Abstract
Objective: The number of children and adolescents (hereafter referred to as “children”) who have been prescribed second-generation antipsychotics (SGAs) has increased over the last decade, but little is known about monitoring practices in pediatric patients who are vulnerable to adverse effects. We examined factors associated with psychiatrists' self-reported monitoring of children who were prescribed SGAs.
Methods: A survey was mailed to a national, randomly selected sample of 1600 child and adolescent psychiatrists from the American Medical Association mailing list. Using logistic regression, we tested whether psychiatrist characteristics, attitudes, and practice characteristics were associated with monitoring (baseline and/or periodic) the following: Patient history, height and weight, blood pressure, waist circumference, lipid and glucose levels, and electrocardiogram.
Results: Among the analytic sample of 308, at least two thirds reported monitoring patient history, height and weight, blood pressure, and fasting plasma lipids and glucose; 23% reported monitoring waist circumference; and 12% reported conducting an electrocardiogram. More than one third stated that they routinely monitored thyroid levels and more than half reported monitoring complete blood count and electrolytes/blood urea nitrogen. Psychiatrists reporting that they were able to measure vital signs on site were more likely to measure height and weight. Those who reported feeling comfortable conducting a physical examination were more likely to measure blood pressure. Those answering that the risk of metabolic syndrome was low were less likely to measure blood pressure and waist circumference. Being board certified and able to measure vital signs on site were associated with more monitoring of glucose and lipid levels. Conversely, years in practice and feeling that patients were nonadherent with blood work were associated with less monitoring of glucose and lipid levels.
Conclusions: In this sample, inconsistent monitoring patterns of children prescribed SGAs were found. Efforts to communicate guidelines' evidence base and improve office capacity to measure and track adverse effects are needed to increase appropriate adverse effect monitoring in children who have been prescribed SGAs.
Introduction
Over the last decade, research has indicated a dramatic growth in the use of second-generation antipsychotics (SGAs) across all age categories, including among children and adolescents (hereafter referred to as “children”) (Olfson et al. 2002, 2006, 2014). Studies suggest that SGA use has been disproportionally increasing in males and in children with Medicaid insurance or in foster care (Patel et al. 2002; Curtis et al. 2005; Zito et al. 2008; Crystal et al. 2009).
This increased use of SGAs is partly explained by a growing evidence base and subsequent Food and Drug Administration (FDA) approval (Agency for Healthcare Research and Quality 2010; Correll et al. 2011) for SGA use in children with schizophrenia (Kumar et al. 2013) or bipolar disorder I with mixed or manic episodes (Vitiello et al. 2009; Correll et al. 2010), or for irritability and aggression in children with autism spectrum disorders (Gagliano et al. 2004; McDougle et al. 2005; Luby et al. 2006; Zuddas et al. 2011) and borderline intelligence quotient (IQ) (Buitelaar et al. 2001). However, the increase in SGA use also reflects off-label use to manage a variety of symptoms, most commonly aggression and other symptoms in children with attention-deficit/hyperactivity disorder (ADHD) and disruptive behavior disorders (Shekelle et al. 2007; Crystal et al. 2009; Birnbaum et al. 2013; Rodday et al. 2014).
The recent growth in the use of SGAs among children has generated growing concern about the limited knowledge regarding the long-term safety of these medications in children. Initially, the SGAs were thought to have a better neurological safety profile than the first-generation antipsychotics (Davis et al. 2003). Studies with adults and pediatric populations over the last decade, however, suggest a variety of cardiometabolic and endocrine adverse effects common to most of the SGAs, including weight gain, hyperglycemia, dyslipidemia, hypertension, type 2 diabetes, and hyperprolactinemia (Remschmidt et al. 2000; Ratzoni et al. 2002; Safer 2004; Correll 2008; Maayan and Correll 2011; Bobo et al. 2012; Nielsen et al. 2014). Specific cardiac adverse effects also have been documented with individual medications, including prolongation of the QTc interval with ziprasidone and tachycardia and orthostatic hypotension with clozapine (Yip et al. 1998; Blair et al. 2005; Jensen et al. 2015). In response to accumulating data about weight gain and metabolic adverse effects, the FDA mandated updated labeling for SGAs in 2003 (Rosack 2003; United States Food and Drug Administration 2004), and in 2004, the American Diabetes Association (ADA), in concert with the American Psychiatric Association (APA) and the American Association for Clinical Endocrinologists, published monitoring recommendations primarily focused on adults (American Diabetes Association et al. 2004).
Of particular concern for pediatric care are findings over the last decade suggesting that SGA-related adverse events may be greater in children than in adults (Safer 2004; Correll et al. 2006, 2009). Children taking SGAs are also undergoing significant developmental changes, both physically and emotionally, and safety information extrapolated from adult studies may not adequately describe potential risks for children.
In response, expert panels have published recommendations for the use and monitoring of SGAs in children. In 2003, the Treatment Recommendations for Use of Antipsychotics for Aggressive Youth (TRAAY) were published by an expert panel (Pappadopulos et al. 2003; Schur et al. 2003). In 2007, the American Academy of Child and Adolescent Psychiatry (AACAP)-sponsored Preschool Psychopharmacology Working Group published its guidelines for psychopharmacological treatment for young children (Gleason et al. 2007), affirming the ADA guidelines. Between 2006 and 2008, several review articles were published providing guidance on monitoring for SGA-related adverse events in children (Correll et al. 2006; Correll 2008; Correll et al. 2009). In 2011, monitoring recommendations for pediatric SGA use were published by both the AACAP (American Academy of Child and Adolescent Psychiatry 2011) and the Canadian Alliance for Monitoring Effectiveness and Safety of Antipsychotics in Children (CAMESA) (Pringsheim et al. 2011). Although there are minor differences across these published articles and guidelines, their recommended baseline and periodic monitoring consists of some combination of: 1) Patient history and physical examination; 2) measurement of height, body weight, or body mass index (BMI) with some guidelines including waist circumference; 3) blood pressure; 4) fasting plasma glucose and lipid levels; and 5) the potential, precautionary inclusion of an electrocardiogram (ECG) to identify QTc prolongation (Table 1). The recommendations regarding monitoring with SGA use in children are increasingly recognized by accreditation groups; the United States-based National Committee for Quality Assurance (NCQA) has proposed that a new set of Healthcare Effectiveness Data and Information Set (HEDIS) measures regarding SGA metabolic monitoring for children and adolescents (e.g., glucose or glycohemoglobin [HbAIc], cholesterol) be implemented in 2015 (National Committee for Quality Assurance 2014).
Table 1.
Summary of Recommended SGA Monitoring
| American Diabetes Association (ADA) and American Psychiatric Association (APA), Consensus Development Conference on Antipsychotic Drugs and Obesity and Diabetes. (American Diabetes Association et al. 2004) | American Academy of Child and Adolescent Psychiatry (AACAP), 2011 Practice Parameter for the Use of Atypical Antipsychotic Medications in Children and Adolescents (American Academy of Child and Adolescent Psychiatry 2011)a | Canadian Alliance for Monitoring Effectiveness and Safety of Antipsychotics in Children (CAMESA), 2011 Evidence-Based Recommendations for Monitoring Safety in SGAs in Children and Youth (Pringsheim et al. 2011)b | |
|---|---|---|---|
| Physical | |||
| Patient history and physical (including neurological)examination | B, annually | B, regularly | B, regularly |
| Height and weight/BMI | B, 4w, 8w, 12w, quarterly | B, regularly | B, regularly |
| Blood pressure | B, 12w, annually | B, regularly | B, regularly |
| Waist circumference | B, annually | — | B, regularly |
| Eye examination | — | If quetiapine: B, periodic | — |
| Laboratory | |||
| Fasting glucose | B, 12w, annually | B, regularly | B, 3m, 6m, 12m |
| Fasting insulin | — | — | B, 3m, 6m, 12m (not recommended for aripiprazole; weak recommendation for ziprasidone) |
| Fasting lipid profile (HDL, LDL, TG, TC) | B, 12w, every 5 years | If weight change or family history: B, regularly | B, 3m, 6m, 12m (weak recommendation for ziiprasidone) |
| Electrocardiogram (ECG) | — | If family or personal history; B, regularly If ziprasidone, given risk of QTc changes: B and stable dose |
— |
| Other | |||
| Prolactin | — | Only if symptoms | B, annually or clinical symptoms |
| Thyroid stimulating hormone | — | — | B, 12m (quetiapine only) |
| Liver functions (AST/ALG) | — | — | B, 6m, 12m |
| Hemoglobin A1c (HgbA1c) | — | As needed | — |
| Electroencephalography (EEG) | — | If clozapine: B, stable dose, behavioral changes | — |
Recommends following the ADA-APA Guidelines.
