Abstract
Mass or body-size measures of ‘condition’ are of central importance to the study of ecology and evolution, and it is often assumed that differences in condition measures are positively and linearly related to fitness. Using examples drawn from ecological studies, we show that indices of condition frequently are unlikely to be related to fitness in a linear fashion. Researchers need to be more explicit in acknowledging the limitations of mass-based condition measures and accept that, under some circumstances, they may not relate to fitness as traditionally assumed. Any relationship between a particular condition measure and fitness should first be empirically validated before condition is used as a proxy for fitness. In the absence of such evidence, researchers should explicitly acknowledge that assuming such a relationship may be unrealistic.
Keywords: birds, condition, fat reserves, fitness, individual phenotypic quality, insects, mammals, mass reserves
Introduction
The concept of ‘condition’, which is often based on a mass- and body-size measure, is of central importance in the study of ecology and evolution for two reasons: 1) animals in better condition are better able to withstand harsh environmental events (e.g., Evans 1969) and 2) sexual selection theory posits that the development and expression of secondary sexual traits often are condition dependent (e.g., Andersson 1986). Moreover, whilst most biologists have an understanding of condition, they frequently differ in how they define it. Here, we use Rowe & Houle's (1996) definition of condition, which is an animal's bodily energy reserves after maintenance costs have been accounted for. This definition emphasises the trade-offs that animals must make to exploit and respond to their environment. Therefore, animals in better condition ought to have more body fat because excess energy is stored as fat. Ecologists have, thus, traditionally weighed animals to be able to estimate their fat reserves and infer relative differences in Darwinian fitness among individuals (Hayes & Shonkwiler, 2001; Labocha & Hayes, 2012). This focus has engendered two points of concern in the literature: 1) how do measures of mass reflect body composition and fat levels, and 2) whether mass levels vary and whether this variation is related to fitness. Here, we deal with the second point as others have dealt with the first (e.g., Green, 2001; Hayes & Shonkwiler, 2001; Schulte-Holstedde et al., 2005; Salewski et al., 2009; Schamber et al., 2009; Labocha and Hayes, 2012).
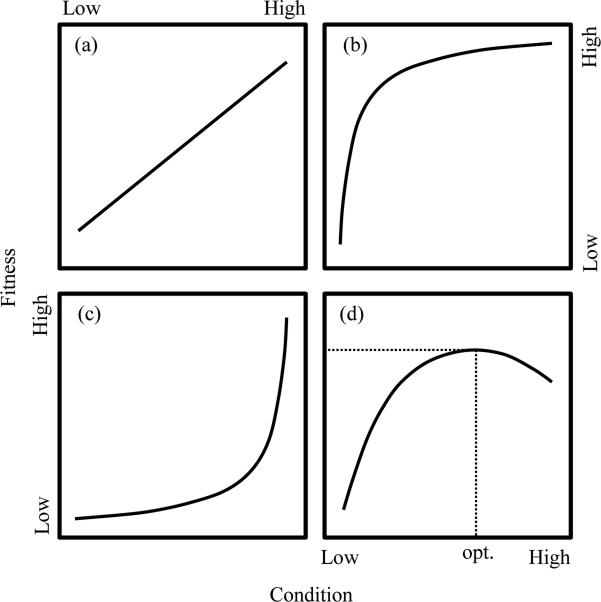
Normally, condition is assumed to relate to fitness as a positive, linear function (e.g., Wilson and Nussey, 2010) (Figure 1a). This relationship between condition and Darwinian fitness is likely based on the wider belief that phenotypic variability represents underlying genetic variation (Lloyd, 1977; Maynard Smith, 1978; Grafen, 1984; Cheverud, 1988; Roff, 1996). However, is this assumption realistic? For example, differences in mating systems could have important implications for the nature of the relationship between condition and fitness. In monogamous species with low rates of extra-pair paternity, most males recruited into the breeding population are likely to reproduce despite potentially large differences in condition among individuals (Figure 1b). Conversely, the relationship between fitness and condition of males in polygynous species is likely to be the opposite of that in monogamous species (Figure 1c), with females choosing only males in the best condition as mates and many males failing to reproduce irrespective of their condition. Despite examples of such non-linear relations between condition and reproductive success, the relationships are still positive (Figure 1b,c). However, there are also situations where the relation between fitness and condition might not be positive (Figure 1d). We focus on some examples to illustrate this point and show that maximal condition might not always yield maximal fitness. In doing so, we hope to question this normally assumed positive relation between fitness and condition.
