Highlights
-
•
Conventional definitions of STIC do not capture all lesions associated with serous neoplasia and the presence of abnormal p53 expression may be helpful diagnostically.
-
•
The management of STICs and tubal atypias remain uncertain.
-
•
The POINT Project is a registry set up to address this critical gap in knowledge.
Keywords: Serous tubal intraepithelial carcinoma, Tubal atypia, BRCA, Serous carcinoma, Prophylactic salpingo-oophorectomy
Introduction
A young woman with a prior diagnosis of bilateral breast cancers, BRCA1 germline mutation was found to have an atypical, proliferative lesion in the fimbrial end of a fallopian tube found at the time of bilateral salpingectomy (BSO). This tubal intra-epithelial lesion did not meet the criteria for high-grade serous tubal intraepithelial carcinoma (STIC) as it lacked high-grade atypia and had a low proliferative index (less than 10%). It did, however, show strong diffuse p53 immunostaining indicative of a TP53 missense mutation. This was followed by the development of advanced stage low-grade serous cancer 18 months later. This carcinoma, very atypically for a low-grade serous carcinoma, showed strong diffuse p53 immuno-reactivity. This case demonstrates the potential for occasional low-grade serous carcinomas, and “low-grade STIC” lesions, to arise in the setting of germline BRCA mutation. The presence of abnormal p53 expression may be helpful, diagnostically, in such cases.
Case
Our patient was diagnosed with a stage IIA, triple negative, invasive ductal carcinoma of the breast at age 33. Genetic testing confirmed a BRCA1 Exon2 185 delAG mutation. Pelvic ultrasound was normal. She received adjuvant systemic chemotherapy and adjuvant radiation to her affected breast. Four years later she was diagnosed with a contralateral T1aN0M0 infiltrating ductal carcinoma and bilateral mastectomies were performed.
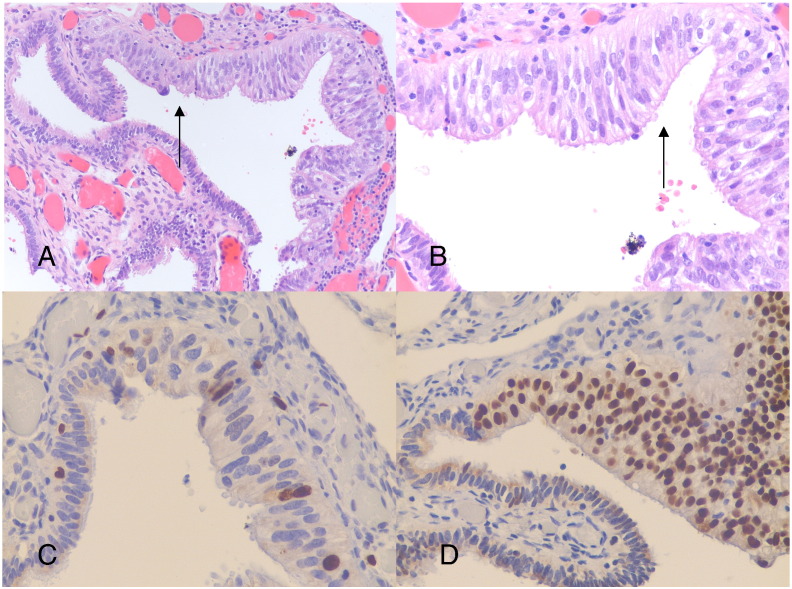
She accepted a prophylactic BSO 7 years after her initial breast cancer diagnosis. Pathologic evaluation revealed a single microscopic focus of epithelial stratification, mild cellular atypia, and intense p53 immuno-reactivity within the fimbria in one fallopian tube (Fig. 1). Peritoneal washings were negative. This unusual histology of a low grade lesion with intense p53 staining was extensively discussed at our center with the consensus that these findings did not fulfill the criteria for STIC, as it lacked the marked atypia and high proliferative index of STIC (Visvanathan et al., 2011). The lesion was diagnosed as tubal epithelial atypia of uncertain significance. Surveillance was the only post operative recommendation.
Fig. 1.
Single microscopic focus of fimbrial epithelium that exhibits stratification, mild atypia and intense p53 immunoreactivity, (A) at 20 × magnification, and (B) at 40 × magnification, (C) low level of immunostaining for Ki-67, and (D) showing intense and diffuse immunostaining for p53.
Eighteen months following the BSO, a CT of the abdomen and pelvis revealed peritoneal carcinomatosis. A core biopsy of an omental nodule confirmed the presence of a low grade papillary serous neoplasm, ER positive, with high p53 (Fig. 2). Chemotherapy followed by surgical debulking was performed. Final histology showed a low-grade serous carcinoma (Fig. 3). However, her disease progressed 5 months later and she was initiated on a PARP inhibitor and has remained clinically stable.