CAMESA relied primarily on screening and monitoring tool (Tables 4 and 5) (Pringsheim et al. 2011).
SGA, second generation antipsychotics; BMI, body mass index; B, baseline; w, weeks; m, months; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TG, triglycerides; TC, total cholesterol; AST, alanine transaminase; ALG, antilymphocyte globulin.
Despite growing public consensus, retrospective claims and chart reviews have demonstrated that monitoring for weight, blood pressure, and glucose and lipid levels have remained infrequent for pediatric patients (Haupt et al. 2009; Morrato et al. 2010; Honey et al. 2013). A few, small-sample survey studies have been conducted with Canadian and Australian psychiatrists. These studies document a high level of awareness of potential metabolic and cardiac side effects associated the SGAs, but a limited use of monitoring practices (Doey et al. 2007; Walter et al. 2008; Morrato et al. 2009). Little is known about SGA monitoring beliefs and patterns of United States-based child and adolescent psychiatrists, although recent research has shown that practice setting and physician attitudes influence off-label SGA use in children (Rodday et al. 2014).
We conducted a national survey of child and adolescent psychiatrists to examine monitoring practices for children prescribed SGAs. In addition, we explored factors—such as psychiatrist characteristics, psychiatrist attitudes, and practice characteristics—that may be associated with SGA monitoring.
Methods
Using the American Medical Association's (AMA) mailing list from 2012, a total of 6156 child and adolescent psychiatrists were identified from which 1600 were randomly selected. Psychiatrists who were not based in the United States, or who were retired or were residents/fellows were excluded from this sample. Psychiatrists meeting the following self-reported criteria were included. 1) They provided care for children 3–18 years of age, 2) prescribed SGAs to any of their patients 3–18 years of age, and 3) specialized in child and/or adolescent psychiatry. Of 362 respondents (24%), 92% met our eligibility criteria, yielding a final sample of 334 psychiatrists (20.9%) who prescribed SGAs.
Data were collected using a survey developed by the study investigators. Survey sections included: 1) Respondent and practice setting characteristics, 2) self-reported rates of SGA prescribing by child age and disorder, 3) attitudes toward SGA use and SGA side effects, and (4) monitoring behaviors. The institutional review board (IRB)-approved survey was mailed twice at 3 week intervals from February to March 2012.
Data analysis
Descriptive statistics, including means, standard deviations (SD), medians (25th–75th percentiles), frequencies and percentages, were used to describe the respondent and practice characteristics, attitudes and use of SGAs, and SGA monitoring behaviors, stratified by practice setting. The χ2 test or the Fisher exact test was used to compare categorical variables, and analysis of variance (ANOVA) was used to compare continuous variables across practice settings.
Because the AACAP and Canadian guidelines had not been published at the time of the survey, we utilized existing ADA/APA monitoring recommendations (American Diabetes Association et al. 2004) to create six binary outcome variables to indicate whether or not the respondent routinely monitored the following at least once per year: 1) Patient history, 2) height and weight, 3) blood pressure, 4) waist circumference, 5) lipid and glucose levels, and 6) ECG. We also explored monitoring behaviors for the following practices that were not routinely included in published monitoring recommendations: Prolactin, thyroid stimulating hormone (TSH), complete blood count (CBC), electrolytes, and blood urea nitrogen (BUN). Differences in monitoring frequency by practice setting were examined for the monitoring activities not routinely recommended.
To control for multiple comparisons, the significance level was set at a p value of 0.01. We built separate logistic regression models to estimate odds ratios (ORs) and 99% confidence intervals (CI) for each of the six recommended monitoring activities. The following categories of covariates were tested in univariate analysis: Psychiatrist characteristics (i.e., sex, race/ethnicity, board certification in child and adolescent psychiatry, years in practice); psychiatrist attitudes toward SGA use and side effects; and practice characteristics (i.e., practice setting, population density, ≥50% of patients with private insurance versus <50%, number of visits per week, availability of electronic health records [EHR], availability of monitoring capabilities). Given prior published work suggesting that practice setting influenced off-label SGA use (Rodday et al. 2014), practice setting was included in each model. Types of practice setting included private practice, community mental health centers, and inpatient/outpatient facilities (i.e., general hospital, academic medical center, residential facility). Other covariates with p<0.2 were considered in multivariable modeling; backwards selection eliminated covariates with p<0.1. Multicollinearity (i.e., the high correlation of two or more variables in the model) was assessed using variance inflation factors (VIF). SAS version 9.4 (Cary, NC) was used.
Results
Study sample
Among the final sample of 334 eligible respondents, the breakdown of practice setting was as follows: Private practice (42%), community mental health center (25%), inpatient/outpatient facility (29%), and other settings (5%), which included 11 multispecialty clinics, three state affiliated clinics, and one military clinic. Given the small sample size, those in other practice settings and those missing a practice setting indication (n=11) were excluded from the remaining analyses, resulting in a final sample size of 308 (19%).
Sample characteristics
The mean age of respondents was 54 (SD=11) years, and 56% were males (Table 2). These psychiatrists had been in practice for an average of 19 (SD=11) years. In terms of their practice patterns and attitudes, approximately one third reported off-label SGA use (36%), felt comfortable performing a physical examination (37%), and felt that the risk of metabolic syndrome was sufficiently low to warrant SGA use (33%). Roughly half (53%) had concerns about adherence with blood draws among patients and their families. Regarding practice characteristics, slightly less than half of the respondents reported that >50% of their patients had private insurance (46%), and used EHRs (41%). Although 85% had the capacity to measure vital signs and height and weight at their setting, only 26% reported being able to draw laboratory blood samples. The median number of visits per week was 40 (25th to 75th percentile: 20–60). Nearly half of respondents were located in urban areas (49%), followed by suburban (41%), and rural (10%) areas.
Table 2.
