture Model, Lonza |
| Epithelium | Trans-well permeable membrane | Commercial product | LabCyte Cornea-Model, Japan Tissue Engineering Co., Ltd. |
| Epithelium | None | Commercial product | EpiOcular™, MatTek Corporation |
Future Perspectives
In an effort to address the need for viable human corneas, significant advances in tissue engineering have been made in recent years. Both synthetic and naturally derived biomaterials in combination with primary cells have been used to regenerate and replace partial or full-thickness pathological corneas, starting from epithelial, stromal, endothelial layers, and full-thickness corneal tissues. Naturally derived corneal substitutes hold promise for long-term success having reached human clinical trials. In addition, purely cell-based approaches may also be viable alternatives for both cornea tissue replacements and in the context of in vitro tissue models to significantly reduce animal testing. Some additional attention will be needed to better match current systems to human physiological relevance and requirements, to match systems to suitable remodeling/regeneration time in vivo to provide a suitable crossover time frame between implants and the regeneration of native cornea tissue, and to allow the study of cornea diseases in vitro as discovery tools for new treatment options.
Acknowledgments
We thank the NIH (R01 EY020856 and P41 EB002520) for support for this work. We also thank our many collaborators who have been involved in our own studies of cornea tissue engineering, including Eun Seok Gil, Biman Mandal, Jeff Marchant, and James L. Funderburgh.
Disclosure Statement
No competing financial interests exist.
References
- 1.Whitcher J.P., Srinivasan M., and Upadhyay M.P. Corneal blindness: a global perspective. Bull World Health Organ 79, 214, 2001 [PMC free article] [PubMed] [Google Scholar]
- 2.Pellegrini G., and De Luca M. Eyes on the prize: limbal stem cells and corneal restoration. Cell Stem Cell 15, 121, 2014 [DOI] [PubMed] [Google Scholar]
- 3.Jacobs J., and Taravella M. Corneal graft rejection. eMedicine 1, 2005 [Google Scholar]
- 4.Tan D.T., Dart J.K., Holland E.J., and Kinoshita S. Corneal transplantation. Lancet 379, 1749, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Boisjoly H.M., Tourigny R., Bazin R., Laughrea P.A., Dube I., Chamberland G., et al. Risk factors of corneal graft failure. Ophthalmology 100, 1728, 1993 [DOI] [PubMed] [Google Scholar]
- 6.Williams K.A., Lowe M., Bartlett C., Kelly T.L., Coster D.J., and All C. Risk factors for human corneal graft failure within the Australian corneal graft registry. Transplantation 86, 1720, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Wagoner M.D., Ba-Abbad R., Al-Mohaimeed M., Al-Swailem S., Zimmerman M.B., and King Khaled Eye Specialist Hospital Corneal Transplant Study Group. Postoperative complications after primary adult optical penetrating keratoplasty: prevalence and impact on graft survival. Cornea 28, 385, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Tan D.T.H., Janardhanan P., Zhou H.J., Chan Y.H., Htoon H.M., Ang L.P.K., et al Penetrating keratoplasty in Asian eyes—the Singapore corneal transplant study. Ophthalmology 115, 975, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Jhanji V., Mehta J.S., Sharma N., Sharma B., and Vajpayee R.B. Targeted corneal transplantation. Curr Opin Ophthalmol 23, 324, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Tan A., Tan D.T., Tan X.-W., and Mehta J.S. Osteo-odonto keratoprosthesis: systematic review of surgical outcomes and complication rates. Ocul Surf 10, 15, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Directive, E.C. Review of Cosmetics Directive 76/768/EEC. 2003
