Abstract
Objective
Electroencephalographic (EEG) neurofeedback training has been shown to produce plastic modulations in salience network and default mode network functional connectivity in healthy individuals. In this study, we investigated whether a single session of neurofeedback training aimed at the voluntary reduction of alpha rhythm (8–12 Hz) amplitude would be related to differences in EEG network oscillations, functional MRI (fMRI) connectivity, and subjective measures of state anxiety and arousal in a group of individuals with PTSD.
Method
21 individuals with PTSD related to childhood abuse underwent 30 minutes of EEG neurofeedback training preceded and followed by a resting-state fMRI scan.
Results
Alpha desynchronizing neurofeedback was associated with decreased alpha amplitude during training, followed by a significant increase (‘rebound’) in resting-state alpha synchronization. This rebound was linked to increased calmness, greater salience network connectivity with the right insula, and enhanced default mode network connectivity with bilateral posterior cingulate, right middle frontal gyrus, and left medial prefrontal cortex.
Conclusion
Our study represents a first step in elucidating the potential neurobehavioral mechanisms mediating the effects of neurofeedback treatment on regulatory systems in PTSD. Moreover, it documents for the first time a spontaneous EEG ‘rebound’ after neurofeedback, pointing to homeostatic/compensatory mechanisms operating in the brain.
Keywords: Electroencephalogram (EEG), Neurofeedback , Posttraumatic stress disorder (PTSD), Functional MRI (fMRI), Functional connectivity
Introduction
Posttraumatic stress disorder (PTSD) is characterized by symptoms of re-experiencing, cognitive and behavioral avoidance, emotional numbing and hyperarousal (1). More generally, individuals with PTSD often face difficulties in attention and arousal regulation, emotional/self-awareness and social emotional processing (2–6). Research increasingly lends support to the view that the aforementioned processes strongly depend on the functional and structural integrity of large-scale neural networks which are thought to be compromised in psychopathology (7–9). Previous investigations have focused on the ‘salience network’ (SN), anchored by the anterior insula and dorsal anterior cingulate cortex (dACC) (10), and the ‘default mode network’ (DMN), which includes the posterior cingulate cortex (PCC), precuneus, medial prefrontal cortex (mPFC), and lateral temporal cortices (11, 12). Many of these same network nodes (e.g., dACC, mPFC, insula, amygdala) are implicated in the neurocircuitry of PTSD (13, 14). In addition, network analyses employing functional magnetic resonance imaging (fMRI) during the resting-state (15, 16) and various cognitive-emotional tasks (17, 18) have revealed alterations in the functional connectivity of the SN and DMN in patients with PTSD. Specifically, patients with PTSD have previously shown altered SN-connectivity with the insula (18, 19), which has been implicated in salience detection (10), interoceptive awareness (20, 21), and the switching between different large-scale networks, thought to facilitate adaptive shifting between externally and internally oriented cognitive and affective processing (22, 23). Moreover, recent studies suggest that the successful control and regulation of threat-related information, which is also impaired in PTSD patients, may depend on the integrity of functional connections within the SN (17, 24, 25). With regard to the DMN, which is thought to play a crucial role in self-referential and social emotional processing (26, 27), patients with PTSD have previously shown reduced DMN connectivity between the posterior cingulate cortex / precuneus and the medial prefrontal cortex (15, 16). Thus, finding a means to modify network connectivity may be a beneficial step in the amelioration of PTSD patients’ observed deficits in the aforementioned functions.
Electroencephalographic (EEG) neurofeedback training is one approach to noninvasively modulate brain network dynamics (28). While specific cortical oscillations would normally defy voluntary control due to our lack of awareness thereof, providing someone with real-time feedback from their EEG can enable them to learn to reliably influence their neuronal activity. For instance, previous studies have shown that activity in the alpha band can be successfully synchronized and/or desynchronized (i.e., increased and/or decreased) by naïve participants through neurofeedback and that this may be beneficial in the treatment of anxiety and attention problems (29–32). The human alpha rhythm is defined as electrical oscillations in the 8- to 12-Hz range, most prominently expressed over posterior regions during a state of resting wakefulness with eyes closed (33, 34). In particular, a desynchronization (reduction) of the alpha rhythm has been linked to directed attention and cognitive processing (35). Recent simultaneous EEG-fMRI studies have further related spontaneous fluctuations in EEG alpha power to blood-oxygenation-level-dependent (BOLD) fMRI signal changes in the aforementioned brain networks thought to mediate salience detection, attention allocation, and self-referential processing (36–40).
Importantly, we recently demonstrated that the functional connectivity of the SN and DMN could be plastically altered following a single session of alpha desynchronization neurofeedback (28). Compared to a sham-feedback group, participants receiving veridical feedback showed an increase in connectivity within the SN, mainly involving the dACC, and a positive correlation between alpha rhythm resting-state changes and DMN connectivity. These plastic connectivity changes were detected during an fMRI session held approximately 30 minutes after termination of training.
Aims of the study
Based on our findings in healthy participants, we designed a proof-of-principle study to assess the impact of alpha desynchronizing neurofeedback in patients with PTSD related to childhood abuse. Our goal was to investigate possible short-term changes in resting-state fMRI connectivity within the salience network (SN) and default mode network (DMN) as well as related changes in EEG network oscillations and subjective measures of state anxiety and arousal following a single session of neurofeedback training aimed at the voluntary reduction of alpha rhythm (8–12 Hz) amplitude.
We propose that examining the neurobehavioral correlates of self-regulating alpha amplitude represents a first step in elucidating the neurobehavioral mechanisms potentially mediating the effects of a full course of neurofeedback treatment in PTSD. Given recent reports linking alpha oscillations to BOLD activity in large-scale brain networks (36–41) and previous findings of neurofeedback-related connectivity changes within the SN and DMN in healthy participants (28), we anticipated that alpha desynchronization would be associated with plastic alterations in DMN and SN connectivity in a group of individuals with PTSD related to childhood abuse. Specifically, we hypothesized to find differences between the two fMRI scans in the connectivity patterns of the SN and DMN with brain regions previously implicated in PTSD, such as the insula, ACC/medial prefrontal cortex and PCC/precuneus, and that the magnitude of these differences would be related to a participant’s performance during the neurofeedback.
Material and methods
Participants
The study was approved by the Research Ethics Board of the University of Western Ontario. We included 21 participants (18 female; mean age = 39.86 years, SD = 13.69 years) who all met the DSM-IV (1) criteria for a primary diagnosis of PTSD. All participants had experienced childhood sexual and/or physical abuse. Axis I diagnoses were assessed by a trained psychologist using the Structured Clinical Interview for DSM-IV Axis-I Disorders (42) and the Clinician Administered PTSD Scale (cut-off score >50) (43). All participants further completed the Childhood Trauma Questionnaire (44). Exclusion criteria comprised a lifetime diagnosis of a psychotic disorder, bipolar disorder, substance use disorders within the last six months, a history of head trauma, serious medical or neurological illness, and any counter-indications for MRI. Eleven participants were currently taking psychotropic medications including Citalopram (2), Fluoxetine (3), Sertraline (1), Clonazepam (3), Trazodone (1), Zopiclone (1), Clozapine (1), Quetiapine (1), Cipralex (3) and Mirtazapine (1). Participants were recruited by advertisements in the community and within the health care network in London, Ontario. After receiving a detailed description of the experimental procedures, all participants provided written informed consent to participate in the study. Demographic and psychometric data are summarized in Table 1.
Table 1.
