Abstract
Our approach for fabricating tissue-engineered vascular grafts (TEVG), applied in the surgical management of congenital heart disease, is accomplished by seeding isolated bone marrow-derived mononuclear cells (BM-MNCs) onto biodegradable scaffolds. The current method used for isolation of BM-MNCs is density centrifugation in Ficoll. This is a time-consuming, labor-intensive, and operator-dependent method. We previously demonstrated that a simpler, faster, and operator-independent method for isolating BM-MNCs using a filter elution technique was feasible. In this study, we compare the use of each technique to determine if the BM-MNCs isolated by the filtration elution method are biologically equivalent to BM-MNCs isolated using density centrifugation. Scaffolds were constructed from a nonwoven poly(glycolic acid) fiber mesh coated with 50:50 poly(l-lactide-co-ɛ-caprolactone) sealant. BM-MNCs were isolated from the bone marrow of syngeneic C57BL/6 mice by either density centrifugation with Ficoll or filtration (Ficoll vs. Filter), then statically seeded onto scaffolds, and incubated overnight. The TEVG were implanted in 10-week-old C57BL/6 mice (n=23 for each group) as inferior vena cava interposition grafts and explanted at 14 days for analysis. At 14 days after implantation, there were no significant differences in graft patency between groups (Ficoll: 87% vs. Filter: 78%, p=0.45). Morphometric analysis by hematoxylin and eosin staining showed no difference of graft luminal diameter or neointimal thickness between groups (luminal diameter, Ficoll: 620.3±82.9 μm vs. Filter: 633.3±131.0 μm, p=0.72; neointimal thickness, Ficoll: 37.9±7.8 μm vs. Filter: 37.9±11.2 μm, p=0.99). Histologic examination demonstrated similar degrees of cellular infiltration and extracellular matrix deposition, and endothelial cell coverage on the luminal surface, in either group. Macrophage infiltration showed no difference in the number of F4/80-positive cells or macrophage phenotypes between the two experimental groups (Ficoll: 2041±1048 cells/mm2 vs. Filter: 1887±907.7 cells/mm2, p=0.18). We confirmed the biological equivalence of BM-MNCs, isolated using either density centrifugation or filtration, for making TEVG.
Introduction
Current surgical management of congenital heart disease involves the use of synthetic vascular grafts, an approach that is limited by the absence of graft growth, high incidence of graft thrombosis, development of graft stenosis, ectopic calcification, and risk of infection.1 To address the shortcomings of currently available synthetic grafts, tissue engineering techniques have emerged as a means of creating biologically active blood vessels: tissue-engineered vascular grafts (TEVG). TEVG offer the potential for surgeons to implant grafts that are capable of growth, remodeling, and repair. We have successfully implanted TEVG in humans with congenital heart defects.2 Long-term follow-up has now shown that TEVG are safe and effective to use in pediatric patients.3
We utilize bone marrow-derived mononuclear cells (BM-MNCs) for cell seeding, which is a crucial step in fabricating our TEVG. The use of BM-MNCs instead of primary somatic cells (mesenchymal stromal cells, endothelial cells, or smooth muscle cells) eliminated the need for cell expansion in culture ex vivo, which decreases the risk of contamination and minimizes the amount of tissue needed to create the TEVG. However, current techniques for the construction of TEVG use density centrifugation as a means of isolating BM-MNCs and are limited by the risk of bacterial contamination, loss of cell viability, and need for a specialized clean room.4 The development of simpler, more rapid, and operator-independent methods for manufacturing TEVG using closed disposable systems could overcome these disadvantages and facilitate more widespread use of TEVG products. We previously demonstrated the feasibility of a novel filtration system to isolate BM-MNCs from human bone marrow5 and applied this technique to a closed disposable system for construction of TEVG by using an ovine model.6
In the present study, we isolated BM-MNCs in a murine model using two different methods: the conventional density centrifugation technique with Ficoll and the new filtration system. We then seeded the isolated BM-MNCs onto biodegradable scaffolds and then implanted these TEVG as inferior vena cava (IVC) interposition grafts in C57BL/6 mice. The objective of this study is to evaluate tissue remodeling as a means of assessing biological equivalence between the two methods in the construction of TEVG.