- 12.Rüfer F., Schröder A., and Erb C. White-to-white corneal diameter: normal values in healthy humans obtained with the Orbscan II topography system. Cornea 24, 259, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Ruberti J.W., Roy A.S., and Roberts C.J. Corneal biomechanics and biomaterials. Annu Rev Biomed Eng 13, 269, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Ethier C.R., Johnson M., and Ruberti J. Ocular biomechanics and biotransport. Annu Rev Biomed Eng 6, 249, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Li F., Carlsson D., Lohmann C., Suuronen E., Vascotto S., Kobuch K., et al. Cellular and nerve regeneration within a biosynthetic extracellular matrix for corneal transplantation. Proc Natl Acad Sci U S A 100, 15346, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DelMonte D.W., and Kim T. Anatomy and physiology of the cornea. J Cataract Refract Surg 37, 588, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Winkler M., Chai D., Kriling S., Nien C.J., Brown D.J., Jester B., et al. Nonlinear optical macroscopic assessment of 3-D corneal collagen organization and axial biomechanics. Invest Ophthalmol Vis Sci 52, 8818, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cintron C., Covington H., and Kublin C.L. Morphogenesis of rabbit corneal stroma. Invest Ophthalmol Vis Sci 24, 543, 1983 [PubMed] [Google Scholar]
- 19.Shah S., and Laiquzzaman M. Comparison of corneal biomechanics in pre and post-refractive surgery and keratoconic eyes by Ocular Response Analyser. Cont Lens Anterior Eye 32, 129, quiz 51, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Bron A.J. The architecture of the corneal stroma. Br J Ophthalmol 85, 379, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Birk D.E., Fitch J.M., Babiarz J.P., Doane K.J., and Linsenmayer T.F. Collagen fibrillogenesis in vitro: interaction of types I and V collagen regulates fibril diameter. J Cell Sci 95 (Pt 4), 649, 1990 [DOI] [PubMed] [Google Scholar]
- 22.Birk D.E., Fitch J.M., Babiarz J.P., and Linsenmayer T.F. Collagen type I and type V are present in the same fibril in the avian corneal stroma. J Cell Biol 106, 999, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donohue D.J., Stoyanov B.J., McCally R.L., and Farrell R.A. Numerical modeling of the cornea's lamellar structure and birefringence properties. J Opt Soc Am A Opt Image Sci Vis 12, 1425, 1995 [DOI] [PubMed] [Google Scholar]
- 24.Gipson I.K., Spurr-Michaud S.J., and Tisdale A.S. Hemidesmosomes and anchoring fibril collagen appear synchronously during development and wound healing. Dev Biol 126, 253, 1988 [DOI] [PubMed] [Google Scholar]
- 25.Geroski D.H., Matsuda M., Yee R.W., Edelhauser H.F., and Sugar J. Pump function of the human corneal endothelium—effects of age and cornea guttata. Ophthalmology 92, 759, 1985 [DOI] [PubMed] [Google Scholar]
- 26.Hedbys B.O., Mishima S., and Maurice D.M. The imbibition pressure of the corneal stroma. Exp Eye Res 2, 99, 1963 [DOI] [PubMed] [Google Scholar]
- 27.Marshall J. Radiation and the ageing eye. Ophthalmic Physiol Opt 5, 241, 1985 [PubMed] [Google Scholar]
- 28.Kobayashi T., Kan K., Nishida K., Yamato M., and Okano T. Corneal regeneration by transplantation of corneal epithelial cell sheets fabricated with automated cell culture system in rabbit model. Biomaterials 34, 9010, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Levis H.J., Massie I., Dziasko M.A., Kaasi A., and Daniels J.T. Rapid tissue engineering of biomimetic human corneal limbal crypts with 3D niche architecture. Biomaterials 34, 8860, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Bardag-Gorce F., Oliva J., Wood A., Niihara H., Makalinao A., Sabino S., et al. Microarray analysis of oral mucosal epithelial cell sheet. Tissue Eng Regen Med 10, 362, 2013 [Google Scholar]