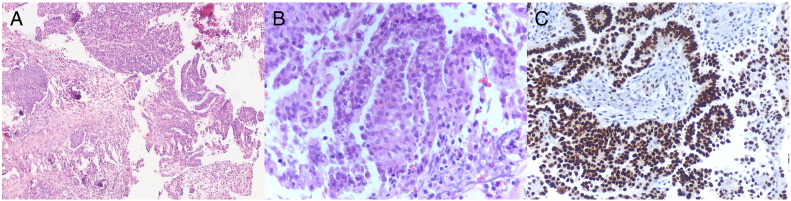
Fig. 2.
Omental biopsy showing metastatic deposits of invasive low-grade serous carcinoma at low power (A), and high power (B), demonstrating the uniform nuclei and low mitotic index characteristic of low-grade serous carcinoma. There is strong diffuse p53 immunoreactivity (C).
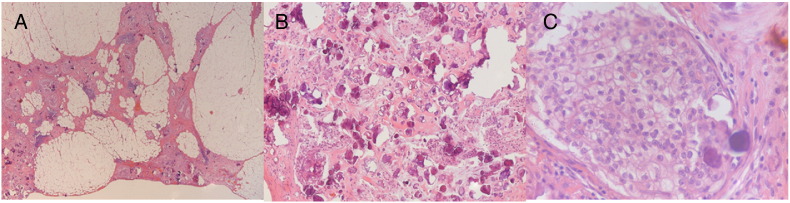
Fig. 3.
Omental biopsy showing metastatic deposits of invasive papillary serous adenocarcinoma with numerous psammoma bodies, (A) at low power, (B) at medium power and (C) at high power. Note the uniform tumor cell nuclei and low mitotic index.
Discussion
Recent evidence on the pathogenesis of serous ovarian cancer points towards there being two diseases: high-grade serous carcinomas and low-grade serous carcinomas, with the fallopian tube epithelium as the origin of most high-grade serous cancers, while most low-grade serous cancers arise from serous borderline tumors. The identification of incidental high-grade serous tubal intraepithelial carcinomas (STIC) in the fallopian tubes of women with a genetic predisposition of ovarian cancer led to the recognition of the tubal origin of HGSC (Kauff et al., 2002).
Prophylactic salpingo-oophorectomy is currently strongly recommended as a risk reduction surgery in women with a high risk for the development of ovarian cancer due to an inherited mutation (e.g. BRCA 1 and BRCA2) (National Comprehensive Cancer Network (NCCN), 2013; National Institute of Health and Care Excellence (NICE), 2013). The prevalence of occult carcinomas in individuals undergoing a prophylactic oophorectomy at age more than 40 years old doubles as compared to individuals less than 40 years of age (Finch et al., 2014). Such findings lend support to current recommendations for prophylactic surgery at age 35 years old or once child bearing is completed. The cancers that arise in patients with BRCA1 or BRCA2 germline mutations are almost invariably of high-grade serous type, when modern diagnostic criteria are applied, with only occasional examples of other cancer cell types reported (McAlpine et al., 2012, Shaw et al., 2002).
Recognition of occult tubal lesions can be challenging. The rising use of the Sectioning and Extensively Examining the Fimbria (SEE-FIM) protocol has resulted in the increased frequency of fallopian tube abnormalities reported (Rabban et al., 2009). The diagnostic criteria for STIC have also evolved, but the consensus is that STIC lesions are uniformly high-grade, with a high proliferative index (Visvanathan et al., 2011). Immunostains for the proliferation marker Ki-67 and p53 have been shown to improve diagnostic reproducibility for STIC in cases that lack unequivocal high-grade cytological atypia; to support a diagnosis of STIC there should be high proliferative index (greater than 10%) and abnormal p53 expression (either complete loss of expression or diffuse intense nuclear immunoreactivity, with both patterns indicating an underlying TP53 mutation).
Opinions on the treatment of precursor lesions like STICs remain uncertain. Few data exist on the clinical outcomes of isolated serous tubal intraepithelial carcinoma (STICS). Wethington et al. reported the favorable short term clinical outcomes in patients with isolated STICS where four patients with isolated STICS and negative washings had gone on to receive adjuvant chemotherapy and there have been no documented recurrences in this small cohort (Wethington et al., 2013). There is an increasing move towards the use of staging in STICS. The favorable short term clinical outcome of cases with negative surgical staging makes observation without adjuvant chemotherapy reasonable (Wethington et al., 2013). Even less is known about tubal atypias that fall short of STIC. The identification of p53 mutations in the Cancer Genome Atlas Project suggested a possible role of p53 aberrations as a precursor of STIC and serous carcinomas (Integrated Genomic Analysis of Ovarian Carcinoma, 2011). Cass et al. reported additional alterations are required in existing p53 overexpressing epithelium for malignant transformation (Cass et al., 2014). Additional information is required to evaluate the role of p53 alterations in the development of serous carcinoma.
