Highlights
-
•
Prophylactic oophorectomy can prevent small cell carcinoma of the ovary, hypercalcemic type in carriers of germline SMARCA4 mutations.
-
•
Unaffected SMARCA4 mutation carriers who desire children may be best served by oocyte cryopreservation prior to prophylactic oophorectomy.
Keywords: Ovarian cancer, SMARCA4 mutation, Prophylactic oophorectomy
Introduction
Small-cell carcinoma of the ovary, hypercalcemic type (SCCOHT) is a highly lethal ovarian malignancy that occurs in young women (average age 24) (Young et al., 1994). The early age of onset and familial aggregation of some cases suggest a hereditary cause (Young et al., 1994). These undifferentiated cancers are comprised of small hyperchromatic cells with brisk mitotic activity, and can be difficult to distinguish morphologically from other undifferentiated tumors. Based on their microscopic appearance, SCCOHTs have been referred to as rhabdoid tumors, however, their cell of origin is unknown. Approximately 60% of SCCOHTs present with hypercalcemia and some have been shown to express parathyroid hormone-related protein (Young et al., 1994). The primary ovarian tumors are almost always unilateral and roughly half are metastatic at diagnosis. Even among stage IA cases, five year survival is only 33% (Young et al., 1994).
Our group and others recently reported that inherited and sporadic mutations in the SMARCA4 gene, which encodes the BRG1 protein (a component of the SWI/SNF chromatin remodeling complex), are the major cause of SCCOHT (Witkowski et al., 2014). SCCOHT arising in SMARCA4 mutation carriers does not express immunohistochemically detectable BRG1. It also has been shown that other members of the SWI/SNF family are frequently mutated in various types of cancers (Wang et al., 2014). Of relevance to SCCOHT, SMARCA4, or the related SMARCB1 gene, are mutated in virtually all rhabdoid tumors, with germline mutations causing the disease in some of the cases (Foulkes et al., 2014). This report describes the first instance of genetic counseling and prophylactic bilateral oophorectomy based on mutational analysis of the SMARCA4 gene in familial SCCOHT.
Case
This patient is a 33 year old Caucasian mother of three teenage boys. Three female relatives had previously been diagnosed with SCCOHT (ages 26, 24 and 12 years) and two had died of the disease (Witkowski et al., 2014). We recently reported identification of the causative SMARCA4 mutation in the affected women in this family, c.2617-3 C > T (Witkowski et al., 2014). This mutation results in a truncated protein that is subject to nonsense-mediated decay and was accompanied by a deleterious mutation in the other allele in the tumor. In view of the position of this patient in the pedigree and the known age distribution at diagnosis of SCCOHT (14 months to 47 years) (Young et al., 1994), we offered her genetic testing, resulting in the identification of the known familial mutation. Prior to genetic testing, we discussed the possibility of prophylactic bilateral oophorectomy. We explained that the risk of SCCOHT for carriers of deleterious mutations in SMARCA4 has not yet been well quantified, but the penetrance appears to be fairly high. We also noted the three cases of SCCOHT in this family, all confirmed by pathology report (Witkowski et al., 2014). The patient elected to undergo total laparoscopic hysterectomy and bilateral salpingo-oophorectomy, and the procedure was completed uneventfully. She received estrogen replacement therapy post-operatively. The ovaries were serially sectioned and examined by a gynecologic pathologist to look for evidence of premalignant or early malignant changes, but none were found. Immunohistochemical staining of multiple sections of both ovaries did not reveal loss of BRG1 expression in the ovarian stroma, follicles, or epithelium.
Discussion
Current evidence suggests that inherited forms of SCCOHT may be more common than previously thought (Foulkes et al., 2014). Given the rarity of these tumors, expert pathology consultation is appropriate in verifying the diagnosis, which can be facilitated by demonstration of loss of BRG1 immunostaining in tumor nuclei. Although in many tumors both SMARCA4 mutations are somatic, in some cases one mutation is germline and others in the family may also be carriers. In view of this, genetic counseling and mutational analysis of the SMARCA4 gene should be offered to families affected by SCCOHT. Mutational analysis can be performed in academic centers and commercially and should be covered by insurance. The penetrance of germline mutations for SCCOHT is difficult to estimate, but appears to be considerable. Identification and counseling of male SMARCA4 mutation carriers are also important, as their daughters may inherit the mutation. It is unclear whether germline SMARCA4 mutations increase the risk of other types of cancers in women or men, but somatic mutations in this gene have been found in lung cancer, medulloblastoma and Burkitt lymphoma (Wang et al., 2014).
