Abstract
Cranial window implants in head-fixed rodents are becoming a preparation of choice for stable optical access to large areas of cortex over extended periods of time. Here, we provide a highly detailed and reliable surgical protocol for a cranial window implantation procedure for chronic widefield and cellular imaging in awake, head-fixed mice, which enables subsequent window removal and replacement in the weeks and months following the initial craniotomy. This protocol has facilitated awake, chronic imaging in adolescent as well as adult mice over several months from a large number of cortical brain regions; targeted virus and tracer injections from data obtained using prior awake functional mapping; and functionally-targeted two-photon imaging across all cortical layers in awake mice using a microprism attachment to the cranial window. Collectively, these procedures extend the reach of chronic imaging of cortical function and dysfunction in behaving animals.
INTRODUCTION
The current generation of optical imaging and optogenetic tools provide unprecedented brain access for selectively mapping and manipulating various microcircuit elements across cortical brain regions. As a result, these methods are growing in popularity for studying the relationship between neuronal activity and behavior in awake, behaving mice. However, it can often be difficult to focus light onto the mouse brain through the skull and collect high-resolution two-photon images over long periods of time (weeks to months), due to light scattering caused by skull irregularities or regrowth1–3. Additionally, skull thinning methods can be limited to a small number of imaging sessions due to requirements for repeated skin closing and resection4. Consequently, glass cranial windows are currently the method of choice for studying the activity of neuronal populations over extended periods of time5–8 (but see ref. 9). Yet, the existence of standardized, detailed and versatile surgical protocols for chronic window implants geared to functional imaging in awake mice are scarce. In particular, it remains challenging to combine imaging with multiple procedures that require intermittent access to the brain
Previous cranial window implant methods have offered useful strategies for reducing brain motion during long-term imaging in anesthetized6 or awake5 mice, by applying a viscous substance between the brain and the coverglass to increase adhesion and/or pressure (agarose6 or Quick Sil5). While effective, these methods make it difficult to subsequently remove and replace the cranial window without damaging the brain. The modified cranial window implant approach that we describe here enables the removal and replacement of the cranial window. This approach facilitated our previous chronic two-photon imaging studies in awake, adult mice13–15, for the following reasons. First, window implant prior to subsequent removal for viral injection initially enabled high-resolution intrinsic autofluorescence mapping of multiple visual areas in awake mice (providing high-resolution multi-areal maps with strong activation of higher visual areas). Following functional mapping, removal of the window was critical for targeting viral infection13–15 of a genetically encoded calcium indicator (AAV2/1-GCaMP3) to a focal region within a small visual cortical area (e.g. GCaMP3 expression extending <300 μm within the borders of lateromedial cortical area LM, see ref. 15; see also Anticipated Results). Second, removal of the cranial window at or after the time of viral injection allowed removal of skull regrowth and/or thickened dura, thus providing enhanced clarity while extending the lifetime of imaging through the window. Third, removal of the cranial window enabled replacement with an assembly consisting of a 1 mm microprism coupled to a replacement cranial window at a precise location and orientation. This method facilitated functionally-targeted two-photon calcium imaging across all cortical layers, simultaneously, in awake mice. While cranial window removal and replacement has not been commonly used by other labs during chronic cortical imaging in awake mice, similar procedures are routinely used and highly valuable for chronic cortical imaging studies in primates17.
Here, we provide detailed protocols developed and standardized in our labs for a set of related procedures to achieve long-term cortical imaging, together with interspersed and repeated surgical access to the brain. A flowchart of these surgical procedures and their estimated durations is provided in Fig. 1. These procedures have evolved to maximize experimental flexibility, consistency across surgeons, and imaging success rates.
Figure 1. Experimental paradigm and surgical procedures involved in chronic imaging in awake mice.
Flowchart showing the sequence of events that may be encountered during chronic imaging in awake, behaving mice. Surgical procedures (solid blue boxes) are described in detail in this protocol (see PROCEDURE). Numbers and approximate timings refer to corresponding steps in PROCEDURE section. Asterisks indicate that neither prior handling of mice nor EEG implantation are essential. Note that cranial window removal and replacement following initial imaging allows for several additional procedures that can extend the lifetime of the cranial window and allow flexible timing, increased brain stability, and reduced surgery times as compared to performing these procedures immediately following the initial craniotomy.