Psychiatrist Characteristics and Attitudes by Practice Setting
| Total, n=308 | Private, n=135 | Community mental health center, n=80 | Inpatient/outpatient facility, n=93 | p value | |
|---|---|---|---|---|---|
| Psychiatrist characteristics | |||||
| Male, n (%) | 171 (55.9%) | 85 (63.4%) | 42 (52.5%) | 44 (47.8%) | 0.05 |
| White, non-Hispanic, n (%) | 215 (70.5%) | 101 (75.9%) | 46 (58.2%) | 68 (73.1%) | 0.02 |
| Board certified in Child and Adolescent Psychiatry, n (%) | 223 (74.6%) | 99 (76.2%) | 50 (64.9%) | 74 (80.4%) | 0.06 |
| Age, mean (SD) | 53.7 (11.4) | 56.2 (11.2) | 53.4 (12.4) | 50.5 (10.1) | <0.001 |
| Years in practice, mean (SD) | 18.8 (11.3) | 21.3 (11.5) | 17.9 (11.8) | 15.9 (9.7) | 0.001 |
| Psychiatrist attitudes and use of SGAs | |||||
| Off-label SGA use, n (%) | 103 (35.9%) | 36 (28.4%) | 32 (41.0%) | 35 (42.7%) | 0.06 |
| Feel the risk of metabolic syndrome is low enough to warrant the use of SGAs, n (%) | 102 (33.3%) | 56 (41.8%) | 23 (28.8%) | 23 (25.0%) | 0.02 |
| Are comfortable performing a physical examination on patients, n (%) | 112 (36.7%) | 36 (27.1%) | 27 (34.2%) | 49 (52.7%) | <0.001 |
| Feel patients are noncompliant with blood work, n (%) | 162 (53.3%) | 55 (41.7%) | 57 (72.2%) | 50 (53.8%) | <0.001 |
| Practice characteristics | |||||
| More than 50% of patients have private insurance, n (%) | 127 (45.7%) | 102 (82.9%) | 3 (4.1%) | 22 (26.8%) | <0.001 |
| Electronic health records, n (%) | 125 (41.1%) | 23 (17.3%) | 48 (60.8%) | 54 (58.7%) | <0.001 |
| Can measure vital signs, height, and weight on site, n (%) | 262 (85.3%) | 94 (70.2%) | 76 (95.0%) | 92 (98.9%) | <0.001 |
| Can get blood drawn on site, n (%) | 79 (26.0%) | 11 (8.2%) | 13 (16.9%) | 55 (59.1%) | <0.001 |
| Visits per week, median (25th–75th percentile) | 40.0 (20.0, 60.0) | 40.0 (30,0, 60.0) | 50.0 (30.0, 75.0) | 20.0 (10.0, 40.0) | 0.002 |
| Population density, n (%) | <0.001 | ||||
| Urban | 144 (48.5%) | 50 (37.6%) | 40 (51.3%) | 54 (62.8%) | |
| Suburban | 123 (41.4%) | 72 (54.1%) | 23 (29.5%) | 28 (32.6%) | |
| Rural | 30 (10.1%) | 11 (8.3%) | 15 (19.2%) | 4 (4.7%) | |
SGA, second generation antipsychotics.
Practice settings
Because of previous work that suggested differences in SGA use by practice setting (Rodday et al. 2014), we examined characteristics of providers by setting type (Table 2). In terms of psychiatrist characteristics, the most striking differences were for age and years in practice, with respondents in private practice being older (p<0.001) and in practice for longer (p=0.001) than respondents in other settings. Regarding attitudes, respondents practicing at inpatient/outpatient facilities were most likely to feel comfortable performing a physical examination (p<0.001). Respondents at community mental health centers were most likely to agree with the statement that patients were noncompliant with blood work (p<0.001). Variations by practice setting were noted for all of the practice characteristics, including insurance type, number of patient visits/week, and urbanicity. It is of note that having EHRs (p<0.001) and being able to measure vital signs, height, and weight on site (p<0.001) were more common at community mental health centers and inpatient/outpatient facilities than in private practice. Those at inpatient/outpatient facilities were more likely to report that they could get blood drawn on site than were those in other practice settings (p<0.001).
Routine SGA monitoring
Overall, 66% reported routinely monitoring patient history, 92% reported routinely monitoring height and weight, 76% reported routinely monitoring blood pressure, 23% reported routinely monitoring waist circumference, 81% reported routinely monitoring lipid and glucose levels, and 12% reported routinely performing an ECG (Table 3). There were no statistically significant differences by practice settings in routine monitoring for the following: Patient history, waist circumference, fasting plasma glucose and lipid levels, and ECG. By contrast, private practices had the lowest reported rates for measuring height and weight (p=0.001) and blood pressure (p<0.001), while inpatient/outpatient facilities had the highest rates.
Table 3.
| Total, n=308 | Private, n=135 | Community mental health center, n=80 | Inpatient/outpatient facility, n=93 | p value | |
|---|---|---|---|---|---|
| Patient history | 194 (65.8%) | 84 (65.1%) | 51 (65.4%) | 59 (67.1%) | 0.95 |
| Height and body weight | 276 (92.0%) | 113 (85.6%) | 77 (96.3%) | 86 (97.7%) | 0.001 |
| Blood pressure | 230 (76.2%) | 87 (64.9%) | 62 (78.5%) | 81 (91.0%) | <0.001 |
| Waist circumference | 68 (22.5%) | 31 (23.3%) | 20 (25.0%) | 17 (19.1%) | 0.63 |
| Fasting plasma glucose and lipid profile | 242 (80.7%) | 98 (74.8%) | 66 (83.5%) | 78 (86.7%) | 0.07 |
| ECG | 35 (11.7%) | 11 (8.5%) | 9 (11.3%) | 15 (17.1%) | 0.15 |
Routinely indicates 1–3x/year or every visit.
May rely on other physicians to conduct monitoring test.
SGA, second generation antipsychotic; ECG, electrocardiogram.
Other reported monitoring behaviors
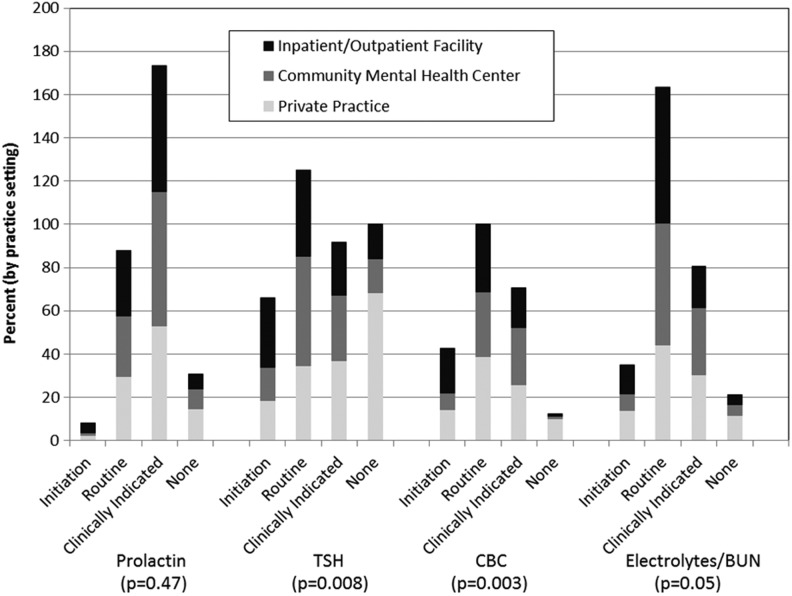
Psychiatrists also reported other SGA monitoring practices, such as measuring prolactin, TSH, CBC, electrolytes and BUN. At SGA initiation, 3% reported measuring prolactin, 22% reported measuring TSH, 14% reported performing a CBC, and 12% reported measuring electrolytes and BUN. With regard to routine monitoring, 29% monitored prolactin, 40% monitored TSH, 57% monitored CBC, and 53% monitored electrolytes and BUN. Others reported monitoring only if indicated based on the history or physical examination: 57% for prolactin, 31% for TSH, 24% for CBC, and 27% for electrolytes and BUN. There were no differences in frequency of monitoring prolactin (p=0.47) or electrolytes and BUN (p=0.05) by practice setting, but there were differences in monitoring TSH (p=0.008) and CBC (p=0.003), which were performed significantly more often by psychiatrists working in inpatient/outpatient facilities (Fig. 1).
FIG. 1.
Monitoring not supported by evidence, by practice setting. TSH, thyroid stimulating hormone; CBC, complete blood count; BUN, blood urea nitrogen.
