Abstract
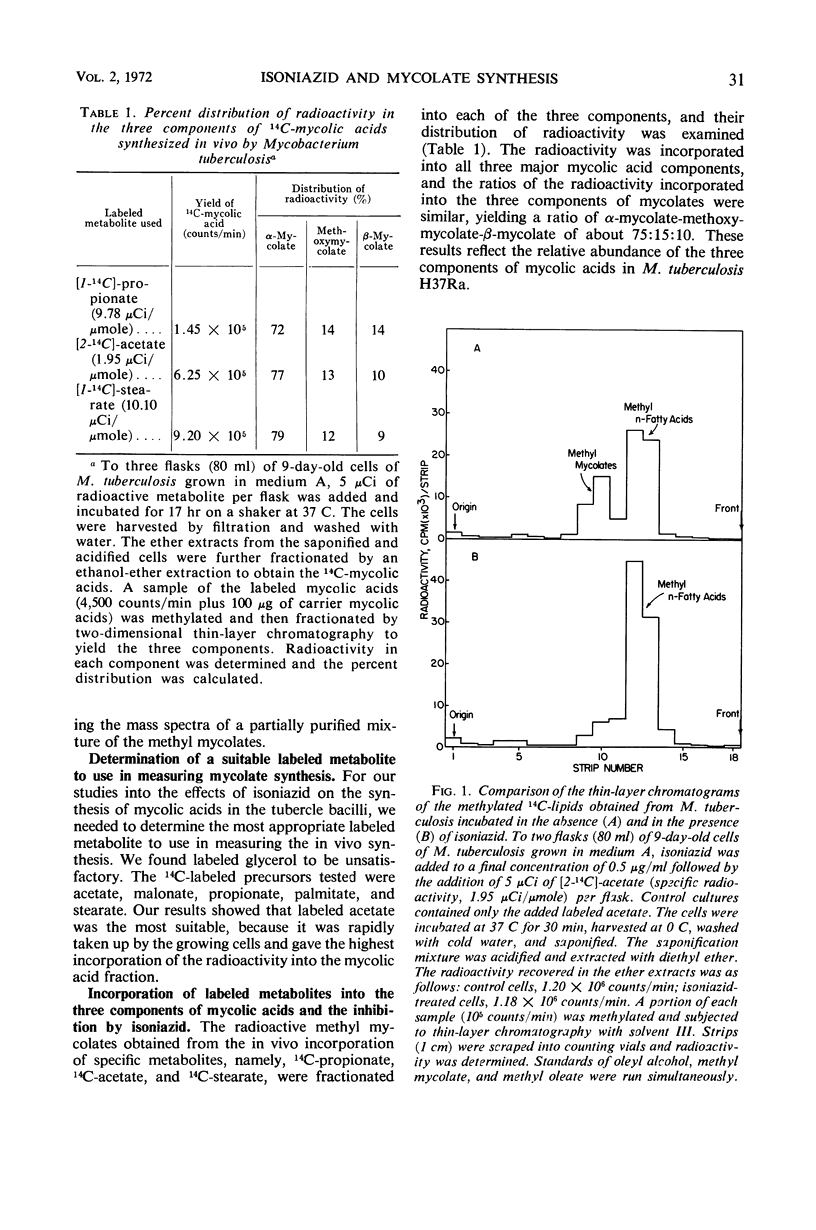
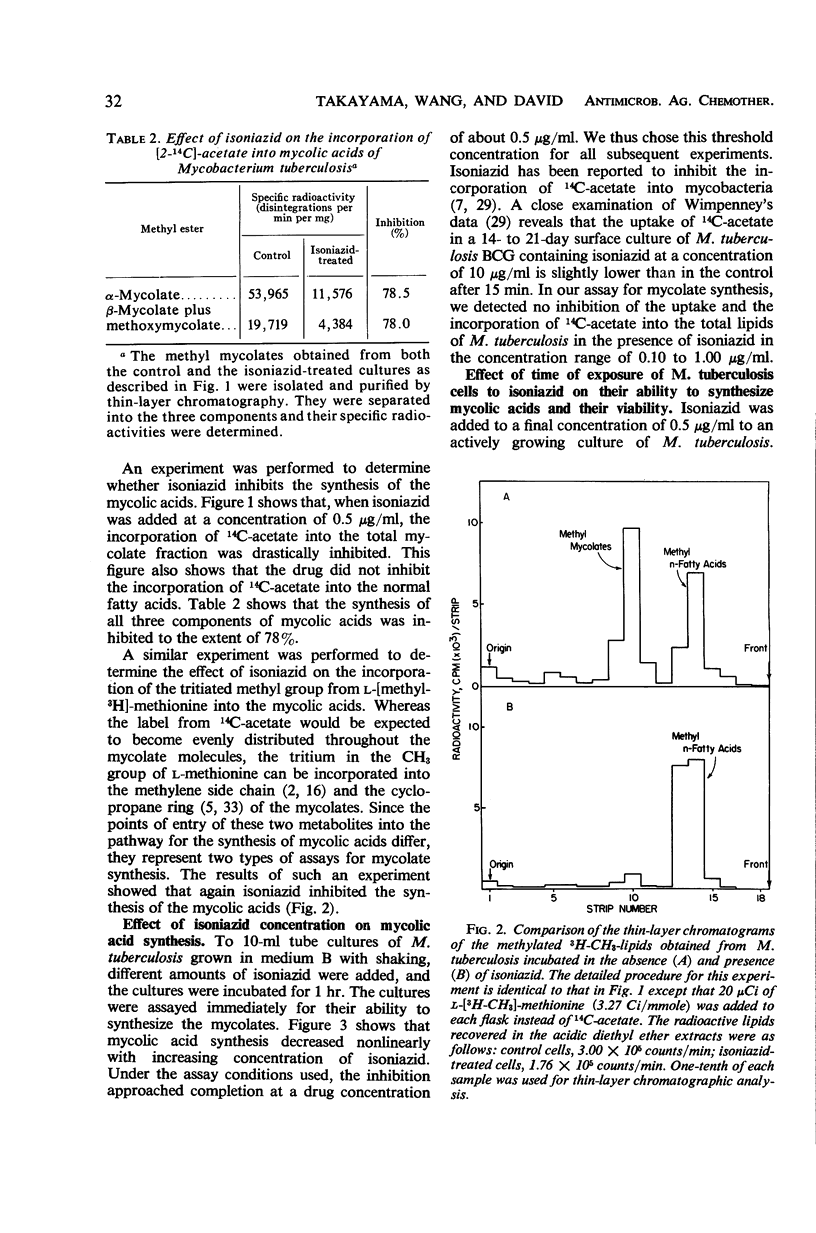
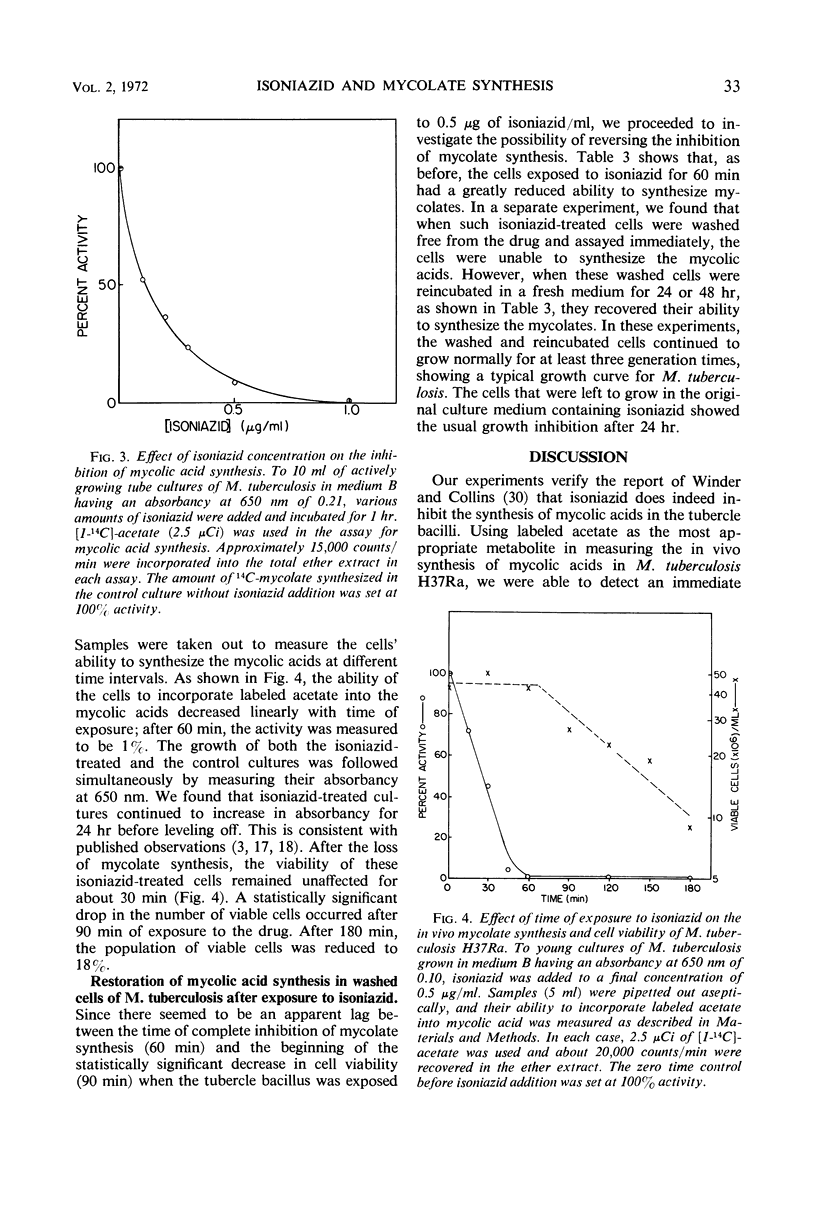
When an actively growing culture of the H37Ra strain of Mycobacterium tuberculosis was exposed to isoniazid at a concentration of 0.5 μg/ml, the cells began to lose their ability to synthesize mycolic acids immediately. After 60 min, the cells had completely lost this ability. The synthesis of the three mycolate components—α-mycolate, methoxymycolate, and β-mycolate—was inhibited. The viability of the isoniazid-treated cells was unaffected up to about 60 min of exposure, after which time there was a gradual decline in the viability to about 18% after 180 min. Correspondingly, growth of the drug-treated cells slowed down and stopped after 24 hr. The inhibition of the synthesis of mycolic acids was reversible if the drug was removed before the loss of viability set in. Incubation of the viable cells in the absence of the drug for 24 hr restored the mycolate synthesis. These results strongly suggest that the inhibition of the synthesis of the mycolic acids is closely associated with the primary mechanism of action of isoniazid on the tubercle bacilli. The sequence of events which leads to the loss of viability of cells exposed to isoniazid is described.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acharya P. V., Goldman D. S. Chemical composition of the cell wall of the H37Ra strain of Mycobacterium tuberculosis. J Bacteriol. 