Figure 1.
The relationship between condition (e.g., mass) and fitness is generally assumed to be positive and linear (a). However, in cases of highly monogamous species with low rates of extra-pair paternity (b) and polygynous species (c), the relationship may be positive, but non-linear. In some circumstances, the relationship between condition and fitness could be more complicated than in a–c. For example, the trade-off between mass and predation risk may make it costly for birds to gain mass because it increases their chances of being caught by predators (d). The dotted line indicates the optimal fitness yield for condition.
Our examples are drawn mainly from vertebrate taxa; however, we recognise that invertebrates also provide some compelling examples of the complexities of studying condition. For example, as with vertebrate taxa, some authors suggest that fat reserves are a primary determinant of condition in insects (e.g., Moya-Laraño et al. 2008; Arrese & Soulages 2010). However, reduction in the availability of a protein (apolipophorin III) limits the ability of crickets (Gryllus texensis) to utilize fat stores after energetically demanding activities (e.g., flight). This, in turn, reduces the immune response (mortality of individuals when injected with heat-killed bacteria [Serratia marcescens]) of the crickets (Adamo et al. 2008). Therefore, many other factors besides fat reserves (or mass) may determine condition in insects as well as in vertebrates.
Some problems with condition
The recent debate on individual quality, a concept that often uses measures of condition as a proxy (Wilson & Nussey, 2010; Bergeron et al., 2011; Lailvaux & Kasumovic, 2011), calls attention to an important point: whether traditional condition measures (especially mass-based) are good measures of condition and whether they are related to an animal's Darwinian fitness at all. We consider this point by presenting five examples that together call into question the continued, uncritical use of traditional condition indices under a range of commonly encountered situations.
First, because there is a trade-off between mass and predation-risk, small birds rarely carry their physiological maximum of body fat. When birds eat more than is necessary for maintenance, they store the excess energy as fat, which is reflected in mass gain. This reduces their probability of starving to death, but also increases their risk of capture by predators (Lima, 1986). Therefore, once a bird has gained sufficient mass to avoid starvation, there is no advantage to gaining further mass because of the increase in the risk of predation (dotted lines in Figure 1d). This suggests that the presence of predators can have a significant effect on the body mass of their prey (Gosler, 1996). For example, in parts of the U.K., the abundance of sparrow hawks (Accipiter nisus) declined during the 1960s as a result of widespread pesticide use; concomitantly, the mass of their prey, great tits (Parus major), increased. The tits then became lighter again as sparrow hawk numbers increased in the 1970s and 1980s to the point where they were again comparable to those recorded in the early 1950s (Gosler et al., 1995). Because the dispersion pattern of predators is not uniform within and among environments, their effects will not be perceived equally among individuals in prey populations. Therefore, assuming that perceived and actual predation risk is equal among individuals or populations may lead to misinterpretation of the fitness-related differences in mass-based measures of condition.
Second, social status can affect individuals' food access and, therefore, the amounts of fat that they should carry. In many species, dominant individuals carry less body fat (and are thus lighter) than subordinates (Krams et al., 2010; Ratikainen & Wright, 2013). Socially dominant great tits under normal conditions are lighter than subordinate individuals (Krams et al., 2010) because subordinates must carry fat to avoid starvation when dominants deny them access to food. In contrast, dominant individuals with free access to food do not need to store fat as a buffer (Krams et al., 2010; Ratikainen & Wright, 2013). However, when the temperature is very low or when day-length becomes short, the benefits of body reserves become more important and dominant individuals increase their fat reserves relative to subordinates (Krams et al., 2010). This interaction between social status and the environment complicates the relation between mass-based condition measures and fitness, a complication that is rarely considered.