This case demonstrates that the conventional morphological definitions of STIC and high-grade serous carcinoma do not capture all lesions associated with the molecular phenotype of serous neoplasia in patients with hereditary breast and ovarian cancers (HBOC). Although the majority of serous neoplasia in these patients will be conventional STIC or high-grade serous carcinoma (based on routine morphological assessment), occasional cases overlap morphologically with low-grade serous carcinoma, despite having the p53 molecular abnormalities characteristic of high-grade serous neoplasia. The disease recurrence and clinical course in our patient further suggests that the presence of intense p53 was highly suspicious of a precursor of malignancy despite the relatively low grade and low proliferative index, findings that persisted in the invasive serous carcinoma that developed subsequently. The index of suspicion for malignancy should be high in high risk individuals who presents with tubal atypia having abnormal p53 staining. Regular surveillance and follow-up should be emphasized. The Pelvic-Ovarian cancer Interception (POINT) Project (http://pointproject.org/POINT/) is a registry set up to address this critical gap in knowledge and add to our understanding of the behavior and outcomes of all tubal abnormalities (serous tubal carcinoma in situ and tubal atypia). This will address the pressing need for more robust guidelines and greatly aid in the optimal management of these patients.
Conflicts of interest statement
The authors declare that there are no conflicts of interests.
Acknowledgments
Concept and ideas: Karen A Gelmon, Anna V. Tinker, C. Blake Gilks.
Manuscript writing: All authors.
References
- Cass I., Walts A.E., Barbuto D. A cautious view of putative precursors of serous carcinomas in the fallopian tubes of BRCA mutation carriers. Gynecol. Oncol. 2014;134(3):492–497. doi: 10.1016/j.ygyno.2014.07.084. (Sep) [DOI] [PubMed] [Google Scholar]
- Finch A.P., Lubinshi J., Moller P., Singer C.F., Karlan B., Senter L., Rosen B., Maehle L., Ghadirian P., Cybulski C., Huzarski T., Eisen A., Foulkes W.D., Kim-Sing C., Ainsworth P., Tung N., Lynch H.T., Neuhausen S., Metcalfe K.A., Thompson I., Murphy J., Sun P., Narod S.A. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J. Clin. Oncol. 2014;32(15):1547–1553. doi: 10.1200/JCO.2013.53.2820. (May 20) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Integrated Genomic Analysis of Ovarian Carcinoma Cancer genomic atlas research network. Nature. 2011;474(7353):609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauff N.D., Satagopan J.M., Robson M.E. Risk-reducing salpingo-oophorectomy in women with a BRCA1 or BRCA2 mutation. N. Engl. J. Med. 2002;346(21):1609–1615. doi: 10.1056/NEJMoa020119. (Epub 2002 May 20) [DOI] [PubMed] [Google Scholar]
- McAlpine J.N., Porter H., Kobel M., Nelson B.H., Prentice L.M., Kalloger S.E., Senz J., Milne K., Ding J., Shah S.P., Huntsman D.G., Gilks C.B. BRCA1 and BRCA2 mutations correlate with TP53 abnormalities and presence of immune cell infiltrates in ovarian high-grade serous carcinoma. Mod. Pathol. 2012;25(5):740–750. doi: 10.1038/modpathol.2011.211. (May, Epub 2012 Jan 27) [DOI] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network (NCCN) vol. 4. 2013. (Genetic/High-Risk familial Assessment: Breast and Ovarian 2013). (Available from: http://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf) [Google Scholar]
- National Institute of Health and Care Excellence (NICE) National Institute for Health and Care Excellence; London: 2013. Familial Breast Cancer: Classification and Care of People at Risk of Familial Breast Cancer and Management of Breast Cancer and Related Risks in People with a Family History of Breast Cancer. (Available from: http://www.nice.org.uk/guidance/cg164) [PubMed] [Google Scholar]
- Rabban J.T., Krasik E., Chen L.M. Multistep level sections to detect occult fallopian tube carcinoma in risk-reducing salpingo-oophorectomies from women with BRCA mutations: implications for defining an optimal specimen dissection protocol. Am. J. Surg. Pathol. 2009;33(12):1878–1885. doi: 10.1097/PAS.0b013e3181bc6059. (Dec) [DOI] [PubMed] [Google Scholar]
- Shaw P.A., McLaughlin J.R., Zweemer R.P., Narod S.A., Risch H., Verheijen R.H., Ryan A., Menko F.H., Kenemans P., Jacobs I.J. Histopathologic features of genetically determined ovarian cancer. Int. J. Gynecol. Pathol. 2002;21(4):407–411. doi: 10.1097/00004347-200210000-00011. (Oct) [DOI] [PubMed] [Google Scholar]
- Visvanathan K., Vang R., Shaw P. Diagnosis of serous tubal intraepithelial carcinoma based on morphologic and immunohistochemical features: a reproducibility study. Am. J. Surg. Pathol. 2011;35(12):1766–1775. doi: 10.1097/PAS.0b013e31822f58bc. (Dec) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wethington S.L., Park K.J., Soslow R.A., Kauff N.D., Brown C.L., Dao F., Otegbeye E., Sonoda Y., Abu-Rustum N.R., Barakat R.R., Levine D.A., Garner G.J. Clinical Outcome of Isolated Serous Tubal Intraepthelial carcinomas (STIC) Int. J. Gynecol. Cancer. 2013;23:1603–1611. doi: 10.1097/IGC.0b013e3182a80ac8. [DOI] [PMC free article] [PubMed] [Google Scholar]