Demographic and psychometric data
| Patients with PTSD (n = 21) | |
|---|---|
| Age, mean ± SD, years | 39.9 ± 13.7 |
| CAPS score, mean ± SD | 80.62 ± 14.01 |
| CTQ Emotional abuse score, mean ± SD | 17.48 ± 5.57 |
| CTQ Physical abuse score, mean ± SD | 11.05 ± 5.30 |
| CTQ Sexual abuse score, mean ± SD | 15.29 ± 8.21 |
| CTQ Emotional neglect score, mean ± SD | 16.33 ± 5.22 |
| CTQ Physical neglect score, mean ± SD | 12.57 ± 5.49 |
| Axis I comorbidity (current [past] No.) | Major depressive disorder (8 [10]) |
| Dysthymic disorder (1) | |
| Panic disorder with agoraphobia (1 [3) | |
| Panic disorder without agoraphobia (3 [2]) | |
| Agoraphobia without panic disorder (2 [1]) | |
| Social phobia (3) | |
| Obsessive—compulsive disorder (0 [1]) | |
| Somatization disorder (2 [1]) | |
| Undifferentiated somatoform disorder (5) | |
| Anorexia nervosa (0 [3]) | |
| Bulimia nervosa (1 [1]) | |
| Eating disorder NOS (1 [1]) |
PTSD, post-traumatic stress disorder; SD, standard deviation; CAPS, Clinician-Administered PTSD Scale; CTQ, Child Trauma Questionnaire; NOS, not otherwise specified.
Experimental Procedures
Following a detailed diagnostic assessment conducted on a separate day, the experimental protocol consisted of three sequential parts that occurred within the same daytime visit: (1) MRI scan before neurofeedback, (2) EEG neurofeedback, and (3) MRI scan after neurofeedback. Including time for detailed instructions and setup, the total experiment lasted about three hours, with approximately 30 minutes between the respective MRI and neurofeedback sessions. Immediately before and after the neurofeedback, participants completed Spielberger's State Anxiety Inventory (STAI) (45) and Thayer's Activation-Deactivation Adjective Checklist (32) in order to measure state anxiety and arousal. After the feedback training, we also asked participants whether they had had a sense of control over the neurofeedback game, what strategy, if any, they found effective for gaining points during the game, and how the experience made them feel.
Unlike in our study in healthy individuals (28), we decided not to include a sham-feedback procedure for ethical reasons, so as not to augment any feelings of frustration and failure often experienced by PTSD patients (46). Moreover, there is some evidence (47) suggesting that effective learning of voluntary control over brain rhythms may be compromised after receiving false feedback, making it more difficult to learn from real feedback in the future. Without a clear mean of mitigating this issue, we elected a within-subject study design.
EEG neurofeedback paradigm
We used the same EEG neurofeedback training protocol as described in our previous study in healthy participants (28). Briefly, the EEG session took place outside of the MRI room and consisted of a 3-minute baseline, followed by 30 minutes of neurofeedback and another 3-minute baseline immediately after training. For the duration of the (feedback-free) baseline recordings, participants were asked to relax with their eyes open, refrain from excessive eye movements and gaze at a blank wall. During the neurofeedback session, participants attempted to suppress real-time alpha (8–12 Hz) amplitude, which was recorded from midline parietal cortex (electrode Pz). Rather than feeding back the global alpha signal averaged across multiple electrodes, which may lead to a mixing of local cortical dynamics, we decided to feed back the signal recorded from Pz for neurofeedback control (28). We selected this electrode based on its location overlying a major hub of the DMN, namely the PCC/precuneus, whose BOLD signal changes have been linked to EEG alpha rhythm modulations (38, 39).
All participants interacted with a "SpaceRace" game where they received continuous visual feedback in the form of a moving spaceship and a dynamic bar graph whose height was inversely proportional to real-time alpha amplitude fluctuations. Participants were told that the spaceship would move forward whenever they were “in-the-zone” of their target brain activity (i.e. alpha lower than threshold), and that it would stop when they were “out-of-the-zone” (i.e. alpha higher than threshold). The aim of the training was to use the feedback they received during the game to learn to keep the spaceship travelling through space. However, in order to prevent demand characteristics from affecting the results of the training, participants neither received any explicit instructions or mental strategies from the experimenter on how to achieve control over their spaceship, nor were they informed about the type of EEG parameter or frequency that was being rewarded. Likewise, neither the letter of information nor the informed consent form contained any indication of the behavioral hypotheses or the specific EEG parameter/frequency that participants were supposed to regulate. Instead, they were told that our research group was interested in whether individuals with and without PTSD could learn to control their brain activity and if so, how they would go about achieving it.
For the purpose of online neurofeedback training, the EEG signal was infinite impulse response band-pass filtered to extract alpha with an epoch size of 0.5 seconds. Participants were rewarded upon suppression of their absolute alpha amplitude. For each participant, the reward threshold was initially set so that their alpha amplitude would fall below the initial 3-min baseline average approximately 60% of the time (i.e., they received negative-feedback about 40% of the time). To ensure that all participants received comparable frequencies of reward, we readjusted their reward thresholds to meet the desired ratio, when they achieved disproportionately higher (> 80%) or lower (< 40%) rates of reward during feedback. The readjustments were then made at the beginning of the next training period based on the EEG of the preceding 30 seconds (Ros et al., 2012). The entire neurofeedback session was divided into three-minute training periods with a short break (10 seconds) after each period. During the breaks, their score for the preceding period was displayed, and participants were asked whether they wanted to continue.
EEG recording and preprocessing
Using a Mitsar amplifier (Mitsar-201, CE0537, Mitsar, Ltd.) and WinEEG software (Version 2.84.44, Mitsar Ltd.), a standardized 19-channel EEG according to the International 10–20 System was recorded at a sampling rate of 250 Hz, using a linked-earlobe referential montage. Low and high pass filters were set to 0.5 and 40 Hz, respectively, with a 55–65 Hz notch filter. Recorded EEG was analyzed offline using a weighted average Laplacian reference montage. Elimination of artifacts was performed by visual inspection and artifact correction procedures based on independent component analysis (ICA) decomposition and spatial filtering. Impedance at all channels was reduced to below 5 kOhms.
EEG spectral analysis
Given our a priori hypotheses, we calculated EEG spectral amplitudes offline via Short Time Fourier Transform in 4-second epochs (50% overlapping with Hanning window) in the alpha frequency (8–12 Hz) band only. To test whether participants had significantly lowered their absolute alpha amplitude during neurofeedback compared to the first (pre-neurofeedback) baseline, paired t-tests were conducted between absolute alpha amplitudes averaged over the first baseline and absolute alpha amplitudes averaged over the entire neurofeedback session. Next, to determine whether participants had plastically changed their absolute alpha amplitude from the first to the second (post-neurofeedback) baseline, paired t-tests were conducted between absolute alpha amplitudes averaged over the first and second baseline EEG, respectively.
For each participant, we also normalized alpha (de)synchronization values by estimating the % signal change using the ratio of the average alpha amplitude during neurofeedback to the average alpha amplitude during the first baseline (subsequently referred to as ‘training alpha change’). This was also done for the ratio of the average alpha amplitude during the second baseline to the average alpha amplitude during the first baseline, and referred to it as ‘resting alpha change’. With changes in alpha amplitude thus normalized values greater than 0 denote a relative percent increase in alpha amplitude, while values less than 0 mark a percent decrease. To further examine the relationship between the absolute alpha amplitude during baseline 1, ‘training alpha change’ and ‘resting alpha change’, we individually computed Pearson product moment correlations between the two change scores and the absolute alpha amplitude for baseline 1 using SPSS. To test whether the correlation results would be qualitatively different for local versus global changes in alpha amplitude, the indices of alpha amplitude were first derived from the neurofeedback electrode (Pz) alone and secondly, as an average across all 19 electrodes (global).
fMRI Paradigm
Imaging data were acquired using a 3-Tesla Siemens Magnetom Verio scanner with a 32-channel phase array head coil. During each MRI session (pre- and post-neurofeedback, respectively), participants underwent a high-resolution T1-weighted anatomical scan, followed by a 6-minute resting-state functional scan, for which they were instructed to close their eyes, relax and let their mind wander as previously described by Fox et al. (48) and Bluhm et al. (15). High-resolution T1-weighted structural images were acquired using a 3-D magnetization-prepared rapid acquisition gradient-echo sequence with TR = 2000 ms, TE = 4 ms, TI = 900 ms, flip angle = 9°, field of view = 256mm × 256 mm, 1-mm isotropic resolution, 176 slices, no gap, GRAPPA acceleration = 2. Whole-brain T2-weighted functional images were obtained using a gradient echo-planar imaging sequence with TR = 3000 ms, TE = 20 ms, flip angle = 90°, field of view = 256 mm, voxel size = 2 × 2 × 2 mm3. Sampling consisted of 60 interleaved slices, slice thickness = 2 mm, no gap, parallel to the anterior commissure - posterior commissure line. The first four images of each run were automatically discarded to minimize T1 equilibration effects.
