Abstract
Aims
Nucleotides are important paracrine regulators of vascular tone. We previously demonstrated that activation of P2Y2 receptors causes an acute, NO-independent decrease in blood pressure, indicating this signalling pathway requires an endothelial-derived hyperpolarization (EDH) response. To define the mechanisms by which activation of P2Y2 receptors initiates EDH and vasodilation, we studied intermediate-conductance (KCa3.1, expressed in endothelial cells) and big-conductance potassium channels (KCa1.1, expressed in smooth muscle cells) as well as components of the myoendothelial gap junction, connexins 37 and 40 (Cx37, Cx40), all hypothesized to be part of the EDH response.
Methods
We compared the effects of a P2Y2/4 receptor agonist in wild-type (WT) mice and in mice lacking KCa3.1, KCa1.1, Cx37 or Cx40 under anaesthesia, while monitoring intra-arterial blood pressure and heart rate.
Results
Acute activation of P2Y2/4 receptors (0.01–3 mg kg−1 body weight i.v.) caused a biphasic blood pressure response characterized by a dose-dependent and rapid decrease in blood pressure in WT (maximal response % of baseline at 3 mg kg−1: −38 ± 1%) followed by a consecutive increase in blood pressure (+44 ± 11%). The maximal responses in KCa3.1−/− and Cx37−/− were impaired (−13 ± 5, +17 ± 7 and −27 ± 1, +13 ± 3% respectively), whereas the maximal blood pressure decrease in response to acetylcholine at 3 µg kg−1 was not significantly different (WT: −53 ± 3%; KCa3.1−/−: −52 ± 3; Cx37−/−: −53 ± 3%). KCa1.1−/− and Cx40−/− showed an identical biphasic response to P2Y2/4 receptor activation compared to WT.
Conclusions
The data suggest that the P2Y2/4 receptor activation elicits blood pressure responses via distinct mechanisms involving KCa3.1 and Cx37.
Keywords: gap junction, hyperpolarization, K+ channels, myogenic tone, P2 receptors, signalling
Vascular tone can be regulated via nucleotides like ATP and UTP that are derived from erythrocytes or platelets or released from the endothelium (Ralevic & Burnstock 2003). It is known that ATP is an agonist of P2Y2 receptors and UTP is an agonist of P2Y2/4 receptors (Abbracchio et al. 2006). A decrease in blood pressure most likely results from ATP inducing endothelium-dependent relaxation (von Kugelgen et al. 1987, von Kugelgen & Starke 1990, Ralevic & Burnstock 1991a, 2003). Evidence suggests that this vasodilatory response can be mediated by nitric oxide (NO; Ralevic & Burnstock 1991a), endothelial-derived hyperpolarization (EDH; Malmsjo et al. 1999, 2002, Wihlborg et al. 2003) and/or prostacyclin (Hammer et al. 2003, Wihlborg et al. 2003). P2Y2 receptors are found on the endothelium and it was proposed that their activation stimulates the synthesis and release of NO (Ralevic & Burnstock 1991b, Buvinic et al. 2002, Burnstock 2009). Moreover, aortic rings from P2Y2 receptor knockout mice (P2Y2−/−) exhibit impaired vasorelaxation in response to ATP, which suggests that NO release is subsequent to P2Y2 receptor activation (Guns et al. 2005, 2006). Of note, mechanical destruction of the endothelium abolishes the ATP induced vasodilatory effect and produces a direct vasoconstrictory response on vascular smooth muscle cells (Kennedy & Burnstock 1985, Kennedy et al. 1985, Ralevic & Burnstock 1996b). Activation of P2Y4 receptors is associated with vasoconstriction (Dietrich et al. 1996, McMillan et al. 1999, Rubino et al. 1999). In contrast to ATP causing vasodilation, UTP was found to vasoconstrict mouse aortic rings (Boarder & Hourani 1998, Kauffenstein et al. 2010) and rabbit inner ear arteries (von Kugelgen et al. 1987). However, depending on route of administration, species, localization within the vascular tree or vessel type, UTP can cause vasoconstriction, vasodilation or both (Ralevic & Burnstock 1996a,b, Janigro et al. 1997, Horiuchi et al. 2001, Guns et al. 2005, Rayment et al. 2007, Inscho 2009).
In recent studies, we demonstrated that P2Y2 receptors play a physiological role in blood pressure regulation and, as a consequence, P2Y2−/− mice were found to have salt-resistant hypertension (Rieg et al. 2007a, Pochynyuk et al. 2010). Direct intra-arterial blood pressure measurements indicated that the blood pressure responses to a P2Y2/4 agonist result in vasodilation via a NO-independent mechanism that possibly involves EDH release subsequently to P2Y2 receptor activation. This was concluded because endothelial NO synthase knockout mice (eNOS−/−) showed an identical blood pressure effect in response to P2Y2/4 receptor activation compared to WT mice. In contrast to WT mice, P2Y2−/− mice responded to P2Y2/4 receptor activation with an increase in blood pressure, possibly a direct effect of P2Y4 receptor activation on vascular smooth muscle cells, which is independent of P2Y2 receptors (Rieg et al. 2011).
The vasodilation mediated by EDH requires activation of calcium-activated potassium channels, including KCa2.3 (small-conductance) and KCa3.1 (intermediate-conductance), which are expressed in most endothelial cells (Kohler & Ruth 2010). In contrast, KCa1.1 (big-conductance) is expressed in vascular smooth muscle cells (Feletou 2009, Kohler & Ruth 2010). The activation of calcium-activated potassium channels is speculated to produce hyperpolarization of the endothelium, which is then transmitted (possibly via connexins, see below) to underlying vascular smooth muscle cells causing vasodilation via EDH. Functional studies employing blockers of calcium-activated potassium channels have demonstrated the role of these channels in EDH and subsequent vascular smooth muscle relaxation (Adeagbo & Triggle 1993, Holzmann et al. 1994, Waldron & Garland 1994, Zygmunt & Hogestatt 1996, Eichler et al. 2003).
Vascular smooth muscle cells and endothelial cells are functionally linked, and the point of contact between the two cells, the myoendothelial gap junction (MEGJ), plays a key role in the regulation of vascular function (Figueroa & Duling 2009). Initially, it was assumed that a diffusible endothelial factor was the mechanism resulting in hyperpolarization; however, this view was later questioned by experiments demonstrating the involvement of the MEGJ (Griffith et al. 2002, Dora et al. 2003, Chaytor et al. 2005, Mather et al. 2005, Sokoya et al. 2006). It was concluded from these studies that EDH is transferred from the endothelium to the smooth muscle by direct charge transfer through the MEGJ (de Wit & Wolfle 2007, Feletou & Vanhoutte 2009, Grgic et al. 2009, Edwards et al. 2010, de Wit & Griffith 2010, Garland et al. 2011). Gap junction proteins found in the vasculature include as follows: Cx37, Cx40, Cx43 and Cx45 (Figueroa et al. 2004, Lohman et al. 2012); however, the MEGJ is specifically comprised of Cx37 and Cx40 which are speculated to conduct the EDH response as an electrical signal between endothelial and vascular smooth muscle cells (Chaytor et al. 2005, Isakson & Duling 2005, Haddock et al. 2006).
To further define the underlying mechanism(s) involved in the P2Y2 receptor-initiated blood pressure responses, possibly via EDH, we studied blood pressure and heart rate in WT, Cx37, Cx40, KCa1.1 and KCa3.1 knockout (−/−) mice in response to systemic application of a P2Y2/4 receptor agonist. We report that P2Y2/4 receptor activation generates a similar biphasic blood pressure response in all mice except for KCa3.1−/− and Cx37−/− mice, which show impaired vascular reactivity. This implies that both KCa3.1 and Cx37 are required for full vascular reactivity in response to P2Y2/4 receptor activation.
Materials and methods
This study is conform with the guidelines for Acta Physiologica (Persson 2013). Cx37−/− (Fang et al. 2011), Cx40−/− (Fang et al. 2012) and KCa1.1−/− (Sausbier et al. 2004, Rieg et al. 2007b) mice were generated and maintained as described previously. Breeder pairs of KCa3.1−/− mice (Begenisich et al. 2004) were kindly provided by Dr. Melvin, National Institutes of Health (Bethesda, MD, USA). Ear tissue DNA was used for genotyping by polymerase chain reaction using gene-specific primers (Fenton et al. 2014). All mice have been reproduced by heterozygous crossing and are on a hybrid SV129/C57BL6 background. Endothelial NO synthase knockout mice (eNOS−/−) were from The Jackson Laboratory (Bar Harbor, ME, USA). Experiments were performed in male adult mice. Mice were housed in the same animal room with a 12 : 12-h light–dark cycle and free access to food (7001, Harlan Teklad, Indianapolis, IN, USA) and tap water.
The compound INS45973 (Inspire Pharmaceuticals, Raleigh, NC, USA), P1-(inosine 5′-)P4-(uridine 5′-)tetraphosphate tetrasodium salt or Ip4U × 4 Na+, was previously described (Mizumori et al. 2009, Rieg et al. 2011, Trabanelli et al. 2012) and dissolved in 0.85% NaCl solution. The EC50 values for INS45973 are as follows: P2Y2: approx. 280 nmol L−1; P2Y4: approx. 280 nmol L−1; P2Y6: >10 µmol L−1; other P2X/P2Y receptors are not activated (Min et al. 2003, Mizumori et al. 2009). INS45973 hydrolysis by ectonucleotide pyrophosphatase/phosphodiesterase possibly results in a ≤1 min in vivo half-life (Vollmayer et al. 2003). As INS45973 does not contain adenine, there is no metabolism to adenosine and consequently adenosine receptors are not activated (Shaver et al. 2005).
Blood pressure and heart rate experiments
Mice were anesthetized with thiobatubarbital and ketamine as previously described (Rieg et al. 2004, 2005). Body temperature was maintained at 37.5 °C by a servo-controlled heating plate as part of the operating table. Mice underwent cannulation of the trachea and were exposed to 100% oxygen throughout the experiment. Cannulation of the jugular vein allowed for i.v. bolus applications and maintenance infusion of 2.25% bovine serum albumin in 0.85% NaCl at a rate of 0.4 mL h−1 × 30−1 g bw. Blood pressure was recorded via a catheter placed in the femoral artery. Urine drainage was allowed via a bladder catheter. Following surgery, mice underwent a 60-min stabilization period prior to starting experiments. Vehicle (0.5 µL g−1 bw of 0.85% NaCl) or INS45973 (0.01, 0.03, 0.1, 0.3, 1 or 3 mg kg−1 bw in escalating doses) were administered via the jugular vein catheter at a rate of 2.4 mL h−1 × 30−1 g bw over 25 s and acute blood pressure responses were measured. The interval between applications was 10 min. For certain experiments, acetylcholine (Sigma-Aldrich, St. Louis, MO, USA) was applied following the above-described protocol (0.01, 0.03, 0.1, 0.3, 1, or 3 µg kg−1 bw in escalating doses).
