Abstract
Background and Aims
Neurotensin (NT) promotes colon cancer and inflammation via NT receptor-1 (NTR1). MicroRNAs regulate protein synthesis by targeting mRNAs. We determined the microRNA signature of NTR1 stimulation on human colonic (NCM460) epithelial cells.
Methods
RNA from NT-stimulated NCM460 cells overexpressing NTR1 was used for microarray expression analysis. NF-κB binding sites were identified by sequence homology, ChIP-assay and qPCR. Tumorigenesis was assessed by the soft agar assay and HCT-116 tumor xenografts in SCID mice. Down-stream targets of NT-regulated microRNAs were identified via bioinformatics, real time PCR and Western blot.
Results
NT stimulated differential expression of 38 microRNAs. We identified NF-κB binding sites on miR-21 and miR-155, previously implicated in tumor growth. NT increased the number of colonies of HCT-116 cells and antisense-microRNAs against miR-21 and/or miR-155 inhibited this response (p<0.001). NT administration (i.p.) increased the rate of tumor growth in xenograft tumors while miR-21 and/or miR-155 antisense attenuated this response. Since potential downstream targets of miR-21 and miR-155 are PTEN and SOCS1, respectively, and both are upstream of Akt, we investigated the effect of NT on Akt activation. NT activated Akt in HCT-116 cells, an effect inhibited by miR-21 and/or miR-155 antisense (p<0.001). We report for the first time PPP2CA phosphatase as a miR-155 target exerting the effects of NT on Akt.
Conclusions
NT stimulates miR-21 and miR-155 expression in colonocytes via Akt and NF-κB, and both microRNAs mediate colon tumor growth in response to NT. Importantly, this NT-microRNA circuit is found perturbated in human colon cancers and correlates with tumor stage, suggesting its relevance to human colon carcinogenesis.
Keywords: neuropeptides, intestinal inflammation, colon cancer
Introduction
During the last decade, hundreds of genes encoding small 19–25 nucleotide single-stranded RNA molecules called microRNAs (miRNAs) have been discovered1. miRNAs negatively regulate gene expression by binding 3′ untranslated regions (UTRs) of transcripts2, leading to messenger RNA (mRNA) degradation, or inhibition of translation into protein3. More than one-third of human genes are regulated by miRNAs4. miRNAs are reported to play a role in the pathogenesis of diseases associated with disordered genome function such as cancer5, 6. In fact, the expression pattern of 217 miRNAs defines a cancer type better than 16,000 mRNAs, providing a much more efficient tool for diagnosis and targeted treatment7. Thus, modulation of miRNA expression may influence human disease states, including colon cancer8.
Neurotensin (NT), a 13aa neuropeptide, originally isolated from the calf hypothalamus9, is expressed in the central nervous system and the gastrointestinal tract10. In the latter, NT acts through its cognate receptor NTR1 to stimulate colonic cell proliferation, MAP kinase and NF-κB activation11, promoting colon cancer12,13 and intestinal inflammation14,15. On intestinal cells, NT also regulates expression of several genes15.
The ability of neuropeptides to modulate expression of microRNAs in colonocytes has never been studied. Here, we used microRNA microarray expression analysis to detect the miRNA signature of NTR1 activation in human colonic NCM460 epithelial cells overexpressing NTR1. Following NT-treatment we found functional NF-κB-binding sites on miR-21 and miR-155, known modulators of human cancer16, 17. Selective inhibition of these miRNAs inhibited the ability of NT to promote colon cancer in vitro and in vivo.
Materials and Methods
Antibodies and reagents
Phospho-NF-κB/p65 (Ser536) (1:500, 3031, Cell Signaling Inc), NF-κB p65 (1:1,000, ab7970, Abcam Inc), PTEN (1:300, 9559, Cell Signaling Inc), PPP2CA (1:1000, TA300307, Origene), SOCS1 (1:750, sc-9021, Santa Cruz Biotech Inc), β-Actin (1:1000, 130065, Santa Cruz Biotech Inc). BAY-117082 (Calbiochem, cat no. 196870), neurotensin (NT) (PhoenixBiotech Inc, cat.no 048-03).
Transduction of NCM460 Cell Line with NTR1 (NCM460-NTR1)
Human NTSR1 gene was isolated from pCR2.118 with Eco RV and inserted into lentiviral backbone CMV-IRES-GFP-PGK-Puro at Eco RV site, 5′ to IRES. Lentiviral particles expressing NTRS1 were generated by transient co-transfection of 293T cells with a three-plasmid combination, as described previously19. Following transduction NCM460-NTR1 cells were maintained as described above.
MicroRNA Expression Analysis
Expression of 365 microRNAs were evaluated with microRNA profiling assays (TLDA human miRNA v1.0) at the Dana Farber Molecular Diagnostics Facility. Results were validated using mirVana qRT–PCR miRNA Detection Kit and qRT–PCR Primer Sets, according to the manufacturer's instructions (Ambion Inc, TX, USA). Internal control: RNU48 expression.
Transfection Experiments
HCT-116 and DLD1 cells were transfected with 100 nM siRNA against NTR1#1 (s9767, Ambion Inc, TX, USA) or 100 nM siRNA against NTR1#2 (s9769, Ambion) or 100 nM of antisense microRNAs for miR-21 (as-miR-21) and miR-155 (as-miR-155) (Ambion) using siPORT NeoFX (Ambion). Transfection with 90 nM siRNA control (AM4611, Ambion) or 100nM antisense miR-negative control (AM17010, Ambion) was used as control. No cell toxicity was detected due to the transfection agent. RNA was extracted at different time points after siRNA transfection for real-time PCR analysis. Beta actin was used for loading control and transfections were performed in triplicate.
Luciferase assays
HCT-116 and DLD1 cells were transfected using Lipofectamine 2000 (Invitrogen) with firefly luciferase reporter constructs containing the 3′UTR of PTEN (HmiT015535, Genecopoeia Inc) or PPP2CA (HmiT014115, Genecopoeia) or SOCS1 (S203417, Switchgear Genomics) and treated with NT (100nM). Cell extracts were prepared 24h post-transfection, and luciferase activity was measured using Dual Luciferase Reporter Assay System (Promega, WI, USA).
Identification of NF-κB sites in microRNA regulatory areas
Lever and PhylCRM algorithms were developed previously20. To identify NF-κB binding motifs (5kb upstream and 2kb downstream of microRNAs) we 1) used Lever algorithm to assess motifs enriched in cis-regulatory modules (CRMs), 2) incorporated phylogenetic information from 12 mammalian species (mouse, rat, human, rabbit, chimp, macaque, cow, dog, armadillo, tenrec, opossum and elephant) and selected high conservation score sites (>100) with PhylCRM algorithm20, 3) mapped conserved binding sites in regions of interest. Loci of the top predicted and conserved NF-κB binding motifs are shown in Supp. Table 1.
Chromatin Immunoprecipitation (ChIP)
Using published procedures21, chromatin fragments from untreated and NT-treated (0.5, 1, 6h) HCT-116 and DLD1 cells were immunoprecipitated with 8ug of antibody against NF-κB/p65 (ab7970, Abcam Inc), DNA was extracted (Qiagen Purification Kit) and real-time-PCR was performed for NF-κB/p65 binding sites in microRNA promoters. Primers: miR-21 forward 5′-AAATTGGGAGGACTCCAAGC-3′, reverse 5′-GAAGGAAAAAGTATGTCAGTGCAA-3′ (PCR product: 119bp); miR-210 forward 5′-GGCAGAAACACACAGAAGCA-3′, reverse 5′-TGGTCATATCTTCAGCCAACA-3′ (PCR product: 102bp); miR-155 forward 5′-GAATAATATATTTCCTTGGTTTGGAA-3′, reverse 5′-TTCAAAAGAAACGTATGAAGTAAAA-3′ (PCR product: 102bp). Negative control: ChIP in the HNRPA2 gene, described previously22. HNRPA2 primers: forward 5′-ACGGCCTGACGTAGCGGAA-3′, reverse 5′-CAACTCTGCGAGGAGCACCT-3′.
