Thalidomide is effective in the treatment of cutaneous lupus erythematosus (CLE) however its use is greatly limited by its side effect profile. Peripheral neuropathy characterized by symmetric painful paresthesias and sensory loss of the distal upper and lower extremities is a well-recognized adverse effect that is most common in the first year of treatment [1].
A 40-year-old woman developed a red, itchy eruption on her chest and back after 2 months of terbinafine use for onychomycosis. She presented to clinic 2 months after discontinuing terbinafine with a persistent rash that involved her arms, chest, and back, as well as recent hair loss. She had a long-standing history of Raynaud’s phenomenon and photosensitivity, but denied fevers, arthritis, muscle weakness and oral ulcers. Her past medical history was significant for 2 first-trimester miscarriages and an elective oopherectomy and hysterectomy due to BRCA2 positivity. Physical examination revealed annular erythematous plaques with peripheral scale on her chest, upper back and arms (Fig. 1A). Laboratory studies demonstrated an antinuclear antibody titer of 1:320, elevated anti-SSA antibodies and IgG beta-2-glycopretein. Anti-ribonuclear antibodies, anticardiolipin antibodies, Russells Viper Venom test, and anti-deoxyribonucleic acid antibodies were all negative. Results of her urinalysis, complete blood count and comprehensive metabolic panel were within normal limits. A punch biopsy specimen of a characteristic lesion on her right arm showed vacuolar interface dermatitis, dermal mucin deposition and a predominantly lymphocytic infiltrate along neurovascular structures in the superficial and deep dermis. Based on these findings the patient was diagnosed with subacute cutaneous lupus erythematosus (SCLE).
Figure 1.
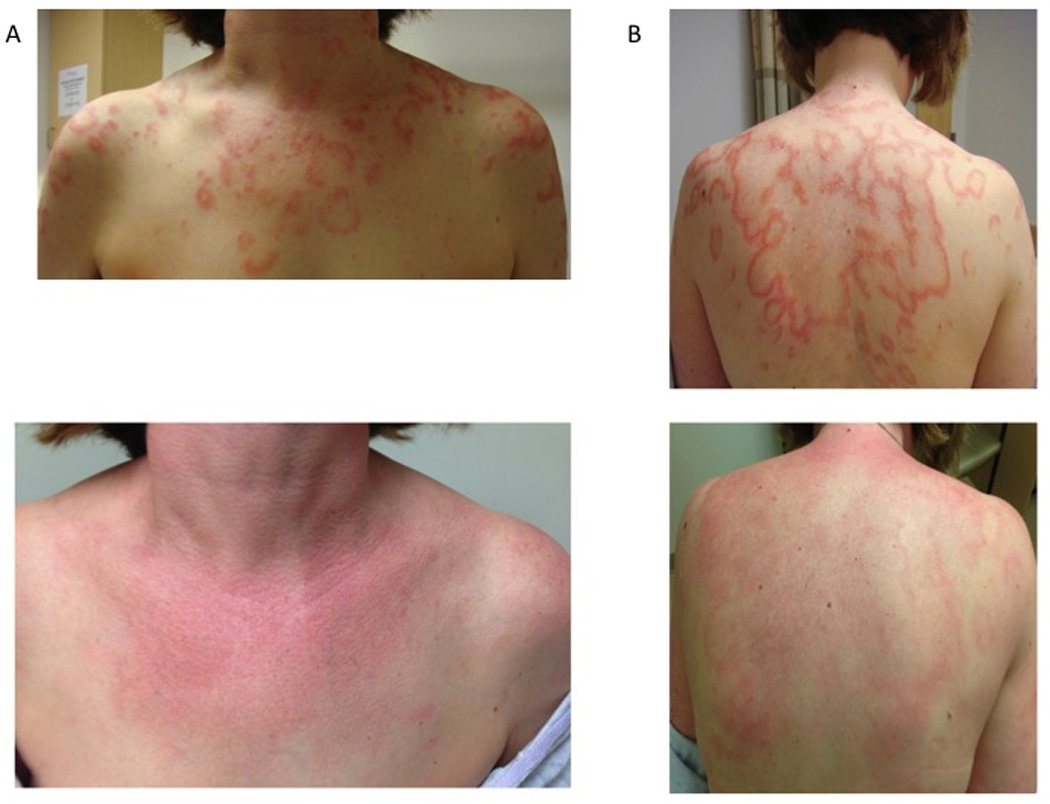
Effect of thalidomide on a patient with terbinafine-induced subacute cutaneous lupus erythematosus. A. Annular plaques on chest and back. B. Residual pink patches on chest and back 1 month after starting thalidomide.
The patient obtained baseline nerve conduction velocity and visual fields tests and started hydroxychloroquine 400 mg/day, thalidomide 100 mg/day, and aspirin 81 mg/day. After 13 doses of thalidomide she complained of numbness of her lips, tongue and face. Her symptoms began with a tingling sensation of her upper and lower lips that expanded to involve her peri-oral face and tip of her tongue. These areas eventually became numb, there were no motor deficits and no other areas were involved. All symptoms resolved with discontinuation of thalidomide. Ten days later she resumed use of thalidomide and three days after a single dose her symptoms recurred in the same pattern but with a more rapid onset. The paresthesias cleared within two days of thalidomide withdrawal. At this time her skin lesions were significantly improved. On physical exam there were pink patches with scale on her arms, legs and chest (Fig. 1B). The patient continued hydroxychloroquine 400 mg/day after thalidomide was discontinued. She has not complained of numbness in the 10 weeks since her last thalidomide dose.