Comparison with previous cranial window protocols
A critical first step for all procedures described above involves the initial chronic implant of a large (5 mm) cranial window, which we have used in previous studies for imaging the posterior cortex of anesthetized10,11 or awake12–15 adult mice across several days to months, and for extending the reach of chronic imaging in awake mice to earlier ages overlapping the visual critical period16 (Fig. 2). This window implant procedure (PROCEDURE Steps 1-78 of the protocol) has some similarities to previous methods4-8, but differs in several key aspects:
As discussed above, our initial window implant (PROCEDURE Steps 60-75) combines the previous ability to achieve high brain stability during awake imaging5 with the capacity for subsequent removal and replacement of the cranial window.
In our experience, moving the mouse to headpost clamps (PROCEDURE Steps 32-37; as opposed to using earbars throughout the surgery) is extremely useful for precise drilling and for decreasing the risk of brain insult during the craniotomy (PROCEDURE Steps 49-59), as the head is more stable than when drilling while the head is held with earbars. It is also our subjective impression that this approach improves visibility during surgery as the skull has been rotated such that the entire craniotomized region remains in focus. This also facilitates subsequent functional imaging by ensuring that the cranial window is always roughly co-planar with the headpost.
The protocol describes how to effectively combine the large headpost/window surgery with electroencephalography (EEG) recordings12 (PROCEDURE Steps 32-37).
Several early steps in this initial implant procedure contain extensive detail that we feel have increased our likelihood of maintaining long-term sterility (e.g. PROCEDURE Steps 19-27) and stability (PROCEDURE Steps 30, 38-40) during chronic awake imaging, and have minimized the risk of intra-surgical fatalities (PROCEDURE Steps 1-18, 76-78).
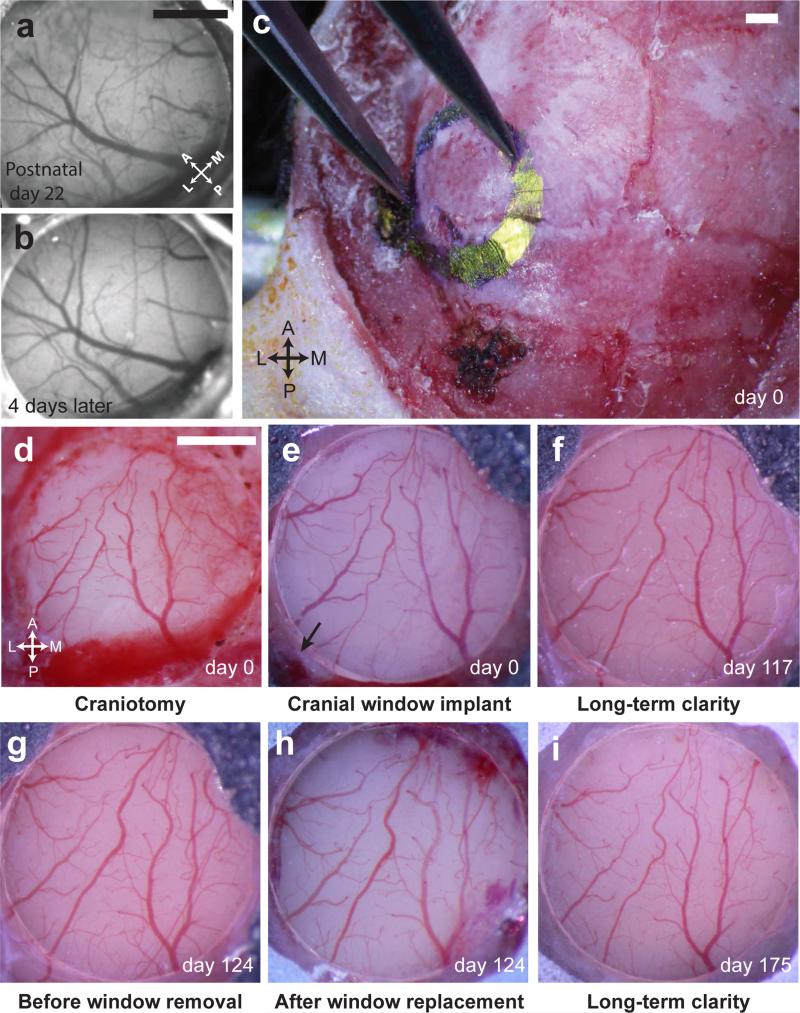
Figure 2. Illustration of chronic window implants for awake imaging in adolescent and adult mice.