Models for routine SGA monitoring
Univariate analyses demonstrated which variables were associated with routinely monitoring patient history, height and weight, blood pressure, waist circumference, lipid and glucose levels, and ECG, and informed variable inclusion in the multivariable modeling (Tables 4 and 5). It is of note that no association with practice setting was noted for any multivariable model; rather, specific practice characteristics were associated with outcomes as will be described subsequently.
Table 4.
Univariate Analyses of Self-Reported SGA Monitoring (“Routinely” Monitoreda), Part 1
| Patient history | Height and weight | Blood pressure | ||||
|---|---|---|---|---|---|---|
| OR (99% CI) | p value | OR (99% CI) | p value | OR (99% CI) | p-value | |
| Psychiatrist characteristics | ||||||
| Male | 0.85 (0.45, 1.61) | 0.50 | 0.31 (0.08, 1.15) | 0.02 | 0.41 (0.19, 0.88) | 0.003 |
| White, non-Hispanic | 1.36 (0.68, 2.70) | 0.26 | 1.53 (0.49, 4.78) | 0.34 | 1.26 (0.59, 2.67) | 0.44 |
| Board certified in child and adolescent psychiatry | 1.70 (0.82, 3.49) | 0.06 | 0.76 (0.20, 2.90) | 0.59 | 1.38 (0.63, 3.01) | 0.30 |
| Years in practice | 0.99 (0.96, 1.02) | 0.29 | 0.94 (0.89, 0.98) | <0.001 | 0.97 (0.94, 1.00) | 0.005 |
| Psychiatrist attitudes and use of SGAs | ||||||
| Off-label SGA use | 0.58 (0.30, 1.14) | 0.04 | 0.52 (0.17, 1.57) | 0.13 | 0.99 (0.47, 2.07) | 0.96 |
| Feel the risk of metabolic syndrome is low enough to warrant the use of SGAs | 0.89 (0.46, 1.74) | 0.66 | 0.46 (0.15, 1.40) | 0.07 | 0.35 (0.17, 0.72) | <0.001 |
| Are comfortable performing a physical examination on patients | 1.33 (0.68, 2.63) | 0.27 | 2.91 (0.68, 12.42) | 0.06 | 2.86 (1.23, 6.64) | 0.001 |
| Feel patients are noncompliant with blood work. | 1.18 (0.63, 2.24) | 0.50 | 1.05 (0.34, 3.23) | 0.90 | 1.05 (0.52, 2.12) | 0.86 |
| Practice characteristics | ||||||
| Practice settingb | ||||||
| Private | 0.92 (0.43, 1.95) | 0.77 | 0.14 (0.02, 0.97) | 0.009 | 0.18 (0.06, 0.53) | <0.001 |
| Community mental health center | 0.93 (0.40, 2.17) | 0.82 | 0.60 (0.06, 6.49) | 0.58 | 0.36 (0.11, 1.18) | 0.03 |
| More than 50% of patients have private insurance | 0.86 (0.44, 1.69) | 0.57 | 0.24 (0.06, 0.94) | 0.007 | 0.46 (0.22, 0.97) | 0.007 |
| Electronic health records | 1.11 (0.58, 2.12) | 0.68 | 8.55 (1.24, 58.81) | 0.004 | 2.56 (1.17, 5.58) | 0.002 |
| Can measure vital signs, height, and weight on site | 1.32 (0.55, 3.17) | 0.41 | 13.61 (4.12, 44.96) | <0.001 | 4.14 (1.72, 9.94) | <0.001 |
| Can get blood drawn on site | 1.00 (0.48, 2.08) | 1.00 | 2.52 (0.49, 12.84) | 0.14 | 2.43 (0.94, 6.33) | 0.02 |
| Visits per week | 0.99 (0.98, 1.00) | 0.12 | 1.00 (0.98, 1.02) | 0.91 | 0.99 (0.98, 1.00) | 0.009 |
| Population densityc | ||||||
| Suburban | 1.25 (0.63, 2.48) | 0.40 | 1.05 (0.32, 3.51) | 0.91 | 0.74 (0.34, 1.59) | 0.31 |
| Rural | 0.82 (0.28, 2.40) | 0.64 | 0.77 (0.13, 4.48) | 0.70 | 0.49 (0.16, 1.53) | 0.11 |
Bolding indicates p<0.01.
Routinely indicates 1–3x/year or every visit.
Reference is inpatient/outpatient facility.
Reference is urban.
SGA, second generation antipsychotic.
Table 5.
Univariate Analyses of Adherence to SGA Monitoring Guidelines (“Routinely” Monitoreda), Part 2
| Waist circumference | Lipid and glucose | ECG | ||||
|---|---|---|---|---|---|---|
| OR (99% CI) | p value | OR (99% CI) | p value | OR (95% CI) | p value | |
| Psychiatrist characteristics | ||||||
| Male | 1.47 (0.71, 3.06) | 0.17 | 0.52 (0.23, 1.17) | 0.04 | 0.65 (0.26, 1.68) | 0.24 |
| White, non-Hispanic | 1.13 (0.51, 2.52) | 0.70 | 1.35 (0.60, 3.04) | 0.35 | 1.16 (0.40, 3.36) | 0.71 |
| Board certified in child and adolescent psychiatry | 0.72 (0.32, 1.59) | 0.28 | 2.04 (0.90, 4.61) | 0.02 | 0.82 (0.28, 2.43) | 0.64 |
| Years in practice | 1.00 (0.97, 1.03) | 0.98 | 0.93 (0.89, 0.96) | <0.001 | 0.97 (0.92, 1.01) | 0.05 |
| Psychiatrist attitudes and use of SGAs | ||||||
| Off-label SGA use | 1.61 (0.76, 3.41) | 0.10 | 0.99 (0.44, 2.24) | 0.96 | 1.31 (0.48, 3.56) | 0.48 |
| Feel the risk of metabolic syndrome is low enough to warrant the use of SGAs | 0.39 (0.16, 0.93) | 0.005 | 0.49 (0.22, 1.07) | 0.02 | 0.48 (0.15, 1.48) | 0.09 |
| Are comfortable performing a physical examination on patients | 1.15 (0.55, 2.39) | 0.63 | 2.50 (1.01, 6.17) | 0.009 | 2.70 (1.05, 6.93) | 0.007 |
| Feel patients are noncompliant with blood work. | 0.96 (0.47, 1.97) | 0.88 | 0.59 (0.27, 1.29) | 0.08 | 0.87 (0.34, 2.30) | 0.70 |
| Practice characteristics | ||||||
| Practice settingb | ||||||
| Private | 1.29 (0.54, 3.08) | 0.46 | 0.46 (0.18, 1.18) | 0.03 | 0.45 (0.15, 1.34) | 0.06 |
| Community mental health center | 1.41 (0.54, 3.69) | 0.36 | 0.78 (0.26, 2.39) | 0.57 | 0.62 (0.19, 1.98) | 0.29 |
| More than 50% of patients have private insurance | 1.09 (0.51, 2.30) | 0.78 | 0.69 (0.31, 1.54) | 0.23 | 0.51 (0.16, 1.59) | 0.13 |
| Electronic health records | 0.85 (0.41, 1.76) | 0.56 | 1.70 (0.76, 3.79) | 0.09 | 1.24 (0.49, 3.16) | 0.55 |
| Can measure vital signs, height, and weight on site | 1.03 (0.38, 2.79) | 0.95 | 4.69 (1.91, 11.52) | <0.001 | 0.82 (0.24, 2.84) | 0.68 |
| Can get blood drawn on site | 0.99 (0.44, 2.25) | 0.97 | 1.48 (0.57, 3.80) | 0.29 | 1.17 (0.42, 3.28) | 0.70 |
| Visits per week | 1.00 (0.99, 1.01) | 0.69 | 1.00 (0.99, 1.01) | 0.95 | 0.99 (0.98, 1.01) | 0.34 |
| Population densityc | ||||||
| Suburban | 0.66 (0.30, 1.43) | 0.17 | 0.57 (0.25, 1.31) | 0.08 | 1.18 (0.46, 3.03) | 0.66 |
| Rural | 0.86 (0.25, 2.89) | 0.74 | 0.68 (0.18, 2.57) | 0.45 | 0.25 (0.02, 3.67) | 0.18 |
Bolding indicates p<0.01.
