Abstract
Neuroblastoma is the most common extracranial solid tumor of childhood and is responsible for over 15% of pediatric cancer deaths. Focal adhesion kinase (FAK) is a non-receptor tyrosine kinase that is important in many facets of neuroblastoma tumor development and progression. The p53 oncogene, although wild type in most neuroblastomas, lacks significant function as a tumor suppressor in these tumors. Recent reports have found that FAK and p53 interact in some tumor types. We have hypothesized FAK and p53 coordinately control each other’s expression and also interact in neuroblastoma. In the current study, we showed that not only do FAK and p53 interact but each one controls the expression of the other. In addition, we also examined the effects of FAK inhibition combined with p53 activation in neuroblastoma and showed that these two, in combination, had a synergistic effect upon neuroblastoma cell survival. The findings from this current study help to further our understanding of the regulation of neuroblastoma tumorigenesis, and may provide novel therapeutic strategies and targets for neuroblastoma and other pediatric solid tumors.
Keywords: FAK, p53, SH-EP, WAC(2), neuroblastoma
Introduction
Neuroblastoma, a tumor of neural crest cells, is the most common extracranial solid tumor of childhood. Children affected by this tumor most often present with advanced stage disease, and despite significant advances in both medical and surgical pediatric oncologic care, continue have a dismal prognosis, with a survival of less than 30% [1]. Clearly, novel therapies will need to be developed to improve the outcomes for children with neuroblastoma.
Focal adhesion kinase (FAK) is a 125 kDa nonreceptor protein tyrosine kinase that has been shown to be important in the tumorigenesis of a number of human tumors. FAK is involved in tumor cell proliferation, motility, survival, and apoptosis [2, 3], and the inhibition of FAK through a number of different means affects tumorigenesis. Inhibition of FAK with RNAi resulted in decreased motility in glioblastoma cells [4]. In other studies, FAK abrogation with antisense oligonucleotides or a dominant-negative FAK protein resulted in decreased melanoma, colon cancer, and breast cancer cell growth [5–7]. Small molecule inhibition of FAK has led to decreased growth and increased apoptosis in ovarian cancer cells [8] and breast cancer cells [9]. Previous studies have shown that FAK mRNA and protein were associated with aggressive neuroblastomas [10] and that FAK inhibition in neuroblastoma through a number of different mechanisms resulted in decreased tumor survival [11, 12] and metastasis [13].
The tumor suppressor protein, p53 (encoded by the human gene, TP53), is a transcription factor that regulates cell signaling pathways involved in cell cycle arrest and apoptosis. Mutations in the TP53 gene are reported to occur in almost every type of cancer at varying rates resulting in mutant expression or inactivation of p53, leading to increased cellular proliferation, avoidance of apoptosis, and resistance to chemotherapy. In neuroblastoma, the majority of tumors are actually wild-type p53, but conflicting data exist about p53 pathway signaling in this tumor, as activity of p53 is often diminished despite its wild type status. It is unknown whether p53 activity is decreased due to upregulation of p53 inhibitors, sequestration of p53 in the cytoplasm, or ubiquitination of p53. Slack reported that Mdm2, the primary inhibitor of p53, is upregulated by MYCN [14], a proto-oncogene that is amplified in many aggressive, treatment resistant neuroblastomas [15]. On a more general level, some investigators have suggested that proteins, such as FAK, may bind p53, thus sequestering it in the cytoplasm of the cell, and preventing it from entering the nucleus and functioning as a transcription factor [16]. Others reported that FAK may facilitate p53 turnover via an MDM2-dependent ubiquitination [17]. There have been recent reports describing the protein-protein interaction between FAK and p53 in breast cancer cell lines, and showing that disruption of this interaction with homologous peptides or small molecules resulted in decreased tumor cell survival [18, 19]. The current studies were designed to test the hypothesis that in neuroblastoma FAK and p53 each coordinately regulate the other’s expression in a biologically significant fashion.
Materials and Methods
Cells, cell culture and transfections
The human neuroblastoma cells line, SH-SY5Y (CRL-2266, American Type Culture Collection, ATCC, Manassas, VA) was maintained in 1:1 mixture of minimum Eagle’s medium and Ham’s F-12 medium with 10% fetal bovine serum, 2 mM L-glutamine, 1 μM non-essential amino acids and 1 μg/mL penicillin / streptomycin. SH-SY5Y cell line was chosen since because this cell line was p53 wild type [20] MDM2 non-amplified [21] and MYCN non-amplified [22]. SH-EP (MYCN-) and the isogenic WAC(2) (MYCN+) human neuroblastoma cell lines were generously provided by Dr. M. Schwab (Deutsches Krebsforschungszentrum, Heidelberg, Germany), and have been described in detail previously [23]. SH-EP and WAC(2) cell lines were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum and 1 μg/mL penicillin / streptomycin. These two cell lines were chosen as they were also p53 wild type [20], MDM2 non-amplified [21] and are isogenic for MYCN, with the SH-EP cells being MYCN negative [23] and the WAC(2) cell line stably transfected with a MYCN vector [23].
For these experiments, transfection of plasmids was completed with Superfect Transfection Reagent (Qiagen Inc., Valencia, CA) as previously described [11]. FAK plasmids, including wild type FAK (pKH3-FAK) and empty vector (pKH3-EV), were generously provided by Dr. JL Guan (University of Cincinnati, Cincinnati, OH) and have been previously described [24]. All plasmids were sequenced at the DNA Sequencing and Analysis Core, Comprehensive Cancer Center, University of Alabama, Birmingham.
Antibodies and reagents
Monoclonal mouse anti-FAK (4.47) and rabbit polyclonal anti-phospho-FAK (Y397) antibodies were obtained from Millipore (1:1000, 05-537, EMD Millipore, Billerica, MA) and Invitrogen (1:1000, 71-7900, Invitrogen Corp., Carlsbad, CA), respectively. Mouse monoclonal antibodies for MDM2 (1:1000, AB-1) and p53 (1:1000, PB53-12) were from Millipore (EMD Millipore) and for p21 from BD Biosciences (1:1000, 2G12, BD Biosciences, San Jose, CA). Anti-MYCN polyclonal rabbit antibody was from Cell Signaling (9405, Cell Signaling Technology, Danvers, MA). Monoclonal mouse anti-GAPDH was from Millipore (1:3000, MAB374, EMD Millipore) and anti-β-actin was from Sigma (1:2000, A1978, Sigma-Aldrich Corp., St. Louis, MO).
The small molecules were as follows: PF-573,228 (C22H20F3N5O3S) from Pfizer (New York, NY); pifithrin-α from Sigma; fluorouracil (5-FU) from EMD Biosciences Millipore; and nutlin-3 from Enzo Life Sciences (Farmingdale, NY).
Antibodies used for immunofluorescence were as listed: primary antibody to FAK (4.47) was a rabbit polyclonal (1:1000, C-20, Santa Cruz, Santa Cruz, CA) and to p53 a mouse monoclonal (1:1000, BP53-12, EMD Millipore). Secondary antibodies for immunofluorescence were from Invitrogen and included goat anti-rabbit Alexa Fluor 488 (green) (1:200, A-11008) and goat anti-mouse Alexa Fluor 594 (red) (1:200, A-21044).
siRNA Transfection
Small interfering RNAs (siRNA) were obtained from Qiagen (Qiagen Inc., Valencia, CA) for the following FAK target sequence: 5′-CCGGTCGAATGATAAGGTGTA-3′. Transfection was carried out as previously described [25, 26]. Briefly, cells were plated (3 × 105 cells per well) on 6-well culture plates and allowed to attach overnight. Cells were treated with HiPerFect® (Qiagen) alone, HiPerFect® plus 20nM Negative Control siRNA (1027310, Qiagen), or HiPerFect® plus FAK siRNA [Hs_PTK2_10 FlexiTube siRNA (NM_005607, Qiagen)] according to manufacturer’s protocol. Cells were incubated for 24 to 48 hours following transfection and then used for experiments. FAK inhibition by siRNA was confirmed using immunoblotting.
