Abstract
Currently available antipsychotic medications lack satisfactory effectiveness against several symptom clusters of schizophrenia, including affective symptoms (e.g., anhedonia) and cognitive deficits (e.g., impulsivity). Translational animal models analogous to these symptoms are necessary to provide insights into the neurobiological events underlying these impairments and allow the development of improved schizophrenia treatments. We investigated the effects of repeated administration of the psychotomimetic phencyclidine (PCP), a noncompetitive N-methyl-D-aspartate receptor antagonist, on performance in the intracranial self-stimulation (ICSS) procedure, a test of reward function. We also explored how chronic treatment with clozapine, an atypical antipsychotic with limited effectiveness on affective and cognitive schizophrenia symptoms, would affect PCP-induced disruptions of ICSS performance. A single injection of 2 mg/kg PCP elevated ICSS thresholds, suggesting a reward deficit. Repeated PCP administration (2 mg/kg once daily for 2 consecutive days followed by a 2-week drug free period, and then 5 consecutive days of 2 mg/kg PCP daily, s.c., 30 min pretreatment) resulted in a small, but significant, lowering of ICSS reward thresholds, indicating increased reward function. Chronic clozapine did not alter the effects of repeated PCP on ICSS thresholds. Repeated PCP also increased the number of extra and timeout responses performed during the ICSS procedure, reflecting disinhibition of inappropriate responding and decreased task efficiency. Chronic clozapine attenuated the increase in extra responses induced by repeated PCP and tended to reduce the PCP-induced increase in timeout responses. These results suggest that repeated PCP administration does not produce an anhedonia-like state resembling that seen in schizophrenia. However, the increased impulsivity and reduced task efficiency seen with repeated PCP administration, and the sensitivity of these effects to attenuation with an atypical antipsychotic, suggest that repeated PCP administration may be a useful inducing condition for eliciting cognitive deficits with relevance to schizophrenia.
Keywords: reward, schizophrenia, anhedonia, phencyclidine, clozapine, ICSS, impulsivity, rat
1. Introduction
Schizophrenia presents a grave public health problem that causes immense personal suffering and societal costs (McEvoy, 2007). Although the positive symptoms of the disorder, such as hallucinations and delusions, can be controlled by medications, the negative symptoms of schizophrenia do not respond well to currently available treatments. These symptoms include affective dysfunction, including emotional flattening and depression-like features, such as anhedonia (i.e., lack of pleasure derived from normally pleasurable experiences) and avolition (i.e., lack of motivation) (Andreasen, 1982). Cognitive deficits also are prominent and affect a wide range of cognitive modalities, including attention, response inhibition/impulsivity, processing speed, and cognitive flexibility (Laurent et al., 1999; Morice, 1990; Nelson et al., 1990; Wykes et al., 2000). The severity of cognitive deficits in schizophrenia is highly correlated with functional impairment and is a major predictor of long-term disability (Green et al., 2004; McGurk and Meltzer, 2000; Sharma and Antonova, 2003). Moreover, the depressive symptoms of schizophrenia likely contribute to the high suicide rate among schizophrenia patients (Meltzer, 2002).
Traditional medications, the so-called typical antipsychotics, do not ameliorate negative or cognitive schizophrenia symptoms, and in some cases aggravate these deficits (Bilder et al., 1992; Jibson and Tandon, 1998; Mortimer, 1997). While some studies show attenuation of negative and cognitive symptoms with the newer atypical antipsychotic compounds (Bilder et al., 2002; Kane et al., 1988; Meltzer and McGurk, 1999; Meltzer, 2002), these improvements remain partial and do not restore satisfactory normal functioning (Sharma and Antonova, 2003). An improved understanding of the neural bases of negative symptoms, as well as cognitive dysfunction, in schizophrenia is needed to develop treatments that are more effective than those currently available. The development and validation of translational animal models of these symptoms are vital in this endeavor.
Phencyclidine (PCP), a dissociative anesthetic that acts as a noncompetitive antagonist at N-methyl-D-aspartate (NMDA) glutamate receptors, has been used to induce schizophrenia-like symptoms in experimental animals. When administered to humans, PCP induces a psychosis-like state that mimics most major symptoms of schizophrenia, including affective and cognitive dysfunction (Javitt and Zukin, 1991). In experimental animals, PCP administration induces several behavioral disruptions with relevance to negative symptoms of schizophrenia, including social withdrawal (Sams-Dodd, 1999) and cognitive impairment (Handelmann et al., 1987; Abdul-Monim et al. 2003). Interestingly, various PCP-induced behavioral deficits are attenuated by atypical, but not typical, antipsychotic treatments (Abdul-Monim et al. 2003; Geyer and Ellenbroek, 2003), paralleling the partial responsiveness of negative and cognitive schizophrenia symptoms to atypical, but not typical, antipsychotics.
Specifically, clozapine, an atypical antipsychotic, partially ameliorates cognitive deficits in schizophrenia (Meltzer and McGurk, 1999; Sharma and Mockler, 1998). Affective flattening, anhedonia, and avolition in schizophrenia patients are also improved by clozapine treatment (Miller et al., 1994). We previously determined that chronic clozapine partially attenuates performance disruptions in a cognitive task induced by repeated administration of moderate doses of PCP to rats (Amitai et al. 2007). In the present study, we assessed whether this same PCP administration regimen induces reward deficits in a test of reward function in rats that may mimic aspects of the negative symptoms of schizophrenia. The intracranial self-stimulation (ICSS) discrete-trial threshold procedure allows for the assessment of brain reward function by determining the minimum current intensity, delivered to a brain site that is part of the brain reward circuit, that an animal perceives as rewarding (the “reward threshold”). The ICSS procedure also provides measures of response inhibition/impulsivity, task efficiency, and vigor of responding. (See Materials and Methods for details.) Further, we assessed how chronic treatment with the atypical antipsychotic medication clozapine alters the behavioral effects of repeated PCP administration in the ICSS task.
2. Materials and Methods
2.1. Animals
Twenty eight male Wistar rats (Charles River Laboratories, Wilmington, MA) were housed two per cage on a 12 h:12 h reverse light/dark schedule (lights off at 8:00 a.m.). Rats had ad libitum access to rodent chow and water at all times except during operant testing. All behavioral testing took place during the dark phase of the light/dark cycle. Rats were allowed to reach a body weight of at least 300 g before initiation of surgeries and behavioral training. All experiments were conducted in accordance with the guidelines of the American Association for the Accreditation of Laboratory Animal Care and the National Research Council’s Guide for Care and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use Committee.