Materials and Methods
Scaffolds
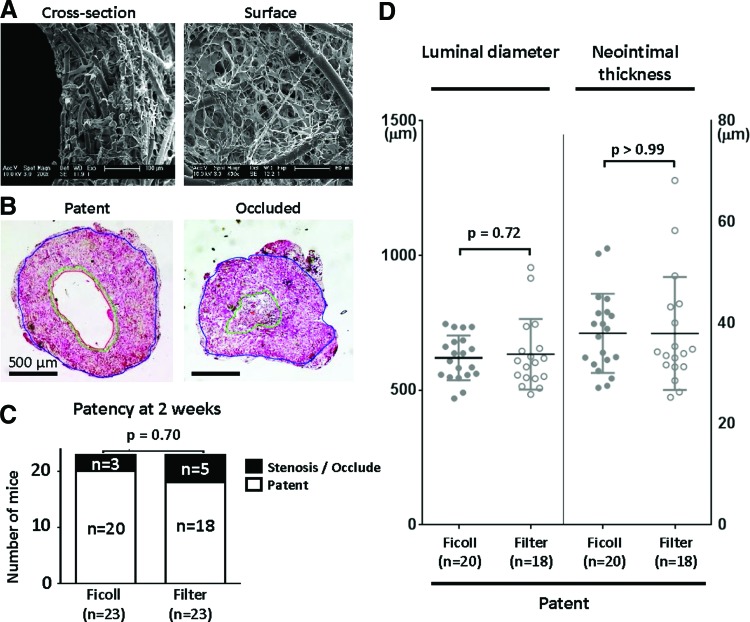
Scaffolds were constructed using a dual cylinder chamber molding system from a nonwoven poly(glycolic acid) fiber mesh (Biomedical Structures, Warwick, RI) coated with a 50:50 copolymer sealant solution of poly(l-lactide-co-ɛ-caprolactone) (Absorbable Polymers International, Birmingham, AL), as previously described.7,8 Scanning electron microscope images of scaffolds are shown in Figure 3A. Each scaffold was 4 mm in length and 0.9 mm in diameter.
FIG. 3.
Comparison of quantitative morphometric analysis of tissue-engineered vascular grafts (TEVG). (A) Representative scanning electron microscope images of scaffolds before cell seeding. (B) Representative hematoxylin and eosin (HE) staining images of patent and occluded TEVG. (C) The adventitia, media, and intima were manually identified and measured on HE staining, and patent was defined as greater than 50% in luminal diameter compared to graft at time of implantation. There was no statistical difference in patency of grafts at 2 weeks between the groups. Data were evaluated by Fisher's exact test. (D) Neither luminal diameter nor neointimal thickness differed between groups. Data were evaluated by Welch's t test. Color images available online at www.liebertpub.com/tec
BM-MNC preparation and seeding onto scaffolds
BM-MNCs were isolated from bone marrow of CB57BL/6 mice by using two techniques: density centrifugation in Ficoll and filtration (Ficoll vs. Filter). For the density centrifugation group, after bone marrow harvest, BM-MNCs were isolated by centrifugation for 30 min with Histopaque-1083 (Sigma, St. Louis, MO). After washing with phosphate-buffered saline (PBS), BM-MNCs were diluted with RPMI 1640 (Sigma).8 For the filtration group, isolation of BM-MNCs was performed as described previously.6 Briefly, the volume of bone marrow plus RMPI 1640 was increased to 15 mL and transferred to an initial chamber using a syringe; this was filtered by a simple gravitational flow using a commercially available cell harvest filter (Pall Corporation, Port Washington, NY). After washing the filter media with PBS twice to reduce contamination by red blood cells, BM-MNCs were recovered by back-flushing the filter with 6 mL of sterile 10% dextran 40/saline solution. The collected solution was centrifuged and the resulting pellet was diluted with RPMI 1640. Finally, 1.0×106 MNCs were prepared by both methods. Also, grafts were seeded by pipetting a concentrated cell suspension through the lumen of the scaffold. Cell-seeded grafts were preconditioned in a CO2 incubator at 37°C for 18–24 h in 1 mL of RPMI 1640 under sterile conditions.8
Cell counts were performed after BM-MNC isolation in each group, and viability of these cells was obtained by trypan blue staining with manual cell count. The cellularity of the seeded scaffolds after overnight incubation was determined by measuring the DNA content with a PicoGreen detection assay (Molecular Probes, Eugene, OR).4
Animal model and surgical implantation
All animals received humane care in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The Institutional Animal Care and Use Committee at Yale University approved the use of animals and all procedures described in this study. C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME).
TEVG were implanted in 10-week-old CB57BL/6 mice (n=23 for each group) as IVC interposition grafts, using a standard microsurgical technique as previously described.7,8 Animals were sacrificed at 14 days and grafts were explanted for analysis after the saline perfusion.