Immunoblotting
Western blots were performed as previously described [27]. Briefly, cells were treated with the agent under study, then lysed on ice for 30 min in a buffer containing 50mM Tris-HCL, (pH 7.5), 150 mM NaCl, 1% Triton-X, 0.5% NaDOC, 0.1% SDS, 5mM EDTA, 50mM NaF, 1 mM NaVO3, 10% glycerol, and protease inhibitors: 10 μg/mL leupeptin, 10 μg/mL PMSF and 1 μg/mL aprotinin. The lysates were cleared by centrifugation at 10 000 rpm for 30 min at 4 °C. Protein concentrations were determined using a Bio-Rad kit (Bio-Rad, Hercules, CA) and proteins were separated by electrophoresis on SDS-PAGE gels. Antibodies were used according to manufacturer’s recommended conditions. Molecular weight markers (Precision Plus Protein Kaleidoscope Standards, Bio-Rad) confirmed the expected size of the target proteins. Immunoblots were developed with Luminata Classico or Crescendo ECL (EMD Millipore). Blots were stripped with stripping solution (Bio-Rad) at 37 °C for 15 minutes and then reprobed with selected antibodies. Immunoblotting with antibody to β-actin or GAPDH provided an internal control for equal protein loading.
Immunofluorescence
Cells were plated on glass chamber slides and allowed to attach for 24 hours after which they were fixed with 3% paraformaldehyde. Cells were permeablized with 0.15% Triton X-100 and the first primary antibody (anti-FAK, C-20, 1:1000, Santa Cruz) was added and incubated at room temperature (RT) for 1 hour followed by the addition of the second primary antibody (anti-p53, BP53-12, 1:1000, Invitrogen) that was also incubated for 1 hour at RT. The Alexa Fluor 488 secondary antibody (1:200) was added for 45 minutes at RT. After washing, the second secondary antibody, Alexa Fluor 594, was added and incubated as above. Prolong Gold antifade reagent with DAPI (P36931, Invitrogen) was used for mounting. Imaging was performed with a Zeiss LSM 710 Confocal Scanning Microscope with Zen 2008 software (Carl Zeiss MicroImaging, LLC, Thornwood, NY) using a 63× objective with a zoom of 0.9. Analysis of the images and detection of confocal overlap was accomplished utilizing the MetaMorph® Microscopy Image Analysis Software (Ver. 7.6, Analytical Technologies, Molecular Devices, Sunnyvale, CA).
Manders’ overlap coefficients were calculated for each of the cell lines [28]. Manders’ M1 and M2 coefficients have a value between 0 and 1 (0 = no overlap; 1 = perfect overlap) and provide the proportion of the overlap of each channel with the other. The M1 and M2 coefficients were calculated as follows: M1 (green) = the sum of the intensities of green pixels that had a red component divided by the total sum green intensities; and M2 (red) = the sum of the intensities of red pixels that had a green component divided by the total sum of red intensities.
Cell viability and apoptosis
Equal numbers of cells were plated and allowed to attach for 24 hours. Cells were treated with PF-573,228 or nutlin-3 in varying concentrations. Cellular viability was measured using alamarBlue® assay. In brief, cells were plated 1.5 × 103 cells per well on 96-well culture plates and allowed to attach. Following treatment, 10 μL of alamarBlue® dye (Invitrogen) was added to 200 μL of cell medium. After 4–6 h, the absorbance at 595 nm was measured using a kinetic microplate reader (BioTek Gen5, BioTek Instruments, Winooski, VT).
Peptides and cell proliferation
Peptides were used to interrupt the binding of FAK and p53. The functional peptide (RP-TAT) and the control peptide (TAT) were synthesized with the assistance of P. Mayakonda at the UAB Protein Synthesis Core. The peptide (RP-TAT) and control (TAT) have been described previously [18]. RP-TAT corresponds to the 7 amino-acid peptide binding site on the N-terminal domain of p53 that interacts with the N-terminal domain of FAK. This peptide with the sequence (RMPEAAP) was coupled to a TAT sequence (YGRKKRRQRRR) to allow for improved cellular penetration. The TAT sequence alone was utilized as a control. Cells were treated with peptides for 48 hours at 10 μM concentrations and then stained with trypan blue to assess proliferation. Results were reported as fold change in the ratio of dead to live cells.
Data analysis
Experiments were repeated at least in triplicate, and data were reported as mean ± standard error of the mean. Densitometry of immunoblots was performed utilizing Scion Image Program (http://www.nist.gov/lispix/imlab/prelim/dnld.html). An ANOVA or Student’s t-test was used as appropriate to compare data between groups. Statistical significance was determined at p ≤ 0.05.
Results
Association of FAK and p53 in neuroblastoma cell lines
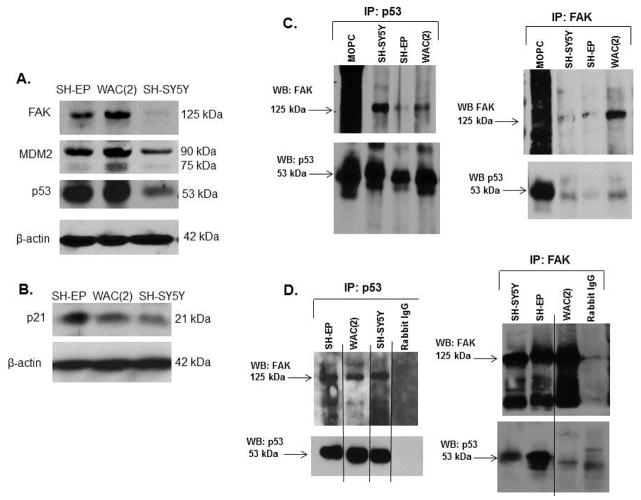
First we documented that the targets of study were present in the cell lines that we chose for the experiments. Immunoblotting was performed on whole cell lysates of SH-EP, WAC(2) and SH-SY5Y cells. FAK, MDM2, p53 (Figure 1A) and p21 (denoting functioning p53) (Figure 1B) were all expressed by these cell lines.
Figure 1.
Association of FAK and p53 in neuroblastoma cell lines. A. Immunoblotting of cell lysates from SH-EP, WAC(2), and SH-SY5Y human neuroblastoma cells for FAK, MDM2 and p53 were performed, confirming that all three proteins were present in the cell lines under study. Immunoblotting for β-actin was used to demonstrate equal protein loading. B. Immunoblotting for p21 in the SH-EP, WAC(2), and SH-SY5Y lysates showed that p21 was present in all three cell lines. Immunoblotting for β-actin demonstrated equal protein loading. C. Immunoprecipitation followed by Western blotting was used to detect FAK and p53 interaction in neuroblastoma cell lines. Immunoprecipitation with p53 antibody followed by Western blotting for FAK showed a band for FAK at the expected 125 kDa in all cell lines examined (left top panel). Immunoblotting the same membrane for p53 detected a band at the expected 53 kDa (left bottom panel). Immunoprecipitation with FAK antibody followed by Western blotting for p53 detected a band at the expected 53 kDa (right bottom panel) and a FAK band at 125 kDa (right top panel) in all cell lines. D. Since the MOPC control band for the immunoprecipitation blots (C) was located near the band for p53, immunoprecipitation experiments were also completed with rabbit IgG as the control. Again, immunoprecipitation with p53 antibody followed by immunoblotting for FAK revealed a band at the expected 125 kDa (upper left panel). The reverse, immunoprecipitation with FAK and Western blotting for p53 showed a band at the expected 53 kDa level (lower right panel). Immunoblotting for the immunoprecipitated protein with their respective antibodies showed bands at the expected levels for p53 and FAK (lower left and upper right panels, respectively).
