Abstract
Chemotherapy remains of limited use for the treatment of prostate cancer with only one drug, docetaxel, demonstrating a modest survival advantage for treatment of late-stage disease. Data from the NCI 60 cell line screen indicated that the castration-resistant prostate cancer cell lines PC3 and DU145 were more sensitive than average to the novel polymeric fluoropyrimidine (FP), F10, despite displaying less than average sensitivity to the widely-used FP, 5FU. Here, we show that F10 treatment of PC3 xenografts results in a significant survival advantage (treatment to control ratio (T/C) days = 18; p < 0.001; n = 16) relative to control mice treated with saline. F10 (40 mg/kg/dose) was administered via jugular vein catheterization 3-times per week for five weeks. This aggressive dosing regimen was completed with no drug-induced weight loss and with no evidence of toxicity. F10 was also shown to sensitize PC3 cells to radiation and F10 was also shown to be a potent radiosensitizer of PC3 xenografts in vivo with F10 in combination with radiation resulting in significantly greater regression of PC3 xenografts than radiation alone. The results indicate that F10 in this pre-clinical setting is an effective chemotherapeutic agent and possesses significant radiosensitizing properties.
Keywords: Radiosensitization, Prostate cancer, Fluoropyrimidine, Thymidylate synthase
Introduction
Prostate cancer is the most frequently diagnosed cancer in men accounting for nearly one-third of all new malignancies in American men and nearly 30,000 deaths [1]. Chemotherapy has limited utility for the treatment of prostate cancer [2] although docetaxel given in combination with prednisone [3] or estramustine [4] provides a survival benefit for treatment of castration-resistant prostate cancer (CRPC). Docetaxel as an adjuvant to radical prostatectomy also provides a survival advantage for men at high-risk for recurrent disease although treatment caused serious toxicities [5]. There is an urgent need for new and more effective chemotherapeutic options with fewer side effects for prostate cancer patients.
F10 is a novel polymeric fluoropyrimidine (FP) that is under pre-clinical development for treatment of acute myeloid leukemia (AML) [6,7], glioblastoma (GBM) [8] and other malignancies (Figure 1). The cytotoxic mechanism of F10 involves dual targeting of thymidylate synthase (TS) and DNA topoisomerase 1 (Top1) causing replication-mediated DNA double-strand breaks (DSBs) [9,10]. Thus, F10 mechanistically resembles the camptothecin (CPT) class of anticancer drugs [11] and is primarily directed towards the DNA locus of FP activity. Results from the NCI-60 cell line screen demonstrated mechanistic similarities to other Top1 poisons but unexpected mechanistic dissimilarities to 5-fluorouracil (5-FU) which is cytotoxic by an RNA-mediated process [9, 10]. The NCI-60 data also showed that the CRPC cell lines PC3 and DU145 cell lines were nearly 1,000 fold more sensitive to F10 than 5-FU suggesting that F10 might be effective for treating CRPC even though 5-FU and capecitabine (a 5-FU pro-drug) are not [12-14]. F10 is very well-tolerated in vivo [6,8], in part because of high specificity for proliferating cells [7], and may be efficacious without reducing patient quality of life.
Figure 1.
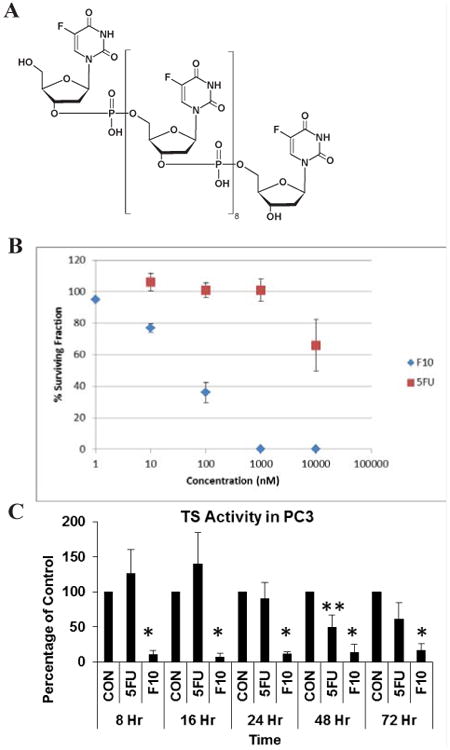
F10 strongly inhibits the clonogenicity of PC3 cells. (A) Structure of F10. (B) Clonogenic survival assay evaluating clonogenic survival of PC3 cells following 72 h treatment with either F10 or 5-FU at the indicated concentration (nM). No surviving colonies were observed following treatment with F10 at 1 μM (1,000 nM). In contrast, treatment with 5FU at 10 μM (10,000 nM) decreased the percent of colonies surviving relative to control by less than 50%. (C) TS activity in PC3 cells at the indicated times following treatment with F10 (10-8 M) or 5-FU (10-6 M) (* p < 0.05 vs control based on Student's two sided t-test).