2.2. Drugs
D-phencyclidine hydrochloride (PCP) was obtained from the National Institute on Drug Abuse (Bethesda, MD), dissolved in sterile 0.9% saline solution, and administered subcutaneously at a concentration of 2 ml/kg because irritation at the injection site has been observed with repeated PCP injections at 1 ml/kg, even when varying the injection site. Clozapine was generously provided by Novartis Pharma AG (Basel, Switzerland). Clozapine was dissolved in a small amount of 0.1 N hydrochloric acid (HCl) and diluted with saline; matched vehicle solution was prepared by dissolving the same amount of HCl in saline. Clozapine and vehicle were administered using two successive 7-day subcutaneous osmotic minipumps (Alzet, Palo Alto, CA). The limited solubility of clozapine at the concentration that was required to deliver 4 mg/kg/day in the maximal volume that could be contained in the minipumps prevented the use of a single 14-day osmotic minipump, and thus two successive 7-day minipumps were used.
2.3. Intracranial self-stimulation surgery
Rats were anesthetized with a 1-3% isoflurane/oxygen vapor mixture and prepared with 11 mm bipolar stainless-steel stimulation electrodes (model MS303/2, Plastics One, Roanoke, VA) into the posterior lateral hypothalamus (anterior/posterior: −0.5 mm, medial/lateral: 1.7 mm, dorsal/ventral: −8.3 mm from dura, with the incisor bar elevated 5 mm above the interaural line) according to the atlas of Pellegrino et al. (1979). Four indentations were made in the skull to accommodate screws that, together with the application of dental acrylic, held the electrode in place. Rats were allowed to recover for at least 3 days after surgery before any behavioral training commenced, and the surgical site was thoroughly inspected to ensure that the animal had recovered sufficiently to permit behavioral training.
2.4. Intracranial self-stimulation apparatus
ICSS training and testing took place in Plexiglas operant chambers (25 × 31 × 24 cm3; Med Associates, St. Albans, VT). The floors of the operant chambers were constructed of parallel aluminum rods spaced 1.25 cm apart. One wall contained a metal wheel manipulandum that required approximately 0.2 Newtons of force (equivalent to the application of a 20 gram weight to the manipulandum) to rotate one-quarter turn. The wheel (5 cm in width) extended 3 cm out from the wall. Each testing chamber was enclosed within a light- and sound-attenuated chamber (62 × 63 × 43 cm3; Med Associates, St. Albans, VT). Intracranial stimulation was delivered by constant current stimulators (Stimtech model 1200; San Diego Instruments, San Diego, CA). Animals were connected to the stimulation circuit through flexible bipolar leads (Plastics One, Roanoke, VA) attached to gold-contact swivel commutators (model SL2C; Plastics One, Roanoke, VA) mounted above the chamber. The stimulation parameters, data collection, and all test session functions were controlled by a computer.
2.5. Intracranial self-stimulation procedure
Reward function was assessed using the discrete-trial current-intensity ICSS procedure based on the procedure originally designed by Kornetsky et al. (1979) and described in detail by Markou and Koob (1992, 1993). Rats were placed in the operant testing chambers and connected via a flexible lead (Plastics One, Roanoke, VA) to the constant current stimulators. The animals were trained (Harrison et al., 2001) to perform operant responses on the wheel manipulandum for delivery of an electrical pulse delivered into the posterior lateral hypothalamus, an area of the brain’s reward circuitry. Above a current intensity threshold, individually determined for each subject, this stimulus is highly reinforcing.
Once stable operant responding for the electrical stimulus had been established, ICSS thresholds were assessed using the following procedure. At the beginning of each trial, rats received a noncontingent stimulus (0.01 μs rectangular cathodal pulses delivered at 100 Hz frequency over 500 ms). Rats were then given the opportunity during a 7.5 s limited hold period to respond on the wheel manipulandum. A quarter turn of the wheel manipulandum was considered a response, and resulted in the delivery of a contingent stimulus that was identical in all parameters to the previous noncontingent stimulus. An intertrial interval averaging 10 s (7.5-12.5 s) followed each response or response-free limited hold period. After completion of this intertrial interval period, a new trial began with the delivery of a new noncontingent stimulus.
By systematically varying the current intensity of the contingent and non-contingent stimulus, the ICSS threshold was determined for each individual subject. The current intensity of the stimuli was varied in steps of 5 μA in a pattern of alternating descending and ascending series of current intensities, with each current intensity presented in three consecutive trials (within each trial, all parameters of the stimulus were the same). Each set of three trials was considered to have provided a “positive” response if the rat responded in at least two out of the three trials in the set. Conversely, if the rat responded to less than two of the three trials in the set, this was considered a “negative” response, suggesting that this particular current intensity was not reinforcing. Two consecutive sets of “negative” trials resulted in termination of the descending series and initiation of the next ascending series. Similarly, two consecutive sets of “positive” trials resulted in the termination of the ascending series and the initiation of a descending series. During each test session, rats underwent two descending and two ascending trials, starting with the descending series and alternating between ascending and descending series. The first descending series started with the current intensity set 30-40 μA above the animal’s estimated average reward threshold; all subsequent series began with the current intensity set 5 μA below (ascending series) or above (descending series) the last stimulus delivered in the previous series. The rats’ reward threshold for a given series was defined as the average current intensity between the two last “positive” sets of trials in the series and the two “negative” sets of trials at the end of the series. The overall reward threshold for a test session was defined as the average of the reward threshold values for the two descending and the two ascending series completed in that session. Elevations in an animal’s reward thresholds indicated that stimulus intensities that were previously perceived as reinforcing were no longer perceived as rewarding, reflecting a decrease in reward function and suggesting an anhedonic or depression-like state. Conversely, lowering of reward thresholds reflected increased reward function.
To discourage responding on the manipulandum that was not time-linked to the presentation of the noncontingent stimulus, responding during the intertrial interval resulted in the resetting of the intertrial interval period to 12.5 s (a length of time that exceeded or was equal to the original random duration of the intertrial interval). These responses that were “penalized” by the re-initiation of the time-out period were recorded as timeout responses, and represented a measure of impulsivity-like disinhibition of responding. Excessive responses (i.e., additional quarter-turns) on the wheel manipulandum within 2 s after the initial response had no consequences and were recorded as extra responses.
Response latency was defined as the time in seconds that elapsed between the delivery of the noncontingent electrical stimulus (beginning of the stimulus) and the animal’s response on the wheel manipulandum. Average response latency for each test session was defined as the mean response latency of all trials for which the animal responded.