Anchorage-independent Growth Assay
Transfections were performed as described above for 48h. Triplicates of 5×105 cells were mixed 4:1 (v/v) with 2.0% agarose in growth medium (final 0.4% agarose). Cell mixtures were plated on a layer of growth medium (0.5% agarose), fed every 6 to 7 days with growth medium (0.4% agarose) and colonies were counted after 15 days. Experiments were repeated thrice. Statistical significance was calculated using Student's t test.
Invasion Assays
HCT-116 and DLD1 cells were treated with NT (100nM) and 100nM antisense-microRNAs (as-miR-21, as-miR-155, as-miR-NC). Assays were conducted according to manufacturer's protocol, using MATRIGEL invasion chambers (PharMingen) and 10% FBS as chemoattractant. Invading cells were fixed and stained with 4′-6-diamidino-2-phenylindole (DAPI, Vector Laboratories Inc.). Ten fields/insert were scored and SD was measured. Assays were repeated thrice. Statistical significance was calculated using Student's t test.
ELISA Assays
HCT-116 and DLD1 cells were exposed to medium or medium containing NT (20, 50, 100nM × 0.5, 1, 3 and 6h). In some experiments cells were treated with control siRNA (100nM) or siRNA-NTR1 (100nM) for 24h. Nuclear extracts were prepared and p65 was detected using the NF-kB/p65 ActivELISA Assay (Cell Signaling) according to manufacturer's instructions. Samples were loaded in triplicate and data are presented as mean ± SD. AKT phosphorylation (S473) (DuoSet IC ELISA, DYC887, R&D Systems), phospho-GSK3β(S9) (DYC-1590, R&D Systems) and phospho-p65(S536) (7834, Cell Signaling) were assessed by ELISA in cells transfected with as-miR-21 (100nM) and/or as-miR-155 (100nM) for 24h and treated with NT (100nM) for 24h.
Mouse experiments
5×106 HCT-116 wild type or transfected with as-miR-NC or as-miR-155 and as-miR-21 were injected subcutaneously in the right flank of athymic nude mice (Charles River Laboratories, n=5 per group). Tumor volume [V(mm3)=a×b2/2 (a=largest diameter, b=perpendicular diameter)] was monitored every five days for 35 days. At ∼100mm3 (15 days), mice were treated with NT (100nM) (days 15, 20, 25). Injections of as-miR-NC, as-miR-155 and as-miR-21 (5mg/kg) were performed next to the tumor every 5 days (starting on day 5), suppressing microRNA expression levels >80%.
RNA Expression Studies from Patient Samples
RNAs purchased: 11 normal colon tissues (Biochain, Hayward, CA) and Origene (Rockville, MD), 14 colon cancer tissues (Origene, Rockville, MD). RNAs extracted: 7 normal colon tissues, 20 colon cancer tissues (Translational Pathology Core Laboratory, Department of Pathology, University of California, Los Angeles). mRNA expression of NTR1, PTEN, SOCS1, PPP2CA, miR-21 and miR-155 were determined by real-time PCR. Samples run in triplicate and data represent mean ± SD.
Results
Neurotensin regulates the expression of microRNAs in colon epithelial cells
NT promotes colon cancer12,13 and intestinal inflammation14,15, by mechanisms involving regulation of gene expression. To assess changes in the expression of miRNAs in response to NT, non-transformed human colonic epithelial NCM460 cells over-expressing NTR1 (NCM460-NTR1) were treated with 10-7M NT for 30 min or 6 hours and miRNA arrays were performed. NT altered the expression levels of 38 miRNAs (8 up-regulated and 30 down-regulated) (Fig. 1A). Hierarchical clustering analysis revealed that 5 different clusters of miRNAs were affected by NT. Specifically, the up-regulated miRNAs were divided into miRNAs that showed early and stable responses and miRNAs that showed dynamic continuous responses (Fig. 1B). Furthermore, NT-associated down-regulated miRNAs were divided into miRNAs showing an early and transient response, miRNAs with a dynamic continuous suppression, and miRNAs with an early and stable response (Fig 1C), suggesting a dynamic interplay between NT signaling and miRNA expression. Validation or these results was performed using a real-time SYBR Green miRNA assay (Supp. Fig. 1).
Figure 1. Neurotensin-regulated microRNAs in colon epithelial cells.
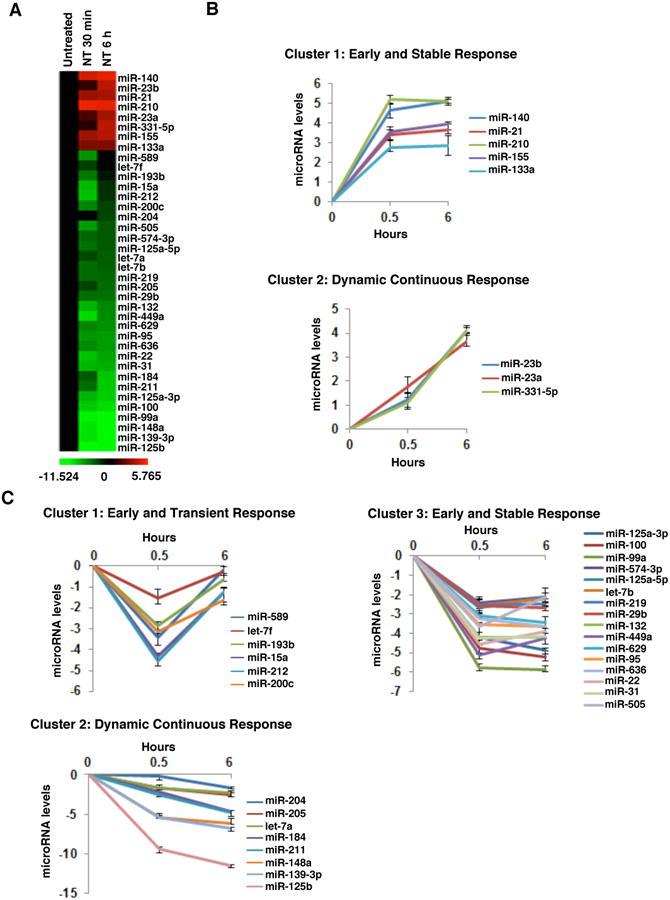
(A) Heatmap representation of differentially expressed microRNAs after NT treatment (0.5, 6h) of NCM460-NTR1 cells. Red: up-regulated microRNAs, green: down-regulated microRNAs. Clustering of NT-induced (B) and NT-suppressed microRNAs (C) according to their response dynamics.
To connect NTR1 stimulation with colon cancer development, we compared the NTR1 expression levels in colon cancer cell lines to NCM460 cells (Fig. 2A). Seven out of the eight cell lines showed a marked increase in NTR1 mRNA levels (5-20-fold) (p<0.00001). We chose the two cancer cell lines with the highest NTR1 mRNA levels (HCT-116, DLD1) and assessed mRNA expression of microRNAs, 0.5 and 6h post NT treatment. The microRNAs tested were the top seven up- and down-regulated microRNAs in response to NT treatment (Fig. 1). Both cell lines showed increased expression levels of miRNAs up-regulated and decreased expression of miRNAs down-regulated in NCM460-NTR1 cells in response to NT (Fig. 2B and Fig. 2C). We next used small interfering RNAs (siRNAs) to knock down NTR1 expression and assessed expression of highly up-regulated miRNAs (miR-21, miR-210 and miR-155) and down-regulated miRNAs (miR-148a, miR-139 and miR-125b) in response to NT. In NTR1 silenced cells, NT did not affect the expression of miRNAs, suggesting that NTR1 is necessary for the effects of NT on the regulation of miRNA expression (Fig. 2E, Supp. Fig. 2).
Figure 2. Neurotensin regulates microRNA expression through NTR1 in colon cancer cells.