Previous publications have documented that FAK and p53 interact in cancer cell lines [17, 29]. We have shown that FAK was differentially expressed in neuroblastoma cell lines and provided a survival advantage in these cell lines [11]. Since both FAK and p53 were present in neuroblastoma cell lines (Figure 1A), we hypothesized that they may also interact in neuroblastoma. Coimmunoprecipitations in both directions (FAK and p53 antibodies) were used to determine whether a FAK and p53 interaction was present. Immunoprecipitation (IP) with p53 antibody followed by immunoblotting for FAK showed co-precipitation of p53 protein with FAK, indicating that an association between these two proteins was present in all three cell lines (Figure 1C, left panel). This association was also detected by immunoprecipitation with FAK antibody followed by immunoblotting for p53 (Figure 1C, right panel). Immunoblotting of the membranes with their respective IP antibody confirmed the immunoprecipitation of the correct protein (Figure 1C, left lower and right upper panels). Further, since the immunoglobulin band for MOPC was located near the band for p53, immunoprecipitation experiments were also completed with rabbit IgG as the control (Figure 1D, right and left panels). These coimmunoprecipitation data confirmed the previous findings of an association between FAK and p53 in neuroblastoma cell lines.
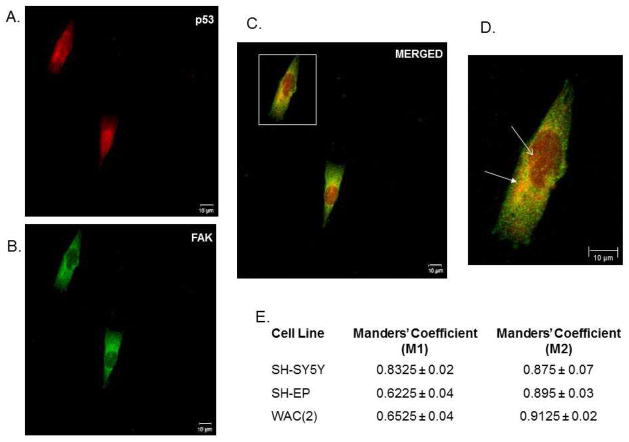
Next, dual immunofluorescence staining and confocal microscopy were employed to further examine the potential association of FAK and p53 (Figure 2A–D). In the SH-SY5Y cell line, FAK and p53 were noted to co-localize in both the cytoplasm (Figure 2D, white closed arrow right panel) primarily in the peri-nuclear area, and in the nucleus (Figure 2D, white open arrow right panel). The same findings were noted for the SH-EP (Supplemental Data Figure 1 A–D) and WAC(2) cell lines (Supplemental Data Figure 2A–D). Manders’ overlap coefficients were calculated for each cell line, with values of 0 indicating no overlap and 1 indicating complete overlap [28]. The overlap coefficients for the FAK and p53 staining indicated a significant amount of overlap, or colocalization of FAK and p53, in all three cell lines (Figure 2E). These confocal microscopy results further demonstrated that FAK and p53 associate in human neuroblastoma cell lines.
Figure 2.
Association of FAK and p53 in neuroblastoma cell lines. Dual immunofluorescence staining followed by confocal microscopy was employed to evaluate FAK and p53 colocalization in the SH-SY5Y cell line. A. Representative photograph of immunofluorescence staining for p53 in SH-SY5Y cells (red). B. These same cells were stained for FAK (green). C. When the stains were merged, there was overlap between the two stains resulting in yellow color. D. Image of overlap staining at higher magnification (white box in C). FAK and p53 were noted to co-localize in both the cytoplasm (white closed arrow) primarily in the peri-nuclear area, and in the nucleus (white open arrow). E. Dual immunofluorescence staining was performed in SH-SY5Y, SH-EP, and WAC(2) cell lines and confocal microscopy was utilized to quantitate the amount of overlap between the two stains by calculating Manders’ coefficients. Manders’ M1 and M2 coefficients have a value between 0 and 1 with zero indicating no overlap and one representing perfect overlap. There was a large amount of overlap detected between FAK and p53 staining in all three cell lines.
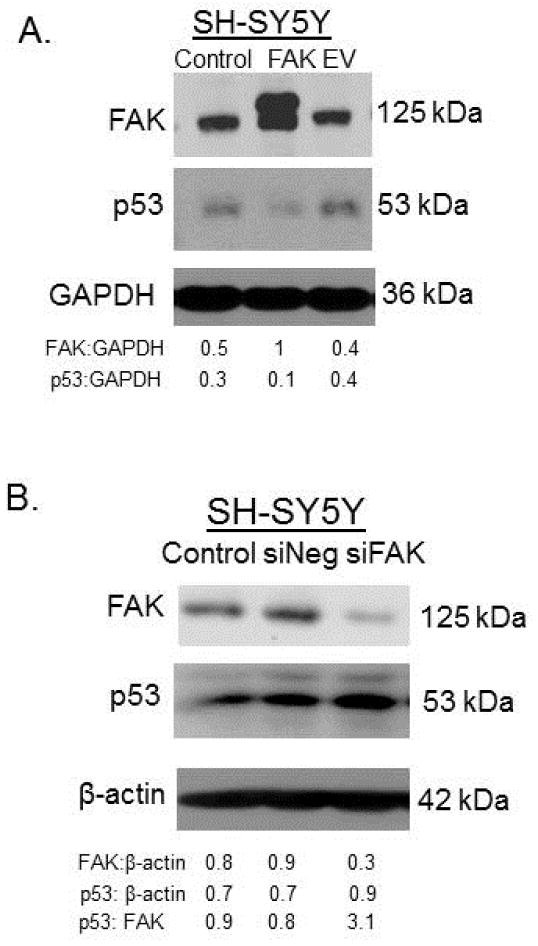
Expression of p53 in the SH-SY5Y cell line was inversely correlated to FAK
Next, we wished to demonstrate that p53 expression was inversely related to FAK expression. SH-SY5Y cells were treated with transfection agent alone (control), with FAK plasmid or with empty vector (EV). Cell lysates were evaluated with immunoblotting for FAK and p53. When FAK expression increased following transfection, the p53 diminished (Figure 3A). Densitometry was performed and confirmed decreased p53 in the FAK transfected cells (Figure 3A). Next, SH-SY5Y cells were transfected with either Lipofectamine alone, negative control siRNA (siNeg) or siRNA to FAK (siFAK) for 48 hours. Cell lysates were examined with immunoblotting. FAK abrogation was confirmed (Figure 3B), and as FAK decreased, p53 expression increased; a finding verified with densitometry (Figure 3B).
Figure 3.
Expression of p53 in the SH-SY5Y cell line was inversely correlated to FAK. A. SH-SY5Y cells were transfected with transfection agent alone (control), FAK plasmid or with empty vector (EV) and cell lysates collected. Immunoblotting was used to detect FAK and p53. Transfection with FAK plasmid resulted in overexpression of FAK protein. Expression of p53 was diminished following FAK overexpression. Densitometry confirmed increased FAK and decreased p53 in the FAK transfected cells. Transfection agent alone or transfection with empty vector had no effect upon FAK or p53 expression. B. SH-SY5Y cells were transfected with either Lipofectamine alone, negative control siRNA (siNeg) or siRNA to FAK (siFAK) for 48 hours. Cell lysates were examined with immunoblotting. FAK expression was decreased with siRNA treatment and p53 expression increased with decreased FAK.