Calculations and statistical analysis
Per cent change in mean arterial pressure (MAP) or heart rate was calculated by the following equation:
Half maximal effective dose (ED50) and mean ± SEM were calculated and analysed using sigmaplot® v11.0 (San Jose, CA, USA) software. Repeated-measures two-way anova was used for comparison of several experimental curves with a control group followed by Dunnett’s test. ED50 values, blood pressure and heart rate measurements were compared by one-way anova followed by Bonferroni test (all data analysed via graphpad prism® v6.05, La Jolla, CA, USA). Significance was considered at P < 0.05.
Results
We have previously shown (Rieg et al. 2011) that acute application of INS45973 in WT mice decreased blood pressure rapidly and dose dependently (within 15 s of starting infusion, early phase, Fig. 1 and Table 1). We now provide a more comprehensive dose–response assessment, which indicates an ED50 of 0.4 ± 0.1 mg kg−1 for the blood pressure decrease. During bolus application in WT mice, blood pressure began to rise producing a biphasic response with a marked increase in blood pressure (late phase) above baseline (maximum response +44 ± 11 mmHg) immediately after the initial decrease with an ED50 of 0.5 ± 0.2 mg kg−1. For comparison, we show P2Y2−/− mice where INS45973 induced a rapid and dose-dependent (within 15 s of starting infusion, early phase) increase in blood pressure, without showing a distinct biphasic response. In hypertensive eNOS−/− mice (Table 1), bolus application of INS45973 rapidly and dose-dependently decreased blood pressure comparable to WT mice; however, the maximum increase in blood pressure was reduced while the ED50 was not significantly different compared to WT mice (Fig. 2 and Table 1). INS45973 did not affect heart rate in WT, P2Y2−/− and eNOS−/− mice in the early and late phase of the blood pressure responses (Tables 2 and 3). As the P2Y2/4 receptor agonist caused biphasic blood pressure effects within seconds of application without affecting heart rate, this likely indicates that there is a direct effect on peripheral resistance. Along those lines, because the blood pressure responses were not different in eNOS−/− compared to WT mice, we concluded that EDH is possibly required for the decrease in blood pressure.
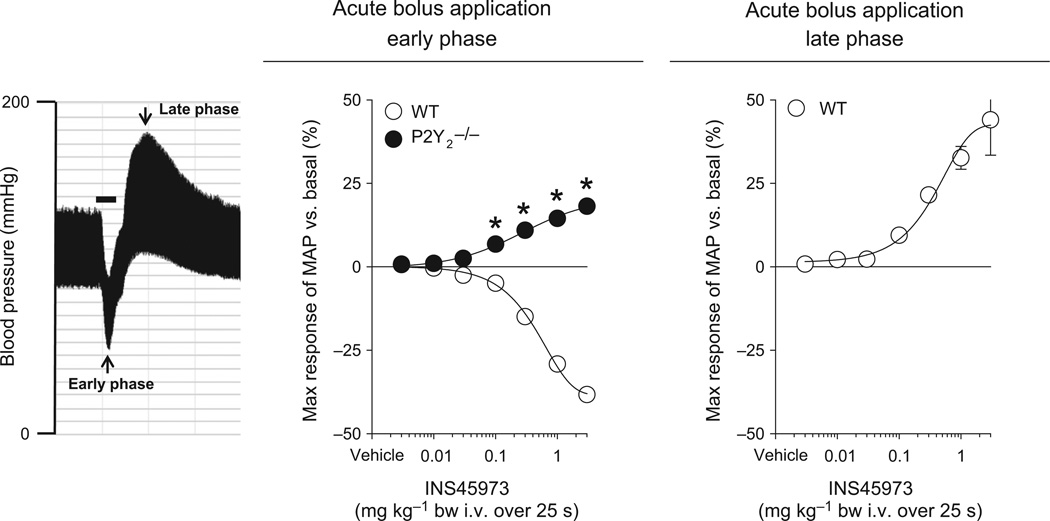
Figure 1.
Maximal responses in mean arterial blood pressure (MAP) to acute bolus application of INS45973 (P2Y2/4 agonist) in wild-type (WT, n = 8) and P2Y2 receptor knockout mice (P2Y2−/−, n = 8). Original recording of INS45973-induced blood pressure effects at 3 mg kg−1 body weight (bar = 25 s) in a WT mouse (left). In WT, application of INS45973 dose-dependently and rapidly decreased blood pressure (middle, early phase), which started to partially recover during drug application and then continued into a dose-dependent increase in blood pressure (right side, late phase). In contrast, INS45973 in P2Y2−/− mice dose-dependently and rapidly increased blood pressure, which was sustained during drug application and thereafter recovered to baseline within 1–2 min, consistent with the short half-life of INS45973 [for an original blood pressure trace see Rieg et al. (2011)]. Some error bars are covered. *P < 0.05 vs. WT (two-way anova with repeated measures followed by Dunnett’s test).
Table 1.
Maximal blood pressure effects at 3 mg kg−1 body weight and ED50 values of wild-type and knockout mice in response to P2Y2/4 receptor activation
| Mean arterial blood pressure at baseline (mmHg) |
Heart rate at baseline (min−1) |
Maximal decrease in %, early phase |
ED50 decrease early phase (mg kg−1) |
Maximal increase in %, late phase |
ED50 increase late phase (mg kg−1) |
|
|---|---|---|---|---|---|---|
| WT | 93 ± 3 | 489 ± 11 | −38 ± 1 | 0.4 ± 0.1 | +44 ± 11 | 0.5 ± 0.2 |
| P2Y2−/− | 111 ± 3* | 489 ± 19 | +18 ± 1* | 0.4 ± 0.1 | – | – |
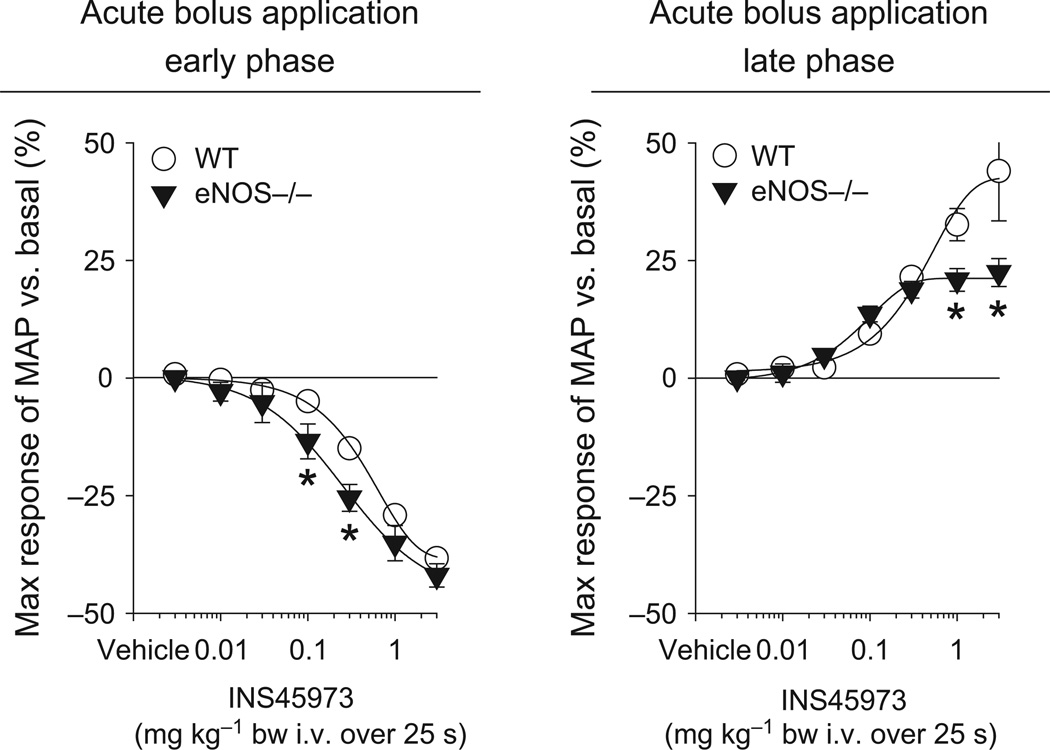
| eNOS−/− | 131 ± 2* | 479 ± 17 | −42 ± 2 | 0.3 ± 0.1 | +22 ± 3* | 0.2 ± 0.1 |
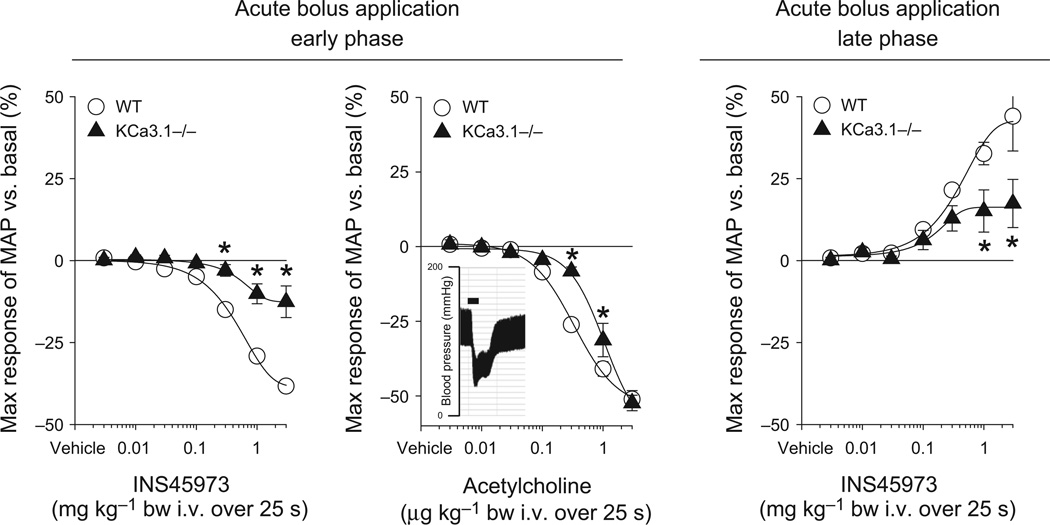
| KCa3.1−/− | 91 ± 6 | 480 ± 28 | −13 ± 5* | 0.6 ± 0.2 | +17 ± 7* | 0.3 ± 0.1 |
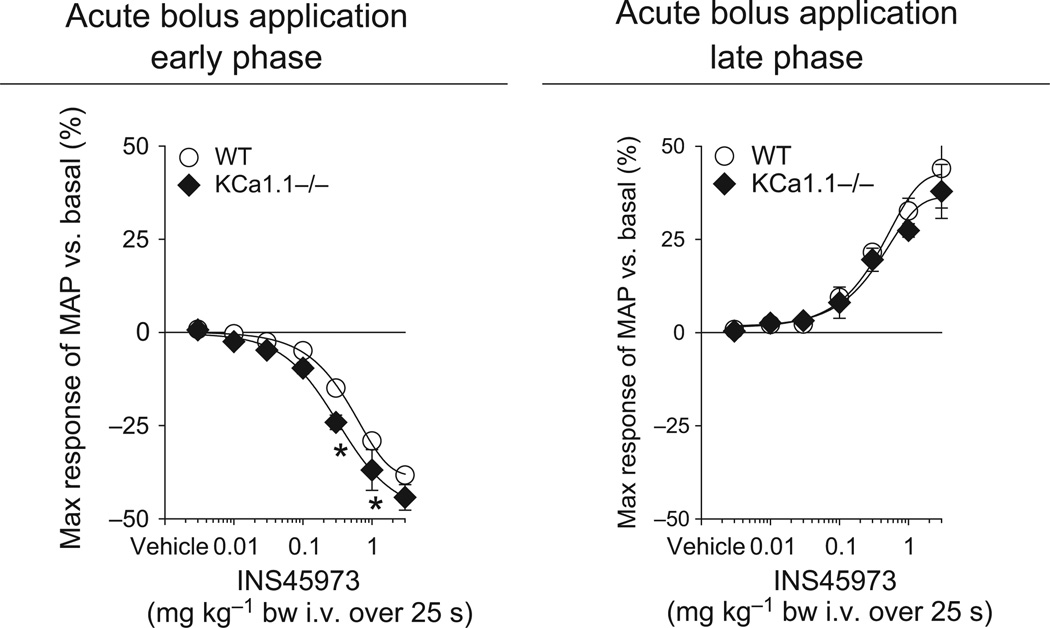
| KCa1.1−/− | 108 ± 2* | 548 ± 23 | −44 ± 3 | 0.4 ± 0.1 | +38 ± 7 | 0.5 ± 0.2 |
| Cx37−/− | 92 ± 4 | 501 ± 31 | −27 ± 1* | 0.6 ± 0.1 | +13 ± 3* | 0.3 ± 0.1 |
| Cx40−/− | 111 ± 5* | 465 ± 33 | −41 ± 2 | 0.3 ± 0.1 | +33 ± 3 | 0.4 ± 0.1 |