Image preprocessing was performed in SPM8 (http://www.fil.ion.ucl.ac.uk/spm/) following methods previously described (Ros et al., 2012). Briefly, the imaging data was subjected to slice-timing correction, motion correction, spatial normalization and smoothing using a full-width at half-maximum (FWHM) Gaussian filter of 8 mm. Data were normalized using the unified segmentation on T1 image pipeline (49), and finally resliced to 3-mm isotropic voxels in Montreal Neurological Institute (MNI) space.
fMRI connectivity analysis
Group spatial ICA (50) was performed using the GIFT toolbox (v1.3i, http://mialab.mrn.org/) in Matlab 7.6 (Mathworks Inc., MA, USA). In order to facilitate comparisons between subjects and sessions (51), we conducted one group spatial ICA across all participants, where we entered the preprocessed resting-state data (120 volumes each) from the pre-neurofeedback fMRI as session 1 and the data from the post-neurofeedback fMRI as session 2. We used the Infomax algorithm and estimated 20 independent components based on our previous study in healthy individuals (28). Using ICASSO (52) as implemented in GIFT, the ICA estimation was repeated 20 times to enhance component reliability. Taken together, this procedure resulted in a set of group aggregate spatial maps (representing which brain regions constitute a network/component) and corresponding time courses of the BOLD signal change across time. For each component, single subject spatial maps and time courses were then back-reconstructed for each participant and converted to z-scores, which denote the strength of each voxel’s contribution to the component’s time course (50, 53).
To identify the components corresponding to the SN and DMN, all spatial maps were first visually inspected for the presence of obvious artifacts, such as edges, ventricles and white matter. Next, we used the spatial sorting function in GIFT to find the components whose spatial pattern showed the highest correlation with two a priori defined masks of the SN and DMN, respectively, as derived from our previous study in healthy participants (28). The resulting components were entered into second-level analyses in SPM8 (http://www.fil.ion.ucl.ac.uk/spm/) to examine differences in the strength of regional functional connectivity between sessions.
For each network, a statistical map was created by entering the single-subject spatial maps (pooled across sessions) into a voxel-wise one-sample t-test. This was thresholded at q<0.05 with false-discovery-rate (FDR) correction and saved as a mask (54, 55) for use in subsequent paired t-tests on the single-subject spatial maps corresponding to pre and post fMRI sessions. This was done to ensure that all findings would be restricted to brain regions actually contributing to the respective component. Given the novelty of our approach to studying the mechanisms of EEG neurofeedback in PTSD, we used a combined intensity and cluster size threshold of P<0.005 with a 20 voxel extent to achieve a reasonable balance between type I and type II errors (56). This extent threshold was in accordance with a cluster-extent correction according to random field theory (57).
Multiple regression analysis
In order to relate individual changes in functional connectivity of the SN and DMN to individual alpha amplitude and STAI/Thayer scale changes, we used the ImCalc function in SPM8 to calculate post-minus-pre z-score connectivity change maps (T2 - T1) for each participant and network, which served as the dependent variable in multiple regression analyses. Specifically, we separately regressed ‘training alpha change’ and ‘resting alpha change’ (using both local and global indices of alpha amplitude) against individual z-score connectivity change maps of the two networks. Regarding STAI and Thayer scale changes, we first conducted paired t-tests to compare the respective measures pre-to-post neurofeedback and only ran multiple regression analyses for the scales that showed a significant change between the two time points. For those, we separately regressed the individual change scores (T2 - T1) against the individual z-score connectivity change maps of the two networks. The results of all multiple regression analyses of z-score connectivity change maps were masked with the respective network mask and deemed significant when passing the aforementioned intensity and cluster size thresholds (56, 57). For scales showing a significant difference in the paired t-test, we also ran Pearson product moment correlations between the individual change scores (T2 - T1) and ‘training alpha change’ and ‘resting alpha change’, respectively.
Results
Subjective results
Analysis of participants’ self-reports suggested that the majority (17 out of 21) had felt a sense of control over the spaceship and that the session had left them feeling more relaxed, calm and clear-minded. Eight participants also reported greater fatigue; although one person specified that this tiredness was “like that…after yoga”. One person experienced mild drowsiness, and three participants reported feeling frustrated when they were unable to make the spaceship move. When asked about strategies employed to make the spaceship move (i.e., achieve a decrease in alpha amplitude), focused (visual) attention was among the most frequently endorsed answers. Finally, a number of participants reported that feeling positive emotions made the spaceship move faster, whereas trauma-related thoughts/memories would bring it to a halt. Notably, participants reported not being overwhelmed by these emotions/images in a way that they usually would be.
On a group level, a paired t-test did not show a significant pre-to-post difference in subjective feelings of state anxiety as assessed with the STAI (P=0.17). However, a comparison of the Thayer subscales between the two time points revealed a significant increase in calmness (t=2.72; df=20; P<0.05) after neurofeedback.
EEG spectral analysis
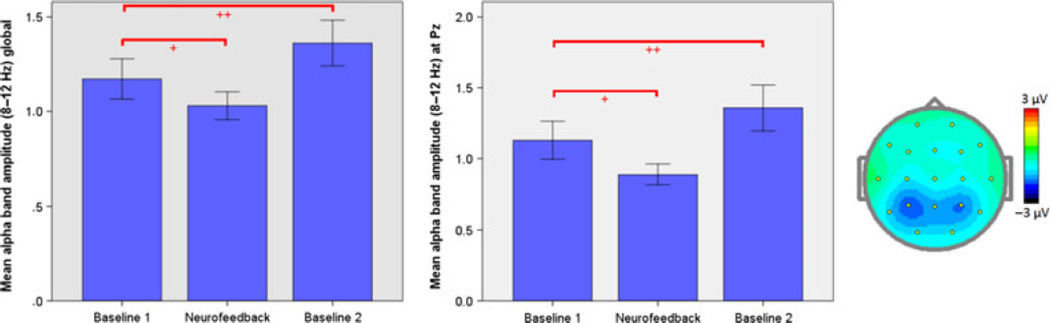
Paired t-tests for both the Pz feedback site as well as the global average showed that on a group level subjects had significantly reduced their absolute alpha band amplitude during neurofeedback compared to the first baseline (Pz: t=−3.19; global: t=−3.21; df=20; P<0.05, Bonferroni-corrected). In contrast, paired t-tests revealed a significant increase in alpha amplitudes from the first to the second baseline (Pz: t=3.54; global: t=3.67; df=20; P<0.05, Bonferroni-corrected), which was also reflected in the ‘resting alpha change’, i.e., the normalized difference between the average alpha amplitudes during the second and first baseline (Figure 1).
Fig. 1.
Left and middle: Bar graphs show group mean alpha (8–12 Hz) amplitudes (calculated offline using a weighted average Laplacian montage) averaged across all subjects for Baseline 1, neurofeedback (NFB), and Baseline 2, respectively, globally over all 19 electrodes (left) and at the Pz feedback site only (middle). Stars indicate the level of statistical significance, with * indicating a threshold of P < 0.05 and ** indicating a threshold of P < 0.005. Error bars represent I standard error of the mean (SEM). Right: Topographic plot of mean alpha amplitude change during neurofeedback relative to the first baseline. Colors indicate magnitude of reductions in amplitude (uV).
Regression analyses showed that absolute alpha amplitude at baseline 1 significantly predicted ‘training alpha change’ both at the Pz feedback site alone (r=0.70, P<0.05) as well as globally across all 19 electrodes (r=0.77, P<0.05). When controlling for absolute alpha amplitude at baseline 1, ‘training alpha change’ was negatively correlated with ‘resting alpha change’ for the global amplitude measure (rpartial=−0.52, P<0.05; Pz: rpartial=−0.42, P=0.06). In other words, the greater the relative reduction in alpha amplitude during neurofeedback, the greater the alpha resting-state ‘rebound’ afterwards.
fMRI connectivity analysis
Independent component identification
For the SN and DMN, components were identified whose spatial properties were highly correlated with the a priori defined mask and included brain regions previously implicated in the two networks (10, 58).