(a-b) Images of a 3 mm cranial window implant over primary visual cortex (V1) of an adolescent mouse, taken on the day of surgery (a; postnatal day P22; anesthetized) and 4 days later (b; awake and head-restrained). (c-i) Images of a 3 mm cranial window implant at a very posterior and lateral location in an adult mouse. (c) Skull outline of proposed craniotomy, extending laterally to the squamosal bone. (d) Image of the exposed brain submerged in sterile saline immediately following the craniotomy. (e) Implant appears clear and brain appears healthy immediately following initial cranial window implant (day 0). Black arrow denotes the intersection of the transverse sinus and the caudal rhinal vein. (f) Image of the same implant as in e, but 117 days later, illustrating sustained window clarity. (g-h) Images prior to (g) and following (h) a demonstration of cranial window removal in the same mouse, 124 days after initial window implant. (i) Image demonstrating window clarity, 51 days after window replacement (see also Fig. 10). Experiments were approved by the IACUC at Harvard Medical School (HMS; panels a-b) and Beth Israel Deaconess Medical Center (BIDMC; panels c-i).
We then describe the procedure we have used for repeated removal of the cranial window (OPTION A), the motivation for which is discussed above. This procedure facilitated several additional surgical procedures also contained in this protocol that we have employed in previous studies (Fig. 1): First, we show how to combine window removal and replacement with durotomy and/or bone growth removal (OPTION B) in situations where thickened dura or skull regrowth have compromised image quality, thus enabling prolonged optical access to the tissue beneath the window (local cleaning of skull regrowth and/or removal of the dura method was used in approximately 70% of mice included in two of our studies involving chronic imaging in awake mice13,15; see below; for a previous example of chronic imaging prior to and following bone regrowth removal, see also Fig. 2A in ref. 14). Second, window removal and replacement has allowed precisely targeted delivery of viral vectors and anatomical tracers to cortical areas that have previously been delineated using epifluorescence imaging in awake mice13-15 (for specific examples of awake functional maps and subsequent targeting of viral injection of calcium indicators, see Anticipated Results and Fig. 1A,C in ref. 13). This procedure also improves the reliability of injections, as substantially less brain edema occurs following window removal as compared to following initial craniotomy (our procedure for volume injections is described in Supplementary Method). Third, by replacing our cranial window with one that is glued to a precisely positioned microprism, we have achieved simultaneous, chronic, awake two-photon imaging of precisely targeted cortical columns14 (OPTION C).
The replaceable cranial window approach we have employed is quite versatile, and should also enable several additional procedures not previously described (and thus not covered in this protocol). For example, temporary replacement with a cranial window with a 120 μm – 1000 μm hole at a targeted location allows pipette access for stable two-photon imaging of a nearby, pressurized cortical area during cell-attached recordings (A. Kerlin and S. Chatterjee, unpublished data), and allows application and washout of various drugs during simultaneous imaging in awake mouse cortex (G. Goldey and M. Andermann, unpublished data).
Experimental Design
Several general and specific considerations discussed below and throughout the protocol can facilitate surgical training and adaptation of the procedures described below. It is helpful to begin training on a window implant surgery with as few additional challenging factors as possible. For example, it is useful to begin surgical training using healthy, young adult mice (P45-P90) wild-type (e.g. C57Bl/6) – in our experience, adolescent mice (e.g. P20-P35) have softer skulls and higher rates of skull growth, while older and/or overweight mice have thicker skulls and often respond more variably to anesthesia. It is also useful to begin by performing craniotomies on dorsal cortical regions that are distant from major sinuses and skull sutures (to avoid the risk of excessive bleeding). Once proficiency is achieved (which typically requires >2-4 weeks of daily practice), one can attempt more challenging adaptations (e.g. craniotomies in adolescent mice with more pliable skulls16, see Figs. 2a-b; or more lateral craniotomies (as employed in ref.15; see also Figs. 2c-i). This protocol focuses on window implants optimized for posterior visual cortex. When adapting these methods to other cortical regions of varying surface curvature, medial/lateral position, and proximity to sinuses, it will be useful to vary both the thickness and diameter of the cranial window (for further discussion, see CUSTOM MATERIAL SETUP). Once appropriate parameters are determined, window clarity can be maintained for several weeks to months following the initial implant, and following window removal/replacement (Figs. 2c-i and ANTICIPATED RESULTS).