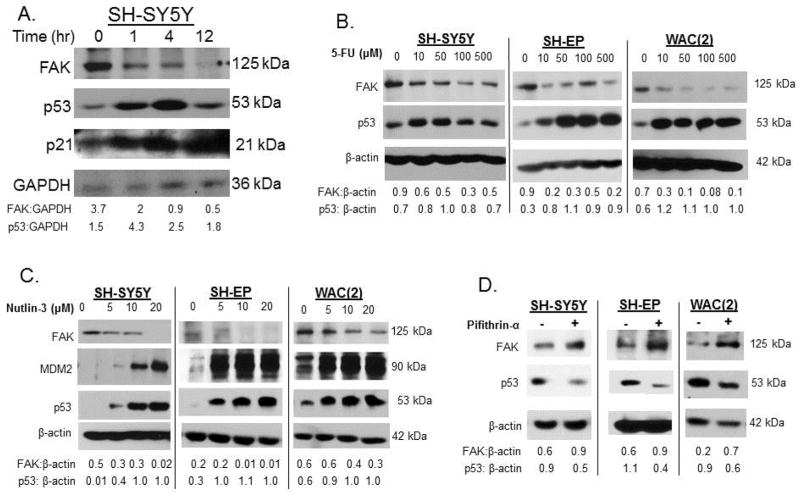
Expression of FAK in neuroblastoma cell lines was inversely correlated to p53
Cellular stressors such as irradiation, hypoxia, or chemotherapy [fluorouracil (5-FU)] [30] upregulate p53 activity. Additionally, the small molecule MDM2 antagonist, nutlin-3 [31], increases the activity of wt p53, while pifithrin-α decreases activity of p53 [32]. To examine the effects of increased p53 upon FAK, we first treated SH-SY5Y cells with irradiation. Cells were irradiated (2 Gy, XRT) collected at increasing times following XRT, and whole cell lysates examined with immunoblotting. A significant increase in p53 was noted as early as 1 hour following XRT (Figure 4A). Inversely, FAK expression decreased following exposure to XRT (Figure 4A). Increased p21 following XRT further showed that p53 was functioning (Figure 4A). We next evaluated pharmacologic manipulation of p53 with 5-fluoruracil (5-FU). SH-SY5Y, SH-EP and WAC(2) neuroblastoma cell lines were treated for 24 hours with increasing concentrations of 5-FU. Cell lysates were examined with immunoblotting. Treatment with 5-FU resulted in a consistent increase in p53 and a decrease in FAK in all three cell lines (Figure 4B). Next, these same cell lines were treated with increasing concentrations nutlin-3, a p53 activator, for 48 hours and immunoblotting was performed on cell lysates. The nutlin-3 treatment led to a significant increase in p53 (Figure 4C), and there was a concomitant decrease in FAK expression with increasing concentrations of nutlin-3 (Figure 4C). To inhibit p53, we utilized pifithrin-α [32] in the SH-SY5Y, SH-EP and WAC(2) cell lines. These cell lines were treated with pifithrin-α for 48 hours and cell lysates examined for FAK and p53. In all three cell lines there was a decrease in p53 expression following pifithrin-α treatment (Figure 4D). There was also an increase in expression of FAK following pifithrin-α treatment in all three cell lines (Figure 4D). All these results demonstrated that FAK and p53 coordinately affect each other’s expression.
Figure 4.
Expression of FAK in neuroblastoma cell lines was inversely correlated to p53. A. SH-SY5Y cells were treated with 2Gy of irradiation to upregulate p53. Cells were harvested and lysates made at increasing times following irradiation. Immunoblotting was performed which showed an increase in p53 and p21 (indicating p53 activity) beginning at 1 hour post-irradiation. As p53 increased following irradiation, FAK protein decreased. B. Pharmacologic manipulation of p53 with 5-fluoruracil (5-FU) was examined. SH-SY5Y, SH-EP and WAC(2) cells were treated for 24 hours with increasing concentrations of 5-FU. Cell lysates were examined with immunoblotting. 5-FU treatment led to increased p53 and a decrease in FAK in all three cell lines. C. SH-SY5Y, SH-EP and WAC(2) cell lines were treated with increasing concentrations nutlin-3 for 48 hours and immunoblotting was performed on cell lysates. Increasing concentrations of nutlin-3 resulted in increased p53, and a concomitant decrease in FAK expression. D. Pifithrin-α was used to inhibit p53 in the SH-SY5Y, SH-EP and WAC(2) cells. Cell lines were treated with pifithrin-α (25 μM) for 48 hours and cell lysates examined for FAK and p53. In all three cell lines there was a decrease in p53 expression following pifithrin-α treatment, and an increase in FAK. Densitometry was performed on all of the blots and verified that p53 and FAK expression were inversely correlated.
Inhibition of FAK and activation of p53 act synergistically to decrease neuroblastoma cell survival
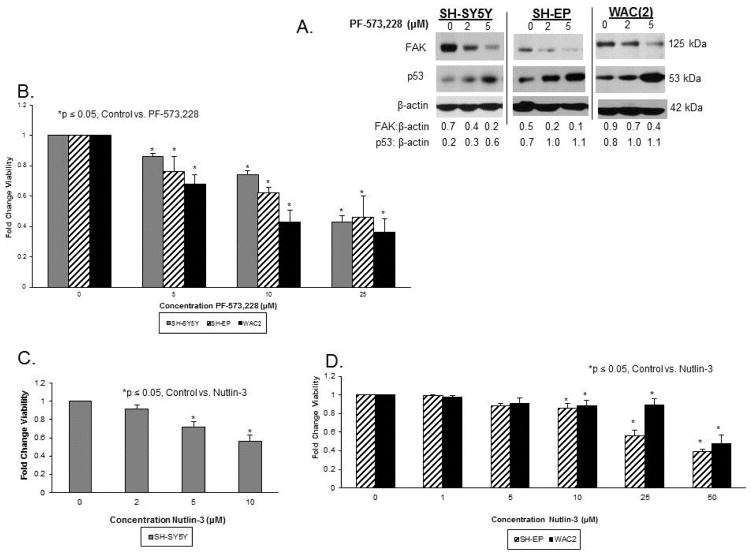
In preparation of pharmacologic dual inhibition studies to follow, we utilized a small molecule FAK inhibitor, PF-573,228 [33]. This small molecule was designed to inhibit FAK phosphorylation, but has been noted to inhibit total FAK expression [34]. This inhibitor decreased FAK expression in the SH-SY5Y, SH-EP and WAC(2) neuroblastoma cell lines (Figure 5A), and p53 was also increased in all three cell lines as FAK diminished, following PF-573,228 treatment (Figure 5A). These results were consistent with those seen previously with FAK inhibition.
Figure 5.
FAK inhibition and p53 activation individually decrease neuroblastoma cell survival. A. SH-SY5Y, SH-EP and WAC(2) cells were treated for 24 hours with increasing concentrations of PF-573,228 and cell lysates were examined for immunoblotting. PF-573,228 treatment resulted in decreased FAK expression in all three cell lines, and as observed previously, p53 expression was inversely related to FAK. B. SH-SY5Y, SH-EP and WAC(2) cells were treated for 24 hours with increasing concentrations of PF-573,228 and cell survival was measured by alamarBlue® assay. There was a significant decrease in cell survival in all three cell lines beginning at 5 μM concentration. C. SH-SY5Y cells were treated with increasing concentrations of nutlin-3 for 24 hours. Cell survival was measured with alamarBlue® assay. There was a significant decrease in SH-SY5Y cell survival with nutlin-3. D. SH-EP and WAC(2) cell lines were treated with increasing concentrations of nutlin-3 for 24 hours. Cell survival was measured with alamarBlue® assay. There was a significant decrease in cell survival with nutlin-3 in these two cell lines also.