Values are mean ± SEM. WT, wild-type mice; P2Y2−/−, P2Y2 receptor knockout mice, eNOS−/−, endothelial NO synthase knockout mice; Cx37−/−, connexin 37 knockout mice; Cx40−/−, connexin 40 knockout mice; KCa1.1−/−, big-conductance potassium channel knockout mice; KCa3.1−/−, intermediate-conductance potassium channel knockout mice. Additional analysis of previously published data (Rieg et al. 2011) from eNOS−/− and P2Y2−/− are included. In contrast, P2Y2−/− mice show an acute blood pressure increase in the early phase and lack a biphasic response therefore not data are shown for the late phase. n = 4–8;
P < 0.05 vs. WT (one-way ANOVA followed by Bonferroni test for blood pressure, heart rate and ED50 values, two-way ANOVA with repeated measures followed by Dunnett’s test for maximal responses).
Figure 2.
Maximal responses in mean arterial blood pressure (MAP) to acute bolus application of INS45973 (P2Y2/4 agonist) in endothelial NO synthase knockout mice (eNOS−/−, n = 5). In eNOS−/− mice, application of INS45973 induced a dose-dependent, rapid decrease in blood pressure (left side, early phase). The dose-dependent rise in blood pressure above basal values following the initial decrease in eNOS−/− mice was significantly impaired compared to WT mice (right side, late phase). Some error bars are covered. *P < 0.05 vs. WT (two-way anova with repeated measures followed by Dunnett’s test).
Table 2.
Maximum heart rate decrease (early phase) of wild-type and knockout mice in response to P2Y2/4 receptor activation
| Vehicle (%) | INS45973 (%) | ||||||
|---|---|---|---|---|---|---|---|
| 0.01 (mg kg−1) | 0.03 (mg kg−1) | 0.1 (mg kg−1) | 0.3 (mg kg−1) | 1 (mg kg−1) | 3 (mg kg−1) | ||
| WT | −1.1 ± 0.5 | 0.2 ± 0.3 | −0.5 ± 0.3 | −1.1 ± 0.7 | 2.0 ± 1.0 | −0.1 ± 0.6 | 1.2 ± 0.8 |
| P2Y2−/− | 0.9 ± 0.1 | 0.3 ± 0.1 | −1.2 ± 1.4 | 3.2 ± 0.8 | 7.7 ± 2.0 | 3.4 ± 0.9 | 6.1 ± 1.2 |
| eNOS−/− | 0.1 ± 0.5 | −0.1 ± 0.5 | −0.4 ± 1.9 | 0.2 ± 0.5 | 0.6 ± 0.3 | 1.2 ± 0.4 | 1.4 ± 0.4 |
| KCa3.1−/− | 0.8 ± 0.9 | 0.9 ± 0.4 | −1.2 ± 0.5 | 0.8 ± 0.3 | −0.3 ± 0.3 | 0.1 ± 0.6 | −0.8 ± 0.5 |
| KCa1.1−/− | 1.1 ± 0.5 | 0.7 ± 0.9 | 0.2 ± 0.1 | 0.6 ± 0.5 | 0.3 ± 0.6 | 0.4 ± 0.6 | 1.9 ± 1.2 |
| Cx37−/− | 1.9 ± 0.8 | 1.1 ± 0.6 | −0.3 ± 0.4 | 0.2 ± 0.5 | 0.1 ± 0.4 | 0.7 ± 0.4 | 1.2 ± 0.3 |
| Cx40−/− | −0.9 ± 0.6 | 0.4 ± 0.2 | −0.2 ± 0.7 | 0.9 ± 0.5 | 1.4 ± 1.3 | 0.4 ± 1.4 | 0.1 ± 1.1 |
Values are mean ± SEM. WT, wild-type mice; P2Y2−/−, P2Y2 receptor knockout mice, eNOS−/−, endothelial NO synthase knockout mice; Cx37−/−, connexin 37 knockout mice; Cx40−/−, connexin 40 knockout mice; KCa1.1−/−, big-conductance potassium channel knockout mice; KCa3.1−/−, intermediate-conductance potassium channel knockout mice. Additional analysis of previously published data (Rieg et al. 2011) from eNOS−/− and P2Y2−/− are included. n = 4–8 (two-way anova with repeated measures followed by Dunnett’s test).
Table 3.
Maximum heart rate increase (late phase) of wild-type and knockout mice in response to P2Y2/4 receptor activation
| Vehicle (%) | INS45973 (%) | ||||||
|---|---|---|---|---|---|---|---|
| 0.01 (mg kg−1) | 0.03 (mg kg−1) | 0.1 (mg kg−1) | 0.3 (mg kg−1) | 1 (mg kg−1) | 3 (mg kg−1) | ||
| WT | −1.1 ± 0.5 | 0.8 ± 0.7 | 0.4 ± 0.6 | 0.7 ± 0.9 | 2.5 ± 1.6 | 7.2 ± 2.4 | 7.3 ± 2.7 |
| P2Y2−/− | – | – | – | – | – | – | – |
| eNOS−/− | 0.1 ± 0.5 | 0.1 ± 0.6 | −0.1 ± 1.0 | −1.2 ± 0.4 | −0.9 ± 1.2 | 0.7 ± 2.0 | 0.1 ± 5.9 |
| KCa3.1−/− | 0.8 ± 0.9 | 2.2 ± 1.0 | −1.0 ± 0.3 | 0.6 ± 1.1 | −0.5 ± 0.9 | 0.4 ± 2.0 | −0.7 ± 1.6 |
| KCa1.1−/− | 1.1 ± 0.5 | 0.7 ± 0.3 | 1.0 ± 0.6 | 1.8 ± 0.8 | 0.2 ± 1.5 | −1.0 ± 2.4 | −2.0 ± 1.8 |
| Cx37−/− | 1.9 ± 0.8 | 3.8 ± 1.1 | 4.0 ± 2.0 | 0.6 ± 1.7 | −2.5 ± 1.2 | 1.2 ± 2.1 | 3.3 ± 2.4 |
| Cx40−/− | −0.9 ± 0.6 | 0.6 ± 0.6 | 0.2 ± 0.1 | 3.4 ± 2.6 | 2.3 ± 0.9 | 6.1 ± 5.6 | 7.9 ± 4.7 |
Values are mean ± SEM. WT, wild-type mice; P2Y2−/−, P2Y2 receptor knockout mice, eNOS−/−, endothelial NO synthase knockout mice; Cx37−/−, connexin 37 knockout mice; Cx40−/−, connexin 40 knockout mice; KCa1.1−/−, big-conductance potassium channel knockout mice; KCa3.1−/−, intermediate-conductance potassium channel knockout mice. Additional analysis of previously published data (Rieg et al. 2011) from eNOS−/− and P2Y2−/− are included. P2Y2−/− mice show an acute blood pressure increase in the early phase and lack a biphasic response; therefore, data are not shown for the late phase. n = 4–8 (two-way anova with repeated measures followed by Dunnett’s test).