While it may be possible to image immediately following cranial window implant or window replacement (see Fig. 2 in ref. 14), we find it useful to wait at least 10 days after any surgical procedure prior to imaging, for several reasons. First, this allows the animal to recover from anesthesia and any long-lasting effects of drugs employed such as atropine and dexamethasone. Second, while we have not evaluated immune responses systematically across time, we do not find evidence of significant microglial or astroglial activation at several weeks to months following either a single window implant or following window replacement (Supplementary Fig. 1; N=4 mice; see also ref. 6 for additional discussion of potential immune responses following chronic window implant).
Activity of many individual neurons and long-range axonal boutons can be recorded simultaneously across all cortical depths via insertion of a 1 mm reflective glass microprism14. While qualitatively normal visually-evoked responses in mouse V1 can be observed as soon as the day following prism insertion, image clarity continues to improve across weeks14. In our experience, installation of the initial cranial window (PROCEDURE Steps 1-78) during a prior surgery preceding prism implantation is advantageous for several reasons: First, there is far less edema following window removal than during initial craniotomy, thus decreasing bleeding and increasing the precision of the insertion. Second, accurate functional targeting of prism location and angle of entry is possible. Third, separating the craniotomy/headpost and microprism implantation surgeries may decrease the risk to animal health inherent in longer, combined surgeries. As discussed in ref. 14 and in ANTICIPATED RESULTS, a useful control when establishing the microprism implant is to compare neural activity in superficial layers following microprism implant with activity patterns obtained through standard imaging methods from superficial cortex. Similarly, insertion of the prism to image the same columns from different directions (in different mice) can provide insight into the effects of connections that may be severed for any given tangential angle of prism insertion (e.g. prism imaging face pointing medial or lateral).
MATERIALS
REAGENTS
Wild-type (C57Bl/6) or transgenic mice (variable); (Charles River or Jackson Laboratories)
CAUTON. Any experiments using animals must be conducted in accordance with institutional and national guidelines and regulations.
Nair (CVS; item no. 339818)
Envirocide (Moore Medical; item no. 13-3324)
Benzalkonium chloride (Moore Medical; item no. 44018)
Betadine solution, 19% povidone-iodine topical solution (Moore Medical; item no. 77911)
Mineral oil (Fisher BioReagents; item no. BP26291)
70% Ethanol (Acros Organics; item no. 04-355-454)
Methanol (Sigma-Aldrich; item no. 34860)
Black tempora powder paint (Blick; item no. SAR227185)
Kwik-Cast (WPI; item no. Kwik-Cast)
Kwik-Sil (WPI; item no. Kwik-Sil)
Loctite® liquid super glue, clear, 0.14 oz (WB Mason; item no. LOC1647358)
Grip cement kit, powder & solvent (Dentsply; item no. 675570)
C&B metabond quick (Parkell; item no. 242-3200)
Drugs
Sulfatrim (Patterson Veterinary; item no. 50383082316)
Sodium chloride injection [0.9%], fliptop plastic vial, 10 ml (Moore Medical; item no. 55277)
Meloxicam (NSAID analgesic) injection, 5 mg/ml, 20 ml vial (Patterson Veterinary; item no. 07-890-733)
Isoflurane (Patterson Veterinary; item no. 07-878-1636)
CAUTION. Operations involving the use of isoflurane should be conducted in a well-ventilated room to prevent the accumulation of anesthetic vapors.