SN and DMN functional connectivity pre-vs.-post neurofeedback
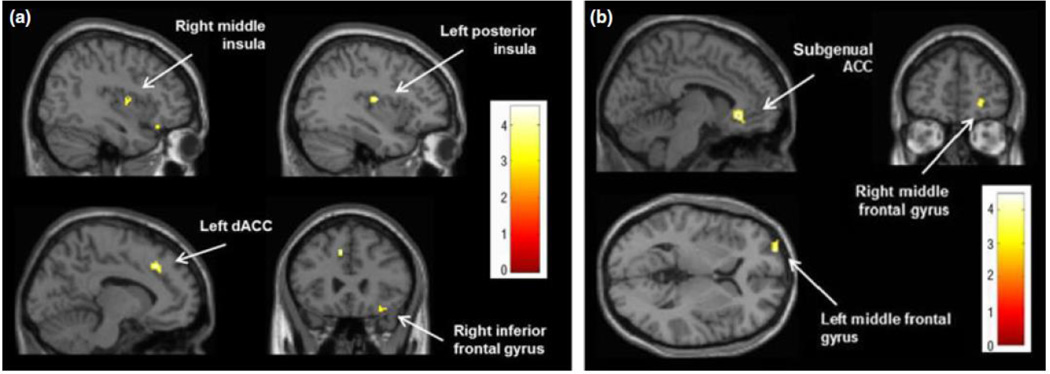
For the SN, a paired t-test revealed significantly increased functional connectivity after neurofeedback with the right middle insula (Montreal Neurological Institute (MNI) coordinates: 40, −2, 10; t=4.49; k=36), the left posterior insula (MNI: −36, −12, 14; t=4.31; k=37), bilateral superior temporal gyri (left: MNI: −62, −40, 20; t=4.34; k=27; right: MNI: 46, 14, −24; t=4.19; k=69), the left dACC (MNI: −10, 26, 40; t=4.15; k=57), and the right inferior frontal gyrus (MNI: 32, 28, −18; t=3.63; k=47) (Figure 2a). No regions showed a significant decrease in functional connectivity with this network after neurofeedback.
Fig. 2.
Clusters showing increased functional connectivity after neurofeedback (P < 0.005, corrected) for the (a) salience network and the (b) default mode network.
For the DMN, a paired t-test showed significantly increased functional connectivity after neurofeedback with bilateral subgenual anterior cingulate (sgACC; MNI: −6, 30, −6; t=4.47; k=130) and bilateral middle frontal gyri (left: MNI: −26, 64, 4; t=4.07; k=41; right: MNI: 24, 50, −2; t=3.70; k=30) (Figure 2b). A significant decrease in DMN functional connectivity was found in the right middle temporal gyrus (MNI: 52, −32, −8; t=4.30; k=40) and PCC (MNI: 12, −54, 24; t=3.74; k=24).
Relationship between changes in alpha amplitude and network connectivity
Salience Network
To investigate whether individual changes in functional connectivity within the SN were related to a participant’s change in alpha amplitude during the neurofeedback, we separately regressed global ‘training alpha change’ as well as ‘resting alpha change’ against individual z-score connectivity change maps of the SN. As can be seen in Table 2, global ‘training alpha change’ was negatively correlated with SN connectivity changes in the right mid-/posterior insula, which was also confirmed when using ‘training alpha change’ at Pz. In other words, the more participants were able to reduce their alpha amplitude during neurofeedback relative to the first baseline, the greater their increase in SN connectivity with the right insula. Additionally, changes in coupling of the right mid-/posterior insula with the rest of the SN were positively correlated with global ‘resting alpha change’, indicating that a strong alpha ‘rebound’ was associated with a larger increase in SN insula connectivity.
Table 2.
Brain regions showing a significant relationship between changes in alpha amplitude (global) and functional connectivity (P < 0.005, corrected)
| Brain region | MNI coordinates |
t Score |
Number of voxels |
|---|---|---|---|
| (a) Salience network | |||
| Positive correlation w/global training alpha change | |||
| – | – | – | – |
| Negative correlation w/global training alpha change | |||
| R mid-/posterior insula (BA 13) | 40, −4, 14 | 4.37 | 92 |
| Positive correlation w/global resting alpha change | |||
| R mid-/posterior insula (BA 13) | 40, −12, 4 | 3.65 | 29 |
| Negative correlation w/global resting alpha change | |||
| R superior frontal gyrus (BA 8) | 4, 12, 54 | 4.22 | 42 |
| L/R anterior/mid-cingulate gyrus (BA 24) | 0, 14, 34 | 3.95 | 63 |
| R superior temporal gyrus (BA 21) | 48, −8, −16 | 3.60 | 34 |
| R dorsal anterior cingulate (BA 32) | 2, 26, 28 | 3.46 | 26 |
| (b) Default mode network | |||
| Positive correlation w/global training alpha change | |||
| L angular gyrus (BA 39) | −46, −70, 34 | 4.47 | 33 |
| R putamen | 30, −2, −12 | 3.37 | 21 |
| Negative correlation w/global training alpha change | |||
| R middle frontal gyrus (BA 11) | 26, 52, −14 | 4.77 | 60 |
| L precuneus (BA 7) | −6, −58, 52 | 4.04 | 36 |
| Positive correlation w/global resting alpha change | |||
| L PCC (BA 29, 30) | −8, −50, 10 | 5.04 | 113 |
| R PCC (BA 30) | 6, −56, 6 | 3.92 | 48 |
| R middle frontal gyrus (BA 10) | 40, 44, 8 | 4.90 | 42 |
| L dorsomedial PFC (BA 9) | −2, 56, 18 | 3.68 | 23 |
| Negative correlation w/global resting alpha change | |||
| R postcentral gyrus (BA 3) | 40, −24, 56 | 5.83 | 115 |
BA, Brodmann area; MNI, Montreal Neurological Institute.
In contrast, global ‘resting alpha change’ was negatively correlated with changes in SN connectivity with the right superior frontal gyrus, the anterior/mid-cingulate gyrus, the right superior temporal gyrus, and the right dACC. For these brain regions, connectivity with the rest of the SN decreased with increasing alpha ‘rebound’ and vice versa (Table 2).
Default Mode Network
When we regressed indices of ‘training alpha change’ against individual z-score connectivity change maps of the DMN, we observed a positive correlation between global (as well as Pz) ‘training alpha change’ and DMN connectivity in the left angular gyrus and the right putamen. DMN connectivity changes in the right middle frontal gyrus and left precuneus were negatively correlated with global and Pz ‘training alpha change’. Both indices of ‘resting alpha change’, on the other hand, were positively correlated with changes in DMN connectivity with bilateral PCC, and the right middle frontal gyrus. In addition, we found a positive correlation for the left mPFC with global ‘resting alpha change’ only. A negative correlation between changes in DMN connectivity and ‘resting alpha change’ was revealed in the right postcentral gyrus for both global and Pz alpha change values (Table 2).
Relationship between calmness and changes in alpha amplitude
We computed Pearson product moment correlations between the individual change scores for Calmness (T2 - T1), versus ‘training alpha change’ and ‘resting alpha change’, respectively. These analyses were non-significant (P=0.91) for ‘training alpha change’, but revealed a trend toward a significant correlation between changes in calmness and “resting alpha change’ in the sense that an increase in alpha amplitude from the first to the second baseline EEG was related to enhanced calmness (global: r=0.41, P=0.07; Pz: r=0.39, P=0.08) after neurofeedback.