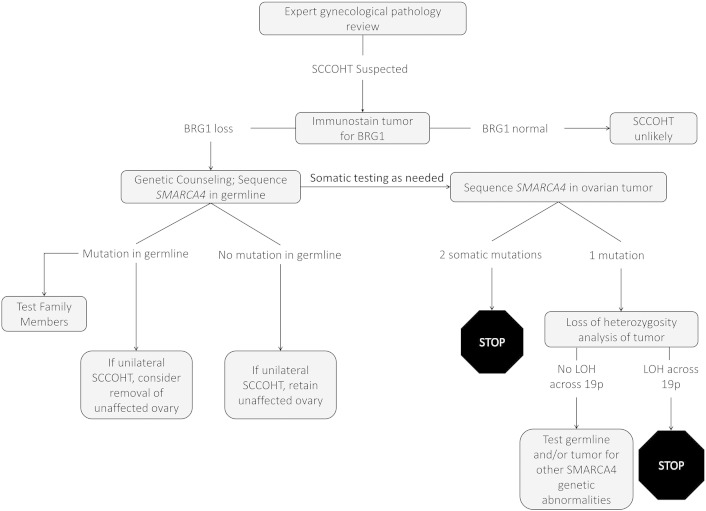
Most patients with SCCOHT present with a pelvic mass. In the absence of a known family history of the disease, unilateral oophorectomy is usually performed in those with stage I disease. A definitive diagnosis of SCCOHT is unlikely to be rendered on intraoperative frozen section and most of these cases occur well prior to the onset of menopause or completion of childbearing. If the final pathology report shows SCCOHT, immunostainining for BRG1 should be performed in the tumor. Germline genetic testing should be recommended if there is a loss of BRG1 expression (Fig. 1). Mutational analysis of the cancer and demonstration of a somatic mutation and loss of heterozygosity in the other allele may also be helpful to identify somatic SMARCA4 mutations and will provide assurance that a germline mutation was not missed (Fig. 1). If the mutation is somatic, a second surgery to remove the other ovary is not needed in stage IA cases, as the contralateral ovary should not be at increased risk for SCCOHT. If a germline mutation is found, removal of the other ovary should be strongly considered.
Fig. 1.
Proposed clinical algorithm for management of small cell carcinoma of the ovary, hypercalcemic type (SCCOHT).
It is instructive to compare the role of prophylactic surgery in SMARCA4 carriers to its more established role in preventing high grade serous cancers of the ovary, fallopian tube and peritoneum in BRCA1/2 mutation carriers. In view of the high lethality of serous cancers and lack of effective early detection methods, it is recommended that prophylactic bilateral salpingo-ophorectomy be performed after age 35, when the risk of high grade serous cancers begins to rise in carriers (Finch et al., 2014). In contrast, delaying prophylactic oophorectomy in those with stage I SCCOHT and in unaffected SMARCA4 carriers until childbearing is complete seems risky in view of the young age of onset and high lethality of this disease. Although screening and early detection using imaging techniques might be an alternative prior to prophylactic bilateral oophorectomy, this may not reduce mortality from a cancer such as SCCOHT with its high propensity for metastasis. However, it is possible that these tumors, which arise as a mass in the ovary, may be easier to detect at an early stage using imaging than BRCA1/2 associated cancers that primarily arise and spread from the fallopian tube epithelium. Since no cases of SCCOHT have been reported after age 45, it may be reasonable to perform preventive oophorectomy only opportunistically in older SMARCA4 mutation carriers who are having surgery for other indications.