Third, the length of time that individuals of different species of animals can survive without eating (i.e., fasting ability) is related to their overall body size (Linstedt & Boyce, 1985; Blem, 1990). Individuals of larger species can survive longer without access to food than individuals of smaller species. Thus, the fat reserves (and therefore condition indices) of smaller animals are more changeable over short periods of time than those of larger animals (Linstedt & Boyce 1985; Krams et al., 2010). For example, condition indices of a small species can change spectacularly over a two-day period, whereas the change in a larger species over the same period is much less dramatic (Blem, 1990). Even within species, size dimorphism may contribute to differences in mass regulation strategies. Furthermore, the reproductive success (and fitness) in animals is the culmination of fitness differences that might persist between individuals within a population over a period of months or years. Therefore, the changeability of mass-based measures of condition in smaller species means that their importance in relation to condition-dependent traits, reproductive success, or fitness is diminished. Despite this, body-size and how it might affect the changeability of condition over time is rarely considered in studies of condition (Krams et al., 2010).
Fourth, the terminal investment life-history strategy may decouple the relation between an animal's apparent condition and its reproductive success (e.g., Bowers et al. 2012). This hypothesis posits that when an animal's residual reproductive value is low, it should increase current reproductive effort because its current reproductive attempt is likely to be its last (Williams, 1966). Such individuals may often be in poor condition, and individuals that are old or immune-challenged have indeed been found to invest more in their current reproductive attempt than others (Velando et al., 2006; Weil et al., 2006; Bowers et al., 2012). Therefore, old or immunologically challenged individuals may have higher reproductive success within breeding attempts than younger or unchallenged individuals. Again, this may be contrary to the relationship that we would normally expect between condition measures and fitness.
Finally, whilst energy availability is an important component affecting reproductive success in many species, it may not be the only constraint limiting individual reproductive effort and success. Rather, animals may be limited by specific components of their diets rather than overall caloric content of their food. For example, female birds may be constrained by calcium availability during egg formation and laying (Pabian & Brittingham, 2011) and bats may also be limited by calcium for milk production when nursing offspring (Barclay, 1994). If reproduction is limited by an animal's access to specific dietary components, mass-based condition indices might lead to erroneous conclusions.
Conclusions and Recommendations
The study of condition is complicated because it assumes there is some relationship between body mass, body size, or fat reserves and Darwinian fitness. We have argued here that using indices of condition as a short-term proxy of fitness is problematic because ecologists often uncritically view differences in condition among individuals as being related to fitness. The relations between short-term measures of condition and life-time reproductive success and Darwinian fitness are still poorly understood in most free-living animals. We do not deny that differences in condition may relate to fitness, but we emphasize that they may do so in unexpected ways.
If there is reason to believe that there is a relationship between a particular condition measure and fitness, researchers should explicitly present evidence when they draw conclusions based on their measures of condition. In the absence of such evidence, researchers might be better to abandon the use of the term ‘condition’ and simply name which phenotypic traits they measured. Researchers should also explicitly acknowledge that assuming that there is a relationship between condition and fitness may be unrealistic because differences in fitness accrue over much longer time-spans than differences in fat reserves and body mass may persist. Mass-based measures of condition are essentially snapshots of animal energy reserves, and condition becomes more unreliable as a proxy of fitness the greater the amount of time separating the measurement of condition and the estimate of fitness (Hõrak et al., 2002; Hatch & Smith, 2010).
This also applies as new measures of condition, such as physiological measures (Wingfield & Farner, 1976; Grubb, 2006; Davis et al., 2008; Willie et al., 2010), become more widely used. These and other approaches (e.g., Milot et al., 2014) hold promise, but they are prone to the same problems as mass-based measures of condition. For example, recent studies show that within individuals different measures of immune responsiveness can be uncorrelated (pied flycatcher, Ficedula hypoleuca; González-Braojos et al., 2013) or even negatively correlated (house wrens, Troglodytes aedon; Forsman et al., 2008). Therefore, without a good understanding of how a particular new measure relates to fitness, we risk making the same mistakes we have been whilst using mass-based measures of condition.
We thus recommend that ecologists avoid assuming that short-term determination of any measure of condition provides an estimate of anything other than short-term condition. This change in approach need not dramatically alter how ecologists conduct research, but it does restrict the conclusions that they might draw from such data. However, until we have more knowledge of how the various measures of condition relate to Darwinian fitness, this is the safest practice.
Acknowledgement
Our work is supported by Grants-in-Aid for JSPS fellows (CAB and TNS) and from the National Science Foundation (IBN-0316580, IOS-1118160) and National Institutes of Health (R15HD076308-01) (SKS and CFT).