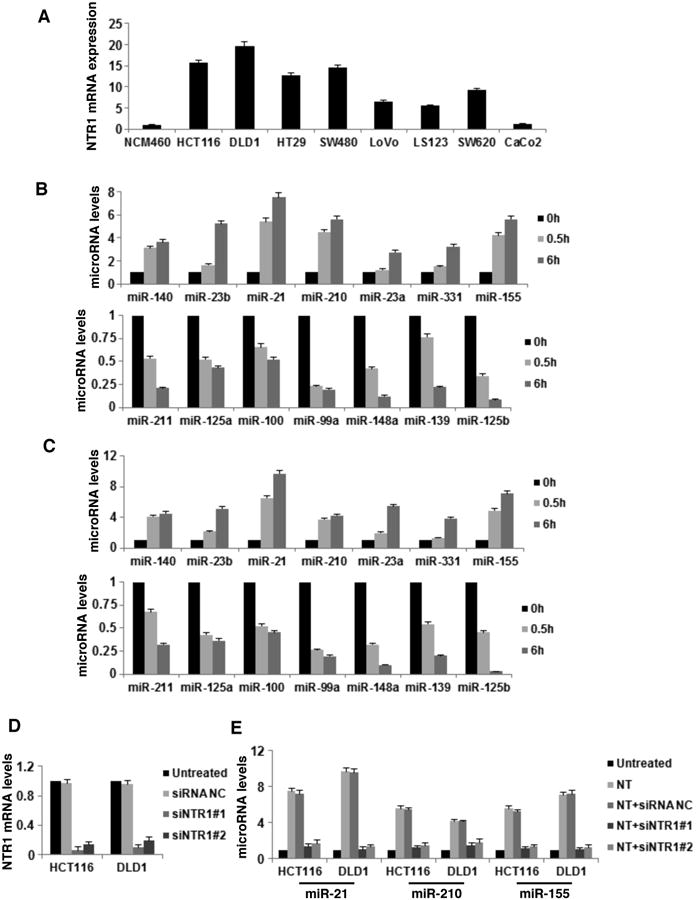
(A) NTR1 mRNA expression levels in non-transformed (NCM460) and cancer cell lines. (B) MicroRNA expression levels in HCT-116 and (C) DLD1 colon cancer cells treated with NT (0.5, 6h), assessed by real-time PCR analysis for the top seven NT up- and down-regulated microRNAs in NCM460-NTR1 cells. (D) NTR1 mRNA expression levels, assessed by real-time PCR analysis, in HCT116 and DLD1 colon cancer cells post siRNA treatment (48h) against NTR1 (siNTR1#1 or siNTR1#2, 100 nM) and negative control (siRNA NC, 100 nM). (E) NTR1 expression was inhibited in HCT116 and DLD1 colon cancer cells by siRNA treatments (siNTR1#1 or siNTR1#2, 100 nM, 48h); cells were treated with NT (6h, 100 nM) and expression levels of miR-21, miR-210 and miR-155 were assessed by real-time PCR analysis. Data show mean ± SD of three independent experiments.
Neurotensin controls microRNA expression through NF-κB activation in colon cancer cells
Activation of NF-κB is observed in many cancers while NF-κB is a known target of NT in colonocytes23. We confirmed this effect (Supp. Fig.3) and investigated whether the differential expression of miRNAs in response to NT involves NF-κB. Using Lever algorithm analysis we confirmed the existence of highly conserved NF-κB binding sites on the promoter region of miR-21, miR-210 and miR-155 (Supp. Table 1). A time course with various concentrations of NT showed a concentration- dependent increase in NF-κB activation detected by ELISA for p65 activity (Fig. 3A). Knock-down of NTR1 reversed this effect (Fig. 3B).
Figure 3. Neurotensin controls microRNA expression through NF-κB activation in colon cancer cells.
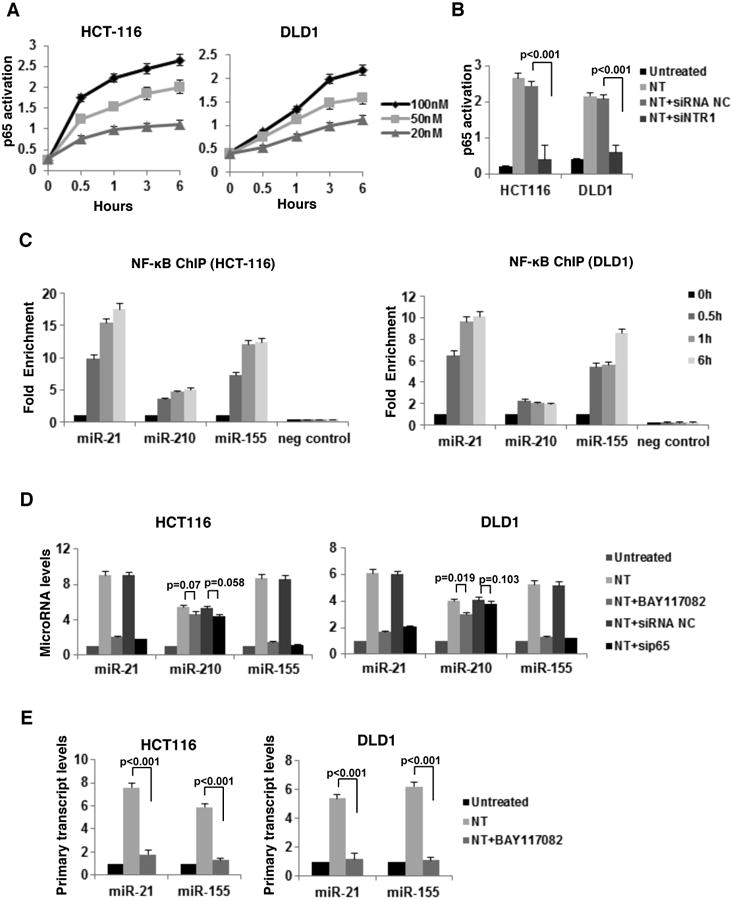
(A) NF-κB phosphorylation assessed by ELISA, after time course treatment of HCT116 and DLD1 cells with NT (20, 50, 100nM). (B) NTR1 expression was suppressed by siRNA in HCT116 and DLD1 cells (48h), cells were treated with NT (100nM, 6h) and NF-κB phosphorylation was assessed by ELISA. (C) ChIP detected enrichment of NF-κB transcription factor in the promoters of miR-21, miR-210, miR-155 and HNRPA2 (negative control) after NT treatment (0.5, 1, 6h) in HCT116 and DLD1 cells. D) Expression levels of miR-21, miR-210 and miR-155 and E) their primary transcripts, assessed by real-time PCR, in HCT116 and DLD cells treated with a pharmacological inhibitor of NF-KB pathway (BAY-117082, 5uM) or an siRNA negative control (siRNA NC, 100nM) or an siRNA against p65 (sip65, 100nM) for 48h and treated with NT (100nM, 6h).
Subsequently, cells were treated with NT (100nM, 0-6h) and the enrichment of NF-κB in the promoter areas of miR-21, miR-210 and miR-155 was assessed by ChIP indicating significant increase for the promoters of miR-21 and miR-155 in both cell lines treated with NT for 0.5 h and 6 h (Fig. 3C). Inhibition of NF-κB either via its pharmacological inhibitor BAY117082 or via sip65 reversed the effect of NT (100nM, 6 h) on the expression levels of mature and primary transcripts of miR-21 and miR-155 in both cell lines (Fig. 3D and Fig. 3E). Conversely, neither BAY117082 nor sip65 altered increased miR-210 expression in response to NT (Fig. 3D). The exact site map of NF-κB binding sites are indicated in Supp. Table 2. Moreover, we confirmed similar NTR1 signaling in relation to data above in LoVo cells, expressing lower levels of NTR1 (Supp. Fig.4)
Neurotensin regulates AKT activity in colon cancer cells through miR-21 and miR-155
We next determined whether NT affects the expression levels of direct targets of miR-21 and miR-155. Previous studies showed that miR-21 suppresses PTEN expression levels and miR-155 suppresses SOCS1 expression levels in cancer cells, through direct binding in their 3′UTRs8,24. We tested whether NT suppresses PTEN and SOCS1 levels in HCT116 and DLD1 cells. We found that NT inhibited the luciferase activities of PTEN and SOCS1 3′UTRs (Fig. 4A) and suppressed PTEN and SOCS1 mRNA and protein levels (Fig 4B, C). Inhibition of miR-155 or miR-21 by antisense-miR-155 and miR-21 blocked the effects of NT on PTEN and SOCS1 (Fig. 4A-C). Since PTEN is a negative regulator of AKT activity, we tested whether NT affects AKT activity in colon cancer cells. Consistent with results from our laboratory23 and others25, we found that NT increased AKT activation, while miR-21 inhibition blocked this effect (Fig 4D). Interestingly, inhibition of miR-155 suppressed the effect of NT on AKT activation and concurrent inhibition of miR-155 and miR-21 had a synergistic effect in suppressing AKT phosphorylation (Fig. 4D).