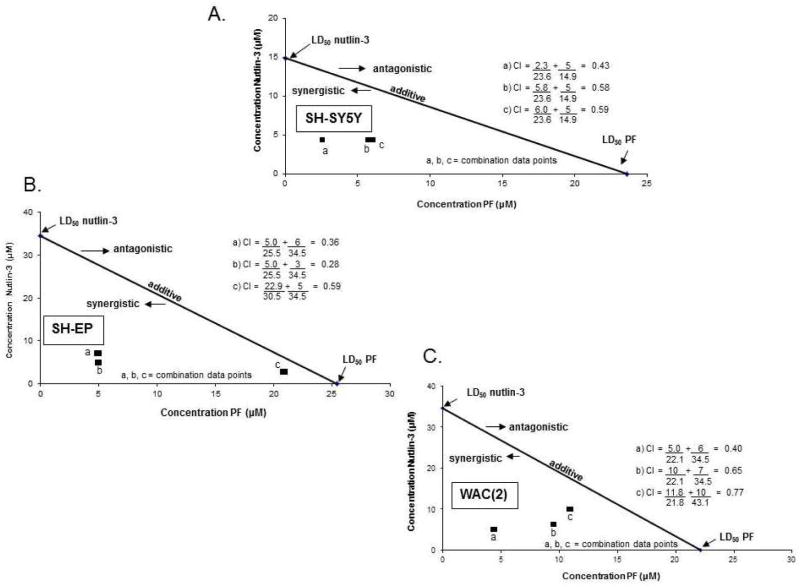
Since there was evidence that p53 and FAK coordinately regulated each of the other’s expression, we wished to determine if FAK inhibition coupled with activation of p53 would lead to increased neuroblastoma cell killing in a synergistic fashion or if it simply occurred as an additive event. Following 24 hour treatment with the FAK inhibitor, PF-573,228 (PF), there was a significant decrease in neuroblastoma cell survival as measured by alamarBlue® assay in SH-SY5Y, SH-EP and WAC(2) cell lines (Figure 5B). In addition, 24 hour treatment with nutlin-3 also resulted in increased cell death in all three cell lines (Figure 5C, D). Next, combined treatments with PF and nutlin-3 were used for 24 hours for inhibition of FAK and upregulation of p53, respectively, in SH-SY5Y, SH-EP and WAC(2) cell lines. AlamarBlue® assay was used to measure cell viability. Using the method of Chou [35] the combination indices (CI) were calculated and isobolograms constructed. The straight line connects the LD50 of each treatment when administered alone. CI values (calculated using the LD50 of the molecules when utilized together) falling above this line suggest an antagonistic effect, those below the line a synergistic effect, and those on the line are thought to represent an additive effect. In all three cell lines, the CI values were less than one (Figure 6A–C) suggesting that there was a synergistic effect upon cell death in neuroblastoma cell lines when FAK inhibition was combined with increases in p53.
Figure 6.
FAK inhibition combined with p53 activation acted synergistically to decrease neuroblastoma cell survival. A. SH-SY5Y cells were treated with PF-573,228 (PF) and nutlin-3 combined for 24 hours and cell survival measured with alamarBlue® assay. Combination indices (CI) were calculated and isobolograms constructed. The straight line connects the LD50 of PF with that of nutlin-3 when each drug was administered alone. CI values above this line suggest an antagonistic effect, those below the line a synergistic effect, and those on the line represent an additive effect. In SH-SY5Y cells, the CIs fall below the line (less than one) suggesting the two treatments together resulted in a synergistic effect. B. As described in A, SH-EP cells were treated with PF and nutlin-3 combined for 24 hours, cell survival detected, and an isobologram constructed. As with the SH-SY5Y cell lines, the CI values were less than one suggesting that the two treatments combined resulted in a synergistic fashion to decrease survival. C. WAC(2) cells were treated as described in A and B. Again, the CI for both drugs combined was less than one and suggested a synergistic effect of the two treatments to decrease survival.
Discussion
Here we showed in multiple neuroblastoma cell lines that the two proteins, FAK and p53, interact. In addition, we demonstrated that these two proteins coordinately regulate each other’s expression. These data are of major importance in neuroblastoma. Children with this tumor have few treatment options, and virtually none that result in long-term survival, so there is a clear need for novel therapies. FAK overexpression has been described in neuroblastoma and has been shown to affect cellular survival and metastasis [10, 13]. In addition, although p53 is wild type in most human neuroblastoma tumors [36], it lacks proper function as a tumor suppressor. The reasons for this apparent lack of tumor suppressor function have not been fully explained in neuroblastoma, and are partly the stimulus for the current study.
In this study, it was demonstrated with both co-immunoprecipitation experiments and dual immunofluorescence staining and confocal microscopy that FAK and p53 proteins interacted in neuroblastoma cell lines. The idea of FAK interacting with another protein in cancer is not new. FAK has been shown to bind to growth factors, tumor suppressors and other proteins. Sieg and colleagues showed that FAK associated with activated PDGF- and EGF-receptors, linking integrin signaling pathways with growth factor receptors [37]. FAK has also been shown to associate with IGF-1R in both pancreatic cancer cell lines MiaPaca-2 and Panc-1, providing a mechanism for increased cellular survival [38]. Previously, our laboratory found that FAK had a direct accociation with VEGFR-3 in human neuroblastoma cell lines, and that interruption of that association with peptides or small molecules resulted in decreased cellular survival in vitro and decreased tumor growth in vivo [39]. In addition to binding to the oncogene p53 [17, 18], FAK has also been shown to bind to the tumor suppressor, neurofibromin [40]. FAK inhibition and disruption of the FAK-neurofibromin interaction resulted in significant cellular detachment and apoptosis, theoretically by increasing expression of neurofibromin tumor suppressor [40]. Finally, Kurenova and others found that FAK directly interacted with the death domain receptor protein, RIP. When this interaction was diminshed through decreased FAK expression, there was a significant increase in cellular apoptosis, showing that the FAK-RIP interaction offered a survival advantage to the cancer cell [41]. Reviewing these studies, it follows that we would find FAK associated with p53 in neuroblastoma cell lines, and that this association may prove to offer a survival advantage to the cells.
The current experiments demonstrated that FAK and p53 coordinately affect the expression of one another. The mechanisms of these findings are the subject of ongoing investigations. Some authors have speculated that the function of p53 as a transcription factor may influence expression of FAK protein. Golubovskaya and others reported that p53 binds directly to the FAK promoter, functioning to directly inhibit the transcription of FAK [42]. In addition, they demonstrated that FAK was more likely to be overexpressed in breast cancers that had p53 mutations [43]. Similarly, Rosado et al found that FAK expression as detected by immunohistochemistry staining in human oral squamous cell carcinoma was inversely correlated with p53 expression in these tumor specimens [44]. Lim et al found that FAK facilitated p53 turnover in fibroblasts through enhanced ubiquitination [17]. FAK expression also inhibited p-53-mediated transcriptional upregulation of p21 in mouse squamous cell carcinoma cells undergoing irradiation by an undescribed mechanism [45]. Our findings cannot be completely explained by any of these mechanisms since we have noted changes in p53 expression that inversely follow changes in FAK expression induced via numerous methods, and vice versa. Therefore, the mechanism for increased or decreased p53 expression in the face of FAK inhibition or overexpression, respectively, is not yet clear and will remain the subject of further investigations.