Relationship between calmness and changes in network connectivity
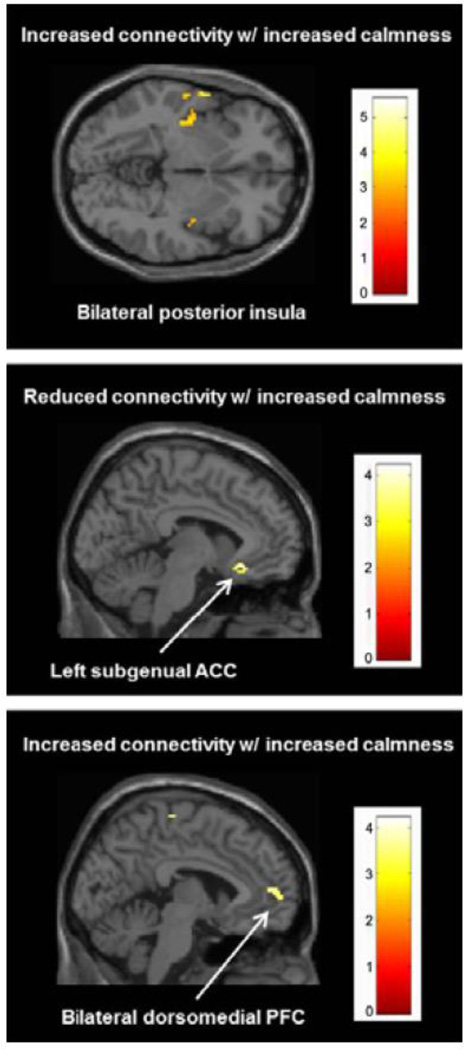
We also performed a regression analysis, in which we regressed the individual change scores for Calmness (T2 - T1) against the individual z-score connectivity change maps of the SN and DMN, respectively. At our combined voxel-wise and cluster-corrected threshold, this analysis revealed that an increase in calmness was associated with an increase in SN connectivity with left posterior insula (left: MNI: −36, −20, 6; t=5.54; k=163), left superior temporal gyrus (MNI: −62, 2, −2; t=4.81; k=23), left middle temporal gyrus (MNI: −50, −22, 12; t=4.51; k=91), left inferior frontal gyrus (MNI: −32, 28, −14; t=4.35; k=27), and right superior frontal gyrus (MNI: 28, 40, 44; t=3.29; k=22) (Figure 3). Although slightly below our predefined extent threshold, a cluster in the right posterior insula (MNI: 38, −6, 0; t=3.95; k=19) also showed greater connectivity with the SN with increasing calmness.
Fig. 3.
Top panel: Clusters exhibiting a significant relationship between changes in calmness (Thayer subscale) and salience network connectivity (P < 0.005, corrected). Middle and bottom panels: Clusters exhibiting a significant relationship between changes in calmness (Thayer subscale) and default mode network connectivity (P < 0.005, corrected).
Regarding the DMN (Figure 3), more calmness was related to an increase in network connectivity with the left superior frontal gyrus (MNI: −18, 40, 36; t=4.12; k=43), bilateral dorsomedial PFC (MNI: −4, 54, 8; t=3.97; k=102), and the right medial frontal gyrus (MNI: 4, −22, 64; t=3.83; k=43). In contrast, decreases in DMN connectivity with increased calmness were found for the left sgACC (MNI: −6, 24, −14; t=4.30; k=45), the left middle temporal gyrus (MNI: −60, −28, −10; t=4.10; k=40), and the left claustrum (MNI: −28, 14, −14; t=3.75; k=40).
Binary covariate analyses with current medication status and comorbid depression
In order to examine whether our findings were influenced by participants’ use of medications, we included current medication status as a binary covariate (0=absent, 1=present) in all of the aforementioned paired t-tests and regression analyses. This did not change the results beyond slight variations in the number of voxels per cluster. Likewise, covarying out a diagnosis of current depression did not significantly change the results for SN and DMN connectivity.
Discussion
In the current study, we examined whether a single session of voluntary reduction of EEG alpha (8–12 Hz) amplitude could induce a significant change in resting-state fMRI connectivity within the salience network (SN) and default-mode network (DMN), and whether these changes would be related to alterations in EEG and subjective measures of calmness and anxiety. Participants were successful in reducing their mean alpha amplitude during neurofeedback as compared to their resting baseline. Intriguingly however, this was followed by a significant increase (or ‘rebound’) in resting alpha synchronization immediately after neurofeedback. Inspecting the resting-state fMRI findings, a comparison of functional connectivity maps revealed significant pre-to-post neurofeedback differences in functional coupling within the SN and DMN, including the bilateral mid-/posterior insula, left dACC, bilateral superior temporal gyri, right inferior frontal gyrus, sgACC, medial prefrontal, and posterior cingulate cortices. These findings replicate in part previous results in healthy individuals who underwent the same neurofeedback protocol and showed enhanced pre-to-post SN connectivity with the dACC during an auditory oddball task (28). Given this concordance and the fact that the study’s sham-control subjects failed to show significant effects, it is plausible that the observed increases in SN connectivity in the PTSD group are directly linked to the neurofeedback intervention and modulation of alpha activity.
This interpretation is further strengthened by the observed negative correlation between greater post-neurofeedback dACC connectivity and resting alpha synchronization, consistent with our previous findings (28). Interestingly however, changes in alpha amplitude during training did not predict changes in pre-to-post training resting alpha amplitude in the same way across studies. In fact, greater alpha desynchronization (i.e., attenuation) during neurofeedback was associated with lower resting state alpha amplitude in healthy individuals pre versus post neurofeedback, while it was linked to a stronger alpha ‘rebound’ (i.e., a post-training increase in alpha amplitude following suppression of the alpha rhythm during training) in patients with PTSD. Such homeostatic rebound phenomena have previously been observed in studies of physical exercise and non-invasive brain stimulation (59, 60). Likewise, motor acts (whether executed, observed or imagined) induced alpha and/or beta desynchronization followed by rebounds of alpha and/or beta power (61).. Interestingly, the observed increase in baseline alpha amplitudes pre-to-post neurofeedback may be linked to the reported increases in calmness and relaxation following neurofeedback aimed to directly increase alpha rhythms (29, 47). Given that baseline alpha power has previously been associated with measures of trait as well as state anxiety (62, 63), it is also conceivable that the observed difference in the levels of post-training alpha between individuals with PTSD and healthy participants may be related to different levels of anxiety. This mechanism is directly supported within our data by an intra-individual correlation of r=0.4 (p=0.07) between post-training alpha changes and increases in calmness. In PTSD, the positive effect of increased resting alpha synchronization is directly in line with the therapeutic benefit of alpha-theta neurofeedback training in combat veterans as pioneered by Peniston and Kulkosky (64–67). An intriguing hypothesis is that this may be due to a ‘re-normalization’ of network oscillations, supported by findings of abnormally decreased alpha power in PTSD (68). Although any interpretation of these findings has to remain speculative, we suggest that the pre-to-post training increase in resting alpha amplitude may reflect an inhibition and decreased arousal of cerebral cortex (69).
On the other hand, changes in cortical oscillations may equally shift network connectivities. In line with previous research, resting alpha synchronization correlated positively with insula connectivity (70). Hence, the stronger the alpha ‘rebound’ after neurofeedback in PTSD patients, the greater the observed increase in SN connectivity with the right insula and the smaller the increase in SN connectivity with the dACC. The proposed rebound mechanism is supported by the fact that decreases in alpha amplitude during neurofeedback also significantly predicted greater pre-to-post neurofeedback connectivity with the right insula. On the other hand, the negative correlation between dACC connectivity and resting alpha synchronization is consistent with the proposed functions of this brain region in attention/alertness modulation and the neurobehavioral correlates of alpha activity (28). Specifically, the dACC has been implicated in the mediation of cognitive control (71), selective attention (72), and emotional arousal (73), which have also been linked to variations of the alpha rhythm (36, 40, 74). Alpha rhythm desynchronization has been reported to reflect a state of externally oriented visual attention (35), while its synchronization is associated with more inwardly directed attention and an inhibition of sensory cortices (69, 75).