Routinely indicates 1–3x/year or every visit.
Reference is inpatient/outpatient facility.
Reference is urban.
SGA, second generation antipsychotic; ECG, electrocardiogram.
On multivariable modeling, no variables met our statistical criteria for association with obtaining a patient history (Table 6). Being able to measure vital signs, height, and weight on site (OR=7.9, p<0.001) was associated with higher odds of monitoring height and weight.
Table 6.
Multivariable Analyses of Adherence to SGA Monitoring Guidelines (“Routinely” Monitoreda), Part 1
| Patient history | Height and weight | Blood pressure | ||||
|---|---|---|---|---|---|---|
| OR (99% CI) | p value | OR (99% CI) | p value | OR (99% CI) | p value | |
| Psychiatrist characteristics | ||||||
| Male | 0.32 (0.07, 1.39) | 0.05 | 0.47 (0.21, 1.08) | 0.02 | ||
| White, non-Hispanic | ||||||
| Board certified in child and adolescent psychiatry | ||||||
| Years in practice | ||||||
| Psychiatrist attitudes and use of SGAs | ||||||
| Off-label SGA use | 0.57 (0.29, 1.13) | 0.04 | 0.33 (0.09, 1.24) | 0.03 | ||
| Feel the risk of metabolic syndrome is low enough to warrant the use of SGAs | 0.36 (0.16, 0.81) | 0.001 | ||||
| Are comfortable performing a physical examination on patients | 2.61 (1.04, 6.54) | 0.007 | ||||
| Feel patients are noncompliant with blood work. | ||||||
| Practice characteristics | ||||||
| Practice settingb | ||||||
| Private | 0.88 (0.40, 1.97) | 0.69 | 0.58 (0.06, 5.50) | 0.53 | 0.32 (0.10, 1.01) | 0.01 |
| Community mental health center | 0.88 (0.36, 2.12) | 0.70 | 0.87 (0.07, 10.49) | 0.88 | 0.35 (0.10, 1.23) | 0.03 |
| More than 50% of patients have private insurance | ||||||
| Electronic health records | 4.48 (0.54, 37.46) | 0.07 | 2.15 (0.85, 5.43) | 0.03 | ||
| Can measure vital signs, height, and weight on site | 7.89 (1.89, 32.97) | <0.001 | ||||
| Can get blood drawn on site | ||||||
| Visits per week | ||||||
| Population densityc | ||||||
| Suburban | ||||||
| Rural | ||||||
Bolding indicates p<0.01.
Routinely indicates 1–3x/year or every visit.
Reference is inpatient/outpatient facility.
Reference is urban.
SGA, second generation antipsychotic.
Respondents who agreed that the risk of metabolic syndrome was low enough to warrant SGA use had lower odds (OR=0.4, p=0.001) of monitoring blood pressure than respondents who disagreed with the statement. Being male (OR=0.5, p=0.02) and being in private practice (OR=0.3, p=0.01) or community mental health centers (OR=0.4, p=0.03) rather than in inpatient/outpatient facilities also were marginally associated with lower odds of monitoring blood pressure. Feeling comfortable performing a physical examination (OR=2.6, p=0.007) was associated with higher odds of monitoring blood pressure; having an EHR (OR=2.2, p=0.03) was also marginally associated with higher odds of monitoring blood pressure.
For the multivariable model for monitoring waist circumference, those responding that the risk of metabolic syndrome was low enough to warrant SGA use (OR=0.3, p<0.001) had lower odds of monitoring waist circumference. Males (OR=1.9, p=0.04) (Table 7) and those reporting off-label SGA use (OR=1.8, p=0.05) were marginally more likely to monitor waist circumference.
Table 7.
Multivariable Analyses of Adherence to SGA Monitoring Guidelines (“Routinely” Monitoreda), Part 2
| Waist circumference | Lipid and glucose | ECG | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |
| Psychiatrist characteristics | ||||||
| Male | 1.88 (0.85, 4.19) | 0.04 | ||||
| White, non-Hispanic | ||||||
| Board certified in child and adolescent psychiatry | 3.15 (1.17, 8.48) | 0.003 | ||||
| Years in practice | 0.92 (0.88, 0.96) | <0.001 | ||||
| Psychiatrist attitudes and use of SGAs | ||||||
| Off-label SGA use | 1.84 (0.84, 4.07) | 0.05 | ||||
| Feel the risk of metabolic syndrome is low enough to warrant the use of SGAs | 0.29 (0.11, 0.76) | <0.001 | ||||
| Are comfortable performing a physical examination on patients | 2.45 (0.93, 6.41) | 0.02 | ||||
| Feel patients are noncompliant with blood work. | 0.41 (0.16, 1.05) | 0.01 | ||||
| Practice characteristics | ||||||
| Practice settingb | ||||||
| Private | 1.80 (0.67, 4.82) | 0.13 | 1.07 (0.32, 3.54) | 0.89 | 0.56 (0.18, 1.71) | 0.18 |
| Community mental health center | 1.73 (0.61, 4.90) | 0.18 | 1.47 (0.42, 5.14) | 0.43 | 0.72 (0.22, 2.35) | 0.47 |
| More than 50% of patients have private insurance | ||||||
| Electronic health records | ||||||
| Can measure vital signs, height, and weight on site | 3.60 (1.12, 11.59) | 0.005 | ||||
| Can get blood drawn on site | ||||||
| Visits per week | ||||||
| Population densityc | ||||||
| Suburban | ||||||
| Rural | ||||||
Bolding indicates p<0.01.
Routinely indicates 1–3x/year or every visit.
Reference is inpatient/outpatient facility
Reference is urban
SGA, second generation antipsychotic; ECG, electrocardiogram.
Being board certified in child and adolescent psychiatry (OR=3.2, p=0.003) and being able to measure vital signs, height, and weight on site (OR=3.6, p=0.005) were both associated with higher odds of monitoring lipid and glucose levels on multivariable modeling. In contrast, more years in practice (OR=0.9, p<0.001) was associated with lower odds of monitoring lipids and glucose, as was reporting that patients were noncompliant with blood work (OR=0.4, p=0.01). Feeling comfortable performing a physical examination (OR=2.5, p=0.02) was marginally associated with higher odds of ECG monitoring.
There was no evidence of multicollinearity in any of the multivariable models (VIFs<2).
Discussion
This article provides important insights into monitoring children who are prescribed SGAs. With regard to components of monitoring, nearly all psychiatrists reported routinely monitoring height and weight, and at least two thirds reported routinely monitoring patient history, blood pressure, and glucose and lipid levels. On the other hand, less than one quarter reported routinely measuring waist circumference, and only 12% reported conducting a monitoring ECG. Interestingly, psychiatrists commonly reported monitoring prolactin, TSH, CBC, electrolytes and BUN.