Serial monitoring of TEVG by ultrasound
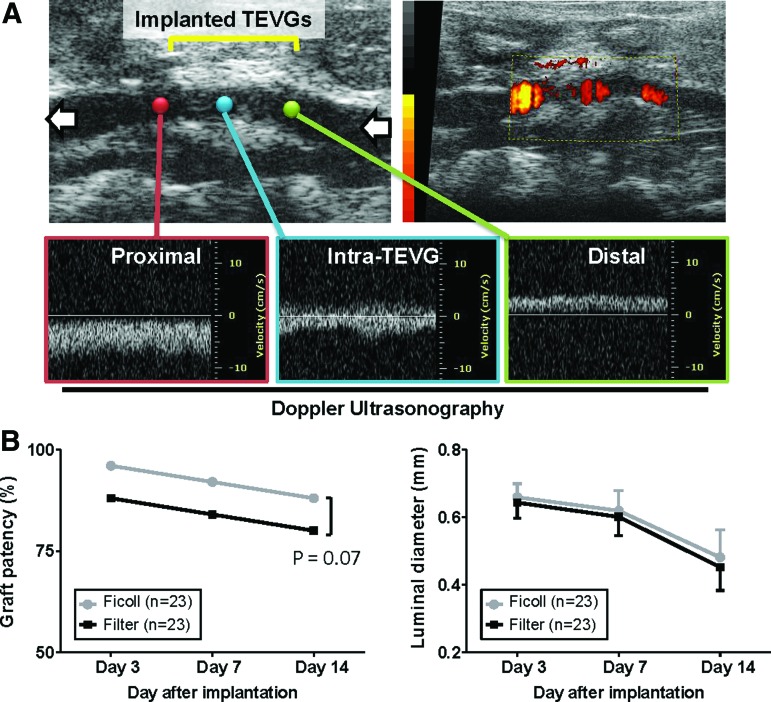
Ultrasonography (Vevo Visualsonics 770; Visualsonics, Toronto, ON) was used to serially monitor patency of grafts at 3, 7, and 14 days after implantation. Before ultrasonography, mice were anesthetized with 1.5% inhaled isoflurane. The graft luminal diameter was determined sonographically, and patency was determined by measuring Doppler flow velocity proximal and distal to the graft (Fig. 2A).
FIG. 2.
Serial monitoring of graft patency and luminal diameter by ultrasonography. (A) Representative images of ultrasound. The graft luminal diameter was determined sonographically and patency was determined by measuring flow velocity proximal and distal to the graft. (B) Serial monitoring by ultrasound demonstrated no difference in graft patency and luminal diameter between groups at each time point. Color images available online at www.liebertpub.com/tec
Histology and immunohistochemistry
Explanted grafts at 14 days after implantation were fixed in 4% paraformaldehyde, embedded in paraffin, sliced (5 μm sections), and stained with hematoxylin and eosin (HE), Masson's trichrome, Elastica van Gieson, Alcian Blue, and von Kossa. The adventitia, media, and intima were manually identified and measured with ZEN lite (Carl Zeiss, Oberkochen, Germany) on HE staining histologically, and patency was defined as >50% in luminal diameter compared to graft at the time of implantation (Fig. 3B).
Identification of smooth muscle cells, endothelial cells, matrix metalloproteinase-2 (MMP-2), and macrophages was done by immunohistochemical staining of paraffin-imbedded explant sections with the anti-smooth muscle actin (SMA) antibody (DAKO, Carpinteria, CA), anti-von Willebrand factor (vWF) antibody (DAKO), anti-matrix metalloproteinase-2 (MMP-2) antibody (Millipore, Billerica, MA), anti-F4/80 antibody (DAKO), anti-inducible nitric oxide synthase (iNOS) antibody (Abcam, Cambridge, MA), and anti-CD206 antibody (Abcam), respectively. Primary antibody binding was detected using biotinylated immunoglobulin G (Vector, Burlingame, CA), and this was followed by the binding of streptavidin-horseradish peroxidase and color development with 3,3-diaminobenzidine.