Amplification of the MYCN oncogene is the most prognostic negative predictor of survival in neuroblastoma [15]. MYCN has been shown to bind to the p53 promoter and positively regulate its expression [46], but whether a significant change in cellular phenotypes result is questionable. For instance, He and colleagues have demonstrated that silencing Mdm2 in MYCN positive cell lines inhibited cell growth in a p53-independent manner, whereas silencing Mdm2 in non-MYCN cell lines triggered p53-dependent apoptosis [47], suggesting the role for other cell survival proteins in the MYCN positive cells with relation to p53. Additionally, Tweddle, in 2001, published that neuroblastoma cell lines without MYCN amplification, but not those that were MYCN amplified, entered G1 arrest following induction of p53 activity with irradiation. These authors suggested a potential abnormality downstream from p53 leading to their findings [20]. These findings could be explained by the association of p53 with other proteins, such as FAK as demonstrated in the current study. Of note, our findings did not suggest a dependence upon MYCN. Treatment of isogenic MYCN cell lines, SH-EP (MYCN negative) and WAC(2) (MYCN positive) (Supplemental Data Figure 3A.) with nutlin-3, an MDM2 antagonist, had similar effects upon both cell lines; and in fact, the LD50 for nutlin-3 was nearly identical for the two cell lines (Figure 6B, C).
In the current study, we demonstrated that in neuroblastoma cell lines, increasing p53 activity in conjunction with FAK inhibition resulted in a decrease in cell survival that was synergistic. This finding is novel in that two small molecules were utilized to determine the effect. A similar result was seen by Kornberg et al who utilized adenoviral dominant-negative inhibitors of FAK and adenoviral vector for overexpression of p53. They reported that co-infection of RPMI 2650 human squamous carcinoma cells with Ad-p53 in conjunction with Ad-FRNK, a dominant-negative FAK inhibitor, resulted in more cellular apoptosis than when this cell line was infected with either agent alone [48]. Recent gene expression profiling showed that disruption of FAK and p53 proteins leads to decreased clonogenicity of colon cancer p53+/+ cell line [49]. On a general level, investigators have suggested that proteins such as FAK may bind to p53, sequestering it in the cytoplasm of the cell, thus preventing it from entering the nucleus and functioning as a transcription factor for cell survival proteins [16]. The variable that remains unknown is whether it is the disruption of the FAK and p53 interaction or the sheer act of combining FAK inhibition with p53 overexpression that were important in neuroblastoma cell killing in our study. Preliminary studies of proliferation in the isogenic MYCN SH-EP and WAC(2) neuroblastoma cell lines utilizing a peptide designed to disrupt the FAK-p53 interaction [18] showed a significant decrease in proliferation in both cell lines treated with the peptide, but the difference was more marked in the MYCN + WAC(2) cells (Supplemental Data Figure 3B). This subject will be the focus of future experiments. We believe that the data from this current study indicate that upregulation of the p53 pathway coupled with inhibition of the FAK pathway would result in a synergistic response and have potential for novel combinatorial therapies. Numerous small molecules and novel compounds are currently under investigation. For example, 5-(1H-benzoimidazol-2-yl)-1H-pyridin-2-one 5-(1H-benzoimidazol-2-yl)-1H-pyridin-2-one (CR389) has recently been shown to induce cell cycle arrest and apoptosis in human ovarian cancer cells by upregulating p53 [50]. There have been a number of inhibitors directed toward FAK including those mentioned. Even more exciting are the significant advances in tyrosine kinase inhibitors, including those not specifically designed to target FAK, but found to have action against FAK, such as tivantinib (ARQ-197) [51]. Obviously, combining these compounds would require validation, but realizing the interactions between oncogenes and kinases may prove to be a source of heretofore unrealized therapeutic targets, and may prove to be a potential therapeutic option for neuroblastoma.
Supplementary Material
Association of FAK and p53 in SH-EP neuroblastoma cell line. Dual immunofluorescence staining followed by confocal microscopy was employed to evaluate FAK and p53 in the SH-EP cell line. A. Representative photograph of immunofluorescence staining for p53 in SH-EP cells (red). B. The same cells were stained for FAK (green). C. When the stains were merged, there was overlap between the two stains resulting in yellow color. D. Image of merged staining at higher magnification (white box C). FAK and p53 were noted to co-localize both in the peri-nuclear area of the cytoplasm (white closed arrow), and in the nucleus (white open arrow).
Association of FAK and p53 in WAC(2) neuroblastoma cell line. Dual immunofluorescence staining followed by confocal microscopy was employed to evaluate FAK and p53 in the WAC(2) cell line. A. Representative photograph of immunofluorescence staining for p53 in SH-EP cells (red). B. The same cells were stained for FAK (green). C. When the stains were merged, there was overlap between the two stains resulting in yellow color. D. Higher magnification of overlap staining (white box C). FAK and p53 were noted to co-localize both in the peri-nuclear area of the cytoplasm (white closed arrow), and in the nucleus (white open arrow).
Disruption of FAK and p53 interaction results in decreased cell survival in neuroblastoma cells. A. SH-EP and WAC(2) cell lysates were examined for MYCN expression. There was significantly more MYCN expression in the MYCN transfected WAC(2) cells than the parent SH-EP (MYCN non-amplified) cell line. B. RP-TAT is a peptide designed to disrupt the FAK / p53 interaction. SH-EP and WAC(2) cells were treated with control (TAT) or RP-TAT peptides for 48 hours. Cell viability was measured with alamarBlue® assay. There was a significant increase in cell death following treatment with RP-TAT in both cell lines, but it was more marked in the MYCN positive WAC(2) cells. Control TAT peptide did not affect survival in either cell line.
Acknowledgments
The authors would like to thank Dr. M Schwab, Deutsches Krebsforschungszentrum, Heidelberg, Germany for his kind gift of the SH-EP and WAC(2) cell lines. We would also like to acknowledge Shawn Williams in the UAB High Resolution Imaging Facility for assistance with confocal microscopy. This work was funded in part by grants from the National Cancer Institute including T32CA091078 (L.A.G., M.L.M., A.M.W.) and K08CA118178 (E.A.B.).
Definitions of Abbreviations
- CI
combination indices
- EDTA
ethylenediaminetetraacetic acid
- FAK
focal adhesion kinase
- 5FU
fluorouracil
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- IP
immunoprecipitation
- LD50
lethal dose 50%
- MDM2
mouse double minute 2
- NaDOC
sodium deoxycholate
- PARP
poly (ADP-ribose) polymerase
- PMFS
phenylmethanesulfonylfluoride
- RT
room temperature
- siRNA
small interfering ribonucleic acid
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- XRT
irradiation
Footnotes
Author Contributions: LAG: experimental design, completion of experiments, manuscript preparation. JES: completion of experiments. MLM: completion of experiments, data evaluation. AMW: completion of experiments. EAB: experimental design, data evaluation, manuscript preparation, manuscript revision.