Figure 4. Neurotensin regulates miR-21 and miR-155 signaling pathways in colon cancer cells.
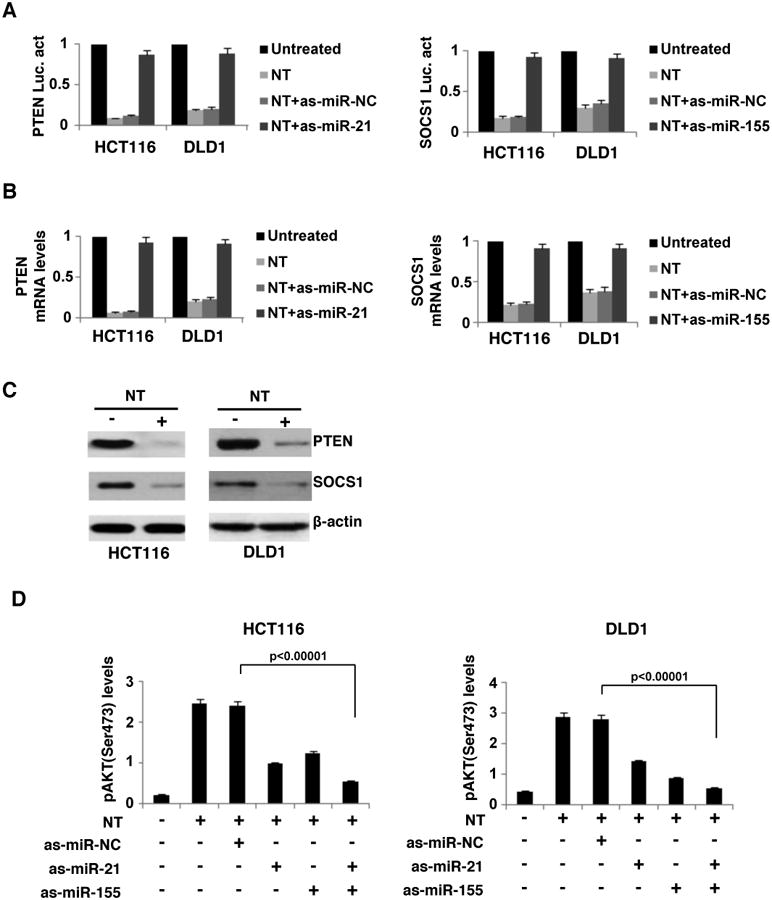
(A) Luciferase activity of the PTEN and SOCS1 3′UTRs after NT treatment (100nM) for 24h in HCT116 and DLD1 cells (untreated or as-miR-NC treated or as-miR-21-treated or as-miR-155-treated). (B) PTEN and SOCS1 mRNA levels, assessed by real-time PCR, in NT-treated (100nM, 6h) HCT116 and DLD1 cells (untreated or as-miR-NC treated or as-miR-21-treated or as-miR-155-treated). (C) PTEN, SOCS1 and β-actin protein levels, assessed by western blot, in NT-treated (100nM, 6h) HCT116 and DLD1 cells. (D) AKT phosphorylation (S473) levels, assessed by ELISA, in HCT116 and DLD1 cells transfected with as-miR-NC (100nM) or as-miR-21 (100nM) and/or as-miR-155 (100nM) for 24h and treated with NT (100nM) for 24h.
Neurotensin activates AKT through miR-155-mediated PPP2CA suppression
Next, we examined how NT affects AKT phosphorylation through miR-155 in colon cancer cells. Bioinformatic analysis revealed that miR-155 may affect AKT activity through binding in the 3′UTR of PPP2CA (Fig. 5A), a well-known suppressor of AKT phosphorylation26. Luciferase activity assay indicated that miR-155 suppresses PPP2CA, while antisense-miR-155 increases PPP2CA (Fig. 5B). Furthermore, NT treatment suppressed PPP2CA mRNA and protein levels in both cell lines (Fig. 5C, D). The increase of AKT phosphorylation by overexpression of miR-155 was comparable to PPP2CA inhibition by siRNA (Fig. 5E).
Figure 5. Neurotensin activates AKT through suppression of PPP2CA by direct interaction with miR-155.
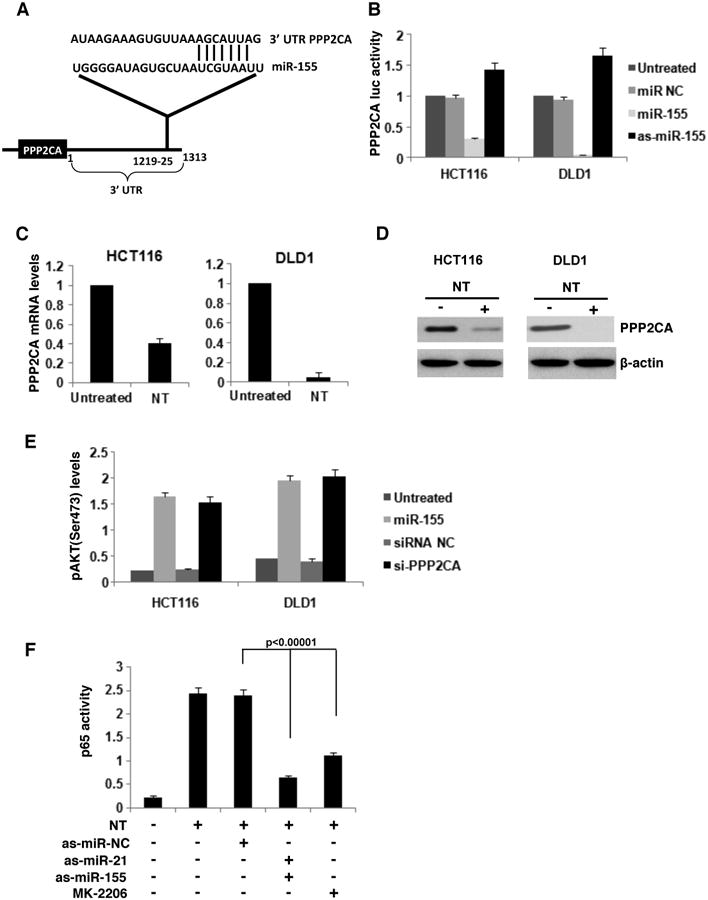
(A) miR-155 binding sites in 3′UTRs of PPP2CA predicted by Lever algorithm analysis. (B) Luciferase activity of the PPP2CA 3′UTRs in HCT116 and DLD1 cells transfected with as-miR-155 (100nM) or as-miR-155 (100nM) for 24h. (C) Luciferase activity of the PPP2CA 3′UTRs in HCT116 and DLD1 cells treated with NT (100nM). (D) PPP2CA and β-actin protein levels, assessed by western blot, in NT-treated (100nM, 6h) HCT116 and DLD1 cells. (E) AKT phosphorylation (S473) levels, assessed by ELISA, in HCT116 and DLD1 cells transfected with miR-155 (100nM) or si-PPP2CA (100nM) for 24h. (F) NF-κB/p65 activity, assessed by ELISA, in HCT cells transfected with NT (100nM) and as-miR-NC (100nM), as-miR-21 (100nM), as-miR-155 (100nM) or with 10nM of an AKT pharmacological inhibitor (MK-2206).