1970 Jun;102(3):733–739. doi: 10.1128/jb.102.3.733-739.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akamatsu Y., Law J. H. Enzymatic alkylenation of phospholipid fatty acid chains by extracts of Mycobacterium phlei. J Biol Chem. 1970 Feb 25;245(4):701–708. [PubMed] [Google Scholar]
- BARCLAY W. R., EBERT R. H., KOCHWESER D. Mode of action of isoniazid. Am Rev Tuberc. 1953 Apr;67(4):490–496. doi: 10.1164/art.1953.67.4.490. [DOI] [PubMed] [Google Scholar]
- Brennan P. J., Rooney S. A., Winder F. G. The lipids of Mycobacterium tuberculosis BCG: fractionation, composition, turnover and the effects of isoniazid. Ir J Med Sci. 1970 Aug;3(8):371–390. doi: 10.1007/BF02956904. [DOI] [PubMed] [Google Scholar]
- CHUNG A. E., LAW J. H. CYCLOPROPANE FATTY ACID SYNTHETASE: PARTIAL PURIFICATION AND PROPERTIES. Biochemistry. 1964 Jul;3:967–974. doi: 10.1021/bi00895a021. [DOI] [PubMed] [Google Scholar]
- Cohen S. S., Barner H. D. STUDIES ON UNBALANCED GROWTH IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1954 Oct;40(10):885–893. doi: 10.1073/pnas.40.10.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etemadi A. H., Lederer E. Sur la structure des acides alpha-mycoliques de la souche humaine test de Mycobacterium tuberculosis. Bull Soc Chim Fr. 1965 Sep;9:2640–2645. [PubMed] [Google Scholar]
- FEINGOLD D. S. ANTIMICROBIAL CHEMOTHERAPEUTIC AGENTS: THE NATURE OF THEIR ACTION AND SELECTIVE TOXICITY. N Engl J Med. 1963 Oct 24;269:900–CONTD. doi: 10.1056/NEJM196310242691706. [DOI] [PubMed] [Google Scholar]