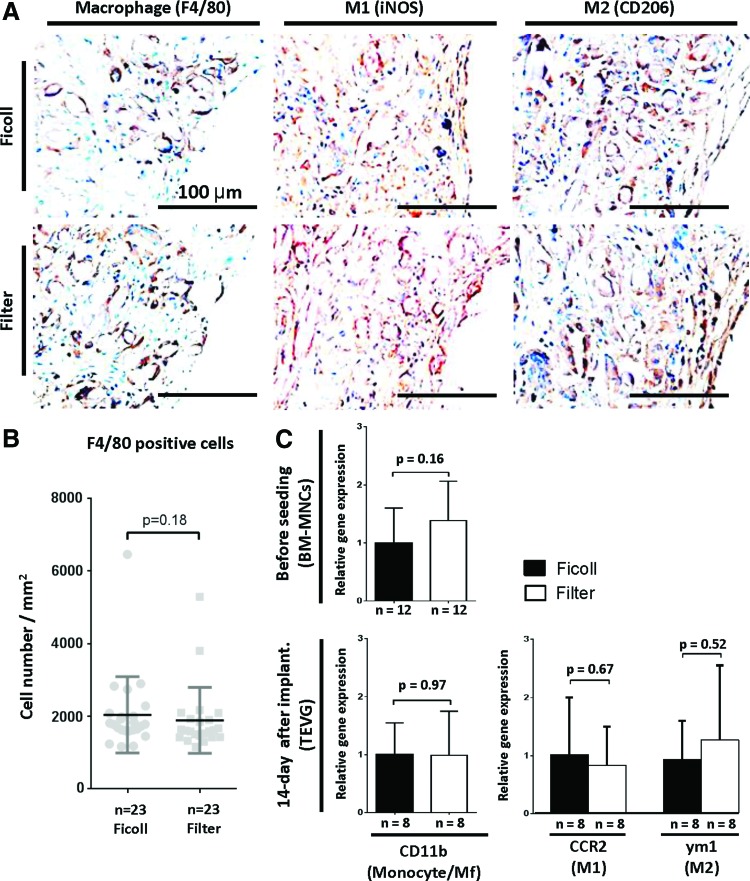
Macrophages identified by positive F4/80 expression were quantified in explanted scaffolds. Two or three separate sections of each explant were stained with F4/80 and imaged at 400× magnification. Each section was divided into eight equal sections and nuclei were counted in three of those regions.
RNA extraction and reverse transcription quantitative polymerase chain reaction
Explanted grafts at 14 days after implantation were frozen in optimal cutting temperature compound (Sakura Finetek, Torrance, CA) and sectioned into twenty 30 μm sections. Total RNA was extracted and purified using the RNeasy mini kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. Reverse transcription was performed using the High Capacity RNA-to-cDNA Kit (Applied Biosystems, Foster City, CA). All reagents and instrumentation for gene expression analysis were obtained from Applied Biosystems. Quantitative polymerase chain reaction (qPCR) was performed with a Step One Plus Real-Time PCR System using the TaqMan Universal PCR Master Mix Kit. Reference numbers for primers are as follows: itgam (Mm00434455_m1), CCR2 (Mm00438270_m1), ym1 (Mm00657889_mH), and HPRT (Mm00446968_m1). The results were analyzed using the comparative threshold cycle method, normalized with HPRT as an endogenous reference, and reported as relative values (ΔΔCT) to those of control native IVC.
Statistical analysis
Our previous study and preliminary observation indicated that the patency rate at 2 weeks after the implantation was 70% for the traditional BM-MNC-seeded group and 30% for the unseeded group. According to these data, a power calculation by Fisher's exact probability test, with 0.05 of alpha-error and 0.8 of power, was completed to decide the sample number.
Numeric values are listed as mean with standard deviation. The number of experiments is shown in each case. Data of continuous variables with normal distribution were evaluated by Student's t test or by Welch's t test in instances when two groups had unequal variance. The nonparametric Mann–Whitney test was performed to detect significant difference of continuous variables with non-normal distributions. Fisher's exact test was used for dichotomous variables. p Values of<0.05 indicated statistical significance.
Results
Comparing isolation of MNCs
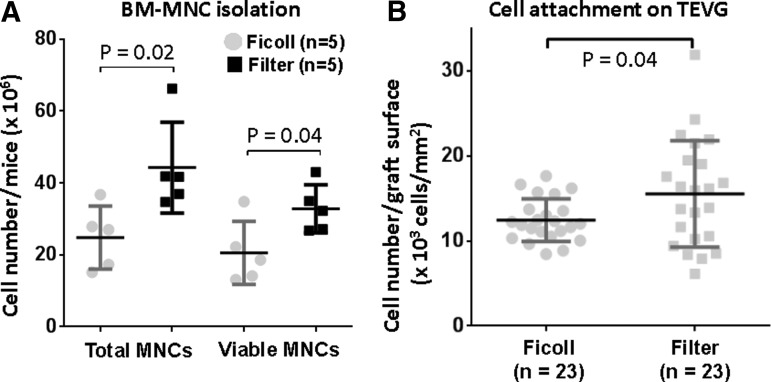
The number of total MNCs and viable MNCs after isolation from bone marrow cells using filtration was statistically higher than that using density centrifugation (total cells, Ficoll: 24.8±8.8×106/mouse vs. Filter: 44.3±12.6×106/mouse, p=0.02; viable cells, Ficoll: 20.6±8.7×106/mouse vs. Filter: 32.8±6.7×106/mouse, p=0.04, respectively; Fig. 1A). Evaluation of cell attachment onto seeded scaffolds by PicoGreen DNA assay showed a statistically significant difference in the number of cells after overnight incubation of scaffold between these two groups (Ficoll: 12.4±2.5×103/mm2 vs. Filter: 15.5±6.3×103/mm2, p=0.04; Fig. 1B).