To test for a possible role of EDH for P2Y2/4 receptor-initiated blood pressure responses, we studied KCa3.1−/− mice because KCa3.1 channels are speculated to initiate EDH. Baseline blood pressure in KCa3.1−/− mice was comparable to WT mice (Table 1). Bolus application of INS45973 to KCa3.1−/− mice showed a severely impaired dose-dependent decrease in blood pressure in the early phase as well as an impaired increase in blood pressure in the late phase compared to WT mice (Fig. 3 and Table 1). The ED50 values of the early and late phase were comparable to WT mice (Table 1). To exclude that the impaired P2Y2/4 receptor-initiated blood pressure responses of KCa3.1−/− mice are not caused by a general vascular dysfunction, we tested in a different set of WT and KCa3.1−/− mice (n = 4 each) blood pressure responses to acetylcholine, a well-described vasodilator (Fig. 3). In WT mice, bolus application of acetylcholine induced a rapid and dose-dependent (within 15 s of starting infusion) decrease in blood pressure (ED50 of 0.4 ± 0.1 µg kg−1) without showing a biphasic response (increase in blood pressure, late phase). Similarly, bolus application of acetylcholine in KCa3.1−/− mice dose-dependently and rapidly decreased blood pressure. Only at doses of 0.3 and 1 µg kg−1 bw was an attenuated acetylcholine-induced decrease in blood pressure observed compared to WT mice; however, the response at the highest dose of 3 µg kg−1 bw was not significantly different from WT mice. There was a significant right-shift of the dose–response curve compared to WT mice (ED50 of 1.0 ± 0.2 µg kg−1, P < 0.05 vs. WT). Acetylcholine did not affect heart rate in WT and KCa3.1−/− mice (not shown). KCa1.1 is expressed in vascular smooth muscle cells and is important for vasodilation and regulation of vessel diameter, contributing to the hypertension found in KCa1.1−/− mice (Sausbier et al. 2005, Rieg et al. 2007b), a finding confirmed in the current study (Table 1). However, KCa1.1−/− mice showed a decrease in blood pressure in the early phase as well as an increase in blood pressure in the late phase that was comparable to WT mice (Fig. 4 and Table 1). Both the early and late phase ED50 values were comparable to WT mice (Table 1). INS45973 did not affect heart rate in WT, KCa3.1−/− and KCa1.1−/− mice in the early and late phase of the blood pressure responses (Tables 2 and 3). These data indicate that KCa3.1 is required for the blood pressure effects following P2Y2/4 receptor activation while KCa1.1 in not part of the signalling pathway activated via P2Y2/4 receptors.
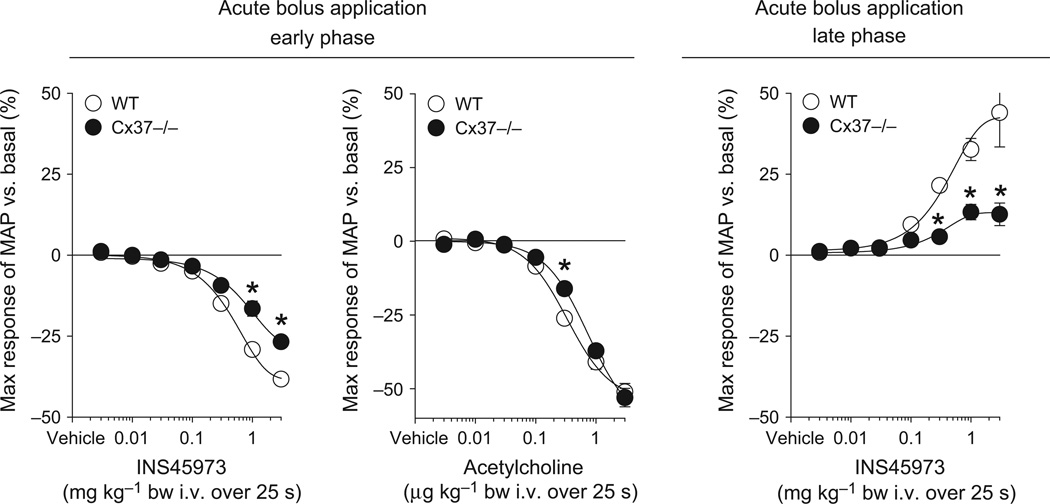
Figure 3.
Maximal responses in mean arterial blood pressure (MAP) to acute bolus application of INS45973 (P2Y2/4 agonist) in intermediate-conductance potassium channel knockout mice (KCa3.1−/−, n = 5). In KCa3.1−/− mice, application of INS45973 induced a dose-dependent, rapid decrease in blood pressure (left side, early phase), which was significantly impaired compared to wild-type (WT) mice. The blood pressure decrease in response to acetylcholine showed a right shift of the dose–response curve in KCa3.1−/− compared to WT mice; however, the maximum response was unaffected. The blood pressure decrease caused by acetylcholine is not followed by an acute blood pressure increase above basal values. Inset: original recording of acetylcholine-induced blood pressure effects at 3 µg kg−1 body weight in a WT mouse (bar = 25 s). The dose-dependent rise in blood pressure in response to INS45973 above basal values following the initial decrease in KCa3.1−/− mice was also significantly impaired compared to WT mice (right side, late phase). Some error bars are covered. *P < 0.05 vs. WT (two-way anova with repeated measures followed by Dunnett’s test).
Figure 4.
Maximal responses in mean arterial blood pressure (MAP) to acute bolus application of INS45973 (P2Y2/4 agonist) in big-conductance potassium channel knockout mice (KCa1.1−/−, n = 4). In KCa1.1−/− mice, application of INS45973 induced a dose-dependent, rapid decrease in blood pressure (left side, early phase). The dose-dependent rise in blood pressure above basal values following the initial decrease in KCa1.1−/− mice was comparable to WT mice (right side, late phase). Some error bars are covered. *P < 0.05 vs. WT (two-way anova with repeated measures followed by Dunnett’s test).
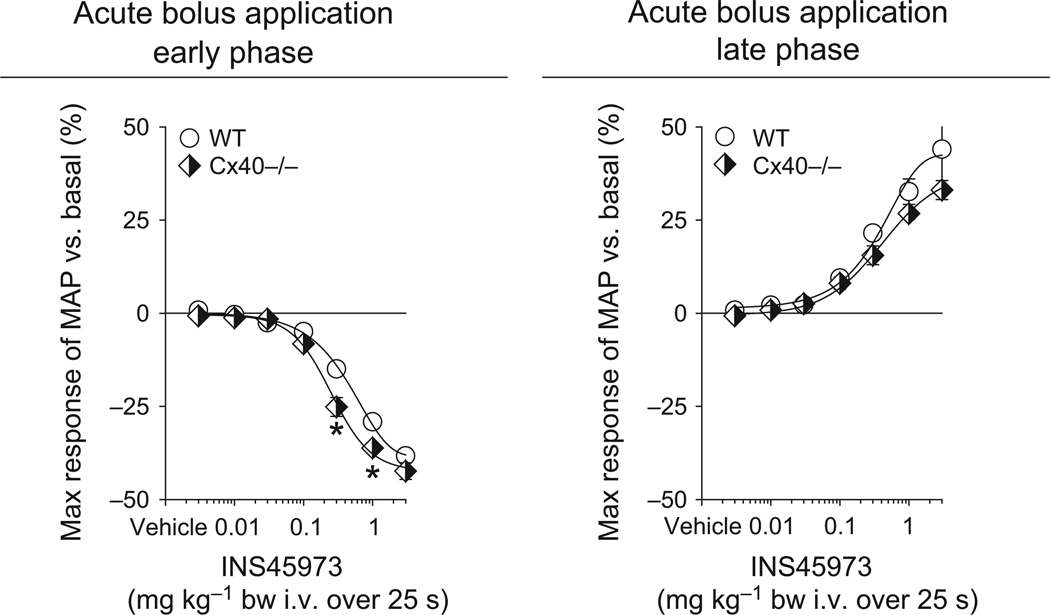
As gap junctions play a critical role in EDH, we studied the blood pressure response to INS45973 in animals lacking either Cx37 or Cx40, the two most abundant connexins found in the MEGJ. Baseline blood pressure in Cx37−/− mice was, as previously described (Figueroa & Duling 2008), comparable to WT mice (Table 1). Bolus application of INS45973 to Cx37−/− mice showed an impaired dose-dependent decrease in blood pressure in the early phase as well as an impaired increase in blood pressure in the late phase (Fig. 5 and Table 1). The early and late phase ED50 values were not significantly different in Cx37−/− vs. WT mice (Table 1). In a different set of Cx37−/− mice (n = 4), blood pressure responses to acetylcholine were tested (Fig. 5). Bolus application of acetylcholine induced a rapid and dose-dependent (within 15 s of starting infusion) decrease in blood pressure (ED50 of 0.6 ± 0.2 µg kg−1, not significant vs. WT) without showing a biphasic response (increase in blood pressure in the late phase). Acetylcholine did not affect heart rate in Cx37−/− mice (not shown). Baseline blood pressure in Cx40−/− mice was significantly higher compared to WT mice (Table 1), a finding possibly related to the activated renin-angiotensin system in these mice (Krattinger et al. 2007). Bolus application of INS45973 to Cx40−/− mice showed a decrease in blood pressure in the early phase as well as an increase in blood pressure in the late phase that was comparable to WT mice (Fig. 6 and Table 1). Both, the early and late phase ED50 values, were comparable to WT mice (Table 1). INS45973 did not affect heart rate in WT, Cx37−/− and Cx40−/− mice in the early and late phase of the blood pressure responses (Tables 2 and 3). These data indicate that Cx37, but not Cx40, takes part in the regulation of vascular tone in response to P2Y2/4 receptor activation.
Figure 5.
Maximal responses in mean arterial blood pressure (MAP) to acute bolus application of INS45973 (P2Y2/4 agonist) in connexin 37 knockout mice (Cx37−/−, n = 5). In Cx37−/− mice, application of INS45973 induced a dose-dependent, rapid decrease in blood pressure (left side, early phase) which was significantly impaired compared to wild-type (WT) mice. The dose-dependent rise in blood pressure above basal values following the initial decrease in Cx37−/− mice was also significantly impaired compared to WT mice (right side, late phase). The dose-dependent blood pressure decrease in response to acetylcholine was comparable between Cx37−/− and WT mice and not followed by an acute blood pressure increase above basal values. Some error bars are covered. *P < 0.05 vs. WT (two-way anova with repeated measures followed by Dunnett’s test).
Figure 6.
Maximal responses in mean arterial blood pressure (MAP) to acute bolus application of INS45973 (P2Y2/4 agonist) in connexin 40 knockout mice (Cx40−/−, n = 4). In Cx40−/− mice, application of INS45973 induced a dose-dependent, rapid decrease in blood pressure (left side, early phase). The dose-dependent rise in blood pressure above basal values following the initial decrease in Cx40−/− mice was comparable to wild-type (WT) mice (right side, late phase). Some error bars are covered. *P < 0.05 vs. WT (two-way anova with repeated measures followed by Dunnett’s test).