Careful gross and microscopic examination should be performed in prophylactically removed ovaries of SMARCA4 carriers to look for a premalignant or malignant lesion. None were seen in this case, nor has this been reported in the literature (Young et al., 1994), but this is the first prophylactic surgery reported for this disease and lesions may be found with additional experience. The cell of origin of SCCOHT is unknown, but these cancers clearly arise from the ovary, perhaps from some type of stem cell. The SMARCA4 mutation carrier who underwent prophylactic surgery in this case report was 33 years old and was fortunate to not have already developed SCCOHT. Hopefully in the future, carriers will be identified at much earlier ages when prophylactic surgery can be performed while the prevention of SCCOHT is likely to be most effective. The degree to which very young unaffected carriers, particularly those who are prepubertal, can participate along with their parents in making this difficult decision will vary based on their age and maturity.
SMARCA4 mutation carriers will increasingly be identified in childhood and early adolescence and should then be counseled about prophylactic oophorectomy, but many will not have chosen a partner or considered conception. Ovarian hyperstimulation and immediate in vitro fertilization are not realistic options in most circumstances. There has been substantial improvement in pregnancy rates using cryopreserved oocytes due to improved methods with vitrification that involve more rapid freezing (Cil and Seli, 2013). Because success rates are similar to those using conventional in vitro fertilization, oocyte cryopreservation is no longer considered experimental for medical indications such as cancer (Loren et al., 2013). This may represent the best approach for many of these patients. A SMARCA4 mutation carrier who is not ready to conceive could undergo ovarian hyperstimulation and oocyte harvest prior to prophylactic bilateral oophorectomy with uterine preservation for future pregnancy via delayed in vitro fertilization. To prevent genetic transmission of the mutation, preimplantation genetic diagnosis of the first polar body prior to oocyte cryopreservation or of a subsequent embryo would be recommended (Keskintepe et al., 2009). Since there are case reports of SCCOHT prior to puberty, prophylactic bilateral oophorectomy could conceivably be considered in some SMARCA4 mutation carriers prior to menarche. In such cases, oocyte harvest and cryopreservation would not be feasible, but the uterus could be left in place for subsequent pregnancy using donor oocytes.
Although pregnancies have been reported after cryopreservation of ovarian tissue with subsequent reimplantation, the success rate is much lower and this is still considered experimental. An additional concern is that this could transplant cancer cells back into the patient. This is particularly problematic in SCCOHT, where the heightened cancer risk is specific to the ovary.
Conflict of interest statement
None of the authors of this paper has any financial or other conflict of interests in relation to this article.
References
- Cil A.P., Seli E. Current trends and progress in clinical applications of oocyte cryopreservation. Curr. Opin. Obstet. Gynecol. 2013;25:247–254. doi: 10.1097/GCO.0b013e32836091f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch A.P., Lubinski J., Moller P. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J. Clin. Oncol. 2014;32:1547–1553. doi: 10.1200/JCO.2013.53.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulkes W.D., Clarke B.A., Hasselblatt M., Majewski J., Albrecht S., McCluggage W.G. No small surprise — small cell carcinoma of the ovary, hypercalcaemic type, is a malignant rhabdoid tumour. J. Pathol. 2014;233:209–214. doi: 10.1002/path.4362. [DOI] [PubMed] [Google Scholar]
- Keskintepe L., Sher G., Machnicka A. Vitrification of human embryos subjected to blastomere biopsy for pre-implantation genetic screening produces higher survival and pregnancy rates than slow freezing. J. Assist. Reprod. Genet. 2009;26:629–635. doi: 10.1007/s10815-009-9369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loren A.W., Mangu P.B., Beck L.N. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J. Clin. Oncol. 2013;31:2500–2510. doi: 10.1200/JCO.2013.49.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Haswell J.R., Roberts C.W. Molecular pathways: SWI/SNF (BAF) complexes are frequently mutated in cancer—mechanisms and potential therapeutic insights. Clin. Cancer Res. 2014;20:21–27. doi: 10.1158/1078-0432.CCR-13-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkowski L., Carrot-Zhang J., Albrecht S. Germline and somatic SMARCA4 mutations characterize small cell carcinoma of the ovary, hypercalcemic type. Nat. Genet. 2014;46:438–443. doi: 10.1038/ng.2931. [DOI] [PubMed] [Google Scholar]
- Young R.H., Oliva E., Scully R.E. Small cell carcinoma of the ovary, hypercalcemic type. A clinicopathological analysis of 150 cases. Am. J. Surg. Pathol. 1994;18:1102–1116. doi: 10.1097/00000478-199411000-00004. [DOI] [PubMed] [Google Scholar]