The content of this manuscript was solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Author Disclosures
The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cotterill SJ, Parker L, More L, Craft AW. Neuroblastoma: changing incidence and survival in young people aged 0–24 years. A report from the North of England Young Persons’ Malignant Disease Registry. Med Pediatr Oncol. 2001;36:231–4. doi: 10.1002/1096-911X(20010101)36:1<231::AID-MPO1056>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 2.Xu LH, Yang XH, Craven RJ, Cance WG. The COOH-terminal domain of the focal adhesion kinase induces loss of adhesion and cell death in human tumor cells. Cell Growth Differ. 1998;9:999–1005. [PubMed] [Google Scholar]
- 3.Xu LH, Owens LV, Sturge GC, Yang X, Liu ET, Craven RJ, et al. Attenuation of the expression of the focal adhesion kinase induces apoptosis in tumor cells. Cell Growth Diff. 1996;7:413–8. [PubMed] [Google Scholar]
- 4.Lipinski CA, Tran NL, Menashi E, Rohl C, Kloss J, Bay RC, et al. The tyrosine kinase pyk2 promotes migration and invasion of glioma cells. Neoplasia. 2005;7:435–45. doi: 10.1593/neo.04712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith CS, Golubovskaya VM, Peck E, Xu LH, Monia BP, Yang X, et al. Effect of focal adhesion kinase (FAK) downregulation with FAK antisense oligonucleotides and 5-fluorouracil on the viability of melanoma cell lines. Melanoma Res. 2005;15:357–62. doi: 10.1097/00008390-200510000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Golubovskaya VM, Gross S, Kaur AS, Wilson RI, Xu LH, Yang XH, et al. Simultaneous inhibition of focal adhesion kinase and SRC enhances detachment and apoptosis in colon cancer cell lines. Mol Cancer Res. 2003;1:755–64. [PubMed] [Google Scholar]
- 7.Golubovskaya VM, Zheng M, Zhang L, Li JL, Cance WG. The direct effect of focal adhesion kinase (FAK), dominant-negative FAK, FAK-CD and FAK siRNA on gene expression and human MCF-7 breast cancer cell tumorigenesis. BMC Cancer. 2009;9:280. doi: 10.1186/1471-2407-9-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halder J, Lin YG, Merritt WM, Spannuth WA, Nick AM, Honda T, et al. Therapeutic efficacy of a novel focal adhesion kinase inhibitor TAE226 in ovarian carcinoma. Cancer Res. 2007;67:10976–83. doi: 10.1158/0008-5472.CAN-07-2667. [DOI] [PubMed] [Google Scholar]
- 9.Golubovskaya VM, Virnig C, Cance WG. TAE226-induced apoptosis in breast cancer cells with overexpressed Src or EGFR. Mol Carcinog. 2008;47:222–234. doi: 10.1002/mc.20380. [DOI] [PubMed] [Google Scholar]
- 10.Beierle EA, Massoll NA, Hartwich J, Kurenova EV, Golubovskaya VM, Cance WG, et al. Focal adhesion kinase expression in human neuroblastoma: immunohistochemical and real-time PCR analysis. Clin Cancer Res. 2008;49:3299–3305. doi: 10.1158/1078-0432.CCR-07-1511. [DOI] [PubMed] [Google Scholar]
- 11.Beierle EA, Trujillo A, Nagaram A, Kurenova EV, Finch R, Ma X, et al. N-MYC regulates focal adhesion kinase expression in human neuroblastoma. J Biol Chem. 2007;282:12503–16. doi: 10.1074/jbc.M701450200. [DOI] [PubMed] [Google Scholar]
- 12.Beierle EA, Ma X, Trujillo A, Kurenova EV, Cance WG, Golubovskaya VM. Inhibition of focal adhesion kinase and src increases detachment and apoptosis in human neuroblastoma cell lines. Mol Carcinog. 2010;49:224–34. doi: 10.1002/mc.20592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Megison ML, Stewart JE, Nabers HC, Gillory LA, Beierle EA. FAK inhibition decreases cell invasion, migration and metastasis in MYCN amplified neuroblastoma. Clin Exp Metastasis. 2013;30:555–8. doi: 10.1007/s10585-012-9560-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slack A, Chen Z, Tonelli R, Pule M, Hunt L, Pession A, et al. The p53 regulatory gene MDM2 is a direct transcriptional target of MYCN in neuroblastoma. Proc Natl Acad Sci U S A. 2005;102:731–6. doi: 10.1073/pnas.0405495102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brodeur G, Seeger R, Schwab M, Varmus H, Bishop J. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science. 1984;224:1121–4. doi: 10.1126/science.6719137. [DOI] [PubMed] [Google Scholar]
- 16.Golubovskaya VM, Cance W. Focal adhesion kinase and p53 signal transduction pathways in cancer. Front Biosci. 2010;15:901–12. doi: 10.2741/3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim ST, Chen XL, Lim Y, Hanson DA, Vo TT, Howerton K, et al. Nuclear FAK promotes cell proliferation and survival through FERM-enhanced p53 degradation. Mol Cell. 2008;29:9–22. doi: 10.1016/j.molcel.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golubovskaya VM, Finch R, Zheng M, Kurenova EV, Cance WG. The 7-amino-acid site in the proline-rich region of the N-terminal domain of p53 is involved in the interaction with FAK and is critical for p53 functioning. Biochem J. 2008;411:151–60. doi: 10.1042/BJ20071657. [DOI] [PubMed] [Google Scholar]
- 19.Golubovskaya VM, Ho B, Zheng M, Magis A, Ostrov D, Morrison C, et al. Disruption of focal adhesion kinase and p53 interaction with small molecule compound R2 reactivated p53 and blocked tumor growth. BMC Cancer. 2013;13:342. doi: 10.1186/1471-2407-13-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tweddle DA, Malcolm AJ, Cole M, Pearson AD, Lunec J. p53 cellular localization and function in neuroblastoma: evidence for defective G1 arrest despite WAF1 induction in MYCN-amplified cells. Am J Pathol. 2001;158:2067–77. doi: 10.1016/S0002-9440(10)64678-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez-Lopez AM, Xenaki D, Eden TO, Hickman JA, Chresta CM. MDM2 mediated nuclear exclusion of p53 attenuates etoposide-induced apoptosis in neuroblastoma cells. Mol Pharmacol. 2001;59:135–43. doi: 10.1124/mol.59.1.135. [DOI] [PubMed] [Google Scholar]
- 22.Zhu X, Wimmer K, Kuick R, Lamb BJ, Motyka S, Jasty R, et al. N-myc modulates expression of p73 in neuroblastoma. Neoplasia. 2002;4:432–9. doi: 10.1038/sj.neo.7900255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schweigerer L, Breit S, Wenzel A, Tsunamoto K, Ludwig R, Schwab M. Augmented MYCN expression enhances the malignant phenotype of human neuroblastoma cells: evidence for induction of autocrine growth factor activity. Cancer Res. 1990;50:411–6. [PubMed] [Google Scholar]
- 24.Chu PY, Huang LY, Hsu CH, Liang CC, Guan JL, Hung TH, et al. Tyrosine phosphorylation of growth factor receptor-bound protein-7 by focal adhesion kinase in the regulation of cell migration, proliferation, and tumorigenesis. J Biol Chem. 2009;284:20215–26. doi: 10.1074/jbc.M109.018259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gillory LA, Stewart JE, Megison ML, Nabers HC, Mroczek-Musulman E, Beierle EA. FAK inhibition decreases hepatoblastoma survival both in vitro and in vivo. Transl Oncol. 2013;6:206–15. doi: 10.1593/tlo.12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Megison ML, Gillory LA, Stewart JE, Nabers HC, Mrozcek-Musulman E, Beierle EA. FAK inhibition abrogates the malignant phenotype in aggressive pediatric renal tumors. Mol Cancer Res. 2014;12:514–26. doi: 10.1158/1541-7786.MCR-13-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beierle E, Ma X, Trujillo A, Kurenova E, Cance W, Golubovskaya V. Inhibition of focal adhesion kinase and src increases detachment and apoptosis in human neuroblastoma cell lines. Mol Carcinog. 2010;49:224–34. doi: 10.1002/mc.20592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manders EMM, Verbeek FJ, Aten JA. Measurement of colocalization of objects in dual-color confocal images. J Microsc. 1993;169:375–82. doi: 10.1111/j.1365-2818.1993.tb03313.x. [DOI] [PubMed] [Google Scholar]