Cefazolin, 1 gram vial (Patterson Veterinary; item no. 07-802-5851)
Puralube ophthalmic ointment (Patterson Veterinary; item no. 07-888-2572)
Dexamethasone injection [4mg/ml] (Patterson Veterinary; item no. 07-839-1921)
Atropine sulfate injection [1/120 grain] (Patterson Veterinary; item no. 07-869-6061)
Mannitol [20%] injection (Patterson Veterinary; item no. 07-869-7681)
Medical grade oxygen (LifeGas; item no. varies on canister size)
Gel foam (Moore Medical; item no. 2928)
Vetbond (Patterson Veterinary; item no. 07-805-5031)
Consumable supplies
Ultra-Fine™ II short needle insulin syringe (Moore Medical; item no. 77589)
1.0 ml syringes, luer-lok (Moore Medical; item no. 51415)
1.0 ml syringes, luer-slip (Moore Medical; item no. 96300)
BD 10ml sterile syringes (Moore Medical; item no. 8821)
BD 25G 1” needle (Moore Medical; item no. 28099)
BD 27G 0.5” needle (Moore Medical; item no. 8838)
BD Brand isopropyl alcohol swabs (Fisher Scientific; item no. 13-680-63)
EF4 drill bits (Microcopy Dental; item no. EF4)
FG1/4 drill bits (Microcopy Dental; item no. FG1/4)
FG4 drill bits (Microcopy Dental; item no. FG4)
1.0mm MicroPrism, Al-coated hypotenuse (Tower Optical; item no. MPCH-1.0)
Teflon coated, platinum iridium EEG (Plastics One; item no. MS333/9A)
EEG mounting holder (Plastics One; item no. MH-325-E)
Surgical skin marker (Fine Science Tools; item no. 18000-30)
Double, one end tapered cotton tip medical applicator (Quick Medical; item no. 821-WCDBL)
Non-woven surgical sponges (Patterson Veterinary; item no. 07-847-3539)
Kimwipes (Fisher Scientific; item no. 06-666)
3 1/2″ × 9″ sterilization pouch (Moore Medical; item no. 40390)
5 1/4″ × 10″ sterilization pouch (Moore Medical; item no. 40391)
1″ autoclave tape (Moore Medical; item no. 17411)
Sterile Towel Drapes 18″ × 26″ (Moore Medical; item no. 14170)
Corning 24 well cell culture cluster tray (Fisher Scientific; item no. 09-761-146)
Sofloop face masks (Moore Medical; item no. 53858)
Sterile surgical gloves (Moore Medical; item number is size-specific)
Surgical gown (Moore Medical; item number is size-specific)
Surgical tie-back cap (Moore Medical; item no. 53501)
Glass microscope slides (Fisher Scientific; item no. S95352)
Surgical tools
Bonn Strabismus scissors, ToughCut®, straight (Fine Science Tools; item no. 14084-09)
Vannas spring scissors, 3mm cutting edge, straight (Fine Science Tools; item no. 15000-00)
Dumont #5 forceps, standard, Dumoxel (Fine Science Tools; item no. 11251-30)
Dumont #5SF forceps (Fine Science Tools; item no. 11252-00)
Dumont #5/45 forceps (Fine Science Tools; item no. 11251-35)
Iris forceps, straight (Fine Science Tools; item no. 11064-07)
Bonn micro probe, angled 45° (Fine Science Tools; item no. 10032-13)
Scalpel handle, item no. 3 (Fine Science Tools; item no. 10003-12)
Scalpel blades, item no. 10 (Fine Science Tools; item no. 10010-00)
Hot bead sterilizer, 8cm (Fine Science Tools; item no. 18000-45)
Replacement beads for hot bead sterilizer (Fine Science Tools; item no. 18000-46)
Stainless steel instrument tray and solid flat cover (Patterson Veterinary; item no. 07-804-0008)
Calipers (McMaster-Carr; item no. 2325A75)
Castroviejo surgical calipers (Fine Science Tools; item no. 18000-35)
EQUIPMENT
CUSTOM MATERIALS
• Titanium headposts
○ Custom designed titanium headposts (see Supplementary Fig. 2; H.E. Parmer). While stainless steel or aluminum headposts may also be used, we chose to us titanium because it demonstrates increased biocompatibility and decreased corrosion during long-term implantation18-19, while remaining cost-effective (~$4 each when purchased in bulk) and precisely replicable across labs.