AKT activation leads to activation of the NF-κB pathway and p65 phosphorylation27. We found that inhibition of AKT in NT-treated colon cancer cells inhibited NF-κB activity (Fig 5F). Inhibition of miR-155 and miR-21 in NT-treated cells suppressed NF-κB phosphorylation (Fig. 5F). Thus, NT induces a positive feedback loop between AKT and NF-κB in colon cancer cells.
Neurotensin affects the tumorigenicity and invasiveness of colon cancer cells through regulation of miR-21 and miR-155 pathways
Both miR-21 and miR-155 are implicated in cancer cell proliferation and invasiveness28-30. Here, we assessed cancer cell colony formation in response to NT treatment and examined participation of these miRNAs. HCT-116, DLD1 and SW480 cells were treated with NT (100nM) in the presence or absence of as-miR21 and/or as-miR-155 and the number of colonies was assessed 15 days post-treatment. NT significantly increased the number of colonies for both cell lines while knock-down of miR-21 and/or miR-155 partially reversed this effect (Fig. 6A). NT (100nM) also significantly increased the number of invading HCT-116, DLD1 and SW480 cells, that was partially reversed when miR-21 and/or miR155 were knocked down (Fig. 6B).
Figure 6. Neurotensin affects the tumorigenicity and invasiveness of colon cancer cells through regulation of miR-21 and miR-155 pathways.
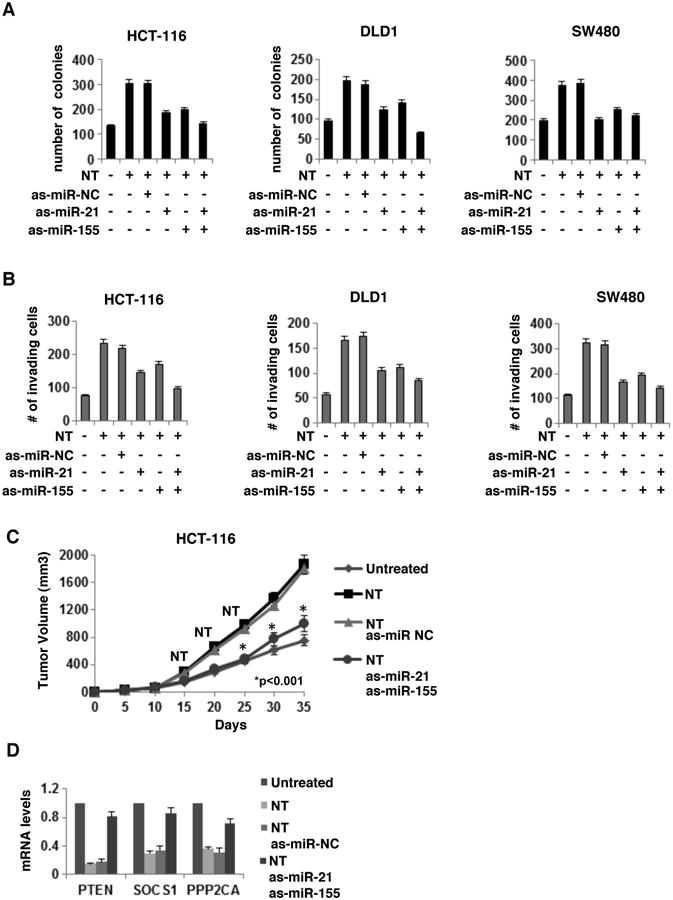
(A) Number of colonies and (B) invading cells in NT-treated HCT116, DLD1 and SW480 cells where miR-21 and/or miR-155 were knocked-down. (C) NT effects on tumor volume in a HCT116-xenograft model described in the Materials and Methods. P value indicates differences between NT/as-miR-NC treated mice vs NT/as-miR-21/as-miR-155 treated mice. (D) PTEN, SOCS1 and PPP2CA mRNA levels in NT-treated HCT-116-xenograft tumors (day 35).
We then tested the effects NT on tumor growth in vivo. We injected athymic nude mice subcutaneously with wild type or as-miR-155 and as-miR-21 transfected HCT-116 cells and monitored tumor size for 35 days. Where appropriate, NT (100nM) was administered intraperitoneously, 15, 20 and 25 days post-injection with HCT-116 cells. NT injection significantly increased the rate of tumor growth as compared to untreated animals while knockdown of miR-155 and miR-21 in the tumor forming cells partially reversed the effect (Fig. 6C). NT inhibited PTEN, SOCS1 and PPP2CA mRNA levels in these tumors (day 35), but was not effective in tumors where miR-155 and miR-21 were suppressed by antisense-miR-155 and antisense-miR-21 (Fig. 6D).
Neurotensin-microRNA signaling pathway in human colon cancers
In an attempt to assess the potential physiologic significance of our findings in human tumors, we quantified the expression levels of both, miR-21 and miR-155 as well as their downstream targets PTEN, PPP2CA and SOCS1 in 18 normal and 34 colon cancer human specimens. We identified that NTR1, miR-21 and miR-155 were significantly up-regulated while PPP2CA, SOCS1 and PTEN mRNA levels were significantly reduced in colon tumors relative to normal colon tissues (Fig. 7A). Testing the correlation between NTR1 mRNA levels and the expression levels of the other genes in the circuit, we identified that NTR1 was positively correlated with miR-21 (r=0.9364) and miR-155 (r=0.8713) while NTR1 levels were negatively correlated with PTEN (r=-0.746), SOCS1 (r=-0.891) and PPP2CA (r=-0.8051) (Supp. Figure 5). Importantly, the expression levels of all the genes correlated with tumor stage (Fig. 7B).
Figure 7. Neurotensin-microRNA signaling pathway in human colon cancers.
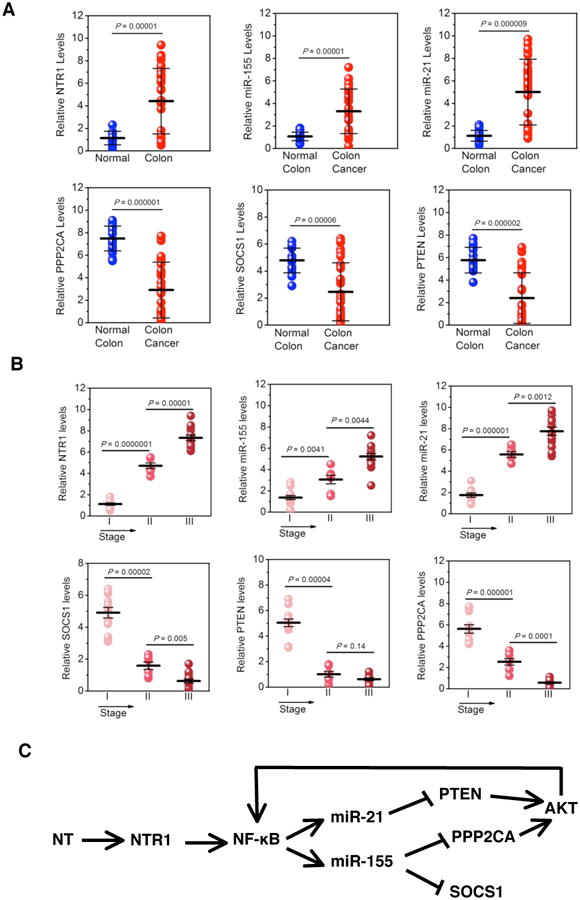
(A) NTR1, PTEN, SOCS1, miR-21 and miR-155 expression levels assessed by real-time PCR in 18 normal and 34 (13 stage I, 7 stage II and 14 stage III) colon cancer tissues. (B) Correlation between NTR1, PTEN, SOCS1 mRNA levels and miR-21, miR-155 expression levels and colon tumor stage. (C) NT-microRNA signaling pathway in colon cancer.