FIG. 1.
Comparison of isolated bone marrow-derived mononuclear cells (BM-MNCs) and cell attachment after seeding. (A) Total and viable cell count of BM-MNCs was performed by trypan blue staining with manual cell count. The filter method isolated significantly more BM-MNCs in both total and viable cells. Data were evaluated by Student t test. (B) Cell attachment on the graft was evaluated by PicoGreen DNA quantification after overnight incubation. The number of attached cells in the filter group was higher than that in the Ficoll group. Data were evaluated by Welch's t test.
Monitoring of TEVG by ultrasound
Serial ultrasonographic imaging demonstrated no difference in graft patency and luminal diameter between the density centrifugation and filtration groups, and these parameters gradually decreased in both groups during the observation period (Fig. 2B). While Figure 2B illustrates decreased graft patency in the filtration group at each time point, this difference was not statistically different. There was no aneurysm formation, hemorrhagic complications, or ectopic calcification in either group.
Quantitative morphometric analysis of TEVG
At 14 days after implantation, there was no difference between groups in terms of graft patency with the Ficoll group achieving 87% patency and the Filter group achieving 78% patency evaluated with HE staining (p=0.70; Fig. 3C). Morphometric analysis of patent grafts demonstrated no statistically significant difference in the luminal diameter (Ficoll: 620±82.9 μm vs. Filter: 633±131 μm, p=0.72) or neointimal thickness (Ficoll: 37.9±7.8 μm vs. Filter: 37.9±11.2 μm, p>0.99) (Fig. 3D).
Histological analysis of TEVG
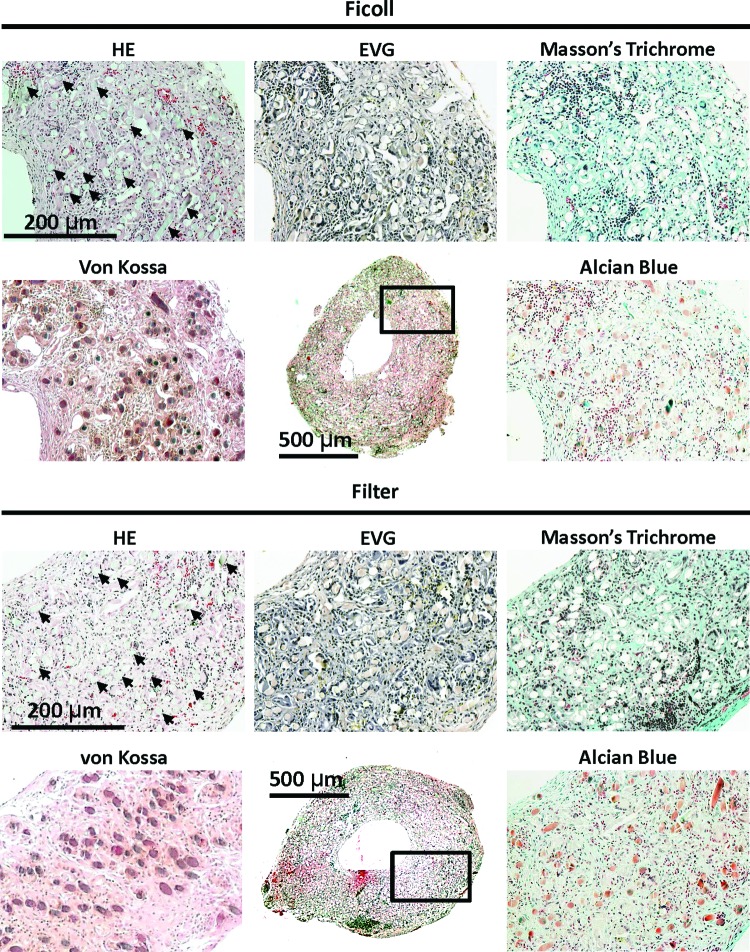
HE staining of TEVG at 14 days after implantation revealed similarities in neovessel development, with similar degree of cellular infiltration within the TEVG, and no differences in cellular distribution or architecture between the two groups (Ficoll and Filter) (Fig. 4). Extracellular matrix stains using Masson's trichrome and Alcian blue stain displayed a robust deposition of collagen throughout TEVG in both groups (Fig. 4). There was no evidence of elastin in 14-day explants of both groups. The von Kossa stain revealed no instances of calcification in either group (Fig. 4). Poly(glycolic acid) fibers, which may appear as vacuoles or capillaries, still existed in the TEVG at the 14-day time point and caused nonspecific staining of both von Kossa and Alcian blue stain.