In addition to their use in chemotherapy, FPs and Top1 poisons have significant radiosensitizing properties [15,16]. As radiation therapy is used for treating localized prostate cancer and preventing disease recurrence [17] information on the radiosensitization properties of F10 is important for translational efforts. In this article, we present data showing that F10 inhibits the growth of PC3 xenografts as a single agent and that the combination of F10 and radiation synergistically inhibits growth of PC3 cells in tissue culture and in vivo. PC3 cells are derived from bone metastases and have been used as a cellular model of CRPC [18]. We demonstrate that F10 is extremely well-tolerated in vivo with much more extensive dosing possible with F10 than with widely-used FPs, such as 5FU. The results obtained indicate that F10 should be evaluated for in vivo efficacy and radiosensitization for the clinical treatment of prostate cancer.
Materials and Methods
Cell culture and reagents
The human prostate cancer cell line PC3 was obtained from the American Type Culture Collection (Rockville, MD). PC3 cells were cultured at 37 °C in 5% CO2 atmosphere in RPMI-1640 medium (Gibco/Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum, 2 mM L-glutamine, and 1% penicillin-streptomycin. Cells were passaged every 3 to 5 days upon reaching 80% confluence using 0.25% trypsin/0.05% EDTA. F10 was synthesized and purified under a National Cancer Institute (NCI) contract to support the RAID project. Concentrations of F10 solutions were determined from UV absorption at 260 nm using 30μg/OD.
TS catalytic activity assays
PC3 cells were plated at a density of 1.5 ×106 cells in 100 mm2 plates. Cells were grown overnight in RPMI 1640 medium with 10% FBS. Cells were treated with 5-FU or F10 at the indicated concentrations and incubated for 0-48 h, harvested, and lysed by freeze-fracturing. Following centrifugation of cell lysates, supernatants were assayed for protein content and TS catalytic activity as previously described [7].
Clonogenic Assay. PC3 cells were cultured as described above, and were passaged three days prior to plating the cells in 60 × 15 mm Petri dishes for the clonogenic assays. Cells at 500 and 750 cells/mL were plated for F10 concentrations of 10-10 to 10-6 M while 250 and 500 cell/mL were plated for 5-FU concentrations from 10-8 to 10-5 M. Cells were allowed to attach for 24 h prior to treatment for 72 h. Each experiment was done in duplicate. The mean value and standard deviation were determined for each drug concentration. Following treatment, the medium was removed and replaced with fresh medium. Cells were then incubated for seven days, and stained with crystal violet. Colonies were counted manually, with a minimal colony diameter of approximately 1 mm required for counting.
Radiation enhancement
The effects of F10 on the response of PC3 cells to radiation were also evaluated using clonogenic assay. PC3 cells were plated as described above for F10-only treatment at densities of 200 and 400 cells/mL (0 Gy), 400 and 800 cells/mL (2 Gy), 800 and 1,600 cells/mL (4 Gy), 1,600 and 3,200 cells/mL (6 Gy) and 3,200 and 6,400 cells/mL (8 Gy). Cells were incubated with F10 for 24 h and then irradiated using a 300 kV orthovoltage x-ray irradiator (Precision x-ray, Inc., North Brantford, CT). Following irradiation, cells were further incubated to complete the entire drug exposure period of 72 h. The surviving fraction was normalized to F10-only treatment to determine to what extent F10 enhanced radiation-mediated cytotoxicity apart from drug-induced effects.