- 31.Nishida K., Yamato M., Hayashida Y., Watanabe K., Yamamoto K., Adachi E., et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med 351, 1187, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Du Y., Chen J., Funderburgh J.L., Zhu X., and Li L. Functional reconstruction of rabbit corneal epithelium by human limbal cells cultured on amniotic membrane. Mol Vis 9, 635, 2003 [PMC free article] [PubMed] [Google Scholar]
- 33.Luo H., Lu Y., Wu T., Zhang M., Zhang Y., and Jin Y. Construction of tissue-engineered cornea composed of amniotic epithelial cells and acellular porcine cornea for treating corneal alkali burn. Biomaterials 34, 6748, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Ma Y., Xu Y., Xiao Z., Yang W., Zhang C., Song E., et al. Reconstruction of chemically burned rat corneal surface by bone marrow-derived human mesenchymal stem cells. Stem Cells 24, 315, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Mi S., Yang X., Zhao Q., Qu L., Chen S., Meek M.K., et al. Reconstruction of corneal epithelium with cryopreserved corneal limbal stem cells in a goat model. Mol Reprod Dev 75, 1607, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Qu L., Yang X., Wang X., Zhao M., Mi S., Dou Z., et al. Reconstruction of corneal epithelium with cryopreserved corneal limbal stem cells in a rabbit model. Vet J 179, 392, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Hopkinson A., McIntosh R.S., Tighe P.J., James D.K., and Dua H.S. Amniotic membrane for ocular surface reconstruction: donor variations and the effect of handling on TGF-beta content. Invest Ophthalmol Vis Sci 47, 4316, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Connon C.J., Doutch J., Chen B., Hopkinson A., Mehta J.S., Nakamura T., et al. The variation in transparency of amniotic membrane used in ocular surface regeneration. Br J Ophthalmol 94, 1057, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Connon C.J., Nakamura T., Hopkinson A., Quantock A., Yagi N., Doutch J., et al. The biomechanics of amnion rupture: an X-ray diffraction study. PLoS One 2, e1147, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin J., Yoon K.-C., Zhang L., Su Z., Lu R., Ma P., et al. A native-like corneal construct using donor corneal stroma for tissue engineering. PLoS One 7, e49571, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mi S., Chen B., Wright B., and Connon C.J. Ex vivo construction of an artificial ocular surface by combination of corneal limbal epithelial cells and a compressed collagen scaffold containing keratocytes. Tissue Eng Part A 16, 2091, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Duan X., and Sheardown H. Dendrimer crosslinked collagen as a corneal tissue engineering scaffold: mechanical properties and corneal epithelial cell interactions. Biomaterials 27, 4608, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Omenetto F.G., and Kaplan D.L. New opportunities for an ancient material. Science 329, 528, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu J., Lawrence B.D., Liu A., Schwab I.R., Oliveira L.A., and Rosenblatt M.I. Silk fibroin as a biomaterial substrate for corneal epithelial cell sheet generation. Invest Ophthalmol Vis Sci 53, 4130, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lawrence B.D., Pan Z., Liu A., Kaplan D.L., and Rosenblatt M.I. Human corneal limbal epithelial cell response to varying silk film geometric topography in vitro. Acta Biomater 8, 3732, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bray L.J., George K.A., Ainscough S.L., Hutmacher D.W., Chirila T.V., and Harkin D.G. Human corneal epithelial equivalents constructed on Bombyx mori silk fibroin membranes. Biomaterials 32, 5086, 2011 [DOI] [PubMed] [Google Scholar]
- 47.Lawrence B.D., Pan Z., and Rosenblatt M.I. Silk film topography directs collective epithelial cell migration. PLoS One 7, e50190, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reichl S., Borrelli M., and Geerling G. Keratin films for ocular surface reconstruction. Biomaterials 32, 3375, 2011 [DOI] [PubMed] [Google Scholar]