• Imaging wells
○ Buna-N O-Rings, size 015 (McMaster-Carr; item no. 9452K59)
○ Buna-N O-Rings, size 017 (McMaster-Carr; item no. 9452K71)
○ Cyanoacrylate (WB Mason; item no. LOC1647358)
• Cranial windows and microprism assemblies
○ 3 mm round coverglass (Harvard Biosciences; item no. W4 64-0720)
○ 5 mm round coverglass (Harvard Biosciences; item no. W4 64-0700)
○ 8 mm round coverglass (Harvard Biosciences; item no. W4 64-0701)
○ Norland optical adhesive, no. 71 (Norland Products, Inc.; item no. 7106)
○ Ultraviolet lamp; 365 nm (Cole-Palmer; item no. UX-97603-05)
○ ELC-410 Light curing system (Thorlabs; item no. CS410)
○ Dumont ceramic tip forceps (Fine Science Tools; item no. 11210-60)
○ Incubator (Fisher Scientific; item no. 11-690-625D)
○ Aluminum coated microprism (Tower Optical; item no. MPCH-1.0)
SURGICAL EQUIPMENT
Small animal stereotaxic instrument with digital display console (KOPF; item no. 940)
Mouse gas anesthesia head (KOPF; item no. 1923-B; includes nose cone)
Non-rupture ear bar, mouse (KOPF; item no. 1922)
Universal stereotaxic holder (KOPF; item no. 1772-F2)
Heating pad bundled with probe and temperature controller (CWE inc.; item no. TC-1000)
Adjustable stage platform for 502600 series (WPI; item no. 502600)
Solid aluminum optical breadboard, metric (Thorlabs; item no. varies depending on size)
Clamping fork (Thorlabs; item no. CF125 or CF175)
General purpose clamp (Thorlabs; item no. CL5)
Pedestal post holder, spring-loaded hex-locking thumbscrew (PH30E/M)
12.7 mm stainless steel optical post (Thorlabs; item no. varies depending on size)
Right-angle post clamp (Metric), fixed 90° adapter (Thorlabs; item no. RA90/M)
Plate clamp (Standa; item no. 4PC69)
M6 cap screw and hardware kit (Thorlabs; HW-KIT2/M)
M6 setscrew and hardware kit (Thorlabs; HW-KIT4/M)
Dental drill
Econo dental delivery system (Aseptico; item no. ADU-07)
High speed dental handpiece (Aseptico; item no. AHP-96)
Hosing line to connect to 60-90 PSI compressed air supply
Vacuum line
1-way stopcock, luer lock (WPI; item no. 14038-10)
4-port manifold (WPI; item no. 14055-2)
Surgical suction hose, 6' (Patterson Veterinary; item no. 07-811-7922)
Suction unit plastic canisters with lids, 1200cc (Patterson Veterinary; item no. 07-865-6131)
Tygon tubing (Cole-Parmer; item no. 06408-64)
Luer valve assortment (WPI; item no. 14011)
Barbed tubing assortment (WPI; 5000890)
Monoject cannula, 18G × 1” (Kendall; item no. 8881202348)
Stereoscope
Zeiss stereo discovery V8 zoom microscope (Zeiss; item no. NT58-814)
Binocular phototube ergo stereo 5-45 (Zeiss; item no. 435100-0000-000)
Eyepiece PL 10x/23 Br. Foc. (Zeiss; item no. 444036-9000)
Heavy duty boom stand (Diagnostic Instruments Inc.; item no. SMS20-6)
Objective achromat S 0.5x FWD 134 mm (Zeiss; item no. 435215-9901-000)
Stemi mount with drive for column 32 (Zeiss; item no. 455094-0000-000)
PC computer (Dell or other)
Canon EOS 60D Digital SLR Camera Body (Canon; item no. 4460B003)
Camera adapter 60N-C 2/3″ 0.63x (Zeiss; item no. 426113-0000-000)
Standard lighting for surgical rig
Cold-light source Zeiss CL 6000 LED (Zeiss; item no. 4357009101000000)
Gooseneck dual 4,5/800 ECO (Zeiss; item no. 4355409004000000)
Focusing attachment f/ active d = 8mm (Zeiss; item no. 4170890000000000)
EasyLED ring light (Zeiss; item no. 4170969200000000)
Anesthesia delivery system
V -1 tabletop system (VetEquip; item no. 901806)
Dual delivery system (VetEquip; item no. 921400)
Isoflurane vaporizer (VetEquip; item no. 911103)
Regulator, oxygen (VetEquip; item no. 901305)
Vapor guard filter (VetEquip; item no. 931401)
Wye connector (VetEquip; item no. YO-124DV)
Induction chamber, 7 liter (VetEquip; item no. 941444)
Charcoal-filtered anesthesia scavengers (VetEquip; item no. 931401)
REAGENT SETUP
Atropine sulfate
Dilute 1ml of atropine sulfate (1/120 grain) in 9 ml of sterile saline (0.9% NaCl) for injection, USP; store the solution at room temperature (20° C) and protect the vial from light. Properly stored atropine sulfate solution is stable until the expiration date of the atropine sulfate stock solution.
Cefazolin
Dissolve 1 mg cefazolin powder in 9 ml sterile water for injection, USP. Once the cefazolin powder has been mixed with a diluent it should be stored in the refrigerator and used within 7 to 10 days.
Metacam
Dilute 1 mL of meloxicam (5 mg/ml) (a generic equivalent of metacam) in sterile saline in 9 mL of sterile saline (0.9% NaCl) for injection, USP; store the solution in the refrigerator and protect the container from light and use within 28 days.
