Highlights
-
•
Inflammatory myofibroblastic tumor (IMT) tends to locally recur but rarely metastasizes.
-
•
IMT tends to locally recur but rarely metastasizes.
-
•
IMTs that do not express anaplastic lymphoma kinase (ALK) have worse prognosis.
-
•
Current trials for ALK targeted therapies show promise in treating this neoplasm.
Keywords: Abdominopelvic, Inflammatory myofibroblastic tumor (IMT), Metastasis, Anaplastic lymphoma kinase (ALK) immunohistochemistry
Introduction
Inflammatory myofibroblastic tumor (IMT) is a rare mesenchymal tumor that is more common in children and young adults, with a median age of 9 years old and slight predominance in females (Unni and Hogendoom, 2002). Although IMT can involve any location in the body, the abdominopelvic region, retroperitoneum and lungs are most commonly affected (Coffin et al., 1995). Grossly, IMTs appear firm or fleshy with a white or tan surface and can resemble other benign or malignant tumors in appearance such as leiomyoma, leiomyosarcoma, and inflammatory liposarcoma (Rabban et al., 2005). Histologically, IMTs have spindle cell proliferation with inflammatory infiltrates of plasma cells, lymphocytes, and eosinophils (Gleason and Hornick, 2008). Immunohistochemical (IHC) staining will typically show reactivity for vimentin, smooth muscle actin and desmin (Unni and Hogendoom, 2002). Approximately 50% of IMTs will have IHC cytoplasmic positivity for anaplastic lymphoma kinase (ALK), which can be used to differentiate the tumor from the ALK-negative leiomyoma and leiomyosarcoma (Unni and Hogendoom, 2002, Rabban et al., 2005).
IMT is currently regarded as a neoplasm of intermediate biologic potential because of its tendency to locally recur but rarely metastasize (Coffin et al., 2007). Management of these tumors is challenging because there are no established protocols for primary or recurrent disease. Herein, we present a case of a woman whose abdominopelvic IMT was surgically debulked and treated with various chemotherapeutic agents, yet metastasized to the bone and liver, with extensive peritoneal sarcomatosis, within 15 months after diagnosis.
Case presentation
A previously healthy nulliparous 28-year old Caucasian woman presented to her primary care physician with a two-week history of post-prandial abdominal pain. She was on oral contraceptives and had no family history of cancer. Physical examination revealed a palpable abdominal mass. The patient's CA 125 was 71 U/mL with negative AFP. Ultrasound and computed tomography (CT) scan showed ascites and multiple peritoneal masses (Fig. 1A), with the largest mass in the right upper quadrant measuring 6.5 × 8 cm. She was diagnosed with abdominal carcinomatosis and referred to gynecologic oncology.
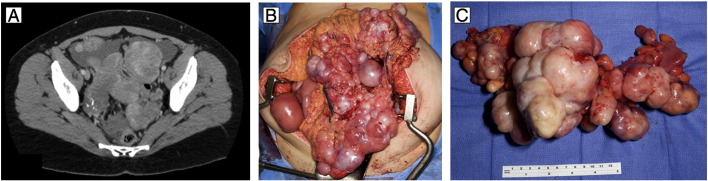
Fig. 1.
A) Preoperative CT image demonstrating multiple solid peritoneal masses. B) Intraoperative findings of multiple solid masses involving pelvic and abdominal viscera. C) Resected sigmoid colon with numerous nodules of inflammatory myofibroblastic tumor ranging from 1.0 cm to 13.5 cm in greatest dimension involving the serosa and mesentery.
Due to the uniform appearance of the masses, the differential diagnoses of disseminated leiomyomatosis and sarcoma were considered. The patient underwent an exploratory laparotomy which revealed extensive solid masses involving multiple viscera with impending obstruction (Fig. 1B). Tumor cytoreduction required resection of the sigmoid colon, two segments of ileum, the omentum, and the appendix. The masses were discrete and well-circumscribed, but deeply infiltrative into the adjacent muscularis of the small bowel and colon, prohibiting local excision without resection of the enteric tract. Despite the previously described resections to prevent obstruction and removal of several 8–10 cm masses from the ileal and jejunal mesenteries, several dozen nodules measuring up to 2 cm in size remained on the mesenteric border throughout the ileum and jejunum, prohibiting further resection. The uterus, ovaries and fallopian tubes were normal in appearance and left in situ as the patient desired fertility preservation.