- GANGADHARAM P. R., HAROLD F. M., SCHAEFER W. B. Selective inhibition of nucleic acid synthesis in Mycobacterium tuberculosis by isoniazid. Nature. 1963 May 18;198:712–714. doi: 10.1038/198712b0. [DOI] [PubMed] [Google Scholar]
- LENNARZ W. J., SCHEUERBRANDT G., BLOCH K. The biosynthesis of oleic and 10-methylstearic acids in Mycobacterium phlei. J Biol Chem. 1962 Mar;237:664–671. [PubMed] [Google Scholar]
- MACKANESS G. B., SMITH N. The bactericidal action of isoniazid, streptomycin and terramycin on extracellular and intracellular tubercle bacilli. Am Rev Tuberc. 1953 Mar;67(3):322–340. doi: 10.1164/art.1953.67.3.322. [DOI] [PubMed] [Google Scholar]
- MIDDLEBROOK G. Sterilization of tubercle bacilli by isonicotinic acid hydrazide and the incidence of variants resistant to the drug in vitro. Am Rev Tuberc. 1952 Jun;65(6):765–767. [PubMed] [Google Scholar]
- Minnikin D. E., Polgar N. Studies on the mycolic acids from human tubercle bacilli. Tetrahedron Lett. 1966 Jun;23:2643–2647. doi: 10.1016/s0040-4039(01)84131-9. [DOI] [PubMed] [Google Scholar]
- PANSY F., STANDER H., DONOVICK R. In vitro studies on isonicotinic acid hydrazide. Am Rev Tuberc. 1952 Jun;65(6):761–764. [PubMed] [Google Scholar]
- RUSSE H. P., BARCLAY W. R. The effect of isoniazid on lipids of the tubercle bacillus. Am Rev Tuberc. 1955 Dec;72(6):713–717. doi: 10.1164/artpd.1955.72.6.713. [DOI] [PubMed] [Google Scholar]