FIG. 4.
Histological analysis of TEVG at 14 days after implantation. Histologic examination showed cellular infiltration into the TEVG, and robust deposition of collagen throughout the TEVG in both experimental groups, although there was no evidence of elastin. von Kossa stain revealed no instances of calcification in either group. Poly(glycolic acid) fibers, which may appear as vacuoles or capillaries, still existed in the TEVG and caused nonspecific staining of both von Kossa and Alcian blue stains. Arrows indicate representative remaining fibers on HE staining. Boxed area highlights the region of TEVG section corresponding to high-magnification sections in each group. Color images available online at www.liebertpub.com/tec
Smooth muscle cells, which were defined by immunohistochemical SMA staining, were primarily localized in the neointima of both experimental groups (Fig. 5, left). Endothelialization of the luminal surface was identified in TEVG in both groups by vWF staining (Fig. 5, middle). MMP-2 is integral to the remodeling process in TEVG and peaks at 2 weeks after implantation in mice.9 In the present study, MMP 2 was positive and similar between the two groups at 2 weeks after implantation (Fig. 5, right).
FIG. 5.
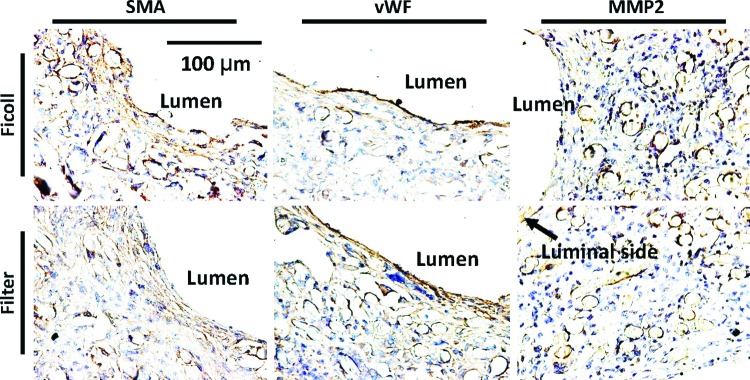
Immunohistological analysis of TEVG at 14 days after implantation. (Left) Smooth muscle cells were shown mainly in neointima of both groups. (Middle) Endothelialization of the luminal surface was found to line the luminal surface of the TEVG by anti-von Willebrand factor (vWF) antibody staining. (Right) Matrix metalloproteinase (MMP)-2 was positive around the remaining fibers in both groups. There was no difference in these results between the two groups. Color images available online at www.liebertpub.com/tec
Macrophage analysis of TEVG
Immunohistochemical characterization with anti-F4/80 antibody demonstrated that macrophage infiltration of TEVG occurred in both groups (Fig. 6A, left). Staining for M1 and M2, which are the two major phenotypes of macrophage, in TEVG in both groups showed no difference between techniques of BM-MNC isolation (Fig. 6A, middle and right). Quantitation of the degree of macrophage infiltration showed no statistically significant difference in the number of F4/80-positive cells between the two experimental groups (Ficoll: 2041±1048 cells/mm2 vs. Filter: 1887±907.7 cells/mm2, p=0.18; Fig. 6B). Gene expression of monocyte/macrophage marker (CD11b) evaluated by qPCR demonstrated that there was no statistical significance between two experimental groups in each time point “before seeding” and “14-day after implantation” (before seeding, Ficoll: 1.00±0.60 vs. Filter: 1.38±0.68, p=0.16; 14-day after implantation, Ficoll: 1.0±0.55 vs. Filter 1.03±0.71, p=0.97, respectively; Fig. 6C). Furthermore, there was no difference in macrophage phenotype (M1 and M2) evaluated by qPCR between groups at 14-day time point (M1, Ficoll: 1.00±0.99 vs. Filter: 0.82±0.67, p=0.67; M2, Ficoll: 0.92±0.67 vs. Filter 1.26±1.30, p=0.52, respectively; Fig. 6C).
FIG. 6.