Final pathologic analysis revealed the sigmoid colon, ileum and omentum to contain numerous nodules of IMT ranging in size from 0.5 cm to 13.5 cm in greatest dimension involving the serosa and mesentery (Fig. 1C). Histologically, the tumor was composed of a cellular proliferation of long, tapered, spindled cells forming loose fascicles in an edematous stroma with prominent lymphoplasmacytic infiltrate. There were occasional mitotic figures, including atypical forms. Some areas of tumor demonstrated ganglion-like round cells. By immunohistochemistry, the neoplastic cells were weakly reactive for smooth muscle actin (SMA) but negative for S100, desmin, KIT, anaplastic lymphoma kinase (ALK-1), CD34 and OSCAR-keratin, consistent with IMT (Fig. 2). The ascitic fluid and multiple lymph nodes from the omentum were negative for malignancy.
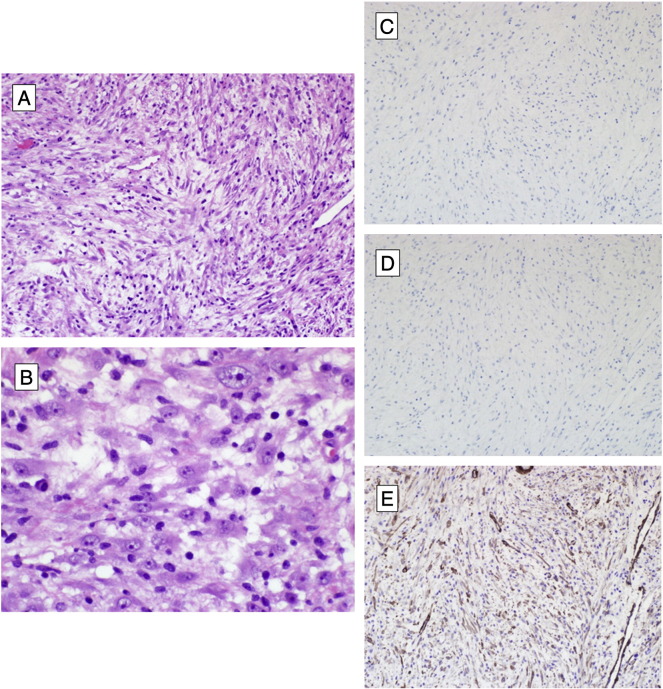
Fig. 2.
Inflammatory myofibroblastic tumor. A) Hematoxylin and eosin stained preparation, 200 × magnification, showing IMT with long, tapered, spindled cells arranged in loose fascicles in an edematous stroma with prominent lymphoplasmacytic infiltrate and occasional atypical mitotic figure. B) Hematoxylin and eosin stain, 600 × magnification, demonstrating ganglion-like tumor cell atypia. C), D), E) Immunohistochemistry, 200 × magnification, showing neoplastic cells to be negative for ALK-1 and desmin, but weakly positive for SMA, respectively.
Postoperatively, the patient was started on prednisone for three months and experienced tumor progression. She was switched to imatinib for a month. During this time, the patient was noted to have significant pelvic pain and ureteral compression; she had bilateral ureteral stents placed with relief of symptoms. She was then started on liposomal doxorubicin; however, she had continued progression. The patient then received 4 cycles of gemcitabine and docetaxel with minimal response. She subsequently was placed on oral pazopanib, initially experiencing stable disease, although the patient began noting increasing back and rib pain. Four months after starting pazopanib, magnetic resonance imaging (MRI) showed metastases to the L4 vertebral body (Fig. 3A). Consequently, the patient was treated with palliative radiation therapy. She completed 5 of a planned 10 fractions when she developed severe abdominal pain for which she presented to the emergency room. CT revealed extensive hepatic metastases and peritoneal sarcomatosis (Fig. 3B). She was also found to have polymicrobial peritonitis secondary to a small bowel perforation from tumor invasion. The patient elected for hospice care and expired 5 days later, 15 months after diagnosis.
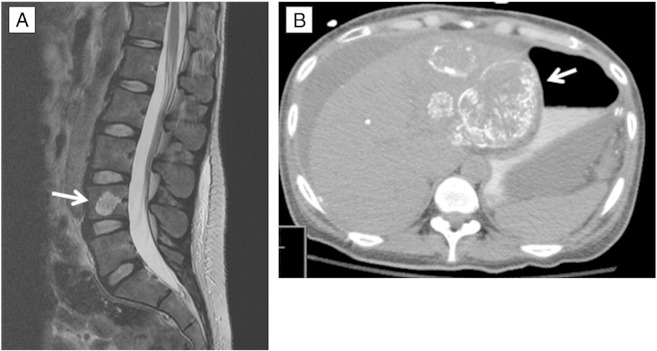
Fig. 3.