It is of note that the AACAP's 2011 recommendations, published after this survey was conducted, do not include waist circumference measurement, but do include an ECG (Gutgesell et al. 1999), citing limited data available regarding adverse events related to the SGAs in children (see Table 1). The CAMESA guidelines, also published after this survey was conducted, however, do include recommendations for monitoring waist circumference, prolactin, TSH, and liver function, but not ECG (Pringsheim et al. 2011). Although some guidelines reference waist circumference, the norms for prepubertal children are not readily available, and their utility is not well established (Cook et al. 2009). Further study on the utility of measuring waist circumference, and the incorporation of waist circumferences references into EHRs, may be warranted. Neither the AACAP nor the CAMESA guidelines recommend routine monitoring of a CBC, electrolytes, or BUN. Although a relationship between specific SGA agents (e.g., risperidone), and prolactin has been described (Findling et al. 2003; Saito et al. 2004), laboratory tests of prolactin levels are not universally recommended in the absence of prolactin-related side effects (Correll 2008; American Academy of Child and Adolescent Psychiatry 2011). Ordering a CBC may reflect concerns about neutropenia and agranulocytosis that have been associated with the use of clozapine, but, again, these laboratory tests are not routinely indicated. Similarly, although laboratory tests of thyroid and electrolyte status are not routinely recommended, we found that a significant proportion of prescribing psychiatrists were ordering them. Given the limited utility and associated healthcare expenditures of routine monitoring of these parameters, reasons for such practices deserve further study.
Although there was some indication of an association between practice setting and routinely monitoring height and weight, blood pressure, and lipid and glucose levels based on univariate analysis, these associations were not demonstrated after controlling for other factors. Two main sets of variables were associated with monitoring practices. First, several variables that reflected capacity to perform the monitoring within the practice setting were associated with reported monitoring practices, specifically the capacity to measure height, weight, and blood pressure on site, and the use of an HER, were marginally associated. Second, physician attitudes about monitoring affected their practices, including comfort level with the physical examination, the evaluation of the risk of SGAs for metabolic syndrome compared with their efficacy, and perceived patient adherence with blood draws.
This study provides some insights regarding mechanisms to improve monitoring in pediatric SGA use. First, practice settings should focus on assuring the capacity to conduct monitoring, including simple, low-cost steps such as the availability of weight scales, stadiometers, and blood pressure cuffs in offices. Decision support can be employed in EHRs to encourage completion of some monitoring strategies (e.g., height, weight, blood pressure) and decrease ordering of other parameters (e.g., prolactin, CBC, TSH, electrolytes, and BUN), unless a clinical indication or concern is present. Ongoing continual medical education can reinforce the need for adverse effect monitoring of SGAs in children; however, the guideline adherence literature continues to document the limited effectiveness of education and training alone (Cabana et al. 1999; Shah et al. 2014). Efforts such as the NCQA to develop measures, may drive greater guideline adherence. Quality improvement activities, such as those called for in the maintenance of certification requirements for demonstration of practice performance improvement (American Board of Medical Specialties 2014), could also focus on monitoring in the office setting and on the implementation of integrated primary/behavioral healthcare models. Interventions might include education, telephone and/or text reminders, on-site laboratory capabilities, EHR-forcing functions that do not permit prescription refills without completing monitoring, or not authorizing refill payment until monitoring is completed (Free et al. 2013; Baker et al. 2014; Department of Maryland Health and Mental Hygiene 2014).
Further research could also explore barriers to monitoring or study the impact of quality improvement initiatives. Engagement of families in these efforts might prove fruitful. For example, the issue regarding familial adherence to requests to return for screening blood work could benefit from inclusion of family perspectives about potential solutions.
Limitations
We acknowledge the study's limitations. The low response rate and inability to compare respondents and nonrespondents limited generalizability. However, our response rate parallels other surveys conducted using the AMA mailing list (Needle et al. 2012) and the sex and age distribution from our sample were similar to that from the American Association of Medical Colleges' 2012 Physician Specialty list. (American Association of Medical Colleges, 2012) Further, research has highlighted different safety profiles across the SGAs (Stigler et al. 2001; Schur et al. 2003; Newcomer et al. 2004; Toren et al. 2004). However, questions about specific monitoring practices with individual SGAs was beyond the scope of this survey. Finally, we were unable to compare reported behaviors with actual performance, and there may be a reporting bias, indicating that the frequencies found in this study are likely optimistic figures.
Conclusions
There appears to be inconsistent monitoring patterns of children and adolescents taking SGAs. We identified factors related to capacity within the practice site and psychiatrist attitudes that were associated with monitoring practices.
Clinical Significance
Given the growing number of children prescribed SGAs, and the potential for SGA-related adverse events, additional efforts are needed to improve monitoring for adverse effects in these children. Interventions related to capacity within the practice site, psychiatrist attitudes and education, and quality improvement may help to improve inappropriately low cardiometabolic monitoring of children prescribed SGAs (Honey et al. 2013; Morrato et al. 2010).
Acknowledgments
We thank Jennifer Bakan and Christina Mule for their assistance with data entry, F. William Rui for his assistance with database development, and Doris Hernandez for her assistance with manuscript preparation.
Disclosures
Dr. Correll has been a consultant and/or advisor to or has received honoraria from: Bristol-Myers Squibb (BMS), Cephalon, Eli Lilly, Genentech, Gerson Lehrman Group, IntraCellular Therapies, Janssen/J&J, Lundbeck, Medavante, Medscape, Merck, Otsuka, Pfizer, ProPhase, Roche, Sunovion, Supernus, and Takeda. He has received grant support from BMS, Janssen/J&J, and Otsuka. Dr. Robb has received grants from BMS, Forest, GlaxoSmithKline, Janssen, Johnson & Johnson, Lundbeck, Merck/Schering Plough, Otsuka America, Pfizer Inc, Sepracor, Supernus, Sunovion, and SyneuRx; served as a grant consultant for Tufts University and Patient-Centered Outcome Research Institute (PCORI); has a research contract with the National Institute of Child Health and Human Development; has served on advisory boards for the organization of Children and Adults with Attention-Deficit/Hyperactivity Disorder, BMS, Forest, Eli Lilly, McNeil Pediatrics, Otsuka America, and Shinogi; has served on speakers bureaus for BMS, Lundbeck, Otsuka, Pfizer Inc., and Takeda; has been a consultant for Lundbeck; and has served on a Safety Data Monitoring Board for Otsuka America. The other authors have nothing to disclose.