Given the proposed functions of the insular cortex, future studies using additional behavioral measures should investigate whether alpha rhythm synchronization and the observed increases in insula connectivity and subjective feelings of calmness are in fact related to enhanced interoceptive/emotional awareness, self-reflection, and/or an improved regulation of affective states in patients with PTSD (20, 21, 76).
With regard to the DMN, we replicated previous findings of a positive relationship between changes in alpha synchronization and functional connectivity/activity (28, 32, 38, 39). Moreover, participants evidenced increased pre-to-post neurofeedback DMN connectivity with bilateral sgACC and middle frontal gyri, as well as pre-to-post decreases in coupling with the right middle temporal gyrus and PCC. These brain regions are part of the neural circuitry subserving the processing of self-relevant stimuli (77–80) and their subjective emotional evaluation (81, 82). Based on previous findings of reduced DMN connectivity in patients with PTSD (15, 16) and the proposed role of prefrontal brain regions in self-reflection and emotion regulation (83, 84), we speculate that the observed increases in DMN coupling may represent a shift towards greater reflective and self-regulatory capacities (85, 86).
An increase in calmness as measured by the Thayer scale predicted a decrease in connectivity with the sgACC, on the one hand, and greater coupling with the dorsomedial PFC and superior frontal gyrus, on the other. At first sight, this negative correlation between post-neurofeedback calmness and DMN connectivity with the sgACC may seem to contradict the previously reported group effect of increased DMN connectivity with this brain region, as revealed by the paired t-test. Given that the sgACC has been implicated in major depression and excessive rumination (87–89), we speculate that increased calmness after neurofeedback may be associated with less negative self-evaluation (82) mediated by reduced connectivity with the sgACC, on the one hand, and enhanced self-reflection mediated by an additional recruitment of the dorsomedial PFC (and insula) (21, 85), on the other. In other words, while the paired t-test result of enhanced sgACC connectivity may reflect an increase in the brain’s general capacity to engage in self-reflection and emotion regulation (90), the content of such reflections may, in fact, be less negative with increasing calmness.
Several limitations of our study are worth noting. First, because our neurofeedback protocol has recently been validated in a randomized, placebo-controlled study in healthy individuals, we decided not to include a sham-feedback procedure in the current study with PTSD patients, as we did not want to augment any feelings of frustration and failure and/or hamper participants’ chances of learning from real feedback in the future (47). However, given the lack of a between-subject group comparison (e.g., a healthy control and/or another patient group) as well as a within-subject comparison condition (such as an attentional training or meditation task), we cannot determine whether the observed changes in SN and DMN connectivity are specific to neurofeedback-induced changes in alpha synchronization rather than being dependent on general effects of attentional deployment,. In order to clarify this issue, future studies should include a psychiatric control group with disorders other than PTSD and contrast the effects of alpha desynchronizing neurofeedback with the benefits of general instructions to relax and/or focus one’s attention in a certain way. Moreover, future studies should compare our protocol to the use of other neurofeedback procedures targeting different frequency bands and/or feedback locations. Regarding the question of specificity to PTSD, it is especially noteworthy that the majority of patients in our sample either met criteria for MDD at the time of scanning or at some point in their past (10). Thus, we cannot rule out the possibility that our findings may have been additionally influenced by comorbid depression rather than PTSD per se. In order to partially address this issue, we conducted additional subgroup analyses to compare PTSD patients with and without a current diagnosis of MDD. In the case of EEG alpha changes, we did not find significant group differences – neither for ‘resting alpha change’ nor for ‘training alpha change’, which means that the two groups did not differ in terms of regulation success. Moreover, covarying out a diagnosis of current depression did not significantly change the results for SN and DMN connectivity. However, given that the group of individuals with comorbid MDD also had significantly higher CAPS scores (p<0.05), it will still be up to future studies to disentangle the individual contributions of PTSD and MDD, respectively.
Forthcoming investigations using simultaneous and continuous EEG/fMRI recordings during both the resting-state and neurofeedback are also needed to further our understanding of the relationship between alpha modulations, brain network connectivity, and subjective experiences during the resting state. In addition, it has to be noted that our sample included mainly women, thus limiting the generalizability of our findings to female patients with PTSD. On a related note, it would also be interesting to assess the effects of our neurofeedback protocol in a military population with combat related PTSD, since this population has traditionally been difficult to treat. Finally, it will be important for future studies to explore whether the observed short-term effects generalize to longer time-scales following repeated applications of the neurofeedback protocol.
To conclude, alpha rhythm desynchronizing neurofeedback in PTSD was associated with an alpha rebound, which was linked to increased calmness and greater SN connectivity with the right insula, and enhanced DMN connectivity with bilateral posterior cingulate, right middle frontal gyrus, and left medial prefrontal cortex. This study thus showed that the function of key brain networks involved in mediating affect and cognition can be volitionally modulated in persons with PTSD with measurable outcomes on immediate subjective wellbeing. Future studies examining EEG-based neurofeedback as an adjunctive treatment to established interventions for PTSD are warranted.
Significant outcomes.
This is the first study to show that key brain networks (salience and default mode networks) involved in mediating affect and cognition in PTSD, can be volitionally modulated by EEG neurofeedback with measurable outcomes on immediate subjective wellbeing.
Post-neurofeedback, a significant alpha rhythm rebound was linked to increased calmness and greater salience network connectivity with the right insula, and enhanced default mode network connectivity with bilateral posterior cingulate, right middle frontal gyrus, and left medial prefrontal cortex.
Individuals with PTSD were successful in reducing their mean alpha amplitude during neurofeedback as compared to a resting EEG baseline recorded before the training session.
Limitations.
A sham-control group was not included for ethical reasons, so future studies will need to ascertain the specificity of the neurofeedback effects.
The findings cannot be generalized to individuals who experienced single-incident trauma, or to individuals suffering from combat-related PTSD.
We scanned participants within 30 minutes after completing the neurofeedback training session, therefore limiting our findings to short-term plasticity effects. Future studies will need to address how such changes generalize to longer time-scales following repeated applications of this neurofeedback protocol.
Acknowledgements
The work presented in the manuscript was supported by a grant from the Lawson Health Research Institute and funding from the Canadian Institute for Military and Veteran Health Research. We also thank The Foundation for Neurofeedback and Applied Neuroscience, in particular H. John Fisher, for generously providing the neurofeedback hardware and software. Finally, we are grateful to Suzy Southwell, Stephanie Nevill, Melody Chow, Nancy Mazza, Maria Densmore and John Butler for their technical support and comments.