Discussion
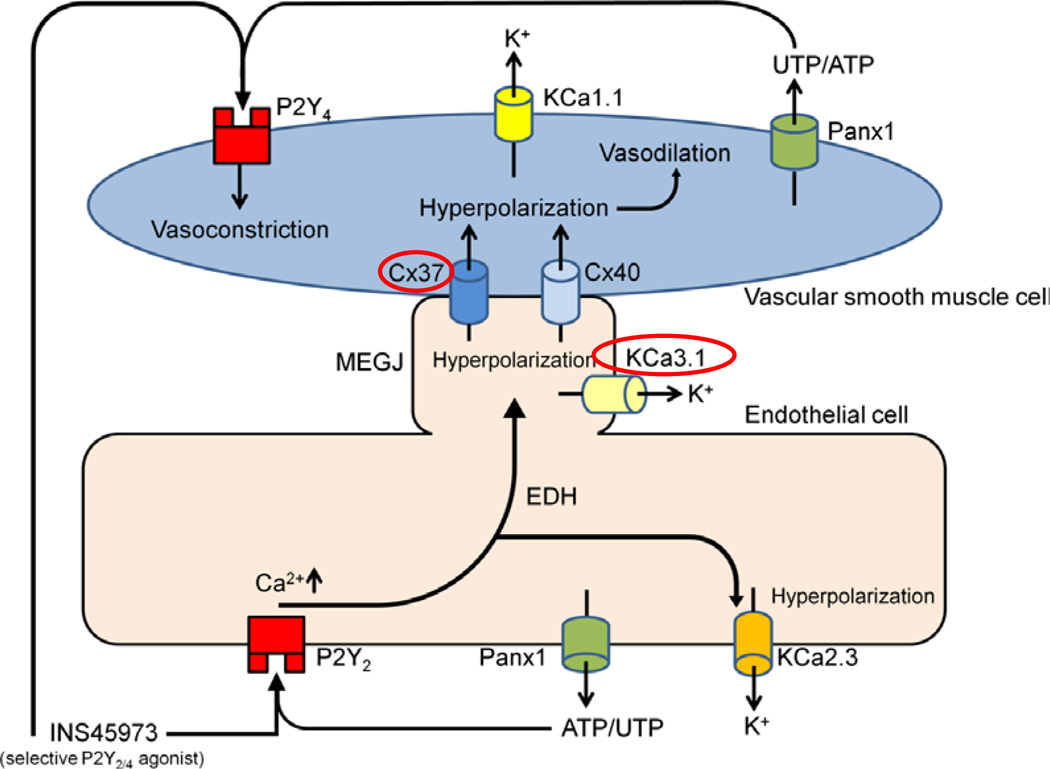
In this study, we utilized a P2Y2/4 receptor agonist and UTP analog, INS45973, to better understand the role of P2Y2 and P2Y4 receptors for acute systemic blood pressure responses. These are, to our knowledge, the first in vivo studies providing direct evidence about the involved proteins mediating vascular effects, possibly via EDH, by P2Y2/4 receptor activation. Our data indicate that KCa3.1 and Cx37 take part in P2Y2/4 receptor-initiated blood pressure responses. As the P2Y2/4 receptor agonist caused biphasic blood pressure effects within seconds of application without affecting heart rate, this likely indicates that there is a direct effect on peripheral resistance. In Figure 7, we depict a model summarizing the blood pressure effects of the studied proteins in this work in response to P2Y2/4 receptor activation.
Figure 7.
A proposed model of murine blood pressure responses to P2Y2/4 receptor activation. Our previous data implicated that the acute vasodilatory response to INS45973, a P2Y2/4 agonist, is mediated by P2Y2 receptor activation on endothelial cells. This hypothesis is supported by experiments in P2Y2 receptor knockout mice which lack the observed initial blood pressure decrease and show instead an immediate increase in blood pressure (Rieg et al. 2011). The blood pressure increase possibly results as a consequence of P2Y4 receptor activation directly on vascular smooth muscle cells resulting in vasoconstriction (Bar et al. 2008). The fact that endothelial NO synthase knockout mice responded with a comparable vasodilation implicated a role for endothelial derived hyperpolarization (EDH) in the acute blood pressure decrease. We speculate that P2Y2 receptor activation, possibly via an increase in intracellular calcium, activates calcium-dependent intermediate-conductance potassium channels, KCa3.1, which induces EDH-type vasodilations. Lack of KCa3.1 impairs this response (red circle). The role of small-conductance potassium channels (KCa2.3) in response to P2Y2/4 receptor activation needs to be determined. Calcium-dependent big-conductance potassium channels (KCa1.1) on vascular smooth muscle cells are not part of P2Y2/4 receptor-initiated blood pressure responses. Gap junction proteins including connexin 37 (Cx37) and connexin 40 (Cx40) contribute to myoendothelial gap junction (MEGJ) communication and electrically conduct EDH activity. Our data suggest that Cx37 is part of such a communication because Cx37 knockout mice show an impaired blood pressure response to P2Y2/4 receptor activation (red circle). In contrast to the distinct response observed in Cx37−/− mice, Cx40 is not part of the P2Y2/4 receptor-initiated blood pressure responses. Endothelial cells and vascular smooth muscle cells are speculated to endogenously release ATP and/or UTP via pannexin 1 (Panx1) channels (Lohman & Isakson 2014) which could consecutively activate P2Y2/4 receptors and contribute to regulation of vascular tone.
In the current study, we confirmed our previous findings (Rieg et al. 2007a) and expanded on the acute effects of P2Y2/4 receptor activation on blood pressure. In mice tested in this study, activation of P2Y2/4 receptors was able to decrease blood pressure. The findings of the current study are conform with other studies where acute i.v. application of ATP and UTP in anesthetized mice induced a dose-dependent decrease in blood pressure, while other receptors like P2Y1, P2Y4 and P2X1 were excluded by the use of pharmacological compounds (Shah & Kadowitz 2002). In aortas of P2Y2−/− mice, ATP-evoked relaxation was impaired (Guns et al. 2006) and, vice versa, ATP- and UTP-induced vasorelaxation were not different in aortas of WT and P2Y4−/− mice (Guns et al. 2005). The hypothesis that EDH may mediate such blood pressure responses comes from studies in eNOS−/− mice which show that the blood pressure decrease in response to P2Y2/4 receptor activation was unaffected compared to WT mice (Rieg et al. 2011), as well as from studies in the human forearm vasculature where eNOS blockade had no effect on UTP-mediated changes in forearm blood flow or vascular resistance (Hrafnkelsdottir et al. 2001, Crecelius et al. 2011). Along those lines, it was proposed that EDH is a major mediator for nucleotide-induced vasodilation in the peripheral vascular bed (Malmsjo et al. 2002) and human vascular endothelial cells (Raqeeb et al. 2011).
Which signalling pathways mediate the P2Y2 receptor-initiated blood pressure decrease? In EDH-related signalling, hyperpolarization via KCa3.1 and KCa2.3 are key steps and are also required to initiate gap junction-dependent vasodilations (Kohler & Ruth 2010). KCa3.1 and KCa2.3 have been shown to be crucial in agonist-induced hyperpolarization and vasodilation, according to experiments employing pharmacologic, electrophysiological and genetic approaches (Si et al. 2006, Brahler et al. 2009). Our experiments in KCa3.1−/− mice provide new evidence for such a contribution: the blood pressure decrease as well as blood pressure increase was significantly impaired in response to selective P2Y2/4 receptor activation. The severely impaired vasodilation indicates that KCa3.1 has a prominent role for electrically mediating this response. Of note, in conscious dogs activation of KCa3.1 via SKA-31, an activator of KCa3.1 (EC50 approx. 0.26 µmol L−1) and KCa2 channels (EC50 approx. 2.9 µmol L−1), rapidly decreased blood pressure by an immediate electrical vasodilator mechanism (Damkjaer et al. 2012) and application of SKA-31 potentiated EDH-mediated vasodilations in carotid arteries of WT mice but not KCa3.1−/− mice (Sankaranarayanan et al. 2009). Our in vivo data are the first linking P2Y2/4 receptors to KCa3.1-mediated hyperpolarization. Our data did not show an increased blood pressure in KCa3.1−/− mice that was described before (Brahler et al. 2009). Of note, the increased blood pressure in KCa3.1−/− mice was restricted to the dark phase (activity phase) of the mice, which could explain why the blood pressure in our KCa3.1−/− mice was not significantly different from WT mice because our experiments were performed during the light phase (quiet phase). Our results confirm other data studying acetylcholine responses in KCa3.1−/− mice. While at intermediate doses the acetylcholine-induced decrease in blood pressure was impaired, the response at the highest dose was not significantly different from WT mice (this study). Consistent with this finding, at lower doses acetylcholine-induced vasodilation of KCa3.1−/− mice cremaster microcirculation and carotid arteries was impaired, while higher doses induced equivalent vasodilation compared to WT mice (Brahler et al. 2009, Wolfle et al. 2009). Along those lines, arguing against the possibility that the abrogated P2Y2/4 agonist-induced decrease in blood pressure is due to an incapability of KCa3.1−/− mice to vasodilate are the findings that sodium nitroprusside- (direct NO donor) and adenosine-induced vasodilation was not impaired in KCa3.1−/− compared to WT mice (Si et al. 2006, Wolfle et al. 2009). In contrast to KCa3.1 localized to endothelial cells, KCa1.1 is localized to vascular smooth muscle cells and was shown to be important for the regulation of myogenic tone (Sausbier et al. 2005, Rieg et al. 2007b, Kohler & Ruth 2010). Even though KCa1.1−/− mice show a complete lack of membrane hyperpolarizing spontaneous potassium outward currents (Sausbier et al. 2005) our data indicate that the acute blood pressure responses via P2Y2/4 receptor activation do not require functional KCa1.1 because neither vasodilation or vasoconstriction were different from WT mice. Along those lines, adenosine-induced relaxation of myogenic tone of tibial arteries in KCa1.1−/− mice was similar to WT mice (Sausbier et al. 2005).
One of the major aims of the current study was to determine whether Cx37 and/or Cx40 are required for P2Y2/4 receptor-initiated blood pressure responses. Our results show that P2Y2/4 receptor activation requires fully functional Cx37 because Cx37−/− mice show impaired vasodilation and vasoconstriction, suggesting that Cx37 possibly contributes to connecting the EDH signal between endothelial and vascular smooth muscle cells. In contrast, the acetylcholine-induced decrease in blood pressure was not different between Cx37−/− and WT mice. Consistent with our data, the acetylcholine-induced local or conducted vasodilator response was unaffected in Cx37−/− mice (Figueroa & Duling 2008). The blood pressure responses in Cx40−/− mice were comparable to WT mice, indicating that P2Y2/4 receptor-initiated EDH-type vasodilatations may be completely independent of Cx40 expression in the MEGJ. Consistent with this finding, arteriolar dilatations induced by SKA-31 were not attenuated in endothelial-specific Cx40−/− mice, indicating that the KCa3.1-mediated EDH signal is able to induce vasodilatation even in the absence of endothelial Cx40 (Radtke et al. 2013). Notably, lack of Cx40 in endothelial cells was associated with a decrease in Cx37 expression (Simon & McWhorter 2003, Jobs et al. 2012). The reason why only Cx37−/− mice and not Cx40−/− mice show impaired blood pressure responses after P2Y2/4 receptor activation remains to be determined.