Jugular vein catheterization
Male NCr Nude (nu/nu) mice were purchased from The National Cancer Institute (Bethesda, MD) and maintained in a WFSM animal facility. All treatments and procedures in mice were conducted according to guidelines approved by the Animal Care and Use Committee of Wake Forest University Health Sciences. Prior to tumor inoculation, polyethylene tubing (PE10) was used to cannulate the jugular vein of mice seven weeks of age. The portion of the catheter, which exited through the animal's skin, was 4-5 cm in length, allowing multiple procedures during the course of the study. Catheters were supplied with a heparin lock and heat-sealed following each procedure. A two component, cloth-covered button kit was used to contain and protect the portion of the catheter outside the mouse. The button was sewn to the mid-scapular region on the mouse's back with the base attached to the mouse and the heat-sealed catheter protected by a cap. For each saline or drug injection, the cap was removed, the catheter was wiped with an alcohol pad and the heat seal removed with a sterile blade. A 30-gauge needle was used to flush the line first with heparinized saline, followed by administration of saline or drug, and followed with a heparin lock and heat-sealing. All injections and all irradiation procedures were carried out with the mice anesthetized with isoflurane. Mice were placed in a Plexiglass induction chamber (25 × 11 × 12 cm) that was filled with 2% isoflurane in O2. Pedal reflex was tested when the mice showed no eye-blink response when the chamber was tapped (∼2 min). Anesthesia was maintained through voluntary breathing of a mixture of 1.5 – 2% isofluorane in O2.
Establishment of tumor xenografts and in vivo experiments
The human prostate cancer cell line PC3 was grown to 80% confluence and harvested. Cells were re-suspended in serum-free RPMI-1640 medium with penicillin and streptomycin, mixed 1:1 with Growth Factor Reduced (GFR) BD Matrigel Basement Membrane Matrix (BD Biosciences, Palo Alto, California). Using a cold syringe and 27-gauge needle, 3.5 × 106 cells were injected subcutaneously into each lateral flank of male athymic nu/nu mice 8 weeks of age. At two weeks post-inoculation, palpable tumors (∼500 mm3) were established and animals were randomized into control and treatment arms consisting of eight to nine animals, respectively, with the latter receiving 40 mg/kg body weight F10 in 100 μL of sterile saline intravenously via jugular vein catheters beginning on Day 0 and alternating with orthovoltage x-ray radiation every 24 hours for five days and continuing each week for 5 weeks. An irradiation schedule of 30 Gy in 10 fractions of 3 Gy each over a 5-week period was used (2 fractions per week), delivered using a 300 kV orthovoltage x-ray irradiator (Precision x-ray, Inc., North Brantford, CT). This irradiation schedule is equivalent to a fractionated dose regimen of approximately 36 Gy in 18 fractions of 2 Gy each or a single fraction of 12-15 Gy. Animal weights were measured each week, and perpendicular tumor diameters were measured using a Vernier scale caliper twice per week until animals were sacrificed, at which time tumors and tissues were harvested. Tumor volume was estimated using the formula: π/6 × length × width × thickness. Animals were euthanized and tumors and other tissues were removed when tumor volume in a single flank exceeded 3000 mm3. Sections of the tumor were fixed in fresh 10% neutral buffered formaldehyde before embedding in paraffin for hematoxylin and eosin staining.
Statistical methods
Survival data and tumor growth curves were compared between groups using parametric and non-parametric methods (i.e. Kaplan-Meier curves and log-rank tests) using SAS (Version 9, Cary, NC). Mean tumor volumes and SE values are shown in figures for comparison and to show trends. Comparisons between the four groups (drug +/- radiation; saline +/- radiation) were made using repeated measures mixed model analysis where mice were considered as random effects and day, group and the day by group interaction were included as fixed effects. The day by group interaction was examined to determine whether the four groups had different changes in tumor volumes over time. In addition to this interaction test, we compared the mean values at different time points to determine at which time longitudinally the four groups began to differ from each other. Median and ranges of tumor volumes at a given date were calculated to determine T/C ratios and these median volume scores were compared using non-parametric two-sample median tests. Survival curves were generated to compare groups and since all animals died at some time during the experiment mean survival times could be compared using two-sample t-tests (i.e. there were no censored data), as well as the median survival times (via the log-rank test).
Results
F10 Inhibits clonogenic survival
The NCI-60 data indicated the CRPC cell lines PC3 and DU145 were highly sensitive to F10 with GI50 values in the nanomolar range. To evaluate the cytotoxicity of F10 towards PC3 cells, we performed clonogenic assays (Figure 1). Treatment with F10 at 100 nM reduced PC3 clonogenic survival >50% while at 1 μM F10 completely inhibited PC3 cell colony formation. In contrast to the results obtained with F10, 5-FU had minimal effect on the clonogenic potential of PC3 cells. Treatment with 1 μM 5FU had no effect on PC3 cell colony formation and even 10 μM 5-FU did not decrease colony formation by 50%. These results are consistent with clinical studies demonstrating that 5-FU is unlikely to be effective for treating prostate cancer but demonstrate that F10 is substantially more potent and should be considered for prostate cancer treatment.