References
- Agency for Healthcare Research and Quality: Comparative effectiveness of first and second generation antipsychotics in the pediatric and young adult populations. Rockville, MD:: Agency for Healthcare Research and Quality; 2010 [Google Scholar]
- American Academy of Child and Adolescent Psychiatry: Practice parameter for the use of atypical antipsychotic medications in children and adolescents. 2011. Available at https://www.aacap.org/App_Themes/AACAP/docs/practice_parameters/Atypical_Antipsychotic_Medications_Web.pdf Accessed November17, 2014
- American Board of Medical Specialties: Support for Board Certification and ABMS MOC. 2014, Available at http://www.abms.org/board-certification/support-for-board-certification-and-abms-moc/ Accessed on November15, 2014
- American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity: Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care 27:596–601, 2004 [DOI] [PubMed] [Google Scholar]
- Association of American Medical Colleges Center for Workforce Studies: 2012 Physician Specialty Data Book. Available at https://www.aamc.org/download/313228/data/2012physicianspecialtydatabook.pdf Accessed April7, 2015
- Baker DW, Brown T, Buchanan DR, Weil J, Balsley K, Ranalli L, Lee JY, Cameron KA, Ferreira MR, Stephens Q, Goldman SN, Rademaker A, Wolf MS: Comparative effectiveness of a multifaceted intervention to improve adherence to annual colorectal cancer screening in community health centers: a randomized clinical trial. JAMA Intern Med 174:1235–1241, 2014 [DOI] [PubMed] [Google Scholar]
- Birnbaum ML, Saito E, Gerhard T, Winterstein A, Olfson M, Kane JM, Correll CU: Pharmacoepidemiology of antipsychotic use in youth with ADHD: Trends and clinical implications. Curr Psychiatry Rep 15:382–395, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair J, Scahill L, State M, Martin A: Electrocardiographic changes in children and adolescents treated with ziprasidone: a prospective study. J Am Acad Child Adolesc Psychiatry 44:73–79, 2005 [DOI] [PubMed] [Google Scholar]
- Bobo WV, Cooper WO, Stein CM, Olfson M, Mounsey J, Daugherty J, Ray WA: Positive predictive value of a case definition for diabetes mellitus using automated administrative health data in children and youth exposed to antipsychotic drugs or control medications: A Tennessee Medicaid study. BMC Med Res Methodol 12:128, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buitelaar JK, van der Gaag RJ, Cohen–Kettenis P, Melman CT: A randomized controlled trial of risperidone in the treatment of aggression in hospitalized adolescents with subaverage cognitive abilities. J Clin Psychiatry 62:239–248, 2001 [DOI] [PubMed] [Google Scholar]
- Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH, Abboud PA, Rubin HR: Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA 282:1458–1465, 1999 [DOI] [PubMed] [Google Scholar]
- Cook S, Auinger P, Huang TT: Growth curves for cardio-metabolic risk factors in children and adolescents. J Pediatr 155:S6–26, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll CU: Antipsychotic use in children and adolescents: Minimizing adverse effects to maximize outcomes. J Am Acad Child Adolesc Psychiatry 47:9–20, 2008 [DOI] [PubMed] [Google Scholar]
- Correll CU, Kratochvil CJ, March JS: Developments in pediatric psychopharmacology: Focus on stimulants, antidepressants, and antipsychotics. J Clin Psychiatry 72:655–670, 2011 [DOI] [PubMed] [Google Scholar]
- Correll CU, Manu P, Olshanskiy V, Napolitano B, Kane JM, Malhotra AK: Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA 302:1765–1773, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll CU, Penzner JB, Parikh UH, Mughal T, Javed T, Carbon M, Malhotra AK: Recognizing and monitoring adverse events of second-generation antipsychotics in children and adolescents. Child Adolesc Psychiatr Clin N Am 15:177–206, 2006 [DOI] [PubMed] [Google Scholar]
- Correll CU, Sheridan EM, DelBello MP: Antipsychotic and mood stabilizer efficacy and tolerability in pediatric and adult patients with bipolar I mania: a comparative analysis of acute, randomized, placebo-controlled trials. Bipolar Disord 12:116–141, 2010 [DOI] [PubMed] [Google Scholar]
- Crystal S, Olfson M, Huang C, Pincus H, Gerhard T: Broadened use of atypical antipsychotics: safety, effectiveness, and policy challenges. Health Aff (Millwood) 28:w770–w781, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis LH, Masselink LE, Ostbye T, Hutchison S, Dans PE, Wright A, Krishnan RR, Schulman KA: Prevalence of atypical antipsychotic drug use among commercially insured youths in the United States. Arch Pediatr Adolesc Med 159:362–366, 2005 [DOI] [PubMed] [Google Scholar]
- Davis JM, Chen N, Glick ID: A meta-analysis of the efficacy of second-generation antipsychotics. Arch Gen Psychiatry 60:553–564, 2003 [DOI] [PubMed] [Google Scholar]
- Department of Maryland Health and Mental Hygiene: Antipsychotic Review Programs. 2014. Available at https://mmcp.dhmh.maryland.gov/pap/SitePages/Antipsychotics%20Review%20Programs.aspx Accessed on November17, 2014
- Doey T, Handelman K, Seabrook JA, Steele M: Survey of atypical antipsychotic prescribing by Canadian child psychiatrists and developmental pediatricians for patients aged under 18 years. Can J Psychiatry 52:363–368, 2007 [DOI] [PubMed] [Google Scholar]
- Findling RL, Kusumakar V, Daneman D, Moshang T, De SG, Binder C: Prolactin levels during long-term risperidone treatment in children and adolescents. J Clin Psychiatry 64:1362–1369, 2003 [DOI] [PubMed] [Google Scholar]
- Free C, Phillips G, Galli L, Watson L, Felix L, Edwards P, Patel V, Haines A: The effectiveness of mobile-health technology-based health behaviour change or disease management interventions for health care consumers: A systematic review. PLoS Med 10:e1001362, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliano A, Germano E, Pustorino G, Impallomeni C, D'Arrigo C, Calamoneri F, Spina E: Risperidone treatment of children with autistic disorder: effectiveness, tolerability, and pharmacokinetic implications. J Child Adolesc Psychopharmacol 14:39–47, 2004 [DOI] [PubMed] [Google Scholar]
- Gleason MM, Egger HL, Emslie GJ, Greenhill LL, Kowatch RA, Lieberman AF, Luby JL, Owens J, Scahill LD, Scheeringa MS, Stafford B, Wise B, Zeanah CH: Psychopharmacological treatment for very young children: contexts and guidelines. J Am Acad Child Adolesc Psychiatry 46:1532–1572, 2007 [DOI] [PubMed] [Google Scholar]
- Gutgesell H, Atkins D, Barst R, Buck M, Franklin W, Humes R, Ringel R, Shaddy R, Taubert KA: AHA Scientific Statement: cardiovascular monitoring of children and adolescents receiving psychotropic drugs. J Am Acad Child Adolesc Psychiatry 38:1047–1050, 1999 [DOI] [PubMed] [Google Scholar]
- Haupt DW, Rosenblatt LC, Kim E, Baker RA, Whitehead R, Newcomer JW: Prevalence and predictors of lipid and glucose monitoring in commercially insured patients treated with second-generation antipsychotic agents. Am J Psychiatry 166:345–353, 2009 [DOI] [PubMed] [Google Scholar]
- Honey BL, Ramos L, Brahm NC: Evaluation of monitoring for metabolic effects in children treated with second generation antipsychotics in a pediatric clinic. J Pediatr Pharmacol Ther 18:292–297, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KG, Juul K, Fink–Jensen A, Correll CU, Pagsberg AK: Corrected QT Changes during antipsychotic treatment of children and adolescents: A systematic review and meta-analysis of clinical trials. 54:25–36, 2015 [DOI] [PubMed]
- Kumar A, Datta SS, Wright SD, Furtado VA, Russell PS: Atypical antipsychotics for psychosis in adolescents. Cochrane Database Syst Rev 10:CD009582, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby J, Mrakotsky C, Stalets MM, Belden A, Heffelfinger A, Williams M, Spitznagel E: Risperidone in preschool children with autistic spectrum disorders: An investigation of safety and efficacy. J Child Adolesc Psychopharmacol 16:575–587, 2006 [DOI] [PubMed] [Google Scholar]