After training in this procedure, rats were tested daily until they had achieved stable baseline thresholds, defined as <10% variation in reward thresholds over 5 consecutive baseline days. The duration of each ICSS session was ~30 min.
2.6. Osmotic minipump implantation and removal
Rats were anesthetized with a 1-3% isoflurane/oxygen vapor mixture, and an osmotic minipump (Alzet model 2ML1 7 day pump, Alza Corporation, Palo Alto, CA, USA) was inserted subcutaneously on the back of the animal parallel to the spine, with the flow moderator directed posteriorly. The wound was stapled, and an antibacterial ointment was applied to the incision area. On day 7, the first minipump was removed, and a second minipump was inserted under anesthesia contralateral to the first minipump (see above). On day 14, the second minipump was removed. For minipump removal, an incision was made under anesthesia, the minipump was removed, the wound was closed with surgical staples, and an antibacterial ointment was applied.
2.7. Experimental design
After establishment of stable baseline thresholds in the ICSS procedure, rats received two initial subcutaneous injections of 2 mg/kg PCP 30 min before ICSS testing, 24 h apart. Rats then were assigned to two groups that did not differ in their average reward thresholds, extra responses, timeout responses, and response latencies both under baseline conditions and during this initial PCP exposure. This group-matching procedure was necessitated by the considerable variability in responsiveness to performance disruptions induced by PCP that cannot be predicted from an animal’s baseline performance. Balancing the groups based on both their performance in the absence of PCP and during PCP exposure ensured that differential responsiveness to PCP would not confound the results after antipsychotic treatment.
One group was prepared with two consecutive 7-day osmotic minipumps delivering 4 mg/kg/day clozapine (salt); the other group received minipumps containing vehicle (n = 14 per group). This clozapine regimen was chosen based on previous findings demonstrating that this dose and treatment duration of clozapine successfully attenuated some of the cognitive deficits induced by repeated PCP administration, measured using the 5-choice serial reaction time test (Amitai et al., 2007). A similar clozapine regimen (6 mg/kg/day for 2 weeks) also attenuated reward deficits associated with amphetamine or nicotine withdrawal, assessed in the ICSS procedure, while inducing little alteration of ICSS performance under baseline conditions (Semenova and Markou, 2003).
During the initial 9 days of minipump exposure, rats received repeated saline injections 30 min before ICSS testing to habituate them to the injection procedure. After 9 days of minipump exposure, rats received five consecutive daily injections of 2 mg/kg PCP 30 min before ICSS testing while minipump exposure continued. This repeated PCP administration regimen was chosen based on previous studies in our laboratory, which indicated that a single injection of PCP produced a general, nonspecific suppression of responding that partially or completely occluded the quantification of specific cognitive and other deficits with relevance to schizophrenia. In contrast, repeated PCP administration according to the regimen outlined here induced robust and selective cognitive deficits that were sensitive to attenuation with chronic clozapine treatment (Amitai et al. 2007).
Overall, this design allowed for the comparison of the effects of PCP vs. those of saline (within-subjects factor) and the effects of clozapine vs. vehicle (between-subjects factor), with all factorial combinations (vehicle/saline, vehicle/PCP, clozapine/saline, clozapine/PCP) explored. The mixed within/between-subjects design was necessitated by the long training times required by the task and the large numbers of animals needed in each group to reduce the considerable variation in the behavioral effects of PCP that is commonly observed.
On day 14 of clozapine/vehicle exposure, minipumps were removed, and rats continued to be tested daily for 10 days to assess whether performance in the ICSS task returned to baseline (see Fig. 1 for a diagram of the experimental design). This design allowed the assessment of the efficacy of an atypical antipsychotic medication in preventing PCP effects, paralleling the prevention of recurrence of a psychotic episode in schizophrenia patients by antipsychotic treatment.
Fig. 1.

Diagram of experimental design. The black underline indicates the period of minipump treatment. ↑ denotes a subcutaneous PCP injection.
Previous studies in our laboratory found no significant alterations in ICSS performance in animals prepared with vehicle minipumps and/or given repeated saline injections over periods of several months (e.g., Lin et al., 1999; Semenova and Markou, 2003; Spielewoy and Markou, 2003). Therefore, any changes in ICSS performance observed in this study after PCP administration are likely due to the actions of the drug, and not to the effects of the injection procedure or the implantation and removal of the minipumps. Furthermore, subjects in any experimental group that showed increased variability in their ICSS performance after minipump implantation were excluded from all analyses (see below).
2.8. Data analyses
Reward threshold and latency data were expressed as percent of baseline, with baseline performance defined as the average performance on the 5 days preceding the first PCP administration. Extra responses and timeout responses were expressed as difference from baseline values (obtained during the last 5 baseline days before PCP administration) because of large individual differences in baseline extra and timeout responses that greatly affect the variability of data when expressed as percent scores. Animals exhibiting more than 10% alteration in reward thresholds after minipump implantation were excluded from all analyses because these animals were considered unstable. Overall, four animals were excluded from the clozapine group and three from the vehicle group, resulting in 10 rats in the clozapine group and 11 rats in the vehicle group.
To assess the effects of the initial two PCP injections before minipump implantation, average values from the 5 days preceding any drug treatment (baseline days) were compared with performance on the 2 days of initial PCP administration. Data were analyzed using one-way repeated-measures analysis of variance (ANOVA), with Baseline/PCP as the within-subjects factor. To assess the effects of repeated PCP administration during minipump exposure, average values from the 9 days of PCP-free minipump treatment were compared with individual values from each of the 5 days of PCP administration during minipump treatment. Data were analyzed using two-way mixed-design ANOVA, with Clozapine/Vehicle as the between-subjects factor and Saline/PCP as the within-subjects factor. When significant effects were found in the ANOVA, post hoc comparisons among means were conducted using Bonferroni tests. The significance level was set at 0.05. Data were analyzed using GraphPad Prism software (GraphPad, San Diego, CA).
3. Results
Baseline values (i.e., average performance on the 5 days preceding the first PCP administration) for the behavioral measures (mean ± S.E.M.) were the following: baseline reward thresholds: 114.41 μA ± 10.05; baseline extra responses: 43.43 ± 9.77; baseline timeout responses: 22.01 ± 3.51; baseline response latencies: 3.20 s ± 0.09. Rats were assigned to clozapine- or saline-treated groups such that no differences were observed among groups in any of these measures.