C&B Metabond
Mix two leveled scoops of powder (opaque or clear) with four drops of quick base and 1 drop of catalyst in a cold ceramic mixing dish that has been stored in the freezer (this will extend the window for application of Metabond).
Black Dental Cement
Mix three parts white dental cement powder (Grip Cement) with one part black Tempora powder paint (for light shielding purposes; some users prefer a 10:1 mixture).
EQUIPMENT SETUP
Titanium head plate
We designed a titanium head plate implant to provide for stable, long-term head fixation. The implant is rigid, lightweight, and machined from Grade 2 (0.063) thickness CP titanium (Supplementary Figs. 2a,c). It is well suited for visual experiments, as the orientation of the bars provide a large unobstructed viewing angle. Prior to use in surgery, sterilize the headpost implant using a hot-bead sterilizer. For details on the custom headpost clamp we use for securing the headpost, see Supplementary Fig. 2. As described below, the skull must be clean and dry (Supplementary Fig. 3) for successful adhesion to head plate.
Imaging well
Imaging wells are needed to form a stable water meniscus under the microscope objective and to aid in the attachment of light-blocking material during imaging. To make the wells, tightly glue two different sized O-rings together using cyanoacrylate (Supplementary Fig. 2a). Once the adhesive has dried, store the imaging wells in benzalkonium chloride and rinse with 70% ethanol prior to use.
EEG recording wires
Stable EEG recordings are important to monitor the animal's global brain state. To prep the EEG wires for implant, strip approximately 1mm of the Teflon insulation from the ends of the EEG wires using a pair of iris forceps, then bend the exposed portion of each wire at a 90° angle. Rinse the entire device with 70% ethanol prior to use.
Cranial windows
The cranial windows we use are designed to provide clear optical access to the brain as well as to control brain motion. A schematic of cranial window construction is shown in Supplementary Fig. 4. The top portion is a single round coverglass (no. 1 thickness) whose outer margins extend beyond the lower portion (the part that rests on the brain) by 1-2 mm and rest on the skull. For the main procedure described in this paper—the headpost and 5 mm cranial window implant centered over lateral V1 (PROCEDURE Steps 1-78)—all cranial windows implanted on the day of the craniotomy are composed of 3 layers of round, no. 1 cover glass. Choices of cranial window dimensions are dependent on the particular surgery, and require consideration of the nature of the field-of-view (e.g., is it near a sinus? does it need to contain multiple regions of interest?), the curvature of the skull and brain surface at the imaging location (less curved regions require thinner cranial windows), and the thickness of the skull (which increases with age). Trial and error are usually required to determine proper window thickness (e.g. layers of coverglass glued together, see below). Insufficient pressure (e.g., using implants that do not extend and put pressure on the dura) usually results in dural thickening, skull regrowth, and increased brain motion. By contrast, excessive pressure can impede cortical blood flow.
For assembly, glue and cure additional layers of glass (one at a time) using optical adhesive and long wavelength UV light (Supplementary Fig. 4c), then warm the fabricated window in an incubator at 50° C for 12 hours to help strengthen the adhesive bonding. Store the cranial window in 70% ethanol at the start of surgery and rinse with sterile saline prior to use. To avoid failure during or after the implant, the cranial window must be screened for imperfections (such as incorrect amounts of optical adhesive or misalignment) under a stereoscope before use. If the amount of optical glue is spread too thin, air spaces can form and cause optical aberrations or coverglass detachment during implantation. Conversely, too much optical adhesive will result in overflow past the edges of the cranial window, effectively creating a larger diameter cranial window than the size of the craniotomy. This can be particularly problematic during cranial window replacements, as the skull tends to regrow and create a tight fit against the edges of the inner coverglasses of the initial cranial window (<100 μm tolerance).
Fashioned cotton-tipped applicator
Cut the end off of a cotton tipped applicator and use sandpaper to thin down the sides of the wooden applicator, starting approximately 5 cm from the end that was just cut. This portion of the cotton tipped applicator should be thinned down so that the applicator will begin to bow when excess pressure is used to hold the cranial window in place at the time of implant, which will prevent the cranial window from breaking and compromising the surgery (Supplementary Figs. 5a-c).