F10 is a potent inhibitor of TS
Thymidylate synthase (TS) is a principal target of fluoropyrimidine (FP) chemotherapy and inhibiting TS is considered central to the anti-tumor activity of FP drugs. We evaluated TS catalytic activity in PC3 cells following treatment with either 5-FU or F10 (Figure 1). F10 significantly reduced TS activity relative to control within 8 h and TS activity remained significantly decreased through 72 h. In contrast 5FU treatment had minimal effect on reducing TS activity with activity levels actually increased at 8 and 16 h for 5-FU treatment relative to control and only the 48 h timepoint showing a significant decrease (∼50% control).
F10 Enhances the Effects of Radiation. We next evaluated to what extent F10 enhanced the effects of radiation at inhibiting the clonogenic survival of PC3 cells (Figure 2). Radiation-only was also effective at reducing colony formation of PC3 cells with 2 Gy reducing colonies to ∼50% control. For all doses of radiation evaluated F10 co-treatment significantly decreased clonogenic potential (Figure 2A). For example 100 nM F10 decreased colony formation at 2 Gy from 50% to 20%. When the data were normalized to separate the F10-only cytotoxic effects from the effects of radiation (Figure 2B) a true radiosensitization effect was apparent for F10. Thus, F10 has potential to be used both for direct anti-tumor effects as well as radiosensitization for treatment of prostate cancer.
Figure 2.
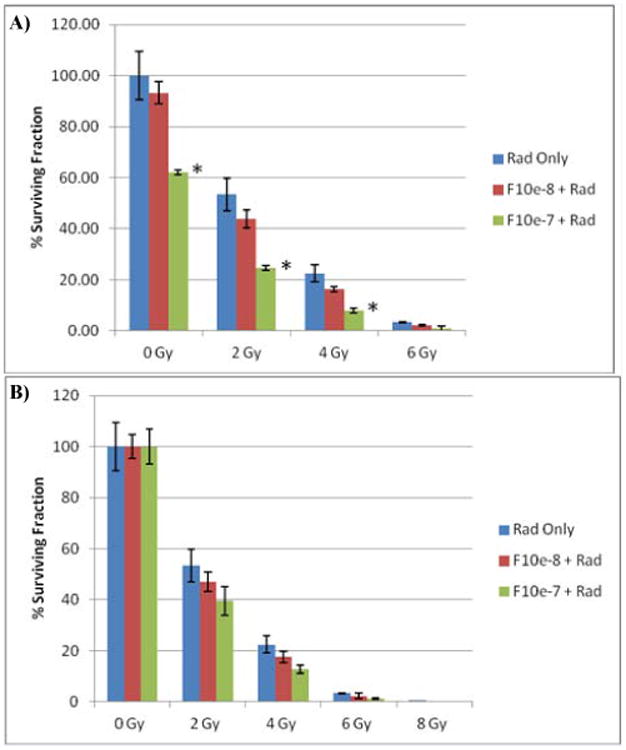
Results of a clonogenic assay evaluating the survival fraction of PC3 cells exposed to radiation or radiation in combination with F10. (A) Graph of surviving fraction as a percent of non-treated cells. (B) Same data as in (A) but normalized for F10-only effects to determine if F10 were radiosensitizing. (* p < 0.05 vs control based on Student's two sided t-test).
F10 inhibits PC3 tumor growth and increases survival
The antitumor activity of F10 was evaluated in NCR nu/nu mice in which PC3 tumor cells had been implanted 14 days previously. Mean initial tumor volumes were approximately 500 mm3. Mice treated with F10 had significantly longer survival times relative to saline-treated controls. The mean survival time for mice treated with F10 was 66 days while the mean survival time for saline-treated control animals was 48 days. Thus, treatment with F10 resulted in increased survival of 18 days (T/C days =18; p < 0.001; n = 16 – Figure 3). In all cases, mice were removed from the study as a result of tumor burden in the non-irradiated flank, thus survival is a direct comparison of the ability of F10 alone to reduce tumor growth. Median survival times were also significantly increased as a result of F10 treatment (p < 0.002; n = 16).
Figure 3.