Discussion
miRNA expression is deregulated across a broad spectrum of human cancers, including colon cancer5, 31-34. NT and its receptor NTR-1 have been implicated in the pathophysiology of colitis14, 15, 35, 36, and colon cancer12, 13 by mechanisms involving regulation of expression of several genes37. However, the pathways involved in the mediation of these responses have not been fully elucidated, and participation of miRNAs in NTR1 signaling has never been evaluated. We report that NT binding to NTR1 in human colonocytes overexpressing this receptor differentially regulates expression of at least 38 miRNAs. We also demonstrate that NT/NTR1 interactions affects colon tumorigenesis in vitro and in vivo through stimulation of an inflammatory-microRNA feedback network. Importantly, we detected that this microRNA circuit is activated in human colon cancer specimens and correlates with colon cancer tumor stage, suggesting the importance of NTR1-associated miRNA regulation in colon cancer pathophysiology.
Increased in endogenous NTR1 expression in colon cancer cells lines is an initial indication of a relationship between NT/NTR1 signaling and colon cancer. Assessment of HCT-116 and DLD-1 cells for expression of miRNAs with the highest dependency on NT treatment seen in NCM460-NTR1 cells showed that NT stimulation of these cells causes miRNA expression phenotypes similar to that of NT-treated NCM460-NTR1 cells.
It is well established that NTR1 coupling activates the transcription factor NF-κB in NCM460-NTR123 and HCT116 human colonic epithelial cells38. Lever algorithm analysis followed by ChIP assay revealed NF-κB binding sites on three of the miRNAs upregulated in response to NT and indicated NF-κB binding following NT treatment on the miR-21 and miR-155 promoter in HCT-116 and DLD-1 cells, in line with previous studies suggesting NF-κB as an important factor for the regulation of miR-21 and miR-15539, 40. Interestingly, miR-21 and miR-155 are upregulated in inflamed intestinal mucosa of patients with ulcerative colitis and Crohn's disease41, 42, while NTR1 expression is elevated in the colon of patients with ulcerative colitis36 and animal models of intestinal inflammation14, 36. Together with studies demonstrating NTR1 as a proinflammatory mediator in the intestine14, 15, these results suggest a possible link between NTR1 and miR-21 and miR-155 in the development of colitis.
Selective inhibition of miR-21 and/or miR-155 reduced the number of colonies formed following NT treatment of HCT-116 and DLD-1 cells and reduced tumor volume of HCT-116 xenografts in nude (nu/nu) mice treated with NT. These results strongly indicate that NT affects the tumorigenicity and invasiveness of colon cancer cells through miR-21- and miR-155-dependent pathways.
NT treatment of colon cancer cells inhibited PTEN and SOCS1 activity and suppressed PTEN and SOCS1 mRNA and protein levels by pathways involving, respectively, miR-21 and miR-155. Previous studies showed that suppression of PTEN and SOCS1 expression levels by miR-21 and miR-155, respectively in cancer cells involves direct binding of these miRNAs in their 3′UTRs8,24. PTEN is a negative regulator of AKT activity and AKT signaling is one of the primary pathways commonly implicated with cancer development43, 44. Consistent with results from our laboratory23 and others25, NT increased AKT activity in colon cancer cells in a manner partially dependent on miR-21 and miR-155, suggesting that NT regulates AKT activity in colon cancer cells through miR-21 and miR-155.
The catalytic subunit of the serine/threonine phosphatase PP2A (PPP2CA) suppresses AKT 26, 45 and may be an intermediate between NT/miRNA signaling and the regulation of AKT activity. Bioinformatics revealed that miR-155 could potentially bind in the 3′UTR of PPP2CA altering its expression and ultimately affecting the regulation of AKT activity. We showed that NT causes AKT phosphorylation in part due to direct interaction between miR-155 and the 3′UTR of PPP2CA that ultimately suppresses the phosphatase. The identification of miR-155 as a direct regulator of PPP2CA, provides a novel link between the NF-κB and AKT pathways in cancer (Fig. 7C).
In this study we have identified a novel microRNA-transcription factor feedback loop network regulating colon cancer growth and invasiveness. Previously, we have described a regulatory feedback loop network between NF-κB, Lin28b, let-7 and IL6 that operates in different cancer cell types, involved in the transformation process46. Recently another study identified a regulatory feed-forward circuit (KRAS-miR-143/miR-145) involved in pancreatic oncogenesis47. These data suggest that microRNA-transcription factor networks are highly important for tumor initiation and progression and their modulation could have therapeutic potential.
Overall, we have identified a combination of increased NTR1, miR-21, miR-155 levels and lowered PTEN, PPP2CA, SOCS1 levels in colon tumors, suggesting the importance of this signaling pathway in human colon carcinogenesis. The expression levels of these genes correlated with tumor stage, suggesting that activation of the NTR1 circuit is potentially important for tumor progression. Our results are consistent with previous reports indicating increased NT expression in human colon cancers48, while NTR1 expression is also elevated in colorectal cancer49, as well as in inflammatory bowel disease-related colon cancer50.
To our knowledge, this is the first report demonstrating the ability of NT or any neuropeptide to modulate expression of specific miRNAs in human colonic epithelium that are functionally linked to colon cancer growth. Also, this is the first report demonstrating PPP2CA phosphatase as a target of miR-155. Further efforts should focus on the study of the regulation of the remaining miRNAs in response to NT, in the sense of other transcription factors involved and different signaling pathways that may be implicated in several intestinal disease states.
Supplementary Material
Suppl. Figure 1. Validation of microRNA array data by SYBR green real-time PCR analysis. The expression of levels of the differentially expressed microRNAs identified by TLDA microRNA sc reen analysis (Applied Biosystems) was validated by real-time PCR analysis in the same time points. The experiments were performed in triplicate and the data represent mean ± SD.
Suppl. Figure 2. Neurotensin suppresses microRNA expression through NTR1 in HCT116 and DLD1 colon cancer cells. NTR1 expression was inhibited in HCT116 and DLD1 colon canc er cells by siRNA treatment for 48h; these cells were treated with neurotensin (100 nM) for 6h and the expression levels of miR-125b, miR-139 and miR-148a were assessed by real-time PCR analysis. All the data show mean ± SD of three independent experiments.
Suppl. Figure 3. NF- B phosphorylation status after neurotensin treatment in colon cancer cells. Evaluation of NF-KB protein expression and phosphorylation levels in HCT116 and DLD1 cells treated with neurotensin (100nM) for 0.5 and 6h.
Suppl Figure 4. Neurotensin circuit in LoVo colon cancer cells. (A) MicroRNA expression levels in LoVo cells 0.5 and 6h post neurotensin treatment. The expression levels were assessed by real-time PCR analysis for the top seven neurotensin up- and down-regulated microRNAs in NCM460-NTR1 cells. (B) NTR1 expression was inhibited in LoVo colon cancer cells by siRNA treatments (siNTR1#1 or siNTR1#2, 100 nM) for 48h; these cells were treated with neurotensin (100 nM) for 6h and the expression levels of miR-21, miR-210 and miR-155 were assessed by real-time PCR analysis. (C) Expression levels of miR-21, miR-210 and miR-155, assessed by realtime PCR, in LoVo cells that were treated with a pharmacological inhibitor of NF-KB pathway (BAY-117082, 5uM) or an siRNA negative control (siRNA NC, 100nM) or an siRNA against p65 (sip65, 100nM) for 48h and then treated with neurotensin (100nM) for 6h. (D) AKT phosphorylation (s473) levels, assessed by ELISA, in LoVo cells transfected with miR-155 (100nM) or si-PPP2CA (100nM) for 24h.
Suppl. Figure 5. Correlation between NTR1 expression levels and miR-21 or miR-155 or PTEN or SOCS1 or PPP2CA in normal and colon cancer tissues.
Acknowledgments
We would like to thank the Vector Core, Department of Cell Biology, UCLA, for providing us with the Lentivirus construct and Dr. Sarah Dry and the Translational Pathology Core Laboratory, Department of Pathology, UCLA, for providing human colon cancer tissue samples for our studies.
Grant support: United States Public Health Service Grant RO1 DK60729 (CP), a Crohn's and Colitis Foundation of America Research Fellowship (IK) and a Medical Research Award from the Broad Foundation (IK). Support was also provided by the Blinder Research Foundation for Crohn's Disease and the Eli and Edythe Broad Chair (CP)
Footnotes
Author Contributions: Kyriaki Bakirtzi: study concept and design, acquisition of data, analysis and data interpretation.