References
- Adamo SA, Roberts JL, Easy RH, Ross NW. Competition between immune function and lipid transport for the protein apolipophorin III leads to stress-induced immunosuppression in crickets. J. Exp. Biol. 2008;211:531–538. doi: 10.1242/jeb.013136. [DOI] [PubMed] [Google Scholar]
- Andersson M. The evolution of condition-dependent sex ornaments and mating preferences: sexual selection based on viability differences. Evolution. 1986;40:804–816. doi: 10.1111/j.1558-5646.1986.tb00540.x. [DOI] [PubMed] [Google Scholar]
- Arrese EL, Soulages JL. Insect fat body: energy, metabolism, and regulation. Annu. Rev. Entomol. 2010;55:207–225. doi: 10.1146/annurev-ento-112408-085356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay RMR. Constraints on reproduction by flying vertebrates: energy and calcium. Am. Nat. 1994;144:1021–1031. [Google Scholar]
- Bergeron P, Baeta R, Pelletier F, Réale D, Garant D. Individual quality: tautology or biological reality? J. Anim. Ecol. 2011;80:361–364. doi: 10.1111/j.1365-2656.2010.01770.x. [DOI] [PubMed] [Google Scholar]
- Blem CR. Avian energy storage. Curr. Ornithol. 1990;7:59–113. [Google Scholar]
- Bowers EK, Smith RA, Hodges CJ, Zimmerman LM, Thompson CF, Sakaluk SK. Sex-biased terminal investment in offspring induced by maternal immune challenge in the house wren (Troglodytes aedon). Proc. Roy. Soc. Lond. B. 2012;279:2891–2898. doi: 10.1098/rspb.2012.0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheverud JM. A comparison of genetic and phenotypic correlations. Evolution. 1988;42:958–968. doi: 10.1111/j.1558-5646.1988.tb02514.x. [DOI] [PubMed] [Google Scholar]
- Davis AK, Maney DL, Maerz JC. The use of leukocyte profiles to measure stress in vertebrates: a review for ecology. Funct. Ecol. 2008;22:760–772. [Google Scholar]
- Evans PR. Winter fat deposition and overnight survival of yellow buntings (Emberiza citronella L.). J. Anim. Ecol. 1969;38:415–423. [Google Scholar]
- Forsman AM, Vogel LA, Sakaluk SK, Grindstaff JL, Thompson CF. Immune-challenged house wren broods differ in the relative strengths of their responses among different axes of the immune system. J. Evol. Biol. 2008;21:873–878. doi: 10.1111/j.1420-9101.2008.01503.x. [DOI] [PubMed] [Google Scholar]
- González-Braojos S, Ruiz-de-Castañeda R, Moreno J. No association between measures of immunity in nestling pied flycatchers (Ficedula hypoleuca). Annu. Zool. Fenn. 2013;50:279–288. [Google Scholar]
- Gosler AG. Environmental and social determinants of winter fat storage in the great tit Parus major. J Anim. Ecol. 1996;65:1–17. [Google Scholar]
- Gosler AG, Greenwood JJD, Perrins C. Predation risk and the cost of being fat. Nature. 1995;377:621–623. [Google Scholar]
- Green AJ. Mass/length residuals: measures of body condition or generators of spurious results? Ecology. 2001;82:1473–1483. [Google Scholar]
- Grafen A. Natural selection, kin selection and group selection. In: Krebs JR, Davies NB, editors. Behavioural ecology: an evolutionary approach. 2nd edn Blackwell; Oxford: 1984. pp. 62–84. [Google Scholar]
- Grubb TC., Jr. Ptilochronology: feather time and the biology of birds. Oxford University Press; Oxford: 2006. [Google Scholar]
- Hatch MI, Smith RJ. Repeatability of hematocrits and body mass of gray catbirds. J. Field Ornithol. 2010;81:64–70. [Google Scholar]
- Hayes JP, Shonkwiler JS. Morphometric indicators of body condition: worthwhile or wishful thinking? In: Speakman JR, editor. In Body composition analysis of animals: a handbook of non-destructive methods. Cambridge University Press; Cambridge: 2001. pp. 8–38. [Google Scholar]
- Hõrak P, Saks L, Ots I, Collist H. Repeatability of condition indices in captive greenfinches (Carduelis chloris). Can. J. Zool. 2002;80:636–643. [Google Scholar]