References
- 1.Association AP. Diagnostic and statistical manual of mental disorders. 4th edition, text revision ed. Washington, DC: Author; 2000. [Google Scholar]
- 2.Frewen PA, Lanius RA. Toward a psychobiology of posttraumatic self-dysregulation: reexperiencing, hyperarousal, dissociation, and emotional numbing. Ann N Y Acad Sci. 2006;1071:110–124. doi: 10.1196/annals.1364.010. [DOI] [PubMed] [Google Scholar]
- 3.Vasterling JJ, Brewin CR. Neuropsychology of PTSD: Biological, Cognitive, and Clinical Perspectives. New York, NY: The Guilford Press; 2005. [Google Scholar]
- 4.Lanius RA, Frewen PA, Vermetten E, Yehuda R. Fear conditioning and early life vulnerabilities: two distinct pathways of emotional dysregulation and brain dysfunction in PTSD. Eur J Psychotraumatol. 2010;1 doi: 10.3402/ejpt.v1i0.5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vasterling JJ, Duke LM, Brailey K, Constans JI, Allain AN, Jr, Sutker PB. Attention, learning, and memory performances and intellectual resources in Vietnam veterans: PTSD and no disorder comparisons. Neuropsychology. 2002;16:5–14. doi: 10.1037//0894-4105.16.1.5. [DOI] [PubMed] [Google Scholar]
- 6.Nazarov A, Frewen P, Parlar M, Oremus C, Macqueen G, Mckinnon M, et al. Theory of mind performance in women with posttraumatic stress disorder related to childhood abuse. Acta Psychiatr Scand. 2013 doi: 10.1111/acps.12142. [DOI] [PubMed] [Google Scholar]
- 7.Bressler SL, Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn Sci. 2010;14:277–290. doi: 10.1016/j.tics.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Uddin LQ, Iacoboni M, Lange C, Keenan JP. The self and social cognition: the role of cortical midline structures and mirror neurons. Trends Cogn Sci. 2007;11:153–157. doi: 10.1016/j.tics.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gusnard DA, Raichle ME, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- 12.Raichle ME, Macleod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayes JP, Hayes SM, Mikedis AM. Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biol Mood Anxiety Disord. 2012;2:9. doi: 10.1186/2045-5380-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel R, Spreng RN, Shin LM, Girard TA. Neurocircuitry models of posttraumatic stress disorder and beyond: A meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2012;36:2130–2142. doi: 10.1016/j.neubiorev.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Bluhm RL, Williamson PC, Osuch EA, Frewen PA, Stevens TK, Boksman K, et al. Alterations in default network connectivity in posttraumatic stress disorder related to early-life trauma. J Psychiatry Neurosci. 2009;34:187–194. [PMC free article] [PubMed] [Google Scholar]
- 16.Sripada RK, King AP, Welsh RC, Garfinkel SN, Wang X, Sripada CS, et al. Neural dysregulation in posttraumatic stress disorder: evidence for disrupted equilibrium between salience and default mode brain networks. Psychosom Med. 2012;74:904–911. doi: 10.1097/PSY.0b013e318273bf33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simmons AN, Paulus MP, Thorp SR, Matthews SC, Norman SB, Stein MB. Functional activation and neural networks in women with posttraumatic stress disorder related to intimate partner violence. Biol Psychiatry. 2008;64:681–690. doi: 10.1016/j.biopsych.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daniels JK, Mcfarlane AC, Bluhm RL, Moores KA, Clark CR, Shaw ME, et al. Switching between executive and default mode networks in posttraumatic stress disorder: alterations in functional connectivity. J Psychiatry Neurosci. 2010;35:258–266. doi: 10.1503/jpn.090175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simmons A, Strigo IA, Matthews SC, Paulus MP, Stein MB. Initial evidence of a failure to activate right anterior insula during affective set shifting in posttraumatic stress disorder. Psychosom Med. 2009;71:373–377. doi: 10.1097/PSY.0b013e3181a56ed8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Critchley HD. The human cortex responds to an interoceptive challenge. Proc Natl Acad Sci U S A. 2004;101:6333–6334. doi: 10.1073/pnas.0401510101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 22.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fonzo GA, Simmons AN, Thorp SR, Norman SB, Paulus MP, Stein MB. Exaggerated and disconnected insular-amygdalar blood oxygenation level-dependent response to threat-related emotional faces in women with intimate-partner violence posttraumatic stress disorder. Biol Psychiatry. 2010;68:433–441. doi: 10.1016/j.biopsych.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klumpp H, Angstadt M, Phan KL. Insula reactivity and connectivity to anterior cingulate cortex when processing threat in generalized social anxiety disorder. Biol Psychol. 2012;89:273–276. doi: 10.1016/j.biopsycho.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- 27.Spreng RN, Grady CL. Patterns of brain activity supporting autobiographical memory, prospection, and theory of mind, and their relationship to the default mode network. J Cogn Neurosci. 2010;22:1112–1123. doi: 10.1162/jocn.2009.21282. [DOI] [PubMed] [Google Scholar]
- 28.Ros T, Theberge J, Frewen PA, Kluetsch R, Densmore M, Calhoun VD, et al. Mind over chatter: Plastic up-regulation of the fMRI salience network directly after EEG neurofeedback. NeuroImage. 2012 doi: 10.1016/j.neuroimage.2012.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hardt JV, Kamiya J. Anxiety change through electroencephalographic alpha feedback seen only in high anxiety subjects. Science. 1978;201:79–81. doi: 10.1126/science.663641. [DOI] [PubMed] [Google Scholar]
- 30.Rasey H, Lubar JF, Mcintyre A, Zoffuto A, Abbott PL. EEG biofeedback for the enhancement of attentional processing in normal college students. Journal of Neurotherapy. 1995;1:15–21. [Google Scholar]
- 31.Ros T, Munneke MA, Ruge D, Gruzelier JH, Rothwell JC. Endogenous control of waking brain rhythms induces neuroplasticity in humans. Eur J Neurosci. 2010;31:770–778. doi: 10.1111/j.1460-9568.2010.07100.x. [DOI] [PubMed] [Google Scholar]
- 32.Musall S, Von Pfostl V, Rauch A, Logothetis NK, Whittingstall K. Effects of Neural Synchrony on Surface EEG. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs389. [DOI] [PubMed] [Google Scholar]
- 33.Berger H. Über das Elektrenkephalogramm des Menschen. Arch Psychiatr Nervenkr. 1929;87:440–471. [Google Scholar]
- 34.Nunez PL, Wingeier BM, Silberstein RB. Spatial-temporal structures of human alpha rhythms: theory, microcurrent sources, multiscale measurements, and global binding of local networks. Hum Brain Mapp. 2001;13:125–164. doi: 10.1002/hbm.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cooper NR, Croft RJ, Dominey SJ, Burgess AP, Gruzelier JH. Paradox lost? Exploring the role of alpha oscillations during externally vs internally directed attention and the implications for idling and inhibition hypotheses. Int J Psychophysiol. 2003;47:65–74. doi: 10.1016/s0167-8760(02)00107-1. [DOI] [PubMed] [Google Scholar]
- 36.Laufs H, Kleinschmidt A, Beyerle A, Eger E, Salek-Haddadi A, Preibisch C, et al. EEG-correlated fMRI of human alpha activity. NeuroImage. 2003;19:1463–1476. doi: 10.1016/s1053-8119(03)00286-6. [DOI] [PubMed] [Google Scholar]
- 37.Laufs H, Krakow K, Sterzer P, Eger E, Beyerle A, Salek-Haddadi A, et al. Electroencephalographic signatures of attentional and cognitive default modes in spontaneous brain activity fluctuations at rest. Proc Natl Acad Sci U S A. 2003;100:11053–11058. doi: 10.1073/pnas.1831638100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mantini D, Perrucci MG, Del Gratta C, Romani GL, Corbetta M. Electrophysiological signatures of resting state networks in the human brain. Proc Natl Acad Sci U S A. 2007;104:13170–13175. doi: 10.1073/pnas.0700668104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jann K, Dierks T, Boesch C, Kottlow M, Strik W, Koenig T. BOLD correlates of EEG alpha phase-locking and the fMRI default mode network. NeuroImage. 2009;45:903–916. doi: 10.1016/j.neuroimage.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 40.Sadaghiani S, Scheeringa R, Lehongre K, Morillon B, Giraud AL, Kleinschmidt A. Intrinsic connectivity networks, alpha oscillations, and tonic alertness: a simultaneous electroencephalography/functional magnetic resonance imaging study. J Neurosci. 2010;30:10243–10250. doi: 10.1523/JNEUROSCI.1004-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu L, Eichele T, Calhoun VD. Reactivity of hemodynamic responses and functional connectivity to different states of alpha synchrony: a concurrent EEG-fMRI study. NeuroImage. 2010;52:1252–1260. doi: 10.1016/j.neuroimage.2010.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for Axis I DSM-IV Disorders–Patient Edition (SCID-I/P) New York, NY: American Psychiatric Press; 1995. [Google Scholar]