In vivo, UTP was found to constrict, independent of the endothelium, murine pial arterioles (Rosenblum & Nelson 1990, Rosenblum et al. 1990) and activation of P2Y2/4 receptors located on vascular smooth muscle cells also causes vasoconstriction (Tölle et al. 2010). Data from our previous study employing the P2Y2/4 agonist in P2Y2−/− mice (shown for comparison in Fig. 1) indicate that activation of P2Y4 receptors can induce vasoconstriction and increase blood pressure (Rieg et al. 2011). The blood pressure increase in P2Y2−/− mice was observed during the timeframe WT mice respond with a blood pressure decrease in the early phase. In all mice tested in this study, except for P2Y2−/− mice, we observed an acute blood pressure decrease that started to recover during administration of the P2Y2/4 agonist, perhaps reflecting an opposing action of P2Y4 receptors on vascular smooth muscle cells. Of note, eNOS−/−, KCa3.1−/− and Cx37−/− mice show an impaired blood pressure increase in the late phase in response to P2Y2/4 receptor activation compared to WT mice. The reason for this might relate to: (i) vasoconstriction consecutively following vasodilation may depend on the action and release of an endothelial-derived vasodilator which, when impaired, results in a reduced vasoconstrictor response; (ii) in eNOS−/− mice vascular tone is increased (Scotland et al. 2001), possibly reducing the maximal vasoconstrictor response via P2Y4 receptor activation. Along those lines, increased EDH activity in eNOS−/− mice in order to normalize myogenic tone may contribute to the reduced vasoconstrictor effect.
We are aware that this study has limitations. Our data only allow us to show in vivo blood pressure effects of P2Y2/4 agonist responses without defining the involved vascular beds or measuring electrical signals. Along those lines, in vivo EDH signalling might be more complex compared to the pharmacological response evoked via P2Y2/4 receptor activation. This might explain why our studies show no involvement of Cx40, as Cx40−/− mice are hypertensive and exhibit diminished conduction of arteriolar dilatation in response to acetylcholine and bradykinin (de Wit et al. 2000). Resistance vasculature in skeletal muscle, which comprises approx. 40% of total body mass in non-obese humans and animals, is essential to blood pressure regulation. However, our data cannot completely exclude that blood pressure actions of the P2Y2/4 agonist are caused by acute effects on cardiac output or changes in nervous activity. Also, our data for P2Y4-mediated vasoconstriction are indirect and future plans include testing in P2Y4−/− mice. The development of selective and stable P2Y2 and P2Y4 agonists, which are not degraded into other vasoactive compounds, will help to test these ideas and additional studies, possibly involving double knockout mice and intravital microscopy, are required to better understand the role or P2Y2/4 receptors in vascular reactivity.
In summary, our results clearly demonstrate that P2Y2 receptor activation in vivo causes an acute blood pressure decrease, which is likely mediated by EDH and independent of endothelial NO. The EDH response after P2Y2/4 receptor activation requires functional KCa3.1 and Cx37 to mediate its full vascular effects. Thus, P2Y2 and P2Y4 receptors may counteract each other in the vasculature and for blood pressure regulation. Effects of P2Y2 receptors on the vasculature as well as in the kidney on renal sodium excretion make P2Y2 agonists a potential target for the treatment of hypertension.
Acknowledgments
This work was supported by the O’Brien Center for Acute Kidney Injury Research Grant P30DK079337 (Pilot award to T.R.), Bastyr University Faculty Research Seed Grant (to J.D.R.), UCSD Academic Senate (to T.R.), Satellite Healthcare, a not-for-profit renal care provider (to T.R.), American Heart Association 15BGIA22410018 (to T.R.) and the Department of Veterans Affairs.
Footnotes
Conflict of interest
No conflicts of interest are declared.
References
- Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adeagbo AS, Triggle CR. Varying extracellular [K+]: a functional approach to separating EDHF- and EDNO-related mechanisms in perfused rat mesenteric arterial bed. J Cardiovasc Pharmacol. 1993;21:423–429. [PubMed] [Google Scholar]
- Bar I, Guns PJ, Metallo J, Cammarata D, Wilkin F, Boeynams JM, Bult H, Robaye B. Knockout mice reveal a role for P2Y6 receptor in macrophages, endothelial cells, and vascular smooth muscle cells. Mol Pharmacol. 2008;74:777–784. doi: 10.1124/mol.108.046904. [DOI] [PubMed] [Google Scholar]
- Begenisich T, Nakamoto T, Ovitt CE, Nehrke K, Brugnara C, Alper SL, Melvin JE. Physiological roles of the intermediate conductance, Ca2+-activated potassium channel Kcnn4. J Biol Chem. 2004;279:47681–47687. doi: 10.1074/jbc.M409627200. [DOI] [PubMed] [Google Scholar]
- Boarder MR, Hourani SMO. The regulation of vascular function by P2 receptors: multiple sites and multiple receptors. Trends Pharmacol Sci. 1998;19:99–107. doi: 10.1016/s0165-6147(98)01170-5. [DOI] [PubMed] [Google Scholar]
- Brahler S, Kaistha A, Schmidt VJ, Wolfle SE, Busch C, Kaistha BP, Kacik M, Hasenau AL, Grgic I, Si H. Genetic deficit of SK3 and IK1 channels disrupts the endothelium-derived hyperpolarizing factor vasodilator pathway and causes hypertension. Circulation. 2009;119:2323–2332. doi: 10.1161/CIRCULATIONAHA.108.846634. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic regulation of vascular tone and remodelling. Auton Autacoid Pharmacol. 2009;29:63–72. doi: 10.1111/j.1474-8673.2009.00435.x. [DOI] [PubMed] [Google Scholar]
- Buvinic S, Briones R, Huidobro-Toro JP. P2Y(1) and P2Y(2) receptors are coupled to the NO/cGMP pathway to vasodilate the rat arterial mesenteric bed. Br J Pharmacol. 2002;136:847–856. doi: 10.1038/sj.bjp.0704789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaytor AT, Bakker LM, Edwards DH, Griffith TM. Connexin-mimetic peptides dissociate electrotonic EDHF-type signalling via myoendothelial and smooth muscle gap junctions in the rabbit iliac artery. Br J Pharmacol. 2005;144:108–114. doi: 10.1038/sj.bjp.0706046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crecelius AR, Kirby BS, Richards JC, Garcia LJ, Voyles WF, Larson DG, Luckasen GJ, Dinenno FA. Mechanisms of ATP-mediated vasodilation in humans: modest role for nitric oxide and vasodilating prostaglandins. Am J Physiol Heart Circ Physiol. 2011;301:H1302–H1310. doi: 10.1152/ajpheart.00469.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damkjaer M, Nielsen G, Bodendiek S, Staehr M, Gramsbergen JB, de Wit C, Jensen BL, Simonsen U, Bie P, Wulff H, Kohler R. Pharmacological activation of KCa3.1/KCa2.3 channels produces endothelial hyperpolarization and lowers blood pressure in conscious dogs. Br J Pharmacol. 2012;165:223–234. doi: 10.1111/j.1476-5381.2011.01546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich HH, Kajita Y, Dacey RG. Local and conducted vasomotor responses in isolated rat cerebral arterioles. Am J Physiol Heart Circ Physiol. 1996;271:H1109–H1116. doi: 10.1152/ajpheart.1996.271.3.H1109. [DOI] [PubMed] [Google Scholar]
- Dora KA, Sandow SL, Gallagher NT, Takano H, Rummery NM, Hill CE, Garland CJ. Myoendothelial gap junctions may provide the pathway for EDHF in mouse mesenteric artery. J Vasc Res. 2003;40:480–490. doi: 10.1159/000074549. [DOI] [PubMed] [Google Scholar]
- Edwards G, Feletou M, Weston AH. Endothelium-derived hyperpolarising factors and associated pathways: a synopsis. Pflugers Arch. 2010;459:863–879. doi: 10.1007/s00424-010-0817-1. [DOI] [PubMed] [Google Scholar]
- Eichler I, Wibawa J, Grgic I, Knorr A, Brakemeier S, Pries AR, Hoyer J, Kohler R. Selective blockade of endothelial Ca2+-activated small- and intermediate-conductance K+-channels suppresses EDHF-mediated vasodilation. Br J Pharmacol. 2003;138:594–601. doi: 10.1038/sj.bjp.0705075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang JS, Angelov SN, Simon AM, Burt JM. Cx37 deletion enhances vascular growth and facilitates ischemic limb recovery. Am J Physiol Heart Circ Physiol. 2011;301:H1872–H1881. doi: 10.1152/ajpheart.00683.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang JS, Angelov SN, Simon AM, Burt JM. Cx40 is required for, cx37 limits, postischemic hindlimb perfusion, survival and recovery. J Vasc Res. 2012;49:2–12. doi: 10.1159/000329616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feletou M. Calcium-activated potassium channels and endothelial dysfunction: therapeutic options? Br J Pharmacol. 2009;156:545–562. doi: 10.1111/j.1476-5381.2009.00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feletou M, Vanhoutte PM. EDHF: an update. Clin Sci (Lond) 2009;117:139–155. doi: 10.1042/CS20090096. [DOI] [PubMed] [Google Scholar]
- Fenton RA, Murray F, Dominguez Rieg JA, Tang T, Levi M, Rieg T. Renal phosphate wasting in the absence of adenylyl cyclase 6. J Am Soc Nephrol. 2014;25:2822–2834. doi: 10.1681/ASN.2013101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa XF, Duling BR. Dissection of two Cx37-independent conducted vasodilator mechanisms by deletion of Cx40: electrotonic versus regenerative conduction. Am J Physiol Heart Circ Physiol. 2008;295:H2001–H2007. doi: 10.1152/ajpheart.00063.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa XF, Duling BR. Gap junctions in the control of vascular function. Antioxid Redox Signal. 2009;11:251–266. doi: 10.1089/ars.2008.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa XF, Isakson BE, Duling BR. Connexins: gaps in our knowledge of vascular function. Physiology (Bethesda) 2004;19:277–284. doi: 10.1152/physiol.00008.2004. [DOI] [PubMed] [Google Scholar]