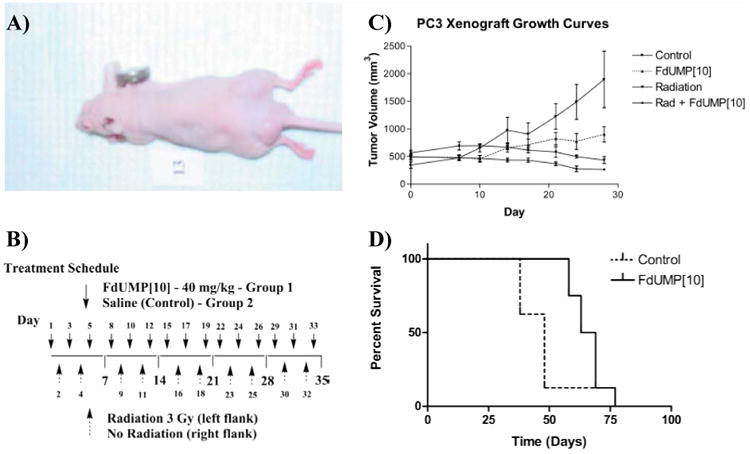
Treatment with F10 significantly reduces the growth of established PC3 cell xenografts. (A) Tumor growth curves for the four tumor groups in the study (left and right flank tumors from each treatment group were analyzed separately). Error bars indicate +/-SEM, n = 8. (B) A typical mouse in the study. Tumors were initiated by s.c. injection of PC3 cells bilaterally. Initial tumor volumes were approximately 500 mm3 in all treatment groups. The left flank tumor in each mouse was selectively irradiated using the 300 kV orthovoltage x-ray irradiator. (C) Treatment schedule for the in vivo experiment. Mice were assigned to one of two treatment groups – either F10 at 40 mg/kg dissolved in 100 μL of sterile saline or saline-only. Treatment was administered through a catheter inserted into the jugular vein. Drug was administered 3× per week for five weeks on the days indicated. A dose of 3 Gy radiation was administered to the left flank of all animals 2× per week for five weeks on the indicated days. (D) Kaplan-Meier survival curves for mice treated with F10 or with saline control. Mice were removed from the study based on tumor size in the non-irradiated flank.
The repeated measures mixed model indicated that there were significant group by day interactions suggesting that the tumor volumes were changing at different rates in the four treatment groups (saline (S); F10 (F); S + radiation (R); and F10 + radiation (F+R). The non-irradiated groups (F and S) began to differ starting at day 20 while the irradiated and non-irradiated (Saline) and the irradiated and non-irradiated (F10) groups began differing significantly at Day 17 (Figure 3). When we compared the median tumor volumes between groups we found using one-tailed comparisons that by day 24 there were significant differences between all groups. The F-irradiated group (F+R) differed from the S-non-irradiated group (S) (p=.002; n = 16), F-non-irradiated (F) versus S-non-irradiated (S) (p=.026; n = 16), and S-irradiated (R) versus S-non-irradiated (S) (p=.0018; n = 16). Although the growth trends clearly indicated continued differentiation among tumor size in the four treatment groups, animals with the largest tumors in the saline group were euthanized due to tumor burden in the non-irradiated flank and direct comparisons among treatment groups were not possible at later timepoints.
Treatment with F10 did not result in weight loss significantly greater than mice treated with vehicle-only. The weight loss in the F10-treated mice was greatest on day 14 with a mean weight loss of 11%. Saline-treated animals had a mean weight loss of 8% at day 14 and a mean weight loss of 11% on day 28 (Figure 4). The weight loss displayed in both the drug-treated and non-drug-treated mice likely resulted from the effects of the anesthesia (isoflurane) that was administered daily prior to either drug-injection or irradiation. The F10-treated mice quickly adjusted to the treatment protocol and began re-gaining weight during the third week of treatment. In contrast, the saline-treated control group continued to lose weight as tumor burden increased in these animals. The present results confirm that extended treatment with F10 is very well-tolerated in vivo and does not result in significant weight-loss at efficacious doses.
Figure 4.