- 49.Feng Y., Borrelli M., Meyer-Ter-Vehn T., Reichl S., Schrader S., and Geerling G. Epithelial wound healing on keratin film, amniotic membrane and polystyrene in vitro. Curr Eye Res 39, 561, 2014 [DOI] [PubMed] [Google Scholar]
- 50.Lawrence B.D., Marchant J.K., Pindrus M.A., Omenetto F.G., and Kaplan D.L. Silk film biomaterials for cornea tissue engineering. Biomaterials 30, 1299, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gil E.S., Park S.H., Marchant J., Omenetto F., and Kaplan D.L. Response of human corneal fibroblasts on silk film surface patterns. Macromol Biosci 10, 664, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Torbet J., Malbouyres M., Builles N., Justin V., Roulet M., Damour O., et al. Tissue engineering of the cornea: orthogonal scaffold of magnetically aligned collagen lamellae for corneal stroma reconstruction. Con Proc IEEE Eng Med Biol Soc 2007, 6400, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Hu X.J., Lui W., Cui L., Wang M., and Cao Y.L. Tissue engineering of nearly transparent corneal stroma. Tissue Eng 11, 1710, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Mimura T., Amano S., Yokoo S., Uchida S., Yamagami S., Usui T., et al. Tissue engineering of corneal stroma with rabbit fibroblast precursors and gelatin hydrogels. Mol Vis 14, 1819, 2008 [PMC free article] [PubMed] [Google Scholar]
- 55.Crabb R.A.B., Chau E.P., Evans M.C., Barocas V.H., and Hubel A. Biomechanical and microstructural characteristics of a collagen film-based corneal stroma equivalent. Tissue Eng 12, 1565, 2006 [DOI] [PubMed] [Google Scholar]
- 56.Wilson S.L., Wimpenny I., Ahearne M., Rauz S., El Haj A.J., and Yang Y. Chemical and topographical effects on cell differentiation and matrix elasticity in a corneal stromal layer model. Adv Funct Mater 22, 3641, 2012 [Google Scholar]
- 57.Builles N., Janin-Manificat H., Malbouyres M., Justin V., Rovère M.-R., Pellegrini G., et al. Use of magnetically oriented orthogonal collagen scaffolds for hemi-corneal reconstruction and regeneration. Biomaterials 31, 8313, 2010 [DOI] [PubMed] [Google Scholar]
- 58.Wu J., Du Y., Mann M.M., Yang E., Funderburgh J.L., and Wagner W.R. Bioengineering organized, multilamellar human corneal stromal tissue by growth factor supplementation on highly aligned synthetic substrates. Tissue Eng Part A 19, 2063, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Griffith M., Fagerholm P., Lagali N., Latorre M.A., Hackett J., and Sheardown H. Regenerative medicine in the cornea. In: Palatal A., Lanza R., Thomson J.A., and Nerem R., eds. Principles of Regenerative Medicine, 2nd Ed. Boston, MA: Academic Press, 2010, pp. 911–924 [Google Scholar]
- 60.Ozcelik B., Brown K.D., Blencowe A., Daniell M., Stevens G.W., and Qiao G.G. Ultrathin chitosan-poly(ethylene glycol) hydrogel films for corneal tissue engineering. Acta Biomater 9, 6594, 2013 [DOI] [PubMed] [Google Scholar]
- 61.Ghezzi C.E., Muja N., Marelli B., and Nazhat S.N. Real time responses of fibroblasts to plastically compressed fibrillar collagen hydrogels. Biomaterials 32, 4761, 2011 [DOI] [PubMed] [Google Scholar]
- 62.Ahearne M., Wilson S.L., Liu K.K., Rauz S., El Haj A.J., and Yang Y. Influence of cell and collagen concentration on the cell-matrix mechanical relationship in a corneal stroma wound healing model. Exp Eye Res 91, 584, 2010 [DOI] [PubMed] [Google Scholar]
- 63.Duncan T.J., Tanaka Y., Shi D., Kubota A., Quantock A.J., and Nishida K. Flow-manipulated, crosslinked collagen gels for use as corneal equivalents. Biomaterials 31, 8996, 2010 [DOI] [PubMed] [Google Scholar]