3.1. Initial PCP injections
The initial two PCP injections significantly elevated reward thresholds [main effect of PCP: F(2, 62) = 10.44, P < 0.001]. Post hoc testing revealed that the first (P < 0.001), but not the second, PCP injection significantly elevated reward thresholds above baseline values (Fig. 2). Extra responses were not affected by the initial two PCP injections (Fig. 3). A significant effect of the initial two PCP injections on timeout responses was detected [F(2, 62) = 3.76, P < 0.05]. This effect was attributable to a significant difference between timeout responses after the first initial PCP injection, after which timeout responses were slightly suppressed, and the second initial PCP injection, after which timeout responses were slightly increased; however, none of these effects reached significance compared with baseline values (Fig. 4). The initial two PCP injections also significantly increased response latencies [F(2, 62) = 14.04, P < 0.0001] after both PCP injections (P < 0.01; data not shown).
Fig. 2.
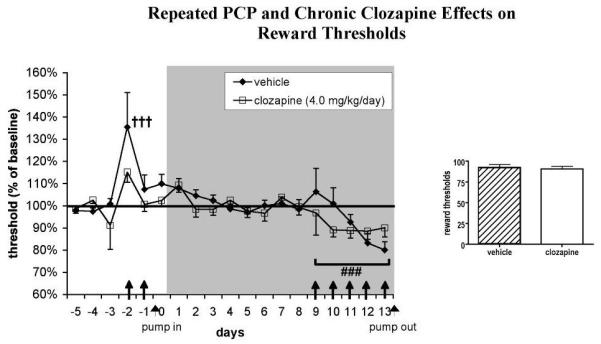
Effects of chronic clozapine and repeated PCP administration on ICSS reward thresholds (n = 10-11 per group). Values (mean ± S.E.M.) are expressed as percent of baseline reward thresholds (average threshold values on the 5 days preceding the first PCP administration). †††P < 0.001, statistically significant difference compared with baseline reward thresholds; ###P < 0.001, statistically significant difference compared with performance during minipump exposure in the absence of PCP administration. The gray shaded area indicates the period of minipump treatment. ↑ denotes a subcutaneous PCP injection.
Fig. 3.
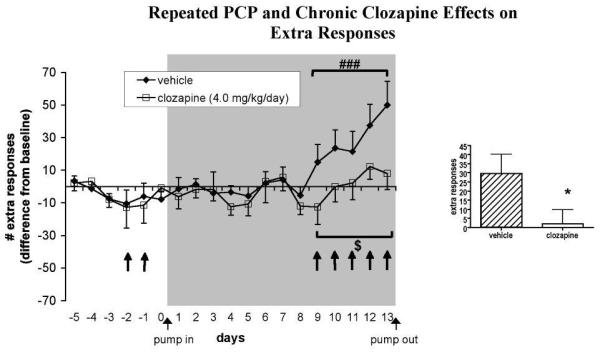
Effects of chronic clozapine and repeated PCP administration on ICSS extra responses (n = 10-11 per group). Values (mean ± S.E.M.) are expressed as difference scores compared with baseline (average number of extra responses on the 5 days preceding the first PCP administration). ###P < 0.001, statistically significant difference compared with performance during minipump exposure in the absence of PCP administration; $P = 0.052, trend toward difference compared with vehicle-treated group; *P < 0.05, statistically significantly difference compared with vehicle-treated group. The gray shaded area indicates the period of minipump treatment. ↑ denotes a subcutaneous PCP injection.
Fig. 4.
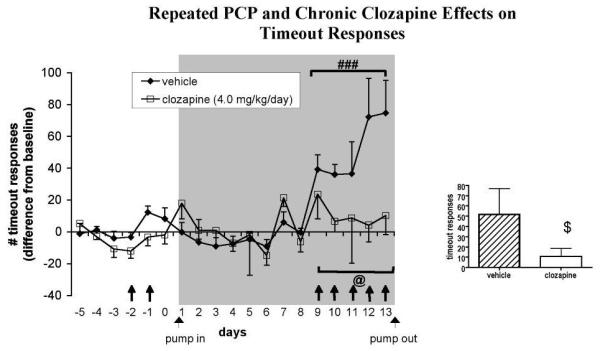
Effects of chronic clozapine and repeated PCP administration on ICSS timeout responses (n = 10-11 per group). Values (mean ± S.E.M.) are expressed as difference scores compared with baseline (average number of timeout responses on the 5 days preceding the first PCP administration). ###P < 0.001, statistically significant difference compared with performance during minipump exposure in the absence of PCP administration; @P = 0.068, trend toward Clozapine × PCP interaction; $P = 0.076, trend toward difference compared with vehicle-treated group. The gray shaded area indicates the period of minipump treatment. ↑ denotes a subcutaneous PCP injection.
3.2. Clozapine/vehicle pretreatment and repeated PCP administration
A two-way mixed-design ANOVA revealed that PCP significantly lowered reward thresholds compared with thresholds after saline [main effect of PCP: F(5, 95) = 4.56, P < 0.001]. No effect of Clozapine and no Clozapine × PCP interaction were detected (Fig. 2).
PCP also increased extra responses [main effect of PCP: F(5, 95) = 7.03, P < 0.0001]. A trend toward a significant main effect of Clozapine was observed [F(1, 95) = 4.34, P = 0.052], suggesting that the increase in extra responding after PCP was attenuated in clozapine-treated rats, although the Clozapine × PCP interaction did not reach significance. Comparison of average values from the 5 days of PCP administration during minipump treatment using a one-tailed t-test showed that clozapine-treated rats made significantly fewer extra responses than vehicle-treated rats (P < 0.05; Fig. 3).
Furthermore, PCP administration increased timeout responses [main effect of PCP: F(5, 95) = 2.31, P < 0.05]. No significant main effect of Clozapine was detected, but a trend toward a significant Clozapine × PCP interaction was observed [F(5, 95) = 2.14, P = 0.068], indicating that clozapine-treated rats exhibited a smaller increase in timeout responding in response to PCP than did vehicle-treated rats. Comparison of average values from the 5 days of PCP administration during minipump treatment using a one-tailed t-test showed a trend toward clozapine-treated rats performing fewer timeout responses than vehicle-treated rats (P = 0.076; Fig. 4).
Response latencies were decreased during PCP administration [main effect of PCP: F(5, 95) = 2.33, P < 0.05]. No effect of Clozapine and no Clozapine × PCP interaction were observed (data not shown).
4. Discussion
Repeated PCP administration induced a small, but significant, lowering of reward thresholds measured by the ICSS discrete-trial threshold procedure. Chronic clozapine did not affect these PCP-induced changes in reward thresholds. Importantly, repeated PCP strongly increased measures of inappropriate responding in the ICSS procedure, an effect that was attenuated by chronic clozapine pretreatment.