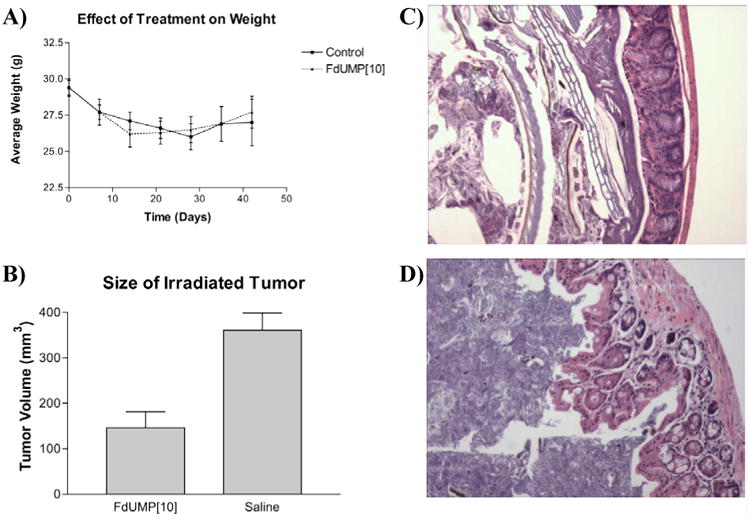
F10 treatment does not result in weight loss greater than control treatment or cause damage to the colonic epithelium. Also, the combination of F10 + radiation results in significantly smaller final tumor size relative to treatment with radiation alone. (A) Average weights for F10-treated and control mice. Error bars indicate +/-SEM, n = 8. (B) Graph of the final mean tumor volumes for left-flank tumors in the study. Tumors treated with F10 + irradiation were significantly smaller than tumors treated with radiation. Graph depicts the mean +/- SEM (n = 8) for left flank tumors from the F10-treated and saline groups. (C) H&E section (10×) of colonic epithelium from an F10-treated mouse. (D) Colonic epithelium from a saline-treated mouse. Both mice were sacrificed as a result of tumor burden to the non-irradiated flank. There is no deterioration of the colonic epithelium in mice from treated with F10.
Histological examination of tissues from animals sacrificed at the conclusion of the study revealed no toxicity to the gastrointestinal tract or to other tissues of drug-treated mice (Figure 4). Mice-treated with F10 also displayed no signs of neutropenia as assessed by histological examination of a cross-section of the femur (data not shown). Other tissues examined included liver, lungs, and kidneys. There was no sign of drug-related toxicity in any tissues. Thus, our results indicate that F10 at a concentration of 40 mg/kg/dose significantly reduces tumor growth; furthermore, doses that are higher than those administered in the present study are likely to be well-tolerated in vivo. Since a dose-response is evident for PC3 cells in culture, it is possible that an even greater reduction in tumor burden could be achieved with higher dosage.
The combination of F10 and radiation potently reduces tumor burden
The antitumor activity of F10 in combination with radiation was compared to radiation only, drug-treatment alone, and no treatment by analyzing the growth curves for the left-flank tumors of mice treated with F10 relative to the other tumor groups (Figure 3). The growth rates for tumors treated with F10 + radiation were significantly less than for tumors treated with radiation-only, drug-only, or saline control. The tumor growth delay (T/C% = 21%) for the F10 + radiation group was significantly reduced relative to control (p < 0.005; n = 16). The tumor growth delay for radiation only was also significant relative to control (T/C% = 36%; p < 0.05; n = 16). The radiation-sensitizing properties of F10 were apparent in comparison of the F10 + radiation group to radiation alone (p < 0.01; n =16).
Although the study design did not permit direct comparison with respect to survival of mice treated with F10 + radiation relative to radiation-only and to drug-only, the final tumor sizes for PC3 xenografts treated with F10 + radiation were significantly smaller, on average, than were the final tumor sizes for PC3 xenografts treated with radiation only (Figure 4). Measurement of final tumor size for the F10 + radiation PC3 xenografts occurred an average of 18 days later than for xenografts treated with radiation-only, as euthanasia of animals was required based on tumor volume for the non-irradiated flank. Average tumor volumes did not become significantly larger for the tumors treated with F10 + radiation during the final weeks of the study indicating that the combination had a long-term effect on tumor growth and animal survival. Histological examination of tumors treated with F10 + radiation revealed marked hypocellularity of the excised tissue (Figure 5). The results indicate that F10 is a potent radiosensitizer and that the combination of F10 + radiation may be highly effective for the treatment of prostate cancer. Histological examination of tumors from the non-irradiated flank of saline-treated and F10-treated mice revealed marked necrosis in the saline-treated animals, but not the F10-treated mice which were euthanized, on average, 18 days later. The results are consistent with tumor re-growth following the conclusion of F10 administration on day 33 (Figure 3).
Figure 5.