Macrophage infiltration into TEVG evaluated by histological assessment and gene expression. (A) Representative images of immunohistochemical staining of F4/80 (entire macrophage), iNOS antibody (M1 macrophage), and CD206 (M2 macrophage). (B) The number of macrophages in TEVG, defined by F4/80-positive cells, at 14 days after implantation showed no difference between groups. Data were evaluated by the Mann–Whitney test. (C), Gene expression was analyzed by reverse transcription quantitative polymerase chain reaction using the ΔΔCT method. Gene expression of monocyte/macrophage marker (CD11b) demonstrated that there was no difference between the two experimental groups in each time point “before seeding” and “14 days after implantation” Furthermore, there was no difference in gene expression of macrophage phenotype (M1: iNOS and M2: CD206) between groups at 14-day time point. Data were evaluated by Student's t test. iNOS, anti-inducible nitric oxide synthase. Color images available online at www.liebertpub.com/tec
Discussion
In our previous studies, we demonstrated the importance of host-derived infiltrating macrophages on neovessel formation.10,11 In studies evaluating vascular neotissue formation in TEVG in animals that were macrophage depleted using clodronate liposomes, we demonstrated that macrophages are essential for vascular neotissue formation and we validated these findings using an inducible CD11b knockout mouse model.11 In this same study, we discovered that the macrophages infiltrating patent TEVG demonstrated a shift away from the M1 phenotype, while the macrophages infiltrating stenotic TEVG demonstrated increased expression of M1 phenotypic markers. We have also previously demonstrated that cell seeding is not essential for vascular neotissue formation, but instead functions to improve patency by modulating the host inflammatory response to the TEVG scaffold by decreasing the number infiltrating macrophages and shifting the macrophage phenotype away from M1 through an as yet undetermined paracrine mechanism.10,11 Thus, we postulate that measuring the degree of macrophage infiltration and characterizing the macrophage phenotype can serve as biomarkers to measure the biological function of the seeded cells in the TEVG.
In the present study, we evaluated and compared the biological activity of BM-MNC isolated using either our conventional density centrifugation method or our novel filtration elution method on vascular neotissue formation in TEVG. We demonstrated no difference in the biological activity on tissue remodeling in implanted TEVG seeded with BM-MNCs isolated using either technique. Specifically, we demonstrated no difference in the incidence of stenosis or the quality of the vascular neotissue. In addition, we demonstrated that the BM-MNCs induced the same degree of macrophage infiltration and M1 polarization despite previously demonstrated differences in the subpopulations of cells that comprise the BM-MNCs based on the method of separation.5
In the clinical arena, traditional methods of isolating BM-MNCs rely on density centrifugation, which is a time-consuming and labor-intensive process with a high degree of operator variability.4 The filter-based method was originally used for isolating MNCs from peripheral blood.12 Due to its success, we applied this technique to our method for construction of TEVG and demonstrated feasibility in isolating MNCs from human bone marrow as an alternative to conventional density centrifugation by showing morphologic equivalence in neovessel formation in the TEVG implantation model.5 However, in that study, we used human bone marrow and implanted the BM-MNC-seeded TEVG in an immunodeficient SCID/bg mouse.5 In the present study, we utilize autologous BM-MNCs for TEVG with implantation into immunocompetent wild-type CB57BL/6 mice and demonstrate biological equivalence of both methodologies in neotissue formation of smooth muscle cells and endothelial cells, both of which have been deemed essential to the structural and functional integrity of neovessels.13,14
In this study, the number of attached cells on scaffolds in the Filter group was higher than the Ficoll group after overnight incubation, and the viable cell number in the Filter group was higher than that in the Ficoll group. These results suggest that the filter method is less harsh compared to the density centrifugation method, in addition to being simpler, faster, and operator independent. Furthermore, our previous study demonstrated the difference in lymphocyte and monocyte populations in isolated cells between these two groups, and this fact might affect the number of attached cells on the scaffold and the viability of isolated cells. However, despite these differences, the two BM-MNC populations had a similar biological effect on TEVG formation.
The feasibility of using filter-based isolation of MNCs for fabricating TEVG was established by our previous study.5 Furthermore, we demonstrated the safety and efficacy of using a closed system to create TEVG in a large animal model.6 In this study, we demonstrated that despite differences in the BM-MNC isolated using either methodology, both BM-MNC populations exerted the same biologic effect and functionality as evidenced by similarities in the degree of cellular infiltration and formation of the TEVG.