4.1. Reward thresholds
A single initial PCP injection significantly elevated reward thresholds (Fig. 2), indicating an anhedonic-like effect reflected in diminished interest in the rewarding electrical stimuli. During the later injections, PCP lowered reward thresholds, indicating enhanced reward function after PCP administration. This effect was relatively small but statistically significant (Fig. 2). In addition, the response latencies increased during the initial PCP injections and decreased during the later injections, indicating early locomotor disruptive and/or sedative effects of PCP, followed by later motor-enhancing effects. Initial motor-impairing effects of PCP that resolve with repeated administration have been detected in our laboratory in other tasks (Amitai et al., 2007). However, it is unlikely that the threshold alterations seen during PCP administration were due to these nonspecific locomotor effects, as previous validation work showed that manipulations that affect motor function alter response latencies without affecting thresholds in this procedure (Harrison et al., 2001; Markou and Koob, 1992).
One possible interpretation is that the first initial PCP injection leads to a variety of effects, including mild dysphoria reflected in the small threshold elevation. However, with subsequent injections, rapid tolerance to this early dysphoric effect may occur, and enhanced reward may emerge with subsequent PCP injections. Previous studies in our laboratory found that PCP administered chronically via subcutaneous minipumps significantly lowered ICSS thresholds (Spielewoy and Markou, 2003). Furthermore, other researchers observed that conditioned place aversion after a single PCP injection was abolished (Iwamoto, 1986) or actually converted into conditioned place preference (Kitaichi et al., 1996) after repeated treatment with PCP. Thus, repeated or chronic administration of PCP may lead to tolerance to its aversive effects and/or potentiation of its rewarding effects.
Variable effects of PCP injections on reward function may also be related to the specific dose administered. Bespalov and colleagues (1999) detected a trend toward lower reward thresholds after administration of 3.2 mg/kg PCP, but a trend towards elevated thresholds after 5.6 mg/kg PCP, in an ICSS progressive-ratio procedure. Similarly, our group observed a trend toward lowered ICSS thresholds with single injections of low doses of PCP (0.3-0.9 mg/kg); an intermediate dose of 1.3 mg/kg of PCP did not affect reward thresholds, and single injections of higher doses (2 or 2.5 mg/kg) elevated reward thresholds (present study; C. Spielewoy and A. Markou, unpublished observations). These findings suggest that lower PCP doses may lead to reward enhancement while higher doses may lead to a negative affective state. While other researchers reported reward facilitation after PCP injections at doses as high as 5 mg/kg (Carlezon and Wise, 1993; Wise et al., 1992), these studies used the ICSS rate-frequency function, an ICSS procedure in which stimulus intensity is determined by the experimenter, and not by the rats themselves as in the previously discussed studies (including the one presented here). The ability of the subject to control the delivery of a stimulus has been shown to have the potential to profoundly alter the consequences of these stimuli (Dworkin et al., 1995; Weiss, 1970). The different responses to higher doses of PCP in the Carlezon and Wise studies may depend, therefore, on the different levels of stimulus control in the ICSS procedures.
Furthermore, the time after injection appears to play an important role in the variable effects of PCP. In the studies outlined above, testing was performed 40 min or less after PCP administration. In contrast, robust elevations of ICSS thresholds were found 8, 12, or 24 h after PCP administration (Spielewoy and Markou, 2003).
Chronic clozapine treatment did not affect the threshold alterations induced by repeated PCP in our study. This lack of effect was not likely due to an insufficient clozapine dose. In a previous study, this clozapine regimen successfully attenuated cognitive deficits induced by repeated PCP administration in the 5-choice serial reaction time test (Amitai et al., 2007). This dose and treatment time are therefore behaviorally active, although the possibility that higher clozapine doses may be effective in reversing PCP-induced changes in reward function cannot be completely ruled out.
Few studies have explored the effect of antipsychotic treatment on PCP-induced alterations of reward function. One study found that PCP-induced reduction in reward function was aggravated by acute clozapine and ameliorated by subchronic clozapine treatment (Turgeon and Hulick, 2007). The considerable differences in tasks used to assess reward function (sucrose consumption in the previous study vs. ICSS thresholds in our study), PCP dose (15 mg/kg vs. 2 mg/kg), and elapsed time between PCP administration and behavioral testing (20 h vs. 30 min) may explain why effects of clozapine treatment on PCP-induced reward alterations were seen in the previous study, but not in the present one. Furthermore, dopaminergic mechanisms are importantly involved in reward function (Fiorillo et al., 2003; Schultz, 1998; Wise, 2004). While clozapine exhibits significant binding at the dopamine D2-type receptor, it has lower affinity for this receptor, and dissociates more quickly from it, than so-called typical antipsychotics (Seeman and Tallerico, 1999). Insufficient occupancy of D2 receptors may therefore play a role in the lack of effect of clozapine on reward thresholds. Indeed, high-affinity antagonists of dopamine D1 and/or D2 receptors block PCP-induced conditioned place aversion (Acquas et al., 1989; Iwamoto, 1986). Investigations of the effects of typical antipsychotics with high affinity for dopamine receptors, such as haloperidol, on PCP-induced reward threshold changes in our model may therefore be of interest. However, it should be noted that in clinical practice, typical antipsychotics do not effectively ameliorate depression-like negative schizophrenia symptoms such as anhedonia (Jibson and Tandon, 1998).
Repeated PCP administration lowered reward thresholds, suggesting that the repeated PCP regimen utilized in the present study does not constitute an adequate inducing condition to produce schizophrenia-like negative affect, such as anhedonia. Notably, most translational animal models of psychiatric or other human disorders do not model all aspects of a disorder. More commonly, specific symptoms or endophenotypes are modeled (Geyer and Markou, 1995; Gray and Hannan, 2007). In the case of an extremely complex syndrome, such as schizophrenia, any one manipulation effectively inducing all facets of the disorder is unlikely. Thus, the fact that the repeated PCP administration regimen used in the present study does not mimic the affective schizophrenia symptoms does not invalidate the usefulness of this PCP administration regimen in inducing cognitive deficits exhibited in schizophrenia (Amitai et al., 2007).