- Krams I, Cirule D, Suraka V, Krama T, Rantala MJ, Ramey G. Fattening strategies of wintering great tits support the optimal body mass hypothesis under conditions of extremely low ambient temperature. Funct. Ecol. 2010;24:172–177. [Google Scholar]
- Labocha MK, Hayes JP. Morphometric indices in body condition in birds: a review. J. Ornithol. 2012;153:1–22. [Google Scholar]
- Lailvaux SP, Kasumovic MM. Defining individual quality over lifetimes and selective contexts. Proc. Roy. Soc. Lond. B. 2011;278:321–328. doi: 10.1098/rspb.2010.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima SL. Predation risk and unpredictable feeding conditions: determinants of body mass in birds. Ecology. 1986;67:377–385. [Google Scholar]
- Lindstedt SL, Boyce MS. Seasonality, fasting endurance, and body size in mammals. Am. Nat. 1985;125:873–878. [Google Scholar]
- Lloyd DG. Genetic and phenotypic models of natural selection. J. Theor. Biol. 1977;69:543–560. doi: 10.1016/0022-5193(77)90155-2. [DOI] [PubMed] [Google Scholar]
- Maynard Smith J. Optimization theory in evolution. Annu. Rev. Ecol. Syst. 1978;9:31–56. [Google Scholar]
- Milot E, Cohen AA, Vézina F, Buehler DM, Matson KD, Piersma T. A novel integrative method for measuring body condition in ecological studies based on physiological dysregulation. Method. Ecol. Evol. 2014;5:146–155. [Google Scholar]
- Moya-Laraño J, Macías-Ordóñez R, Blanckenhorn WU, Fernández-Montraveta C. Analysing body condition: mass, volume or density? J Anim. Ecol. 2008;77:1099–1108. doi: 10.1111/j.1365-2656.2008.01433.x. [DOI] [PubMed] [Google Scholar]
- Pabian SE, Brittingham MC. Soil calcium availability limits forest songbird productivity and density. Auk. 2011;128:441–447. [Google Scholar]
- Ratikainen II, Wright J. Adaptive management of body mass by Siberian jays. Anim. Behav. 2013;85:427–434. [Google Scholar]
- Roff DA. The evolution of genetic correlations: an analysis of patterns. Evolution. 1996;50:1392–1403. doi: 10.1111/j.1558-5646.1996.tb03913.x. [DOI] [PubMed] [Google Scholar]
- Rowe L, Houle D. The lek paradox and the capture of genetic variance by condition dependent traits. Proc. Roy. Soc. Lond. B. 1996;263:1415–1421. [Google Scholar]
- Salewski V, Kéry M, Herremans M, Lietchi F, Jenni L. Estimating fat and protein fuel from fat and muscle scores in passerines. Ibis. 2009;151:640–653. [Google Scholar]
- Schamber JL, Esler D, Flint PL. Estimating the validity of using unverified indices of body condition. J. Avian Biol. 2009;40:49–56. [Google Scholar]
- Schulte-Holstedde AI, Zinner B, Millar JS, Hickling GJ. Restitution of mass-size residuals: validating body condition indices. Ecology. 2005;86:155–163. [Google Scholar]
- Velando A, Drummond H, Torres R. Senescent birds redouble reproductive effort when ill: confirmation of the terminal investment hypothesis. Proc. Roy. Soc. Lond. B. 2006;273:1443–1448. doi: 10.1098/rspb.2006.3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil ZM, Martin LB, Workman JL, Nelson RJ. Immune challenge retards seasonal reproductive regression in rodents: evidence for terminal investment. Biol. Lett. 2006;2:393–396. doi: 10.1098/rsbl.2006.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GC. Adaptation and natural selection. Princeton University Press; Princeton: 1966. [Google Scholar]
- Willie J, Travers M, Williams TD. Female zebra finches (Taeniopygia guttata) are chronically but not cumulatively “anemic” during repeated egg laying in response to experimental nest predation. Physiol. Biochem. Zool. 2010;83:119–126. doi: 10.1086/605478. [DOI] [PubMed] [Google Scholar]
- Wilson AJ, Nussey DH. What is individual quality? An evolutionary perspective. Trends Ecol. Evol. 2010;25:207–214. doi: 10.1016/j.tree.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Farner DS. Avian endocrinology –field investigations and methods. Condor. 1976;78:570–573. [Google Scholar]