SCLE was first recognized as a distinct subset of CLE in 1979 [2]. Since drug-induced SCLE was described in 1985, over 100 medications have been implicated [3,4]. Terbinafine is one of the most commonly reported agents for inducing SCLE [5]. Cutaneous findings of drug-induced SCLE are indistinguishable from those of non-drug related disease. In some cases of drug-induced SCLE symptoms resolve with discontinuation of the inciting agent. When the cutaneous eruption persists clinical management is similar to that of non-drug related SCLE. Thalidomide is a treatment option for CLE patients who are resistant to first-line agents or for acute treatment prior to onset of action of antimalarials.
Potential adverse effects of thalidomide include birth defects, peripheral neuropathy, sedation, rash, constipation, dizziness, and thromboembolic complications. The peripheral neuropathy that ensues is usually characterized by paresthesias and sensory loss of the distal extremities [6]. The mechanism by which thalidomide induces neuropathy is not completely understood. Proposed theories include thalidomide-related toxic distal axonopathy, antiangiogenesis, dysregulation of neurotrophic activity and genetic predispositions [7,8]. In a prospective evaluation of 135 patients treated with thalidomide for various dermatologic indications, the minimal prevalence rate of thalidomide neuropathy was 25% and maximal rate was 56% [1]. In 36% of these cases thalidomide was used for lupus erythematosus. Thalidomide was discontinued in more than half of patients due to a neurologic adverse event. Patients were more likely to develop neuropathy in the first year of treatment. The most important risk factor for thalidomide neuropathy was the daily dose; neuropathy was not observed at doses less than 25 mg daily [1]. Cumulative thalidomide doses and duration of treatment were not statistically different between patients who developed neuropathy and those who did not.
Thalidomide-induced perioral neuropathy has been reported previously in a case of chronic graft-versus-host disease secondary to allogeneic peripheral blood stem cell transplantation [9]. In this case the patient experienced numbness of the face, fingers and toes after 23 days of thalidomide 400 mg/day. Withdrawal of thalidomide produced resolution of symptoms within 8 days. The clinical and histologic findings in our case are consistent with terbinafine-induced SCLE. The patient’s history of miscarriages, livedo pattern on physical exam and high IgG beta-2-glycoprotein levels raise concern for a hypercoagulable state and increased risk of adverse events with thalidomide. Given the acuity of the patient’s presentation, thalidomide was used for short-term relief while waiting for hydroxychloroquine to take effect. In this case the patient’s skin lesions responded dramatically to her therapeutic regimen. The reproducibility of her symptoms with thalidomide re-challenge confirmed that the orofacial numbness represents another form of thalidomide-induced neurotoxicity. Clinicians should be aware of this phenomenon and discontinue thalidomide if suspected.
Key Message.
In addition to the classic peripheral distal sensory neuropathy, thalidomide may induce an orofacial neuropathy.
Acknowledgements
We are indebted to the Department of Veterans Affairs Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development and the National Institutes of Health (NIH K24-AR 02207) (VPW).
Funding/Support: None
Footnotes
Disclosure statement: Anyanwu, Stewart and Werth declare no conflicts of interest.
REFERENCES
- 1.Bastuji-Garin S, Ochonisky S, Bouche P, et al. Incidence and risk factors for thalidomide neuropathy: a prospective study of 135 dermatologic patients. J Invest Dermatol. 2002;119:1020–1026. doi: 10.1046/j.1523-1747.2002.19502.x. [DOI] [PubMed] [Google Scholar]
- 2.Sontheimer RD, Thomas JR, Gilliam JN. Subacute cutaneous lupus erythematosus: a cutaneous marker for a distinct lupus erythematosus subset. Arch Dermatol. 1979;115:1409–1415. [PubMed] [Google Scholar]
- 3.Reed BR, Huff JC, Jones SK, et al. Subacute cutaneous lupus erythematosus associated with hydrochlorothiazide therapy. Ann Intern Med. 1985;103:49–51. doi: 10.7326/0003-4819-103-1-49. [DOI] [PubMed] [Google Scholar]
- 4.Callen JP. Drug-induced subacute cutaneous lupus erythematosus - filling the gap in knowledge [published online July 10, 2013] JAMA Dermatol. doi: 10.1001/jamadermatol.2013.4958. [DOI] [PubMed] [Google Scholar]
- 5.Gronhagen CM, Fored CM, Linder M, et al. Subacute cutaneous lupus erythematosus and its association with drugs: a population-based matched case-control study of 234 patients in Sweden. Br J Dermatol. 2012;167:296–305. doi: 10.1111/j.1365-2133.2012.10969.x. [DOI] [PubMed] [Google Scholar]
- 6.Wu JJ, Huang DB, Pang KR, et al. Thalidomide: dermatological indications, mechanisms of action and side-effects. Br J Dermatol. 2005;153:254–273. doi: 10.1111/j.1365-2133.2005.06747.x. [DOI] [PubMed] [Google Scholar]
- 7.Chaudhry V, Cornblath DR, Corse A, et al. Thalidomide-induced neuropathy. Neurology. 2002;59:1872–1875. doi: 10.1212/01.wnl.0000037480.59194.85. [DOI] [PubMed] [Google Scholar]
- 8.Johnson DC, Corthals SL, Walker BA, et al. Genetic Factors Underlying the Risk of Thalidomide-Related Neuropathy in Patients With Multiple Myeloma. J Clin Oncol. 2011;29:797–804. doi: 10.1200/JCO.2010.28.0792. [DOI] [PubMed] [Google Scholar]
- 9.Elad S, Galili D, Garfunkel AA, et al. Thalidomide-induced perioral neuropathy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84:362–364. doi: 10.1016/s1079-2104(97)90032-9. [DOI] [PubMed] [Google Scholar]