A) MRI shows sclerotic metastases to L4 vertebrae body (arrow) 14 months after initial diagnosis and surgical debulking, despite various systemic therapies. B) CT scan shows hepatic metastases (arrow) and extensive peritoneal sarcomatosis 15 months after diagnosis.
Discussion
In its first description in 1939, IMT was considered a benign disease process (Gleason and Hornick, 2008). In the gynecologic literature, IMT has been described also in the uterus as a benign tumor (Rabban et al., 2005, Fuehrer et al., 2012). However, over the past two decades, case series have illustrated the potentially aggressive course of the disease, including a 37% rate of local recurrence and 11% rate of metastases (Coffin et al., 1995, Meis and Enzinger, 1991). Subsequently, IMT is now classified by the World Health Organization as an intermediate, rarely metastasizing tumor (Unni and Hogendoom, 2002). While IMT can occur in any site in the body, primary abdominopelvic IMT is associated with a higher local recurrence rate of 25% compared to only 2% for primary pulmonary IMT (Gleason and Hornick, 2008, Coffin et al., 2007). When it does metastasize, the most frequent site of metastasis is the lungs and the most common sites of extrapulmonary IMT metastases are the mesentery and omentum (Unni and Hogendoom, 2002).
Patients with abdominopelvic IMT typically present with nonspecific symptoms such as abdominal pain, loss of appetite and weight, and a palpable mass (Unni and Hogendoom, 2002). Clinical presentation may also include fever, iron deficiency anemia and elevated sedimentation rate. With these symptoms, the differential diagnoses of peritoneal carcinomatosis, gastrointestinal stromal tumor (GIST) or lymphoma are often considered (Gleason and Hornick, 2008). In a young woman as in our patient, the additional consideration of disseminated peritoneal leiomyomatosis or leiomyosarcoma should be excluded (Rabban et al., 2005). In particular, the myxoid variant of leiomyosarcoma can be difficult to distinguish from IMT due to a similar well-circumscribed gross appearance and hypocellular pattern (Rabban et al., 2005). In addition, immunohistochemical staining for ALK-1, negative in leiomyosarcoma, may not be helpful in differentiating certain IMT, such as our patient's ALK-1 negative tumors, from leiomyosarcoma (Fuehrer et al., 2012).
The occurrence of extrapulmonary IMT metastases is rare and captured in only a handful of case series and reports (Meis and Enzinger, 1991, Morotti et al., 2005). With potential for IMTs to behave like a sarcoma (Hagenstad et al., 2003), there is a need to distinguish malignant disease from benign to establish appropriate management. Previous studies on prognostic indicators in IMT have examined morphology and genetics, suggesting that aneuploidy, p53 overexpression and cellular atypia are associated with progression of disease (Meis and Enzinger, 1991, Hussong et al., 1999). Histologic features described as associated with aggressive IMT are polygonal/ganglion-like round cells, increased cellularity, vesicular chromatin, prominent nucleoli, and atypical mitotic figures; the presence of atypical mitotic figures has been proposed as the most specific feature of aggressive tumors (Coffin et al., 2007). A recent promising prognostic factor that has been supported is ALK-1 immunohistochemistry. ALK gene rearrangements have been identified in 50–75% of extrapulmonary IMTs and this has strengthened the concept of IMT as a sarcoma variant (Coffin et al., 2001, Coffin et al., 2007). Furthermore, studies have suggested that ALK-negative IMTs may follow a more aggressive disease course with increased risk of metastasis (Coffin et al., 2007, Jiang et al., 2009). Thus, ALK1 immunohistochemistry may be helpful in determining prognosis. Whether ALK-positive and ALK negative tumors are distinct disease entities with overlapping morphologic features is a question of debate.
Surgical debulking with complete excision is the treatment of choice for IMTs and is often curative. However, the ability to achieve complete surgical excision depends on the site of the lesion, multinodularity, involvement of other organs and proximity to vital structures. For the unresectable or recurrent IMT, systemic treatments have provided inconsistent results. Systemic therapies that have been tried with varying success include NSAIDs, cyclooxygenase 2 inhibitors, steroids, radiation therapy, and various chemotherapeutic agents, including cisplatin, Adriamycin, methotrexate, vincristine, carboplatin and paclitaxel (Coffin et al., 1995). Curative options remain limited for patients with aggressive metastatic disease.
Current research has shifted to target gene therapy for treatment. For the IMT expressing ALK, there are now targeted therapies, such as crizotinib, an ALK inhibitor, which has been used to treat aggressive disease in recent case reports (Butrynski et al., 2010). Crizotinib was first approved for the treatment of ALK-positive non-small cell lung cancer (NSCLC) in 2011. In a meta-analysis of six clinical trials for treatment of ALK-positive NSCLC, crizotinib treatment demonstrated an objective response rate (ORR) of 61.2% (Qian et al., 2014) and appears to be a reasonable option for ALK-positive IMT (Butrynski et al., 2010). For the ALK-negative IMT, targeting kinase fusion genes is being explored (Lovly et al., 2014).