- 64.Phu D., Wray L.S., Warren R.V., Haskell R.C., and Orwin E.J. Effect of substrate composition and alignment on corneal cell phenotype. Tissue Eng Part A 17, 799, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gil E.S., Mandal B.B., Park S.H., Marchant J.K., Omenetto F.G., and Kaplan D.L. Helicoidal multi-lamellar features of RGD-functionalized silk biomaterials for corneal tissue engineering. Biomaterials 31, 8953, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shang K., Rnjak-Kovacina J., Lin Y., Hayden R., Tao H., and Kaplan D. Accelerated in vitro degradation of optically clear low beta-sheet silk films by enzyme mediated pretreatment. Trans Vis Sci Technol 2, 2, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu J., Rnjak-Kovacina J., Du Y., Funderburgh M.L., Kaplan D.L., and Funderburgh J.L. Corneal stromal bioequivalents secreted on patterned silk substrates. Biomaterials 35, 3744, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bray L.J., George K.A., Hutmacher D.W., Chirila T.V., and Harkin D.G. A dual-layer silk fibroin scaffold for reconstructing the human corneal limbus. Biomaterials 33, 3529, 2012 [DOI] [PubMed] [Google Scholar]
- 69.Honda N., Mimura T., Usui T., and Amano S. Descemet stripping automated endothelial keratoplasty using cultured corneal endothelial cells in a rabbit model. Arch Ophthalmol 127, 1321, 2009 [DOI] [PubMed] [Google Scholar]
- 70.Watanabe R., Hayashi R., Kimura Y., Tanaka Y., Kageyama T., Hara S., et al. A novel gelatin hydrogel carrier sheet for corneal endothelial transplantation. Tissue Eng Part A 17, 2213, 2011 [DOI] [PubMed] [Google Scholar]
- 71.Liang Y., Liu W., Han B., Yang C., Ma Q., Zhao W., et al. Fabrication and characters of a corneal endothelial cells scaffold based on chitosan. J Mater Sci Mater Med 22, 175, 2011 [DOI] [PubMed] [Google Scholar]
- 72.Teichmann J., Valtink M., Gramm S., Nitschke M., Werner C., Funk R.H.W., et al Human corneal endothelial cell sheets for transplantation: thermo-responsive cell culture carriers to meet cell-specific requirements. Acta Biomater 9, 5031, 2013 [DOI] [PubMed] [Google Scholar]
- 73.Fan T., Ma X., Zhao J., Wen Q., Hu X., Yu H., et al. Transplantation of tissue-engineered human corneal endothelium in cat models. Mol Vis 19, 400, 2013 [PMC free article] [PubMed] [Google Scholar]
- 74.Levis H.J., Peh G.S.L., Toh K.-P., Poh R., Shortt A.J., Drake R.A.L., et al Plastic compressed collagen as a novel carrier for expanded human corneal endothelial cells for transplantation. PLoS One 7, e50993, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li F.F., Carlsson D., Lohmann C., Suuronen E., Vascotto S., Kobuch K., et al. Cellular and nerve regeneration within a biosynthetic extracellular matrix for corneal transplantation. Proc Natl Acad Sci U S A 100, 15346, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lagali N.S., Griffith M., Shinozaki N., Fagerholm P., and Munger R. Innervation of tissue-engineered corneal implants in a porcine model: a 1-year in vivo confocal microscopy study. Invest Ophthalmol Vis Sci 48, 3537, 2007 [DOI] [PubMed] [Google Scholar]
- 77.Liu W., Merrett K., Griffith M., Fagerholm P., Dravida S., Heyne B., et al. Recombinant human collagen for tissue engineered corneal substitutes. Biomaterials 29, 1147, 2008 [DOI] [PubMed] [Google Scholar]
- 78.Rafat M., Li F., Fagerholm P., Lagali N.S., Watsky M.A., Munger R., et al. PEG-stabilized carbodiimide crosslinked collagen-chitosan hydrogels for corneal tissue engineering. Biomaterials 29, 3960, 2008 [DOI] [PubMed] [Google Scholar]
- 79.Griffith M., Osborne R., Munger R., Xiong X., Doillon C.J., Laycock N.L., et al. Functional human corneal equivalents constructed from cell lines. Science 286, 2169, 1999 [DOI] [PubMed] [Google Scholar]
- 80.Alaminos M., Sánchez-Quevedo M.D.C., Muñoz-Ávila J.I., Serrano D., Medialdea S., Carreras I., et al. Construction of a complete rabbit cornea substitute using a fibrin-agarose scaffold. Invest Ophthalmol Vis Sci 47, 3311, 2006 [DOI] [PubMed] [Google Scholar]
- 81.Duan X. Biomaterials 28, 78, 2007 [DOI] [PubMed] [Google Scholar]