- Garland CJ, Hiley CR, Dora KA. EDHF: spreading the influence of the endothelium. Br J Pharmacol. 2011;164:839–852. doi: 10.1111/j.1476-5381.2010.01148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grgic I, Kaistha BP, Hoyer J, Kohler R. Endothelial Ca+-activated K+ channels in normal and impaired EDHF-dilator responses–relevance to cardiovascular pathologies and drug discovery. Br J Pharmacol. 2009;157:509–526. doi: 10.1111/j.1476-5381.2009.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith TM, Chaytor AT, Taylor HJ, Giddings BD, Edwards DH. cAMP facilitates EDHF-type relaxations in conduit arteries by enhancing electrotonic conduction via gap junctions. Proc Natl Acad Sci USA. 2002;99:6392–6397. doi: 10.1073/pnas.092089799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guns PJ, Korda A, Crauwels HM, Van Assche T, Robaye B, Boeynaems JM, Bult H. Pharmacological characterization of nucleotide P2Y receptors on endothelial cells of the mouse aorta. Br J Pharmacol. 2005;146:288–295. doi: 10.1038/sj.bjp.0706326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guns PJ, Van Assche T, Fransen P, Robaye B, Boeynaems JM, Bult H. Endothelium-dependent relaxation evoked by ATP and UTP in the aorta of P2Y2-deficient mice. Br J Pharmacol. 2006;147:569–574. doi: 10.1038/sj.bjp.0706642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock RE, Grayson TH, Brackenbury TD, Meaney KR, Neylon CB, Sandow SL, Hill CE. Endothelial coordination of cerebral vasomotion via myoendothelial gap junctions containing connexins 37 and 40. Am J Physiol Heart Circ Physiol. 2006;291:H2047–H2056. doi: 10.1152/ajpheart.00484.2006. [DOI] [PubMed] [Google Scholar]
- Hammer LW, Overstreet CR, Choi J, Hester RL. ATP stimulates the release of prostacyclin from perfused veins isolated from the hamster hindlimb. Am J Physiol Regul Integr Comp Physiol. 2003;285:R193–R199. doi: 10.1152/ajpregu.00468.2002. [DOI] [PubMed] [Google Scholar]
- Holzmann S, Kukovetz WR, Windischhofer W, Paschke E, Graier WF. Pharmacologic differentiation between endothelium-dependent relaxations sensitive and resistant to nitro-L-arginine in coronary arteries. J Cardiovasc Pharmacol. 1994;23:747–756. doi: 10.1097/00005344-199405000-00009. [DOI] [PubMed] [Google Scholar]
- Horiuchi T, Dietrich HH, Tsugane S, Dacey RG. Analysis of purine- and pyrimidine-induced vascular responses in the isolated rat cerebral arteriole. Am J Physiol Heart Circ Physiol. 2001;280:H767–H776. doi: 10.1152/ajpheart.2001.280.2.H767. [DOI] [PubMed] [Google Scholar]
- Hrafnkelsdottir T, Erlinge D, Jern S. Extracellular nucleotides ATP and UTP induce a marked acute release of tissue-type plasminogen activator in vivo in man. Thromb Haemost. 2001;85:875–881. [PubMed] [Google Scholar]
- Inscho EW. ATP, P2 receptors and the renal microcirculation. Purinergic Signal. 2009;5:447–460. doi: 10.1007/s11302-009-9147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isakson BE, Duling BR. Heterocellular contact at the myoendothelial junction influences gap junction organization. Circ Res. 2005;97:44–51. doi: 10.1161/01.RES.0000173461.36221.2e. [DOI] [PubMed] [Google Scholar]
- Janigro D, Nguyen TS, Meno J, West GA, Winn HR. Endothelium-dependent regulation of cerebrovascular tone by extracellular and intracellular ATP. Am J Physiol Heart Circ Physiol. 1997;273:H878–H885. doi: 10.1152/ajpheart.1997.273.2.H878. [DOI] [PubMed] [Google Scholar]
- Jobs A, Schmidt K, Schmidt VJ, Lubkemeier I, van Veen TA, Kurtz A, Willecke K, de Wit C. Defective Cx40 maintains Cx37 expression but intact Cx40 is crucial for conducted dilations irrespective of hypertension. Hypertension. 2012;60:1422–1429. doi: 10.1161/HYPERTENSIONAHA.112.201194. [DOI] [PubMed] [Google Scholar]
- Kauffenstein G, Drouin A, Thorin-Trescases N, Bachelard H, Robaye B, D’Orleans-Juste P, Marceau F, Thorin E, Sevigny J. NTPDase1 (CD39) controls nucleotide-dependent vasoconstriction in mouse. Cardiovasc Res. 2010;85:204–213. doi: 10.1093/cvr/cvp265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy C, Burnstock G. ATP produces vasodilation via P1 purinoceptors and vasoconstriction via P2 purinoceptors in the isolated rabbit central ear artery. Blood Vessels. 1985;22:145–155. doi: 10.1159/000158592. [DOI] [PubMed] [Google Scholar]
- Kennedy C, Delbro D, Burnstock G. P2-purinoceptors mediate both vasodilation (via the endothelium) and vasoconstriction of the isolated rat femoral artery. Eur J Pharmacol. 1985;107:161–168. doi: 10.1016/0014-2999(85)90055-x. [DOI] [PubMed] [Google Scholar]
- Kohler R, Ruth P. Endothelial dysfunction and blood pressure alterations in K+-channel transgenic mice. Pflugers Arch. 2010;459:969–976. doi: 10.1007/s00424-010-0819-z. [DOI] [PubMed] [Google Scholar]
- Krattinger N, Capponi A, Mazzolai L, Aubert JF, Caille D, Nicod P, Waeber G, Meda P, Haefliger JA. Connexin40 regulates renin production and blood pressure. Kidney Int. 2007;72:814–822. doi: 10.1038/sj.ki.5002423. [DOI] [PubMed] [Google Scholar]
- von Kugelgen I, Starke K. Evidence for two separate vasoconstriction-mediating nucleotide receptors, both distinct from the P2x-receptor, in rabbit basilar artery: a receptor for pyrimidine nucleotides and a receptor for purine nucleotides. Naunyn Schmiedebergs Arch Pharmacol. 1990;341:538–546. doi: 10.1007/BF00171734. [DOI] [PubMed] [Google Scholar]
- von Kugelgen I, Haussinger D, Starke K. Evidence for a vasoconstriction-mediating receptor for UTP, distinct from the P2 purinoceptor, in rabbit ear artery. Naunyn Schmiedebergs Arch Pharmacol. 1987;336:556–560. doi: 10.1007/BF00169313. [DOI] [PubMed] [Google Scholar]
- Lohman AW, Isakson BE. Differentiating connexin hemichannels and pannexin channels in cellular ATP release. FEBS Lett. 2014;588:1379–1388. doi: 10.1016/j.febslet.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohman AW, Billaud M, Isakson BE. Mechanisms of ATP release and signalling in the blood vessel wall. Cardiovasc Res. 2012;95:269–280. doi: 10.1093/cvr/cvs187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmsjo M, Erlinge D, Hogestatt ED, Zygmunt PM. Endothelial P2Y receptors induce hyperpolarisation of vascular smooth muscle by release of endothelium-derived hyperpolarising factor. Eur J Pharmacol. 1999;364:169–173. doi: 10.1016/s0014-2999(98)00848-6. [DOI] [PubMed] [Google Scholar]
- Malmsjo M, Chu ZM, Croft K, Erlinge D, Edvinsson L, Beilin LJ. P2Y receptor-induced EDHF vasodilatation is of primary importance for the regulation of perfusion pressure in the peripheral circulation of the rat. Acta Physiol Scand. 2002;174:301–309. doi: 10.1046/j.1365-201x.2002.00956.x. [DOI] [PubMed] [Google Scholar]
- Mather S, Dora KA, Sandow SL, Winter P, Garland CJ. Rapid endothelial cell-selective loading of connexin 40 antibody blocks endothelium-derived hyperpolarizing factor dilation in rat small mesenteric arteries. Circ Res. 2005;97:399–407. doi: 10.1161/01.RES.0000178008.46759.d0. [DOI] [PubMed] [Google Scholar]
- McMillan MR, Burnstock G, Haworth SG. Vasoconstriction of intrapulmonary arteries to P2-receptor nucleotides in normal and pulmonary hypertensive newborn piglets. Br J Pharmacol. 1999;128:549–555. doi: 10.1038/sj.bjp.0702814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min K, Munarriz R, Yerxa BR, Goldstein I, Shaver SR, Cowlen MS, Traish AM. Selective P2Y2 receptor agonists stimulate vaginal moisture in ovariectomized rabbits. Fertil Steril. 2003;79:393–398. doi: 10.1016/s0015-0282(02)04677-0. [DOI] [PubMed] [Google Scholar]
- Mizumori M, Ham M, Guth PH, Engel E, Kaunitz JD, Akiba Y. Intestinal alkaline phosphatase regulates protective surface microclimate pH in rat duodenum. J Physiol. 2009;587:3651–3663. doi: 10.1113/jphysiol.2009.172270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson PB. Good publication practice in physiology 2013: revised author guidelines for Acta Physiologica. Acta Physiol. 2013;209:250–253. [Google Scholar]
- Pochynyuk O, Rieg T, Bugaj V, Schroth J, Fridman A, Boss GR, Insel PA, Stockand JD, Vallon V. Dietary Na+ inhibits the open probability of the epithelial sodium channel in the kidney by enhancing apical P2Y2-receptor tone. FASEB J. 2010;24:2056–2065. doi: 10.1096/fj.09-151506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radtke J, Schmidt K, Wulff H, Kohler R, de Wit C. Activation of KCa3.1 by SKA-31 induces arteriolar dilatation and lowers blood pressure in normo- and hypertensive connexin40-deficient mice. Br J Pharmacol. 2013;170:293–303. doi: 10.1111/bph.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Effects of purines and pyrimidines on the rat mesenteric arterial bed. Circ Res. 1991a;69:1583–1590. doi: 10.1161/01.res.69.6.1583. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Roles of P2-purinoceptors in the cardiovascular system. Circulation. 1991b;84:1–14. doi: 10.1161/01.cir.84.1.1. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Discrimination by PPADS between endothelial P2Y- and P2U-purinoceptors in the rat isolated mesenteric arterial bed. Br J Pharmacol. 1996a;118:428–434. doi: 10.1111/j.1476-5381.1996.tb15420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Relative contribution of P2U- and P2Y-purinoceptors to endothelium-dependent vasodilatation in the golden hamster isolated mesenteric arterial bed. Br J Pharmacol. 1996b;117:1797–1802. doi: 10.1111/j.1476-5381.1996.tb15357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Involvement of purinergic signaling in cardiovascular diseases. Drug News Perspect. 2003;16:133–140. doi: 10.1358/dnp.2003.16.3.876886. [DOI] [PubMed] [Google Scholar]