- 43.Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, et al. The development of a clinician-administered PTSD scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 44.Bernstein DP, Fink L, Handelsman L, Foote J. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry. 1994;151:1132–1136. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- 45.Spielberger CD. Manual for the state-trait anxiety inventory. Palo Alto, CA: Consulting Psychologists; 1983. [Google Scholar]
- 46.Frewen PA, Dozois DJA, Neufeld RWJ, Densmore M, Stevens TK, Lanius RA. Self-referential processing in women with PTSD: Affective and neural response. Psychological Trauma: Theory, Research, Practice, and Policy. 2011;3:318. [Google Scholar]
- 47.Van Boxtel GJ, Denissen AJ, Jager M, Vernon D, Dekker MK, Mihajlovic V, et al. A novel self-guided approach to alpha activity training. Int J Psychophysiol. 2012;83:282–294. doi: 10.1016/j.ijpsycho.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 48.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 50.Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp. 2001;14:140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Calhoun VD, Liu J, Adali T. A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. NeuroImage. 2009;45:S163–S172. doi: 10.1016/j.neuroimage.2008.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Himberg J, Hyvarinen A, Esposito F. Validating the independent components of neuroimaging time series via clustering and visualization. NeuroImage. 2004;22:1214–1222. doi: 10.1016/j.neuroimage.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 53.Erhardt EB, Rachakonda S, Bedrick EJ, Allen EA, Adali T, Calhoun VD. Comparison of multi-subject ICA methods for analysis of fMRI data. Hum Brain Mapp. 2011;32:2075–2095. doi: 10.1002/hbm.21170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garrity AG, Pearlson GD, Mckiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant "default mode" functional connectivity in schizophrenia. Am J Psychiatry. 2007;164:450–457. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- 55.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 56.Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. Soc Cogn Affect Neur. 2009;4:423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hayasaka S, Nichols TE. Combining voxel intensity and cluster extent with permutation test framework. NeuroImage. 2004;23:54–63. doi: 10.1016/j.neuroimage.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 58.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 59.Hall EE, Ekkekakis P, Petruzzello SJ. Predicting affective responses to exercise using resting EEG frontal asymmetry: does intensity matter? Biol Psychol. 2010;83:201–206. doi: 10.1016/j.biopsycho.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 60.Barr MS, Farzan F, Arenovich T, Chen R, Fitzgerald PB, Daskalakis ZJ. The effect of repetitive transcranial magnetic stimulation on gamma oscillatory activity in schizophrenia. PloS one. 2011;6:e22627. doi: 10.1371/journal.pone.0022627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Neuper C, Pfurtscheller G. Event-related dynamics of cortical rhythms: frequency-specific features and functional correlates. Int J Psychophysiol. 2001;43:41–58. doi: 10.1016/s0167-8760(01)00178-7. [DOI] [PubMed] [Google Scholar]
- 62.Knyazev GG, Savostyanov AN, Levin EA. Alpha oscillations as a correlate of trait anxiety. Int J Psychophysiol. 2004;53:147–160. doi: 10.1016/j.ijpsycho.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 63.Knyazev GG, Savostyanov AN, Levin EA. Anxiety and synchrony of alpha oscillations. Int J Psychophysiol. 2005;57:175–180. doi: 10.1016/j.ijpsycho.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 64.Peniston EG, Kulkosky PJ. Alpha-theta brainwave training and beta-endorphin levels in alcoholics. Alcohol Clin Exp Res. 1989;13:271–279. doi: 10.1111/j.1530-0277.1989.tb00325.x. [DOI] [PubMed] [Google Scholar]
- 65.Peniston EG, Kulkosky PJ. Alpha-theta brainwave neuro-feedback therapy for Vietnam veterans with combat-related post-traumatic stress disorder. Medical Psychotherapy: An International Journal. 1991;4:47–60. [Google Scholar]
- 66.Raymond J, Varney C, Parkinson LA, Gruzelier JH. The effects of alpha/theta neurofeedback on personality and mood. Brain Res Cogn Brain Res. 2005;23:287–292. doi: 10.1016/j.cogbrainres.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 67.Hammond DC. Neurofeedback with anxiety and affective disorders. Child Adolesc Psychiatr Clin N Am. 2005;14:105–123. doi: 10.1016/j.chc.2004.07.008. vii. [DOI] [PubMed] [Google Scholar]
- 68.Jokic-Begic N, Begic D. Quantitative electroencephalogram (qEEG) in combat veterans with post-traumatic stress disorder (PTSD) Nord J Psychiatry. 2003;57:351–355. doi: 10.1080/08039480310002688. [DOI] [PubMed] [Google Scholar]
- 69.Haegens S, Nacher V, Luna R, Romo R, Jensen O. alpha-Oscillations in the monkey sensorimotor network influence discrimination performance by rhythmical inhibition of neuronal spiking. Proc Natl Acad Sci U S A. 2011;108:19377–19382. doi: 10.1073/pnas.1117190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goldman RI, Stern JM, Engel J, Jr, Cohen MS. Simultaneous EEG and fMRI of the alpha rhythm. Neuroreport. 2002;13:2487–2492. doi: 10.1097/01.wnr.0000047685.08940.d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, et al. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- 73.Mcrae K, Reiman EM, Fort CL, Chen K, Lane RD. Association between trait emotional awareness and dorsal anterior cingulate activity during emotion is arousal-dependent. NeuroImage. 2008;41:648–655. doi: 10.1016/j.neuroimage.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Macdonald JS, Mathan S, Yeung N. Trial-by-Trial Variations in Subjective Attentional State are Reflected in Ongoing Prestimulus EEG Alpha Oscillations. Front Psychol. 2011;2:82. doi: 10.3389/fpsyg.2011.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Klimesch W, Sauseng P, Hanslmayr S. EEG alpha oscillations: the inhibition-timing hypothesis. Brain Res Rev. 2007;53:63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 76.Terasawa Y, Fukushima H, Umeda S. How does interoceptive awareness interact with the subjective experience of emotion? An fMRI Study. Hum Brain Mapp. 2011 doi: 10.1002/hbm.21458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. J Cogn Neurosci. 2002;14:785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- 79.Northoff G, Heinzel A, De Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain--a meta-analysis of imaging studies on the self. NeuroImage. 2006;31:440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 80.Rameson LT, Satpute AB, Lieberman MD. The neural correlates of implicit and explicit self-relevant processing. NeuroImage. 2010;50:701–708. doi: 10.1016/j.neuroimage.2009.12.098. [DOI] [PubMed] [Google Scholar]
- 81.Fossati P, Hevenor SJ, Graham SJ, Grady C, Keightley ML, Craik F, et al. In search of the emotional self: an fMRI study using positive and negative emotional words. Am J Psychiatry. 2003;160:1938–1945. doi: 10.1176/appi.ajp.160.11.1938. [DOI] [PubMed] [Google Scholar]
- 82.Moran JM, Macrae CN, Heatherton TF, Wyland CL, Kelley WM. Neuroanatomical evidence for distinct cognitive and affective components of self. J Cogn Neurosci. 2006;18:1586–1594. doi: 10.1162/jocn.2006.18.9.1586. [DOI] [PubMed] [Google Scholar]
- 83.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 84.Bluhm RL, Frewen PA, Coupland NC, Densmore M, Schore AN, Lanius RA. Neural correlates of self-reflection in post-traumatic stress disorder. Acta Psychiatr Scand. 2012;125:238–246. doi: 10.1111/j.1600-0447.2011.01773.x. [DOI] [PubMed] [Google Scholar]
- 85.Frewen PA, Dozois DJ, Neufeld RW, Lane RD, Densmore M, Stevens TK, et al. Emotional numbing in posttraumatic stress disorder: a functional magnetic resonance imaging study. J Clin Psychiatry. 2012;73:431–436. doi: 10.4088/JCP.10m06477. [DOI] [PubMed] [Google Scholar]
- 86.Lanius RA, Bluhm RL, Frewen PA. How understanding the neurobiology of complex post-traumatic stress disorder can inform clinical practice: a social cognitive and affective neuroscience approach. Acta Psychiatr Scand. 2011;124:331–348. doi: 10.1111/j.1600-0447.2011.01755.x. [DOI] [PubMed] [Google Scholar]
- 87.Berman MG, Peltier S, Nee DE, Kross E, Deldin PJ, Jonides J. Depression, rumination and the default network. Soc Cogn Affect Neur. 2011;6:548–555. doi: 10.1093/scan/nsq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS spectrums. 2008;13:663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kohn N, Falkenberg I, Kellermann T, Eickhoff SB, Gur RC, Habel U. Neural correlates of effective and ineffective mood induction. Soc Cogn Affect Neur. 2013 doi: 10.1093/scan/nst055. [DOI] [PMC free article] [PubMed] [Google Scholar]