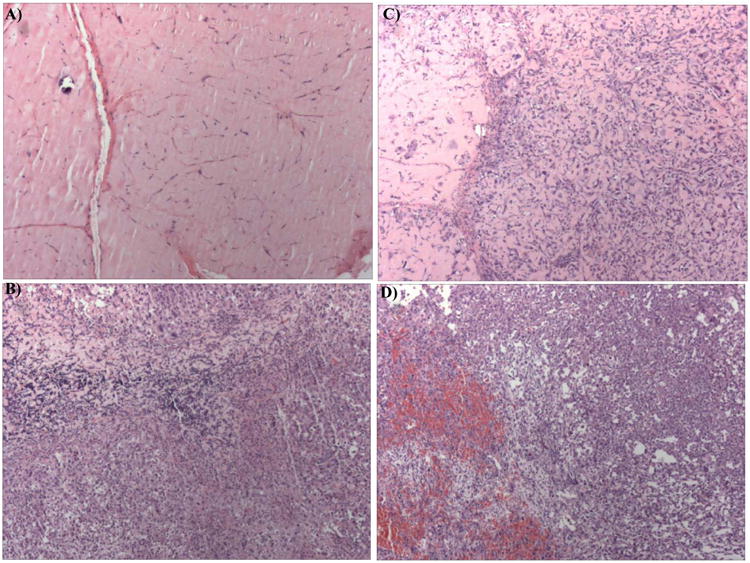
H&E sections (4×) from a left-flank (irradiated) tumor from an (A) F10-treated animal; and (B) Saline-treated animal. Irradiated tumors from the F10-treated group displayed marked hypocellularity relative to saline-treated animals. (C) H&E section from the right-flank (non-irradiated) tumor from an F10-treated animal and (D) from the right-flank of a saline-treated animal. While animals from both groups were sacrificed based on volumes in the right-flank tumors, tumors from the saline group displayed marked necrosis. Animals from the F10-treated group were sacrificed, on average, 18 days later than animals in the saline group (see Figure 3).
Discussion
Advanced prostate cancer remains a challenging disease with few effective chemotherapeutic options. Our studies show the novel FP F10 increases survival of mice with PC3 xenografts and sensitizes PC3 xenografts to radiation. These results are somewhat unexpected in light of previous studies reporting that 5-FU and capecitabine are not efficacious for treating prostate cancer [12-14] and likely reflects selective targeting of the DNA-directed locus of FP activity. Thus, F10 may be effective in the clinical management of prostate cancer both as a chemotherapeutic agent and as a radiosensitizer.
The results of the present study stand in stark contrast to previous studies with 5-FU that determined the maximum tolerated dose of 5-FU to be 45 mg/kg/dose on a once daily, three times per week dosing schedule with higher doses resulting in lethality. In the present study, we were able to continue dosing with F10 at 40 mg/kg/dose 3-times per week for 5 consecutive weeks with no adverse drug-related effects. Animals in the F10-treated group experienced no drug-induced weight loss and histological examination of the GI-tract following animal sacrifice at the end of the study indicated no drug-related damage to the GI-tract or any other tissues. These results are consistent with the GI-tract toxicity of FPs being mainly an RNA-mediated effect [19] and indicate that selectively targeting the DNA-directed locus of FP activity with F10 results in significant antitumor activity and with elimination of the GI-tract toxicity associated with 5-FU treatment. The lack of toxicity at the current dose indicates that higher or more extensive dosing than was employed in the present study is likely to be safe. In light of the observed concentration-dependence of F10 cytotoxicity and radiosensitization, higher dosing may further reduce tumor burden in vivo.
The radiosensitization properties of FPs and Top1 poisons have been documented [15,16]. The extent of radio sensitization observed with F10 in the present study compares favorably with Gossypol [18] and curcumin [20] – two natural products that are being evaluated as radiosensitizers for the treatment of prostate cancer and other malignancies. F10 displays strong anticancer activity as a single agent, is radiosensitizing, and is very well tolerated in vivo. While chemically and mechanistically distinct from conventional FPs, F10 has similarities to these drugs that have been used successfully in the clinic for decades. Future clinical studies can draw upon this longstanding clinical experience as well as new mechanistic insights obtained with this novel FP polymer.
Acknowledgments
This work was supported by DOD PCRP 093606, DOD PCRP 110135, NIH CA102532, NIH P30 CA12197 and the NCI RAID Program. The authors are grateful for technical assistance from Jamie Jennings-Gee, Christopher Stuart, and Stacey Trozzo.
References
- 1.ACS Facts Figures. 2013.