4.2. Extra and timeout responses
Interestingly, the repeated PCP administration regimen used in the present study impaired efficient performance of the ICSS task by disinhibiting both unpunished (extra) and punished (timeout) responses (Fig. 3 and 4). This inability to withhold inappropriate responses resembles the cognitive impulsivity exhibited by schizophrenia patients in cognitive tasks such as the go/no go task (Weisbrod et al., 2000; Wykes et al., 2000). Impaired response inhibition and elevated impulsivity after PCP administration is observed in a variety of cognitive tasks in rats and primates (Amitai et al., 2007; Compton et al., 2001; Jentsch et al., 2000; Le Pen et al., 2003; Sanger and Jackson, 1989). Notably, the response disinhibition observed here followed the same profile as the PCP-induced impulsivity observed previously using the same PCP regimen in the 5-choice serial reaction time task (Amitai et al., 2007). An initial suppression of responding after the first injection was followed by increased levels of inappropriate responding that emerged with repeated injections.
Chronic clozapine treatment attenuated the increase in extra responses in the ICSS task induced by repeated PCP and tended to ameliorate the PCP-induced increase in timeout responses. These results mirror the significant attenuation of PCP-induced impulsivity in the 5-choice serial reaction time task previously reported by our laboratory (Amitai et al., 2007). We thus replicated in two different and quite distinct behavioral tasks the finding that repeated PCP administration induces schizophrenia-like impairments of response inhibition that are sensitive to attenuation by chronic clozapine treatment. Clozapine has been shown to ameliorate the cognitive deficits observed in schizophrenia (Meltzer and McGurk, 1999; Sharma and Mockler, 1998). Thus, the presently used repeated PCP administration regimen constitutes a valid inducing condition that can induce schizophrenia-like cognitive deficits in experimental animals that have predictive validity (Geyer and Markou, 1995). One possible mechanism underlying clozapine’s effectiveness on PCP-induced impulsivity is its strong antagonism of serotonin 5-HT2A receptors. Serotonin neurotransmission has been suggested to play a critical role in impulsivity (Harrison et al., 1997; Winstanley et al., 2003). Selective 5-HT2A receptor antagonists decrease impulsive responding in cognitive tasks under baseline conditions or after NMDA receptor antagonist administration (Higgins et al., 2003; Fletcher et al., 2007).
Attenuation of PCP-induced cognitive impulsivity by clozapine was also observed in a primate study (Jentsch et al., 1997; 2000), although another rat study found no such effects (Compton et al., 2001). However, in the Compton study, clozapine was administered acutely. Chronic administration is required to observe beneficial effects of clozapine in some behavioral paradigms, while acute administration is ineffective (Amitai et al., 2007; Semenova and Markou, 2003). Therefore, the Compton study may not have observed attenuation of PCP-induced impulsivity because clozapine was not administered chronically, a possibility proposed by the authors themselves.
4.3. Conclusions
Although the repeated PCP administration regimen used in the present study did not produce anhedonia-like elevations of reward thresholds in the ICSS discrete-trial threshold procedure, it reduced task efficiency and induced disinhibition of inappropriate responses in a manner that resembled the deficits seen in schizophrenia patients. Chronic clozapine, an atypical antipsychotic with limited effectiveness on cognitive schizophrenia symptoms (Meltzer and McGurk, 1999; Sharma and Mockler, 1998), attenuated some of these impairments and tended to attenuate others. Hence, the repeated PCP administration regimen described here constitutes a useful inducing condition in models of cognitive deficits in schizophrenia.
Acknowledgements
Supported by National Institute of Mental Health grant MH062527 to AM and by Tobacco-Related Disease Research Program Individual Pre-doctoral Fellowship 15DT-0048 from the State of California to NA. The authors would like to thank Ms. Kim Edwards for technical assistance, Mr. Mike Arends for editorial assistance, and Dr. Daniel Hoyer from Novartis Pharma AG for providing us with clozapine.
References
- Abdul-Monim Z, Reynolds GP, Neill JC. The atypical antipsychotic ziprasidone, but not haloperidol, improves phencyclidine-induced cognitive deficits in a reversal learning task in the rat. J. Psychopharmacol. 2003;17:57–65. doi: 10.1177/0269881103017001700. [DOI] [PubMed] [Google Scholar]
- Acquas E, Carboni E, Leone P, Di Chiara G. SCH 23390 blocks drug-conditioned place-preference and place-aversion: anhedonia (lack of reward) or apathy (lack of motivation) after dopamine-receptor blockade? Psychopharmacology (Berl.) 1989;99:151–155. doi: 10.1007/BF00442800. [DOI] [PubMed] [Google Scholar]
- Amitai N, Semenova S, Markou A. Cognitive-disruptive effects of the psychotomimetic phencyclidine and attenuation by atypical antipsychotic medications in rats. Psychopharmacology (Berl.) 2007;193:521–537. doi: 10.1007/s00213-007-0808-x. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. Negative symptoms in schizophrenia: definition and reliability. Arch. Gen. Psychiatry. 1982;39:784–788. doi: 10.1001/archpsyc.1982.04290070020005. [DOI] [PubMed] [Google Scholar]
- Barr GA, Paredes W, Bridger WH. Place conditioning with morphine and phencyclidine: dose-dependent effects. Life Sci. 1985;36:363–368. doi: 10.1016/0024-3205(85)90122-5. [DOI] [PubMed] [Google Scholar]
- Bespalov A, Lebedev A, Panchenko G, Zvartau E. Effects of abused drugs on thresholds and breaking points of intracranial self-stimulation in rats. Eur. Neuropsychopharmacol. 1999;9:377–383. doi: 10.1016/s0924-977x(99)00008-5. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Goldman RS, Volavka J, Czobor P, Hoptman M, Sheitman B, Lindenmayer JP, Citrome L, McEvoy J, Kunz M, Chakos M, Cooper TB, Horowitz TL, Lieberman JA. Neurocognitive effects of clozapine, olanzapine, risperidone, and haloperidol in patients with chronic schizophrenia or schizoaffective disorder. Am. J. Psychiatry. 2002;159:1018–1028. doi: 10.1176/appi.ajp.159.6.1018. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Lieberman JA, Kim Y, Alvir JM, Reiter G. Methylphenidate and neuroleptic effects on oral word production in schizophrenia. Neuropsychiatry Neuropsychol. Behav. Neurol. 1992;5:262–271. [Google Scholar]