- Maayan L, Correll CU: Weight gain and metabolic risks associated with antipsychotic medications in children and adolescents. J Child Adolesc Psychopharmacol 21:517–535, 2011 [DOI] [PubMed] [Google Scholar]
- McDougle CJ, Scahill L, Aman MG, McCracken JT, Tierney E, Davies M, Arnold LE, Posey DJ, Martin A, Ghuman JK, Shah B, Chuang SZ, Swiezy NB, Gonzalez NM, Hollway J, Koenig K, McGough JJ, Ritz L, Vitiello B: Risperidone for the core symptom domains of autism: Results from the study by the autism network of the research units on pediatric psychopharmacology. Am J Psychiatry 162:1142–1148, 2005 [DOI] [PubMed] [Google Scholar]
- Morrato EH, Newcomer JW, Kamat S, Baser O, Harnett J, Cuffel B: Metabolic screening after the American Diabetes Association's consensus statement on antipsychotic drugs and diabetes. Diabetes Care 32:1037–1042, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrato EH, Nicol GE, Maahs D, Druss BG, Hartung DM, Valuck RJ, Campagna E, Newcomer JW: Metabolic screening in children receiving antipsychotic drug treatment. Arch Pediatr Adolesc Med 164:344–351, 2010 [DOI] [PubMed] [Google Scholar]
- National Committee for Quality Assurance: Proposed New Measures for HEDIS®1 2015: Safe and Judicious Antipsychotic Use in Children and Adolescents. 2014. Available at: http://www.ncqa.org/Portals/0/HomePage/Antipsychotics.pdf Accessed April7, 2015
- Needle JS, Mularski RA, Nguyen T, Fromme EK: Influence of personal preferences for life-sustaining treatment on medical decision making among pediatric intensivists. Crit Care Med 40:2464–2469, 2012 [DOI] [PubMed] [Google Scholar]
- Newcomer JW, Nasrallah HA, Loebel AD: The Atypical Antipsychotic Therapy and Metabolic Issues National Survey: Practice patterns and knowledge of psychiatrists. J Clin Psychopharmacol 24:S1–S6, 2004 [DOI] [PubMed] [Google Scholar]
- Nielsen RE, Laursen MF, Vernal DL, Bisgaard C, Jakobsen H, Steinhausen HC, Correll CU: Risk of diabetes in children and adolescents exposed to antipsychotics: A nationwide 12-year case-control study. J Am Acad Child Adolesc Psychiatry 53:971–979, 2014 [DOI] [PubMed] [Google Scholar]
- Olfson M, Blanco C, Liu L, Moreno C, Laje G: National trends in the outpatient treatment of children and adolescents with antipsychotic drugs. Arch Gen Psychiatry 63:679–685, 2006 [DOI] [PubMed] [Google Scholar]
- Olfson M, Blanco C, Wang S, Laje G, Correll CU: National trends in the mental health care of children, adolescents, and adults by office-based physicians. JAMA Psychiatry 71:81–90, 2014 [DOI] [PubMed] [Google Scholar]
- Olfson M, Marcus SC, Weissman MM, Jensen PS: National trends in the use of psychotropic medications by children. J Am Acad Child Adolesc Psychiatry 41:514–521, 2002 [DOI] [PubMed] [Google Scholar]
- Pappadopulos E, Macintyre Ii JC, Crismon ML, Findling RL, Malone RP, Derivan A, Schooler N, Sikich L, Greenhill L, Schur SB, Felton CJ, Kranzler H, Rube DM, Sverd J, Finnerty M, Ketner S, Siennick SE, Jensen PS: Treatment recommendations for the use of antipsychotics for aggressive youth (TRAAY). Part II. J Am Acad Child Adolesc Psychiatry 42:145–161, 2003 [DOI] [PubMed] [Google Scholar]
- Patel NC, Sanchez RJ, Johnsrud MT, Crismon ML: Trends in antipsychotic use in a Texas medicaid population of children and adolescents: 1996 to 2000. J Child Adolesc Psychopharmacol 12:221–229, 2002 [DOI] [PubMed] [Google Scholar]
- Pringsheim T, Panagiotopoulos C, Davidson J, Ho J: Evidence-based recommendations for monitoring safety of second generation antipsychotics in children and youth. J Can Acad Child Adolesc Psychiatry 20:218–233, 2011 [PMC free article] [PubMed] [Google Scholar]
- Ratzoni G, Gothelf D, Brand–Gothelf A, Reidman J, Kikinzon L, Gal G, Phillip M, Apter A, Weizman R: Weight gain associated with olanzapine and risperidone in adolescent patients: a comparative prospective study. J Am Acad Child Adolesc Psychiatry 41:337–343, 2002 [DOI] [PubMed] [Google Scholar]
- Remschmidt H, Hennighausen K, Clement HW, Heiser P, Schulz E: Atypical neuroleptics in child and adolescent psychiatry. Eur Child Adolesc Psychiatry 9 Suppl 1:I9–19, 2000 [DOI] [PubMed] [Google Scholar]
- Rodday AM, Parsons SK, Correll CU, Robb AS, Zima BT, Saunders TS, Leslie LK: Child and adolescent psychiatrists' attitudes and practices prescribing second generation antipsychotics. J Child Adolesc Psychopharmacol 24:90–93, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosack J: FDA to require diabetes warning on antipsychotics. Psychiatric News 38:1, 2003 [Google Scholar]
- Safer DJ: A comparison of risperidone-induced weight gain across the age span. J Clin Psychopharmacol 24:429–436, 2004 [DOI] [PubMed] [Google Scholar]
- Saito E, Correll CU, Gallelli K, McMeniman M, Parikh UH, Malhotra AK, Kafantaris V: A prospective study of hyperprolactinemia in children and adolescents treated with atypical antipsychotic agents. J Child Adolesc Psychopharmacol 14:350–358, 2004 [DOI] [PubMed] [Google Scholar]
- Schur SB, Sikich L, Findling RL, Malone RP, Crismon ML, Derivan A, Macintyre Ii JC, Pappadopulos E, Greenhill L, Schooler N, Van OK, Jensen PS: Treatment recommendations for the use of antipsychotics for aggressive youth (TRAAY). Part I: A review. J Am Acad Child Adolesc Psychiatry 42:132–144, 2003 [DOI] [PubMed] [Google Scholar]
- Shah BR, Bhattacharyya O, Yu CH, Mamdani MM, Parsons JA, Straus SE, Zwarenstein M: Effect of an educational toolkit on quality of care: A pragmatic cluster randomized trial. PLoS Med 11:e1001588, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekelle P, Maglione M, Bagley S, Suttorp M, Mojica WA, Carter J, Rolon C, Hilton L, Zhou A, Chen S, Glassman P: Comparative Effectiveness of Off-Label Use of Atypical Antipsychotics. Comparative Effectiveness Review No 6 (Prepared by the Southern California/RAND Evidence-based Practice Center under Contract No 290-02-0003). 2007. Available at www.effectivehealthcare.ahrq.gov/reports/final.cfm Accessed November17, 2014 [PubMed]
- Stigler KA, Potenza MN, McDougle CJ: Tolerability profile of atypical antipsychotics in children and adolescents. Paediatr Drugs 3:927–942, 2001 [DOI] [PubMed] [Google Scholar]
- Toren P, Ratner S, Laor N, Weizman A: Benefit-risk assessment of atypical antipsychotics in the treatment of schizophrenia and comorbid disorders in children and adolescents. Drug Saf 27:1135–1156, 2004 [DOI] [PubMed] [Google Scholar]
- United States Food and Drug Administration: “Black Box” Warning for Antidepressants. Show #34. FDA Patient Safety News. 2004. Available at http://www.accessdata.fda.gov/psn/transcript.cfm?show=34 Accessed on January5, 2012
- Vitiello B, Correll C, van Zwieten–Boot B, Zuddas A, Parellada M, Arango C: Antipsychotics in children and adolescents: Increasing use, evidence for efficacy and safety concerns. Eur Neuropsychopharmacol 19:629–635, 2009 [DOI] [PubMed] [Google Scholar]
- Walter G, DeLaroche A, Soh N, Hunt G, Cleary M, Malhi G, Lambert T, Correll C, Rey J: Side effects of second-generation antipsychotics: The experiences, views and monitoring practices of Australian child psychiatrists. Australas Psychiatry 16:253–262, 2008 [DOI] [PubMed] [Google Scholar]
- Yip L, Dart RC, Graham K: Olanzapine toxicity in a toddler. Pediatrics 102:1494, 1998 [DOI] [PubMed] [Google Scholar]
- Zito JM, Safer DJ, Sai D, Gardner JF, Thomas D, Coombes P, Dubowski M, Mendez–Lewis M: Psychotropic medication patterns among youth in foster care. Pediatrics 121:e157–e163, 2008 [DOI] [PubMed] [Google Scholar]
- Zuddas A, Zanni R, Usala T: Second generation antipsychotics (SGAs) for non-psychotic disorders in children and adolescents: a review of the randomized controlled studies. Eur Neuropsychopharmacol 21:600–620, 2011 [DOI] [PubMed] [Google Scholar]