- SCHAEFER W. B. Effect of isoniazid on the dehydrogenase activity of Mycobacterium tuberculosis. J Bacteriol. 1960 Feb;79:236–245. doi: 10.1128/jb.79.2.236-245.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEENKEN W., Jr, WOLINSKY E. Antituberculous properties of hydrazines of isonicotinic acid (rimifon, marsilid). Am Rev Tuberc. 1952 Apr;65(4):365–375. doi: 10.1164/art.1952.65.4.365. [DOI] [PubMed] [Google Scholar]
- Takayama K., Goldman D. S. Enzymatic synthesis of mannosyl-1-phosphoryl-decaprenol by a cell-free system of Mycobacterium tuberculosis. J Biol Chem. 1970 Dec 10;245(23):6251–6257. [PubMed] [Google Scholar]
- Wimpenny J. W. Effect of isoniazid on biosynthesis in Mycobacterium tuberculosis var. bovis BCG. J Gen Microbiol. 1967 Jun;47(3):379–388. doi: 10.1099/00221287-47-3-379. [DOI] [PubMed] [Google Scholar]
- Winder F. G., Collins P. B. Inhibition by isoniazid of synthesis of mycolic acids in Mycobacterium tuberculosis. J Gen Microbiol. 1970 Sep;63(1):41–48. doi: 10.1099/00221287-63-1-41. [DOI] [PubMed] [Google Scholar]
- Winder F. G., Rooney S. A. The effects of isoniazid on the carbohydrates of Mycobacterium tuberculosis BCG. Biochem J. 1970 Apr;117(2):355–368. doi: 10.1042/bj1170355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youatt J. A review of the action of isoniazid. Am Rev Respir Dis. 1969 May;99(5):729–749. doi: 10.1164/arrd.1969.99.5.729. [DOI] [PubMed] [Google Scholar]
- ZALKIN H., LAW J. H., GOLDFINE H. Enzymatic synthesis of cyclopropane fatty acids catalyzed by bacterial extracts. J Biol Chem. 1963 Apr;238:1242–1248. [PubMed] [Google Scholar]