- 29.Golubovskaya VM, Finch R, Cance WG. Direct interaction of the N-terminal domain of focal adhesion kinase with the N-terminal transactivation domain of p53. J Biol Chem. 2005;280:25008–21. doi: 10.1074/jbc.M414172200. [DOI] [PubMed] [Google Scholar]
- 30.Huang Y, Zhang J, McHenry KT, Kim MM, Zeng W, Lopez-Pajares V, et al. Induction of cytoplasmic accumulation of p53: a mechanism for low levels of arsenic exposure to predispose cells for malignant transformation. Cancer Res. 2008;68:9131–6. doi: 10.1158/0008-5472.CAN-08-3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844–8. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 32.Komarov PG, Komarova EA, Kondratov RV, Christov-Tselkov K, Coon JS, Chernov MV, et al. A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science. 1999;285:1733–7. doi: 10.1126/science.285.5434.1733. [DOI] [PubMed] [Google Scholar]
- 33.Slack-Davis JK, Martin KH, Tilghman RW, Iwanicki M, Ung EJ, Autry C, et al. Cellular characterization of a novel focal adhesion kinase inhibitor. J Biol Chem. 2007;282:14845–52. doi: 10.1074/jbc.M606695200. [DOI] [PubMed] [Google Scholar]
- 34.Cabrita MA, Jones LM, Quizi JL, Sabourin LA, McKay BC, Addison CL. Focal adhesion kinase inhibitors are potent anti-angiogenic agents. Mol Oncol. 2011;5:517–26. doi: 10.1016/j.molonc.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70:440–6. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 36.Kusafuka T, Fukuzawa M, Oue T, Komoto Y, Yoneda A, Okada A. Mutation analysis of p53 gene in childhood malignant solid tumors. J Pediatr Surg. 1997;32:1175–80. doi: 10.1016/s0022-3468(97)90677-1. [DOI] [PubMed] [Google Scholar]
- 37.Sieg DJ, Hauck CR, Ilic D, Klingbeil CK, Schaefer E, Damsky CH, et al. FAK integrates growth-factor and integrin signals to promote cell migration. Nat Cell Biol. 2000;2:249–56. doi: 10.1038/35010517. [DOI] [PubMed] [Google Scholar]
- 38.Liu W, Bloom D, Cance W, Kurenova E, Golubovskaya V, Hochwald S. FAK and IGF-IR interact to provide survival signals in human pancreatic adenocarcinoma cells. Carcinogenesis. 2008;29:1096–107. doi: 10.1093/carcin/bgn026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stewart JE, Ma X, Megison M, Nabers H, Cance WG, Kurenova EV, et al. Inhibition of FAK and VEGFR-3 binding decreases tumorigenicity in neuroblastoma. Mol Carcinog. 2013 Jul 19; doi: 10.1002/mc.22070. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kweh F, Zheng M, Kurenova E, Wallace M, Golubovskaya V, Cance WG. Neurofibromin physically interacts with the N-terminal domain of focal adhesion kinase. Mol Carcinog. 2009;48:1005–17. doi: 10.1002/mc.20552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kurenova E, Xu LH, Yang X, Baldwin AS, Jr, Craven RJ, Hanks SK, et al. Focal adhesion kinase suppresses apoptosis by binding to the death domain of receptor-interacting protein. Mol Cell Biol. 2004;24:4361–71. doi: 10.1128/MCB.24.10.4361-4371.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Golubovskaya VM, Finch R, Kweh F, Massoll NA, Campbell-Thompson M, Wallace MR, et al. p53 regulates FAK expression in human tumor cells. Mol Carcinog. 2008;47:373–82. doi: 10.1002/mc.20395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Golubovskaya V, Conway-Dorsey K, Edmiston SN, Tse CK, Lark AA, Livasy CA, et al. FAK overexpression and p53 mutations are highly correlated in human breast cancer. Int J Cancer. 2009;125:1735–8. doi: 10.1002/ijc.24486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosado P, Lequerica-Fernández P, Peña I, Alonso-Durán L, de Vicente JC. In oral squamous cell carcinoma, high FAK expression is correlated with low P53 expression. Virchows Arch. 2012;461:163–8. doi: 10.1007/s00428-012-1283-2. [DOI] [PubMed] [Google Scholar]
- 45.Graham K, Moran-Jones K, Sansom OJ, Brunton VG, Frame MC. FAK deletion promotes p53-mediated induction of p21, DNA-damage responses and radio-resistance in advanced squamous cancer cells. PLoS One. 2011;6:e27806. doi: 10.1371/journal.pone.0027806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Slack A, Chen Z, Tonelli R, Pule M, Hunt L, Pession A, et al. The p53 regulatory gene MDM2 is a direct transcriptional target of MYCN in neuroblastoma. Proc Natl Acad Sci U S A. 2005;102:731–6. doi: 10.1073/pnas.0405495102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.He J, Gu L, Zhang H, Zhou M. Crosstalk between MYCN and MDM2-p53 signal pathways regulates tumor cell growth and apoptosis in neuroblastoma. Cell Cycle. 2011;10:2994–3002. doi: 10.4161/cc.10.17.17118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kornberg L. Ad-fRNK and Ad-p53 cooperate to augment drug-induced death of a transformed cell line. Anticancer Res. 2006;26:3025–31. [PubMed] [Google Scholar]
- 49.Golubovskaya VM, Ho B, Conroy J, Liu S, Wang D, Cance WG. Gene Expression Profiling Identifies Important Genes Affected by R2 Compound Disrupting FAK and P53 Complex. Cancers (Basel) 2014;6:166–78. doi: 10.3390/cancers6010166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suh H, Choi KW, Lee CH. CR389, a Benzoimidazolyl Pyridinone Analog, induces Cell Cycle Arrest and Apoptosis via p53 Activation in Human Ovarian Cancer PA-1 Cells. J Microbiol Biotechnol. 2015 Jan 29; doi: 10.4014/jmb.1412.12080. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 51.Xi WH, Yang LY, Cao ZY, Qian Y. Tivantinib (ARQ-197) exhibits anti-tumor activity with down-regulation of FAK in oral squamous cell carcinoma. Biochem Biophys Res Commun. 2015 Jan 24; doi: 10.1016/j.bbrc.2015.01.062. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Association of FAK and p53 in SH-EP neuroblastoma cell line. Dual immunofluorescence staining followed by confocal microscopy was employed to evaluate FAK and p53 in the SH-EP cell line. A. Representative photograph of immunofluorescence staining for p53 in SH-EP cells (red). B. The same cells were stained for FAK (green). C. When the stains were merged, there was overlap between the two stains resulting in yellow color. D. Image of merged staining at higher magnification (white box C). FAK and p53 were noted to co-localize both in the peri-nuclear area of the cytoplasm (white closed arrow), and in the nucleus (white open arrow).
Association of FAK and p53 in WAC(2) neuroblastoma cell line. Dual immunofluorescence staining followed by confocal microscopy was employed to evaluate FAK and p53 in the WAC(2) cell line. A. Representative photograph of immunofluorescence staining for p53 in SH-EP cells (red). B. The same cells were stained for FAK (green). C. When the stains were merged, there was overlap between the two stains resulting in yellow color. D. Higher magnification of overlap staining (white box C). FAK and p53 were noted to co-localize both in the peri-nuclear area of the cytoplasm (white closed arrow), and in the nucleus (white open arrow).
Disruption of FAK and p53 interaction results in decreased cell survival in neuroblastoma cells. A. SH-EP and WAC(2) cell lysates were examined for MYCN expression. There was significantly more MYCN expression in the MYCN transfected WAC(2) cells than the parent SH-EP (MYCN non-amplified) cell line. B. RP-TAT is a peptide designed to disrupt the FAK / p53 interaction. SH-EP and WAC(2) cells were treated with control (TAT) or RP-TAT peptides for 48 hours. Cell viability was measured with alamarBlue® assay. There was a significant increase in cell death following treatment with RP-TAT in both cell lines, but it was more marked in the MYCN positive WAC(2) cells. Control TAT peptide did not affect survival in either cell line.