- Raqeeb A, Sheng J, Ao N, Braun AP. Purinergic P2Y2 receptors mediate rapid Ca(2+) mobilization, membrane hyperpolarization and nitric oxide production in human vascular endothelial cells. Cell Calcium. 2011;49:240–248. doi: 10.1016/j.ceca.2011.02.008. [DOI] [PubMed] [Google Scholar]
- Rayment SJ, Latif ML, Ralevic V, Alexander SPH. Evidence for the Expression of Multiple Uracil Nucleotide-Stimulated P2 Receptors Coupled to Smooth Muscle Contraction in Porcine Isolated Arteries. Br J Pharmacol. 2007;150:604–612. doi: 10.1038/sj.bjp.0707120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieg T, Richter K, Osswald H, Vallon V. Kidney function in mice: thiobutabarbital versus alpha-chloralose anesthesia. Naunyn Schmiedebergs Arch Pharmacol. 2004;370:320–323. doi: 10.1007/s00210-004-0982-x. [DOI] [PubMed] [Google Scholar]
- Rieg T, Steigele H, Schnermann J, Richter K, Osswald H, Vallon V. Requirement of intact adenosine A1 receptors for the diuretic and natriuretic action of the methylxanthines theophylline and caffeine. J Pharmacol Exp Ther. 2005;313:403–409. doi: 10.1124/jpet.104.080432. [DOI] [PubMed] [Google Scholar]
- Rieg T, Bundey RA, Chen Y, Deschenes G, Junger W, Insel PA, Vallon V. Mice lacking P2Y2 receptors have salt-resistant hypertension and facilitated renal Na+ and water reabsorption. FASEB J. 2007a;21:3717–3726. doi: 10.1096/fj.07-8807com. [DOI] [PubMed] [Google Scholar]
- Rieg T, Vallon V, Sausbier M, Sausbier U, Kaissling B, Ruth P, Osswald H. The role of the BK channel in potassium homeostasis and flow-induced renal potassium excretion. Kidney Int. 2007b;72:566–573. doi: 10.1038/sj.ki.5002369. [DOI] [PubMed] [Google Scholar]
- Rieg T, Gerasimova M, Boyer JL, Insel PA, Vallon V. P2Y(2) receptor activation decreases blood pressure and increases renal Na(+) excretion. Am J Physiol Regul Integr Comp Physiol. 2011;301:R510–R518. doi: 10.1152/ajpregu.00148.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum WI, Nelson GH. Tone regulates opposing endothelium-dependent and -independent forces: resistance brain vessels in vivo. Am J Physiol Heart Circ Physiol. 1990;259:H243–H247. doi: 10.1152/ajpheart.1990.259.1.H243. [DOI] [PubMed] [Google Scholar]
- Rosenblum WI, Nelson GH, Weinbrecht P. Histamine elicits competing endothelium-dependent constriction and endothelium-independent dilation in vivo in mouse cerebral arterioles. Stroke. 1990;21:305–309. doi: 10.1161/01.str.21.2.305. [DOI] [PubMed] [Google Scholar]
- Rubino A, Ziabary L, Burnstock G. Regulation of vascular tone by UTP and UDP in isolated rat intrapulmonary arteries. Eur J Pharmacol. 1999;370:139–143. doi: 10.1016/s0014-2999(99)00150-8. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan A, Raman G, Busch C, Schultz T, Zimin PI, Hoyer J, Kohler R, Wulff H. Naphtho[1,2-d]thiazol-2-ylamine (SKA-31), a new activator of KCa2 and KCa3.1 potassium channels, potentiates the endothelium-derived hyperpolarizing factor response and lowers blood pressure. Mol Pharmacol. 2009;75:281–295. doi: 10.1124/mol.108.051425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sausbier M, Hu H, Arntz C, Feil S, Kamm S, Adelsberger H, Sausbier U, Sailer CA, Feil R, Hofmann F. Cerebellar ataxia and Purkinje cell dysfunction caused by Ca2+-activated K+ channel deficiency. Proc Natl Acad Sci USA. 2004;101:9474–9478. doi: 10.1073/pnas.0401702101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sausbier M, Arntz C, Bucurenciu I, Zhao H, Zhou XB, Sausbier U, Feil S, Kamm S, Essin K, Sailer CA, et al. Elevated blood pressure linked to primary hyperaldosteronism and impaired vasodilation in BK channel-deficient mice. Circulation. 2005;112:60–68. doi: 10.1161/01.CIR.0000156448.74296.FE. [DOI] [PubMed] [Google Scholar]
- Scotland RS, Chauhan S, Vallance PJ, Ahluwalia A. An endothelium-derived hyperpolarizing factor-like factor moderates myogenic constriction of mesenteric resistance arteries in the absence of endothelial nitric oxide synthase-derived nitric oxide. Hypertension. 2001;38:833–839. doi: 10.1161/hy1001.092651. [DOI] [PubMed] [Google Scholar]
- Shah MK, Kadowitz PJ. Cyclic adenosine monophosphate-dependent vascular responses to purinergic agonists adenosine triphosphate and uridine triphosphate in the anesthetized mouse. J Cardiovasc Pharmacol. 2002;39:142–149. doi: 10.1097/00005344-200201000-00015. [DOI] [PubMed] [Google Scholar]
- Shaver SR, Rideout JL, Pendergast W, Douglass JG, Brown EG, Boyer JL, Patel RI, Redick CC, Jones AC, Picher M, Yerxa BR. Structure–activity relationships of dinucleotides: Potent and selective agonists of P2Y receptors. Purinergic Signal. 2005;1:183–191. doi: 10.1007/s11302-005-0648-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si H, Heyken WT, Wolfle SE, Tysiac M, Schubert R, Grgic I, Vilianovich L, Giebing G, Maier T, Gross V, Bader M, de Wit C, Hoyer J, Kohler R. Impaired endothelium-derived hyperpolarizing factor-mediated dilations and increased blood pressure in mice deficient of the intermediate-conductance Ca2+-activated K+ channel. Circ Res. 2006;99:537–544. doi: 10.1161/01.RES.0000238377.08219.0c. [DOI] [PubMed] [Google Scholar]
- Simon AM, McWhorter AR. Decreased intercellular dye-transfer and downregulation of non-ablated connexins in aortic endothelium deficient in connexin37 or connexin40. J Cell Sci. 2003;116:2223–2236. doi: 10.1242/jcs.00429. [DOI] [PubMed] [Google Scholar]
- Sokoya EM, Burns AR, Setiawan CT, Coleman HA, Parkington HC, Tare M. Evidence for the involvement of myoendothelial gap junctions in EDHF-mediated relaxation in the rat middle cerebral artery. Am J Physiol Heart Circ Physiol. 2006;291:H385–H393. doi: 10.1152/ajpheart.01047.2005. [DOI] [PubMed] [Google Scholar]
- Tölle M, Schuchardt M, Wiedon A, Huang T, Klöckel L, Jankowski J, Jankowski V, Zidek W, van der Giet M. Differential effects of uridine adenosine tetraphosphateon purinoceptors in the rat isolated perfused kidney. Br J Pharmacol. 2010;161:530–540. doi: 10.1111/j.1476-5381.2010.00914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabanelli S, Ocadlikova D, Gulinelli S, Curti A, Salvestrini V, Vieira RP, Idzko M, Di Virgilio F, Ferrari D, Lemoli RM. Extracellular ATP exerts opposite effects on activated and regulatory CD4+ T cells via purinergic P2 receptor activation. J Immunol. 2012;189:1303–1310. doi: 10.4049/jimmunol.1103800. [DOI] [PubMed] [Google Scholar]
- Vollmayer P, Clair T, Goding JW, Sano K, Servos J, Zimmermann H. Hydrolysis of diadenosine polyphosphates by nucleotide pyrophosphatases/phosphodiesterases. Eur J Biochem. 2003;270:2971–2978. doi: 10.1046/j.1432-1033.2003.03674.x. [DOI] [PubMed] [Google Scholar]
- Waldron GJ, Garland CJ. Contribution of both nitric oxide and a change in membrane potential to acetylcholine-induced relaxation in the rat small mesenteric artery. Br J Pharmacol. 1994;112:831–836. doi: 10.1111/j.1476-5381.1994.tb13154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wihlborg AK, Malmsjo M, Eyjolfsson A, Gustafsson R, Jacobson K, Erlinge D. Extracellular nucleotides induce vasodilatation in human arteries via prostaglandins, nitric oxide and endothelium-derived hyperpolarising factor. Br J Pharmacol. 2003;138:1451–1458. doi: 10.1038/sj.bjp.0705186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit C, Griffith TM. Connexins and gap junctions in the EDHF phenomenon and conducted vasomotor responses. Pflugers Arch. 2010;459:897–914. doi: 10.1007/s00424-010-0830-4. [DOI] [PubMed] [Google Scholar]
- de Wit C, Wolfle SE. EDHF and gap junctions: important regulators of vascular tone within the microcirculation. Curr Pharm Biotechnol. 2007;8:11–25. doi: 10.2174/138920107779941462. [DOI] [PubMed] [Google Scholar]
- de Wit C, Roos F, Bolz SS, Kirchhoff S, Kruger O, Willecke K, Pohl U. Impaired conduction of vasodilation along arterioles in connexin40-deficient mice. Circ Res. 2000;86:649–655. doi: 10.1161/01.res.86.6.649. [DOI] [PubMed] [Google Scholar]
- Wolfle SE, Schmidt VJ, Hoyer J, Kohler R, de Wit C. Prominent role of KCa3.1 in endothelium-derived hyperpolarizing factor-type dilations and conducted responses in the microcirculation in vivo. Cardiovasc Res. 2009;82:476–483. doi: 10.1093/cvr/cvp060. [DOI] [PubMed] [Google Scholar]
- Zygmunt PM, Hogestatt ED. Role of potassium channels in endothelium-dependent relaxation resistant to nitroarginine in the rat hepatic artery. Br J Pharmacol. 1996;117:1600–1606. doi: 10.1111/j.1476-5381.1996.tb15327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]