Maria Hatziapostolou, Iordanes Karagiannides, Christos Polytarchou, Savina Jaeger: acquisition of data, analysis and data interpretation.
Dimitrios Iliopoulos, Charalabos Pothoulakis: study design, manuscript writing, data interpretation and technical support.
Disclosures: Nothing to disclose.
References
- 1.Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294:853–8. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- 2.McKenna LB, Schug J, Vourekas A, McKenna JB, Bramswig NC, Friedman JR, Kaestner KH. MicroRNAs control intestinal epithelial differentiation, architecture, and barrier function. Gastroenterology. 139:1654–64, 1664 e1. doi: 10.1053/j.gastro.2010.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–33. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 5.Akao Y, Nakagawa Y, Naoe T. let-7 microRNA functions as a potential growth suppressor in human colon cancer cells. Biol Pharm Bull. 2006;29:903–6. doi: 10.1248/bpb.29.903. [DOI] [PubMed] [Google Scholar]
- 6.Meltzer PS. Cancer genomics: small RNAs with big impacts. Nature. 2005;435:745–6. doi: 10.1038/435745a. [DOI] [PubMed] [Google Scholar]
- 7.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–8. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 8.Iliopoulos D, Jaeger SA, Hirsch HA, Bulyk ML, Struhl K. STAT3 activation of miR-21 and miR-181b-1 via PTEN and CYLD are part of the epigenetic switch linking inflammation to cancer. Mol Cell. 39:493–506. doi: 10.1016/j.molcel.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carraway R, Leeman SE. The isolation of a new hypotensive peptide, neurotensin, from bovine hypothalami. J Biol Chem. 1973;248:6854–61. [PubMed] [Google Scholar]
- 10.Carraway R, Leeman SE. Characterization of radioimmunoassayable neurotensin in the rat. Its differential distribution in the central nervous system, small intestine, and stomach. J Biol Chem. 1976;251:7045–52. [PubMed] [Google Scholar]
- 11.Zhao D, Zhan Y, Koon HW, Zeng H, Keates S, Moyer MP, Pothoulakis C. Metalloproteinase-dependent transforming growth factor-alpha release mediates neurotensin-stimulated MAP kinase activation in human colonic epithelial cells. J Biol Chem. 2004;279:43547–54. doi: 10.1074/jbc.M401453200. [DOI] [PubMed] [Google Scholar]
- 12.Tasuta M, Iishi H, Baba M, Taniguchi H. Enhancement by neurotensin of experimental carcinogenesis induced in rat colon by azoxymethane. Br J Cancer. 1990;62:368–71. doi: 10.1038/bjc.1990.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maoret JJ, Anini Y, Rouyer-Fessard C, Gully D, Laburthe M. Neurotensin and a non-peptide neurotensin receptor antagonist control human colon cancer cell growth in cell culture and in cells xenografted into nude mice. Int J Cancer. 1999;80:448–54. doi: 10.1002/(sici)1097-0215(19990129)80:3<448::aid-ijc19>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 14.Castagliuolo I, Wang CC, Valenick L, Pasha A, Nikulasson S, Carraway RE, Pothoulakis C. Neurotensin is a proinflammatory neuropeptide in colonic inflammation. J Clin Invest. 1999;103:843–9. doi: 10.1172/JCI4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koon HW, Kim YS, Xu H, Kumar A, Zhao D, Karagiannides I, Dobner PR, Pothoulakis C. Neurotensin induces IL-6 secretion in mouse preadipocytes and adipose tissues during 2,4,6,-trinitrobenzensulphonic acid-induced colitis. Proc Natl Acad Sci U S A. 2009;106:8766–71. doi: 10.1073/pnas.0903499106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nielsen BS, Jorgensen S, Fog JU, Sokilde R, Christensen IJ, Hansen U, Brunner N, Baker A, Moller S, Nielsen HJ. High levels of microRNA-21 in the stroma of colorectal cancers predict short disease-free survival in stage II colon cancer patients. Clin Exp Metastasis. doi: 10.1007/s10585-010-9355-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang XY, Wu MH, Liu F, Li Y, Li N, Li GY, Shen SR. Differential miRNA expression and their target genes between NGX6-positive and negative colon cancer cells. Mol Cell Biochem. 345:283–90. doi: 10.1007/s11010-010-0582-7. [DOI] [PubMed] [Google Scholar]
- 18.Zhao D, Keates AC, Kuhnt-Moore S, Moyer MP, Kelly CP, Pothoulakis C. Signal transduction pathways mediating neurotensin-stimulated interleukin-8 expression in human colonocytes. J Biol Chem. 2001;276:44464–71. doi: 10.1074/jbc.M104942200. [DOI] [PubMed] [Google Scholar]
- 19.Naldini L, Blomer U, Gage FH, Trono D, Verma IM. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci U S A. 1996;93:11382–8. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warner JB, Philippakis AA, Jaeger SA, He FS, Lin J, Bulyk ML. Systematic identification of mammalian regulatory motifs' target genes and functions. Nat Methods. 2008;5:347–53. doi: 10.1038/nmeth.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang A, Zhu Z, Kapranov P, McKeon F, Church GM, Gingeras TR, Struhl K. Relationships between p63 binding, DNA sequence, transcription activity, and biological function in human cells. Mol Cell. 2006;24:593–602. doi: 10.1016/j.molcel.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 22.Iliopoulos D, Lindahl-Allen M, Polytarchou C, Hirsch HA, Tsichlis PN, Struhl K. Loss of miR-200 inhibition of Suz12 leads to polycomb-mediated repression required for the formation and maintenance of cancer stem cells. Mol Cell. 39:761–72. doi: 10.1016/j.molcel.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao D, Bakirtzi K, Zhan Y, Zeng H, Koon HW, Pothoulakis C. Insulin-like growth factor-1 receptor transactivation modulates the inflammatory and proliferative response of neurotensin in human colonic epithelial cells. J Biol Chem. doi: 10.1074/jbc.M110.192534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang S, Zhang HW, Lu MH, He XH, Li Y, Gu H, Liu MF, Wang ED. MicroRNA-155 functions as an OncomiR in breast cancer by targeting the suppressor of cytokine signaling 1 gene. Cancer Res. 70:3119–27. doi: 10.1158/0008-5472.CAN-09-4250. [DOI] [PubMed] [Google Scholar]
- 25.Hassan S, Dobner PR, Carraway RE. Involvement of MAP-kinase, PI3-kinase and EGF-receptor in the stimulatory effect of Neurotensin on DNA synthesis in PC3 cells. Regul Pept. 2004;120:155–66. doi: 10.1016/j.regpep.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Resjo S, Goransson O, Harndahl L, Zolnierowicz S, Manganiello V, Degerman E. Protein phosphatase 2A is the main phosphatase involved in the regulation of protein kinase B in rat adipocytes. Cell Signal. 2002;14:231–8. doi: 10.1016/s0898-6568(01)00238-8. [DOI] [PubMed] [Google Scholar]
- 27.Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–5. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 28.Zhang JG, Wang JJ, Zhao F, Liu Q, Jiang K, Yang GH. MicroRNA-21 (miR-21) represses tumor suppressor PTEN and promotes growth and invasion in non-small cell lung cancer (NSCLC) Clin Chim Acta. 411:846–52. doi: 10.1016/j.cca.2010.02.074. [DOI] [PubMed] [Google Scholar]
- 29.Liang Z, Wu H, Reddy S, Zhu A, Wang S, Blevins D, Yoon Y, Zhang Y, Shim H. Blockade of invasion and metastasis of breast cancer cells via targeting CXCR4 with an artificial microRNA. Biochem Biophys Res Commun. 2007;363:542–6. doi: 10.1016/j.bbrc.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Taby R, Issa JP. Cancer epigenetics. CA Cancer J Clin. 60:376–92. doi: 10.3322/caac.20085. [DOI] [PubMed] [Google Scholar]