In conclusion, IMT is a rare tumor of intermediate malignant potential. Some tumors act in an aggressive fashion. Surgical debulking remains the primary treatment and determination of ALK expression may help triage patients to adjuvant therapies. Additional research on targeted therapies for this disease appears promising.
Consent
This study was deemed exempt by the Mayo Foundation Institutional Review Board.
Conflict of interest statement
The authors declare that there is no conflict of interest.
References
- Butrynski J.E. Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor. N. Engl. J. Med. 2010;363(18):1727–1733. doi: 10.1056/NEJMoa1007056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin C.M. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am. J. Surg. Pathol. 1995;19(8):859–872. doi: 10.1097/00000478-199508000-00001. [DOI] [PubMed] [Google Scholar]
- Coffin C.M. ALK1 and p80 expression and chromosomal rearrangements involving 2p23 in inflammatory myofibroblastic tumor. Mod. Pathol. 2001;14(6):569–576. doi: 10.1038/modpathol.3880352. [DOI] [PubMed] [Google Scholar]
- Coffin C.M., Hornick J.L., Fletcher C.D. Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am. J. Surg. Pathol. 2007;31(4):509–520. doi: 10.1097/01.pas.0000213393.57322.c7. [DOI] [PubMed] [Google Scholar]
- Fuehrer N.E. ALK-1 protein expression and ALK gene rearrangements aid in the diagnosis of inflammatory myofibroblastic tumors of the female genital tract. Arch. Pathol. Lab. Med. 2012;136(6):623–626. doi: 10.5858/arpa.2011-0341-OA. [DOI] [PubMed] [Google Scholar]
- Gleason B.C., Hornick J.L. Inflammatory myofibroblastic tumours: where are we now? J. Clin. Pathol. 2008;61(4):428–437. doi: 10.1136/jcp.2007.049387. [DOI] [PubMed] [Google Scholar]
- Hagenstad C.T. Inflammatory myofibroblastic tumor with bone marrow involvement. A case report and review of the literature. Arch. Pathol. Lab. Med. 2003;127(7):865–867. doi: 10.5858/2003-127-865-IMTWBM. [DOI] [PubMed] [Google Scholar]
- Hussong J.W. Comparison of DNA ploidy, histologic, and immunohistochemical findings with clinical outcome in inflammatory myofibroblastic tumors. Mod. Pathol. 1999;12(3):279–286. [PubMed] [Google Scholar]
- Jiang Y.H. Comparison of the clinical and immunohistochemical features, including anaplastic lymphoma kinase (ALK) and p53, in inflammatory myofibroblastic tumours. J. Int. Med. Res. 2009;37(3):867–877. doi: 10.1177/147323000903700332. [DOI] [PubMed] [Google Scholar]
- Lovly C.M. Inflammatory myofibroblastic tumors harbor multiple potentially actionable kinase fusions. Cancer Discov. 2014;4(8):889–895. doi: 10.1158/2159-8290.CD-14-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meis J.M., Enzinger F.M. Inflammatory fibrosarcoma of the mesentery and retroperitoneum: a tumor closely simulating inflammatory pseudotumor. Am. J. Surg. Pathol. 1991;15(12):1146–1156. doi: 10.1097/00000478-199112000-00005. [DOI] [PubMed] [Google Scholar]
- Morotti R.A. Pediatric inflammatory myofibroblastic tumor with late metastasis to the lung: case report and review of the literature. Pediatr. Dev. Pathol. 2005;8(2):224–229. doi: 10.1007/s10024-004-8088-5. [DOI] [PubMed] [Google Scholar]
- Qian H. The efficacy and safety of crizotinib in the treatment of anaplastic lymphoma kinase-positive non-small cell lung cancer: a meta-analysis of clinical trials. BMC Cancer. 2014;14:683. doi: 10.1186/1471-2407-14-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabban J.T. Inflammatory myofibroblastic tumor of the uterus: a clinicopathologic study of 6 cases emphasizing distinction from aggressive mesenchymal tumors. Am. J. Surg. Pathol. 2005;29(10):1348–1355. doi: 10.1097/01.pas.0000172189.02424.91. [DOI] [PubMed] [Google Scholar]
- Unni K., Hogendoom P. IARC press; Lyon: 2002. World Health Organization Classification of Tumours Pathology and Genetics of Tumours of Soft Tissue and Bone. [Google Scholar]