- 82.Li F., Griffith M., Li Z., Tanodekaew S., Sheardown H., Hakim M., et al. Recruitment of multiple cell lines by collagen-synthetic copolymer matrices in corneal regeneration. Biomaterials 26, 3093, 2005 [DOI] [PubMed] [Google Scholar]
- 83.Liu W., Deng C., McLaughlin C.R., Fagerholm P., Lagali N.S., Heyne B., et al. Collagen-phosphorylcholine interpenetrating network hydrogels as corneal substitutes. Biomaterials 30, 1551, 2009 [DOI] [PubMed] [Google Scholar]
- 84.McLaughlin C.R., Acosta M.C., Luna C., Liu W., Belmonte C., Griffith M., et al. Regeneration of functional nerves within full thickness collagen-phosphorylcholine corneal substitute implants in guinea pigs. Biomaterials 31, 2770, 2010 [DOI] [PubMed] [Google Scholar]
- 85.Myung D. Biomed microdevices 9, 911, 2007 [DOI] [PubMed] [Google Scholar]
- 86.Fagerholm P., Lagali N.S., Merrett K., Jackson W.B., Munger R., Liu Y., et al. A biosynthetic alternative to human donor tissue for inducing corneal regeneration: 24-month follow-up of a phase 1 clinical study. Sci Transl Med 2, 46ra61, 2010 [DOI] [PubMed] [Google Scholar]
- 87.Fagerholm P., Lagali N.S., Ong J.A., Merrett K., Jackson W.B., Polarek J.W., et al. Stable corneal regeneration 4 years after implantation of a cell-free recombinant human collagen scaffold. Biomaterials 35, 2420, 2014 [DOI] [PubMed] [Google Scholar]
- 88.Du Y., Funderburgh M.L., Mann M.M., SundarRaj N., and Funderburgh J.L. Multipotent stem cells in human corneal stroma. Stem Cells 23, 1266, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Funderburgh M.L., Du Y., Mann M.M., SundarRaj N., and Funderburgh J.L. PAX6 expression identifies progenitor cells for corneal keratocytes. FASEB J 19, 1371, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pinnamaneni N., and Funderburgh J.L. Concise review: stem cells in the corneal stroma. Stem Cells 30, 1059, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Du Y., Carlson E.C., Funderburgh M.L., Birk D.E., Pearlman E., Guo N., et al. Stem cell therapy restores transparency to defective murine corneas. Stem Cells 27, 1635, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gibbons M.C., Foley M.A., and Cardinal K.O. Thinking inside the box: keeping tissue-engineered constructs in vitro for use as preclinical models. Tissue Eng Part B Rev 19, 14, 2013 [DOI] [PubMed] [Google Scholar]
- 93.Curren R.D., and Harbell J.W. Ocular safety: a silent (in vitro) success story. Altern Lab Anim 30 Suppl 2, 69, 2002 [DOI] [PubMed] [Google Scholar]
- 94.Tegtmeyer S., Papantoniou I., and Muller-Goymann C.C. Reconstruction of an in vitro cornea and its use for drug permeation studies from different formulations containing pilocarpine hydrochloride. Eur J Pharm Biopharm 51, 119, 2001 [DOI] [PubMed] [Google Scholar]
- 95.Van Goethem, F., Adriaens E., Alepee N., Straube F., De Wever, B., Cappadoro M., et al. Prevalidation of a new in vitro reconstituted human cornea model to assess the eye irritating potential of chemicals. Toxicol In Vitro 20, 1, 2006 [DOI] [PubMed] [Google Scholar]
- 96.Reichl S., Bednarz J., and Muller-Goymann C.C. Human corneal equivalent as cell culture model for in vitro drug permeation studies. Br J Ophthalmol 88, 560, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kitazawa K., Kawasaki S., Shinomiya K., Aoi K., Matsuda A., Funaki T., et al. Establishment of a human corneal epithelial cell line lacking the functional TACSTD2 gene as an in vitro model for gelatinous drop-like dystrophy. Invest Ophthalmol Vis Sci 54, 5701, 2013 [DOI] [PubMed] [Google Scholar]
- 98.Liang H., Baudouin C., Daull P., Garrigue J.S., and Brignole-Baudouin F. Ocular safety of cationic emulsion of cyclosporine in an in vitro corneal wound-healing model and an acute in vivo rabbit model. Mol Vis 18, 2195, 2012 [PMC free article] [PubMed] [Google Scholar]