- 31.Petrocca F, Visone R, Onelli MR, Shah MH, Nicoloso MS, de Martino I, Iliopoulos D, Pilozzi E, Liu CG, Negrini M, Cavazzini L, Volinia S, Alder H, Ruco LP, Baldassarre G, Croce CM, Vecchione A. E2F1-regulated microRNAs impair TGFbeta-dependent cell-cycle arrest and apoptosis in gastric cancer. Cancer Cell. 2008;13:272–86. doi: 10.1016/j.ccr.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 32.Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, Prueitt RL, Yanaihara N, Lanza G, Scarpa A, Vecchione A, Negrini M, Harris CC, Croce CM. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006;103:2257–61. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michael MZ, SM OC, van Holst Pellekaan NG, Young GP, James RJ. Reduced accumulation of specific microRNAs in colorectal neoplasia. Mol Cancer Res. 2003;1:882–91. [PubMed] [Google Scholar]
- 34.Saito Y, Suzuki H, Hibi T. The role of microRNAs in gastrointestinal cancers. J Gastroenterol. 2009;44(Suppl 19):18–22. doi: 10.1007/s00535-008-2285-3. [DOI] [PubMed] [Google Scholar]
- 35.Castagliuolo I, Leeman SE, Bartolak-Suki E, Nikulasson S, Qiu B, Carraway RE, Pothoulakis C. A neurotensin antagonist, SR 48692, inhibits colonic responses to immobilization stress in rats. Proc Natl Acad Sci U S A. 1996;93:12611–5. doi: 10.1073/pnas.93.22.12611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brun P, Mastrotto C, Beggiao E, Stefani A, Barzon L, Sturniolo GC, Palu G, Castagliuolo I. Neuropeptide neurotensin stimulates intestinal wound healing following chronic intestinal inflammation. Am J Physiol Gastrointest Liver Physiol. 2005;288:G621–9. doi: 10.1152/ajpgi.00140.2004. [DOI] [PubMed] [Google Scholar]
- 37.Gross KJ, Pothoulakis C. Role of neuropeptides in inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:918–32. doi: 10.1002/ibd.20129. [DOI] [PubMed] [Google Scholar]
- 38.Wang X, Wang Q, Ives KL, Evers BM. Curcumin inhibits neurotensin-mediated interleukin-8 production and migration of HCT116 human colon cancer cells. Clin Cancer Res. 2006;12:5346–55. doi: 10.1158/1078-0432.CCR-06-0968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thompson RC, Herscovitch M, Zhao I, Ford TJ, Gilmore TD. NF-kappaB down-regulates expression of the B-lymphoma marker CD10 through a miR-155/PU.1 pathway. J Biol Chem. 286:1675–82. doi: 10.1074/jbc.M110.177063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shin VY, Jin H, Ng EK, Cheng AS, Chong WW, Wong CY, Leung WK, Sung JJ, Chu KM. NF-kappaB targets miR-16 and miR-21 in gastric cancer: involvement of prostaglandin E receptors. Carcinogenesis. 32:240–5. doi: 10.1093/carcin/bgq240. [DOI] [PubMed] [Google Scholar]
- 41.Takagi T, Naito Y, Mizushima K, Hirata I, Yagi N, Tomatsuri N, Ando T, Oyamada Y, Isozaki Y, Hongo H, Uchiyama K, Handa O, Kokura S, Ichikawa H, Yoshikawa T. Increased expression of microRNA in the inflamed colonic mucosa of patients with active ulcerative colitis. J Gastroenterol Hepatol. 25(Suppl 1):S129–33. doi: 10.1111/j.1440-1746.2009.06216.x. [DOI] [PubMed] [Google Scholar]
- 42.Wu F, Zhang S, Dassopoulos T, Harris ML, Bayless TM, Meltzer SJ, Brant SR, Kwon JH. Identification of microRNAs associated with ileal and colonic Crohn's disease. Inflamm Bowel Dis. 16:1729–38. doi: 10.1002/ibd.21267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Staal SP. Molecular cloning of the akt oncogene and its human homologues AKT1 and AKT2: amplification of AKT1 in a primary human gastric adenocarcinoma. Proc Natl Acad Sci U S A. 1987;84:5034–7. doi: 10.1073/pnas.84.14.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 45.Ugi S, Imamura T, Maegawa H, Egawa K, Yoshizaki T, Shi K, Obata T, Ebina Y, Kashiwagi A, Olefsky JM. Protein phosphatase 2A negatively regulates insulin's metabolic signaling pathway by inhibiting Akt (protein kinase B) activity in 3T3-L1 adipocytes. Mol Cell Biol. 2004;24:8778–89. doi: 10.1128/MCB.24.19.8778-8789.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139:693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kent OA, Chivukula RR, Mullendore M, Wentzel EA, Feldmann G, Lee KH, Liu S, Leach SD, Maitra A, Mendell JT. Repression of the miR-143/145 cluster by oncogenic Ras initiates a tumor-promoting feed-forward pathway. Genes Dev. 24:2754–9. doi: 10.1101/gad.1950610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Evers BM, Ishizuka J, Chung DH, Townsend CM, Jr, Thompson JC. Neurotensin expression and release in human colon cancers. Ann Surg. 1992;216:423–30. doi: 10.1097/00000658-199210000-00005. discussion 430-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chao C, Tallman ML, Ives KL, Townsend CM, Jr, Hellmich MR. Gastrointestinal hormone receptors in primary human colorectal carcinomas. J Surg Res. 2005;129:313–21. doi: 10.1016/j.jss.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 50.Bossard C, Souaze F, Jarry A, Bezieau S, Mosnier JF, Forgez P, Laboisse CL. Over-expression of neurotensin high-affinity receptor 1 (NTS1) in relation with its ligand neurotensin (NT) and nuclear beta-catenin in inflammatory bowel disease-related oncogenesis. Peptides. 2007;28:2030–5. doi: 10.1016/j.peptides.2007.06.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppl. Figure 1. Validation of microRNA array data by SYBR green real-time PCR analysis. The expression of levels of the differentially expressed microRNAs identified by TLDA microRNA sc reen analysis (Applied Biosystems) was validated by real-time PCR analysis in the same time points. The experiments were performed in triplicate and the data represent mean ± SD.
Suppl. Figure 2. Neurotensin suppresses microRNA expression through NTR1 in HCT116 and DLD1 colon cancer cells. NTR1 expression was inhibited in HCT116 and DLD1 colon canc er cells by siRNA treatment for 48h; these cells were treated with neurotensin (100 nM) for 6h and the expression levels of miR-125b, miR-139 and miR-148a were assessed by real-time PCR analysis. All the data show mean ± SD of three independent experiments.
Suppl. Figure 3. NF- B phosphorylation status after neurotensin treatment in colon cancer cells. Evaluation of NF-KB protein expression and phosphorylation levels in HCT116 and DLD1 cells treated with neurotensin (100nM) for 0.5 and 6h.
Suppl Figure 4. Neurotensin circuit in LoVo colon cancer cells. (A) MicroRNA expression levels in LoVo cells 0.5 and 6h post neurotensin treatment. The expression levels were assessed by real-time PCR analysis for the top seven neurotensin up- and down-regulated microRNAs in NCM460-NTR1 cells. (B) NTR1 expression was inhibited in LoVo colon cancer cells by siRNA treatments (siNTR1#1 or siNTR1#2, 100 nM) for 48h; these cells were treated with neurotensin (100 nM) for 6h and the expression levels of miR-21, miR-210 and miR-155 were assessed by real-time PCR analysis. (C) Expression levels of miR-21, miR-210 and miR-155, assessed by realtime PCR, in LoVo cells that were treated with a pharmacological inhibitor of NF-KB pathway (BAY-117082, 5uM) or an siRNA negative control (siRNA NC, 100nM) or an siRNA against p65 (sip65, 100nM) for 48h and then treated with neurotensin (100nM) for 6h. (D) AKT phosphorylation (s473) levels, assessed by ELISA, in LoVo cells transfected with miR-155 (100nM) or si-PPP2CA (100nM) for 24h.
Suppl. Figure 5. Correlation between NTR1 expression levels and miR-21 or miR-155 or PTEN or SOCS1 or PPP2CA in normal and colon cancer tissues.