- Carlezon WA, Jr., Wise RA. Phencyclidine-induced potentiation of brain stimulation reward: acute effects are not altered by repeated administration. Psychopharmacology (Berl.) 1993;111:402–408. doi: 10.1007/BF02253528. [DOI] [PubMed] [Google Scholar]
- Compton AD, Slemmer JE, Drew MR, Hyman JM, Golden KM, Balster RL, Wiley JL. Combinations of clozapine and phencyclidine: effects on drug discrimination and behavioral inhibition in rats. Neuropharmacology. 2001;40:289–297. doi: 10.1016/s0028-3908(00)00126-x. [DOI] [PubMed] [Google Scholar]
- Dworkin SI, Mirkis S, Smith JE. Response-dependent versus response-independent presentation of cocaine: differences in the lethal effects of the drug. Psychopharmacology (Berl.) 1995;117:262–266. doi: 10.1007/BF02246100. [DOI] [PubMed] [Google Scholar]
- Fiorillo CD, Tobler PN, Schultz W. Discrete coding of reward probability and uncertainty by dopamine neurons. Science. 2003;299:1898–1902. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Tampakeras M, Sinyard J, Higgins GA. Opposing effects of 5-HT(2A) and 5-HT(2C) receptor antagonists in the rat and mouse on premature responding in the five-choice serial reaction time test. Psychopharmacology (Berl.) 2007;195:223–234. doi: 10.1007/s00213-007-0891-z. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Ellenbroek B. Animal behavior models of the mechanisms underlying antipsychotic atypicality. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2003;27:1071–1079. doi: 10.1016/j.pnpbp.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Markou A. Animal models of psychiatric disorders. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The Fourth Generation of Progress. Raven Press; New York: 1995. pp. 787–798. [Google Scholar]
- Gray L, Hannan AJ. Dissecting cause and effect in the pathogenesis of psychiatric disorders: genes, environment and behaviour. Curr. Mol. Med. 2007;7:470–478. doi: 10.2174/156652407781387064. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Heaton RK. Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophr. Res. 2004;72:41–51. doi: 10.1016/j.schres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Handelmann GE, Contreras PC, O’Donohue TL. Selective memory impairment by phencyclidine in rats. Eur. J. Pharmacol. 1987;140:69–73. doi: 10.1016/0014-2999(87)90635-2. [DOI] [PubMed] [Google Scholar]
- Harrison AA, Everitt BJ, Robbins TW. Central 5-HT depletion enhances impulsive responding without affecting the accuracy of attentional performance: interactions with dopaminergic mechanisms. Psychopharmacology (Berl.) 1997;133:329–342. doi: 10.1007/s002130050410. [DOI] [PubMed] [Google Scholar]
- Harrison AA, Liem YT, Markou A. Fluoxetine combined with a serotonin-1A receptor antagonist reversed reward deficits observed during nicotine and amphetamine withdrawal in rats. Neuropsychopharmacology. 2001;25:55–71. doi: 10.1016/S0893-133X(00)00237-2. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Enderlin M, Haman M, Fletcher PJ. The 5-HT2A receptor antagonist M100,907 attenuates motor and impulsive-type behaviours produced by NMDA receptor antagonism. Psychopharmacology (Berl.) 2003;170:309–319. doi: 10.1007/s00213-003-1549-0. [DOI] [PubMed] [Google Scholar]
- Iwamoto ET. Place-aversion conditioned by phencyclidine in rats: development of tolerance and pharmacological antagonism. Alcohol Drug Res. 1986;6:265–276. [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am. J. Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Jibson MD, Tandon R. New atypical antipsychotic medications. J. Psychiatr. Res. 1998;32:215–228. doi: 10.1016/s0022-3956(98)00023-5. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Redmond DE, Jr., Elsworth JD, Taylor JR, Youngren KD, Roth RH. Enduring cognitive deficits and cortical dopamine dysfunction in monkeys after long-term administration of phencyclidine. Science. 1997;277:953–955. doi: 10.1126/science.277.5328.953. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Roth RH, Taylor JR. Object retrieval/detour deficits in monkeys produced by prior subchronic phencyclidine administration: evidence for cognitive impulsivity. Biol. Psychiatry. 2000;48:415–424. doi: 10.1016/s0006-3223(00)00926-4. [DOI] [PubMed] [Google Scholar]
- Kane JM, Honigfeld G, Singer J, Meltzer H. Clozapine in treatment-resistant schizophrenics. Psychopharmacol. Bull. 1988;24:62–67. [PubMed] [Google Scholar]
- Kitaichi K, Noda Y, Hasegawa T, Furukawa H, Nabeshima T. Acute phencyclidine induces aversion, but repeated phencyclidine induces preference in the place conditioning test in rats. Eur. J. Pharmacol. 1996;318:7–9. doi: 10.1016/s0014-2999(96)00875-8. [DOI] [PubMed] [Google Scholar]
- Kornetsky C, Esposito RU, McLean S, Jacobson JO. Intracranial self-stimulation thresholds: a model for the hedonic effects of drugs of abuse. Arch. Gen. Psychiatry. 1979;36:289–292. doi: 10.1001/archpsyc.1979.01780030055004. [DOI] [PubMed] [Google Scholar]
- Laurent A, Saoud M, Bougerol T, D’Amato T, Anchisi AM, Biloa-Tang M, Dalery J, Rochet T. Attentional deficits in patients with schizophrenia and in their non-psychotic first-degree relatives. Psychiatry Res. 1999;89:147–159. doi: 10.1016/s0165-1781(99)00109-2. [DOI] [PubMed] [Google Scholar]
- Le Pen G, Grottick AJ, Higgins GA, Moreau JL. Phencyclidine exacerbates attentional deficits in a neurodevelopmental rat model of schizophrenia. Neuropsychopharmacology. 2003;28:1799–1809. doi: 10.1038/sj.npp.1300208. [DOI] [PubMed] [Google Scholar]
- Lin D, Koob GF, Markou A. Differential effects of withdrawal from chronic amphetamine or fluoxetine administration on brain stimulation reward in the rat: interactions between the two drugs. Psychopharmacology (Berl.) 1999;145:283–294. doi: 10.1007/s002130051060. [DOI] [PubMed] [Google Scholar]