- 2.Hadaschik BA, Sowery RD, Gleave ME. Novel targets and approaches in advanced prostate cancer. Curr Opin Urol. 2007;17:182–187. doi: 10.1097/MOU.0b013e3280dd8a4f. [DOI] [PubMed] [Google Scholar]
- 3.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 4.Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 5.Kibel AS, Rosenbaum E, Kattan MW, Picus J, Dreicer R, Klein EA, et al. Eisenberger, Adjuvant weekly docetaxel for patients with high risk prostate cancer after radical prostatectomy: a multi-institutional pilot study. J Urol. 2007;177:1777–1781. doi: 10.1016/j.juro.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 6.Pardee TS, Gomes E, Jennings-Gee J, Caudell D, Gmeiner WH. Unique dual targeting of thymidylate synthase and topoisomerase1 by FdUMP[10] results in high efficacy against AML and low toxicity. Blood. 2012;119:3561–3570. doi: 10.1182/blood-2011-06-362442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jennings-Gee J, Pardee TS, Gmeiner WH. Replication-dependent irreversible topoisomerase 1 poisoning is responsible for FdUMP[10] anti-leukemic activity. Exp Hematol. 2013;41:180–188. doi: 10.1016/j.exphem.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gmeiner WH, Lema-Tome C, Gibo D, Jennings-Gee J, Milligan C, Debinski W. Selective anti-tumor activity of the novel fluoropyrimidine polymer F10 towards G48a orthotopic GBM tumors. J Neurooncol. 2014;116:447–454. doi: 10.1007/s11060-013-1321-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gmeiner WH, Reinhold WC, Pommier Y. Genome-wide mRNA and microRNA profiling of the NCI 60 cell-line screen and comparison of FdUMP[10] with fluorouracil, floxuridine, and topoisomerase 1 poisons. Mol Cancer Ther. 2010;9:3105–3114. doi: 10.1158/1535-7163.MCT-10-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liao ZY, Sordet O, Zhang HL, Kohlhagen G, Antony S, Gmeiner WH, Pommier Y. A novel polypyrimidine antitumor agent FdUMP[10] induces thymineless death with topoisomerase I-DNA complexes. Cancer Res. 2005;65:4844–4851. doi: 10.1158/0008-5472.CAN-04-1302. [DOI] [PubMed] [Google Scholar]
- 11.Pommier Y, Leo E, Zhang H, Marchand C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem Biol. 2010;17:421–433. doi: 10.1016/j.chembiol.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berlin JD, Propert KJ, Trump D, Wilding G, Hudes G, Glick J, et al. 5-Fluorouracil and leucovorin therapy in patients with hormone refractory prostate cancer: an Eastern Cooperative Oncology Group phase II study (E1889) Am J Clin Oncol. 1998;21:171–176. doi: 10.1097/00000421-199804000-00016. [DOI] [PubMed] [Google Scholar]
- 13.Huan SD, Aitken SE, Stewart DJ. A phase II study of 5-fluorouracil and high dose folinic acid in cisplatin-refractory metastatic bladder cancer. Ann Oncol. 1995;6:836–837. doi: 10.1093/oxfordjournals.annonc.a059325. [DOI] [PubMed] [Google Scholar]
- 14.Morant R, Bernhard J, Dietrich D, Gillessen S, Bonomo M, Borner M, et al. Capecitabine in hormone-resistant metastatic prostatic carcinoma - a phase II trial. Br J Cancer. 2004;90:1312–1317. doi: 10.1038/sj.bjc.6601673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lawrence TS, Davis MA, Tang HY, Maybaum J. Fluorodeoxyuridine-mediated cytotoxicity and radiosensitization require S phase progression. Int J Radiat Biol. 1996;70:273–280. doi: 10.1080/095530096145003. [DOI] [PubMed] [Google Scholar]
- 16.Chen AY, Chou R, Shih SJ, Lau D, Gandara D. Enhancement of radiotherapy with DNA topoisomerase I-targeted drugs. Crit Rev Oncol Hematol. 2004;50:111–119. doi: 10.1016/j.critrevonc.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Sailer SL. Radiation therapy for prostate cancer: external beam, brachytherapy, and salvage. N C Med J. 2006;67:149–153. [PubMed] [Google Scholar]
- 18.Xu L, Yang D, Wang S, Tang W, Liu M, Davis M, et al. (-)-Gossypol enhances response to radiation therapy and results in tumor regression of human prostate cancer. Mol Cancer Ther. 2005;4:197–205. [PubMed] [Google Scholar]
- 19.Pritchard DM, Watson AJ, Potten CS, Jackman AL, Hickman JA. Inhibition by uridine but not thymidine of p53-dependent intestinal apoptosis initiated by 5-fluorouracil: evidence for the involvement of RNA perturbation. Proc Natl Acad Sci U S A. 1997;94:1795–1799. doi: 10.1073/pnas.94.5.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chendil D, Ranga RS, Meigooni D, Sathishkumar S, Ahmed MM. Curcumin confers radiosensitizing effect in prostate cancer cell line PC-3. Oncogene. 2004;23:1599–1607. doi: 10.1038/sj.onc.1207284. [DOI] [PubMed] [Google Scholar]