Acknowledgments
The authors would like to thank Nancy Troiano, Rose Webb, and Christiane Coady of the Yale Core Center for Musculoskeletal Disorders for their technical expertise in processing murine TEVG tissue. Finally, they would also like to acknowledge Martin Smith from the Pall Corporation for his assistance in the design of the prototype for the closed disposable seeding system. The project described was supported by Award Number Grant UL1TR001070 from the National Center for Advancing Translational Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Disclosure Statement
C.K.B. and T.S. receive grant support from Gunze Ltd. (Kyoto, Japan). C.K.B. receives grant support from Pall Corp (NY). S.T. and H.K. were recipients of Banyu Fellowship from Banyu Life Science Foundation International (Tokyo, Japan) (H.K. in 2011 and S.T. in 2012). H.K. was recipient of fellowship from the Shinsenkai Imabari Daiichi Hospital (Ehime, Japan) in 2013.
References
- 1.Kurobe H., Maxfield M.W., Breuer C.K., and Shinoka T. Concise review: tissue-engineered vascular grafts for cardiac surgery: past, present, and future. Stem Cells Transl Med 1, 566, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shin'oka T., Imai Y., and Ikada Y. Transplantation of a tissue-engineered pulmonary artery. N Engl J Med 344, 532, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Hibino N., McGillicuddy E., Matsumura G., Ichihara Y., Naito Y., Breuer C., and Shinoka T. Late-term results of tissue-engineered vascular grafts in humans. J Thorac Cardiovasc Surg 139, 431, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Udelsman B., Hibino N., Villalona G.A., McGillicuddy E., Nieponice A., Sakamoto Y., Matsuda S., Vorp D.A., Shinoka T., and Breuer C.K. Development of an operator-independent method for seeding tissue-engineered vascular grafts. Tissue Eng Part C Methods 17, 731, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hibino N., Nalbandian A., Devine L., Martinez R.S., McGillicuddy E., Yi T., Karandish S., Ortolano G.A., Shin'oka T., Snyder E., and Breuer C.K. Comparison of human bone marrow mononuclear cell isolation methods for creating tissue-engineered vascular grafts: novel filter system versus traditional density centrifugation method. Tissue Eng Part C Methods 17, 993, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurobe H., Maxfield M.W., Naito Y., Cleary M., Stacy M., Solomon D., Rocco K.A., Tara S., Lee A., Sinusas A., Snyder E., Shinoka T., and Breuer C.K. Comparison of a closed system to a standard open technique for preparing tissue engineered vascular grafts. Tissue Eng Part C Methods 2014. [Epub ahead of print]; DOI: 10.1089/ten.tec.2014.0160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roh J.D., Nelson G.N., Brennan M.P., Mirensky T.L., Yi T., Hazlett T.F., Tellides G., Sinusas A.J., Pober J.S., Saltzman W.M., Kyriakides T.R., and Breuer C.K. Small-diameter biodegradable scaffolds for functional vascular tissue engineering in the mouse model. Biomaterials 29, 1454, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee Y.U., Yi T., Tara S., Lee A.Y., Hibino N., Shinoka T., and Breuer C.K. Implantation of inferior vena cava interposition graft in mouse model. J Vis Exp 88, e51632 [Epub ahead of print]; DOI: 10.3791/51632, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naito Y., Williams-Fritze M., Duncan D.R., Church S.N., Hibino N., Madri J.A., Humphrey J.D., Shinoka T., and Breuer C.K. Characterization of the natural history of extracellular matrix production in tissue-engineered vascular grafts during neovessel formation. Cells Tissues Organs 195, 60, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roh J.D., Sawh-Martinez R., Brennan M.P., Jay S.M., Devine L., Rao D.A., Yi T., Mirensky T.L., Nalbandian A., Udelsman B., Hibino N., Shinoka T., Saltzman W.M., Snyder E., Kyriakides T.R., Pober J.S., and Breuer C.K. Tissue-engineered vascular grafts transform into mature blood vessels via an inflammation-mediated process of vascular remodeling. Proc Natl Acad Sci U S A 107, 4669, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hibino N., Yi T., Duncan D.R., Rathore A., Dean E., Naito Y., Dardik A., Kyriakides T., Madri J., Pober J.S., Shinoka T., and Breuer C.K. A critical role for macrophages in neovessel formation and the development of stenosis in tissue-engineered vascular grafts. FASEB J 25, 4253, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teleron A.A., Carlson B., and Young P.P. Blood donor white blood cell reduction filters as a source of human peripheral blood-derived endothelial progenitor cells. Transfusion 45, 21, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Vane J.R., Anggard E.E., and Botting R.M. Regulatory functions of the vascular endothelium. N Engl J Med 323, 27, 1990 [DOI] [PubMed] [Google Scholar]
- 14.Wagenseil J.E., and Mecham R.P. Vascular extracellular matrix and arterial mechanics. Physiol Rev 89, 957, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]