- Markou A, Koob GF. Construct validity of a self-stimulation threshold paradigm: effects of reward and performance manipulations. Physiol. Behav. 1992;51:111–119. doi: 10.1016/0031-9384(92)90211-j. [DOI] [PubMed] [Google Scholar]
- Markou A, Koob GF. Intracranial self-stimulation thresholds as a measure of reward. In: Sahgal A, editor. Behavioural Neuroscience: A Practical Approach. Vol. 2. IRL Press; Oxford: 1993. pp. 93–115. [Google Scholar]
- McEvoy JP. The costs of schizophrenia. J. Clin. Psychiatry. 2007;68(Suppl. 14):4–7. [PubMed] [Google Scholar]
- McGurk SR, Meltzer HY. The role of cognition in vocational functioning in schizophrenia. Schizophr. Res. 2000;45:175–184. doi: 10.1016/s0920-9964(99)00198-x. [DOI] [PubMed] [Google Scholar]
- Meltzer HY. Suicidality in schizophrenia: a review of the evidence for risk factors and treatment options. Curr. Psychiatry Rep. 2002;4:279–283. doi: 10.1007/s11920-996-0047-6. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, McGurk SR. The effects of clozapine, risperidone, and olanzapine on cognitive function in schizophrenia. Schizophr. Bull. 1999;25:233–255. doi: 10.1093/oxfordjournals.schbul.a033376. [DOI] [PubMed] [Google Scholar]
- Miller DD, Perry PJ, Cadoret RJ, Andreasen NC. Clozapine’s effect on negative symptoms in treatment-refractory schizophrenics. Compr. Psychiatry. 1994;35:8–15. doi: 10.1016/0010-440x(94)90164-3. [DOI] [PubMed] [Google Scholar]
- Morice R. Cognitive inflexibility and pre-frontal dysfunction in schizophrenia and mania. Br. J. Psychiatry. 1990;157:50–54. doi: 10.1192/bjp.157.1.50. [DOI] [PubMed] [Google Scholar]
- Mortimer AM. Cognitive function in schizophrenia: do neuroleptics make a difference? Pharmacol. Biochem. Behav. 1997;56:789–795. doi: 10.1016/s0091-3057(96)00425-x. [DOI] [PubMed] [Google Scholar]
- Nelson HE, Pantelis C, Carruthers K, Speller J, Baxendale S, Barnes TRE. Cognitive functioning and symptomatology in chronic schizophrenia. Psychol. Med. 1990;20:357–365. doi: 10.1017/s0033291700017670. [DOI] [PubMed] [Google Scholar]
- Pellegrino LJ, Pellegrino AS, Cushman AJ. A Stereotaxic Atlas of the Rat Brain. 2nd edn. Plenum Press; New York: 1979. [Google Scholar]
- Sams-Dodd F. Phencyclidine in the social interaction test: an animal model of schizophrenia with face and predictive validity. Rev. Neurosci. 1999;10:59–90. doi: 10.1515/revneuro.1999.10.1.59. [DOI] [PubMed] [Google Scholar]
- Sanger DJ, Jackson A. Effects of phencyclidine and other N-methyl-D-aspartate antagonists on the schedule-controlled behavior of rats. J. Pharmacol. Exp. Ther. 1989;248:1215–1221. [PubMed] [Google Scholar]
- Seeman P, Tallerico T. Rapid release of antipsychotic drugs from dopamine D2 receptors: an explanation for low receptor occupancy and early clinical relapse upon withdrawal of clozapine or quetiapine. Am. J. Psychiatry. 1999;156:876–884. doi: 10.1176/ajp.156.6.876. [DOI] [PubMed] [Google Scholar]
- Schaefer GJ, Michael RP. Interactions of naloxone with morphine, amphetamine and phencyclidine on fixed interval responding for intracranial self-stimulation in rats. Psychopharmacology (Berl.) 1990;102:263–268. doi: 10.1007/BF02245931. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J. Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Semenova S, Markou A. Clozapine treatment attenuated somatic and affective signs of nicotine and amphetamine withdrawal in subsets of rats exhibiting hyposensitivity to the initial effects of clozapine. Biol. Psychiatry. 2003;54:1249–1264. doi: 10.1016/s0006-3223(03)00240-3. [DOI] [PubMed] [Google Scholar]
- Sharma T, Antonova L. Cognitive function in schizophrenia: deficits, functional consequences, and future treatment. Psychiatr. Clin. North Am. 2003;26:25–40. doi: 10.1016/s0193-953x(02)00084-9. [DOI] [PubMed] [Google Scholar]
- Sharma T, Mockler D. The cognitive efficacy of atypical antipsychotics in schizophrenia. J. Clin. Psychopharmacol. 1998;18(2 Suppl. 1):12S–19S. doi: 10.1097/00004714-199804001-00004. [DOI] [PubMed] [Google Scholar]
- Spielewoy C, Markou A. Withdrawal from chronic phencyclidine treatment induces long-lasting depression in brain reward function. Neuropsychopharmacology. 2003;28:1106–1116. doi: 10.1038/sj.npp.1300124. [DOI] [PubMed] [Google Scholar]
- Tamlyn D, McKenna PJ, Mortimer AM, Lund CE, Hammond S, Baddeley AD. Memory impairment in schizophrenia: its extent, affiliations and neuropsychological character. Psychol. Med. 1992;22:101–115. doi: 10.1017/s0033291700032773. [DOI] [PubMed] [Google Scholar]
- Turgeon SM, Hulick VC. Differential effects of acute and subchronic clozapine and haloperidol on phencyclidine-induced decreases in voluntary sucrose consumption in rats. Pharmacol. Biochem. Behav. 2007;86:524–530. doi: 10.1016/j.pbb.2007.01.014. [DOI] [PubMed] [Google Scholar]
- Weisbrod M, Kiefer M, Marzinzik F, Spitzer M. Executive control is disturbed in schizophrenia: evidence from event-related potentials in a Go/NoGo task. Biol. Psychiatry. 2000;47:51–60. doi: 10.1016/s0006-3223(99)00218-8. [DOI] [PubMed] [Google Scholar]
- Weiss JM. Somatic effects of predictable and unpredictable shock. Psychonom. Med. 1970;32:397–408. doi: 10.1097/00006842-197007000-00008. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Chudasama Y, Dalley JW, Theobald DE, Glennon JC, Robbins TW. Intra-prefrontal 8-OH-DPAT and M100907 improve visuospatial attention and decrease impulsivity on the five-choice serial reaction time task in rats. Psychopharmacology (Berl.) 2003;167:304–314. doi: 10.1007/s00213-003-1398-x. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat. Rev. Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bauco P, Carlezon WA, Jr., Trojniar W. Self-stimulation and drug reward mechanisms. Ann. N. Y. Acad. Sci. 1992;654:192–198. doi: 10.1111/j.1749-6632.1992.tb25967.x. [DOI] [PubMed] [Google Scholar]
- Wykes T, Reeder C, Corner J. The prevalence and stability of an executive processing deficit, response inhibition, in people with chronic schizophrenia. Schizophr. Res. 2000;46:241–253. doi: 10.1016/s0920-9964(99)00233-9. [DOI] [PubMed] [Google Scholar]