Microprism assembly
For microprism imaging (PROCEDURE OPTION C), the microprism assembly consists of a microprism attached to the bottom of a cranial window (using optical adhesive; Supplementary Fig. 4d,e). Once the imaging location and orientation in the brain has been determined (for details, see ref. 14), plan the final point of insertion of the microprism by creating a template for precise positioning and attachment of the microprism to the bottom of the cranial window. The procedure for creating this template and attaching the prism are described below and illustrated in Supplementary Fig. 4f-m (see also associated figure legend).
First, attach a digital camera to the surgical scope, and position it directly above the implanted cranial window with the objective perpendicular to the cranial window. Using digital illustration software (e.g. Adobe Illustrator), draw a circular template that is the same size as the current cranial window implant (Supplementary Fig. 4f, left). Then load the image of the brain that was taken orthogonal to the window's surface, and rescale it to fit inside the circular template. Next, zoom in on the image to determine where the incision will be made, then draw a line that is the same size of the bottom edge of the prism (Supplementary Fig. 4f, left). When selecting the line, be careful to avoid bisecting large cortical vasculature.
Next, print the image and tape it to the bottom of a glass microscope slide (Supplementary Fig. 4g). Apply a small drop of Kwik-Cast to the top of the slide (Supplementary Fig. 4h), directly over the taped image, align a single coverglass concentric with the image, and allow the Kwik-Cast to cure (Supplementary Fig. 4i). Use a fine-tipped applicator (i.e. broken cotton-tipped applicator) to apply a small drop of optical adhesive over the arrow on the template (Supplementary Fig. 4k), then position the microprism in place with a pair of ceramic-tipped forceps so that the imaging face is pointing in the same direction of the arrow (Supplementary Fig. 4l). Finally, cure the prism to the coverglass using a high-intensity light curing system (Thorlabs; Supplementary Fig. 4m). Carefully remove the coverglass assembly from the Kwik-Cast and clean (by rinsing with methanol) if necessary; then use optical glue and the light curing system to add this layer to a standard cranial window, as shown in Supplementary Fig. 4e (bottom panel).
Handle the microprism-window implant carefully to avoid scratches, store in 70% ethanol at the start of surgery, and rinse with sterile saline prior to use.
Custom stereotax setup
For a detailed description of how to prepare the custom modifications to our Kopf Stereotax in order to transfer between earbars and headpost clamps in the same surgical plane, please see Supplementary Fig. 2d-f and associated text. Other brands of stereotaxic equipment (e.g. Stoelting, Narishige) can be similarly adapted.
Equipment setup for intracerebral injections
Pull a custom micropipette from a 1.0 mm outer diameter (OD) glass capillary tube so length of the needle's taper (i.e. neck-to-tip) is > 2 cm and the needle tip has a small OD (~1 μm). Break the needle tip using a fine pair of forceps and use a light microscope (equipped with a reticle) to confirm that the OD of the tip is between 12.5-25 μm. While evaluating the OD under the microscope, check to make sure that the break is clean. Once the custom-pulled micropipette needle has been deemed acceptable for use, load it with the substance that is to be injected into the cortex using the Hamilton syringe priming kit.
PROCEDURE
CRITICAL. Any experiments using animals must be conducted in accordance with institutional and national guidelines and regulations.
HEADPOST & CRANIAL WINDOW IMPLANT (2-4 HOURS)
1| Pre-operative care To prevent neural edema during or after the craniotomy, administer the anti-inflammatory synthetic glucocorticoid dexamethasone sodium phosphate (4.8 mg/kg, 4 mg/ml concentration) intramuscularly (IM) into the quadriceps, 4-8 hours prior to surgery, with a short needle insulin syringe.
CAUTION. All experiments using animals should be in accordance with institutional and national guidelines.
2| Check isoflurane and oxygen levels to make sure there is enough for the entire duration of the surgery.
3| Turn on heating pad, LED and gooseneck lights for surgery.
4| Anesthesia Direct the airflow of the anesthesia system to the induction chamber and turn it on, allowing it to charge for one minute at 3% isoflurane in 100% oxygen (0.5-1 L/min). CRITICAL STEP As an alternative to steps 4-5, animals can be pre-anesthetized with an intraperitoneal injection of ketamine (100 mg/kg) and xylazine (15 mg/kg), which lasts longer than isoflurane (tens of minutes) and can facilitate placement of the mouse into the earbars (see below).
5| Place the mouse inside the induction chamber and allow the anesthetic to take effect. Thorough sedation typically occurs between 60-90 s, although this depends on the age and size of the mouse. Hallmark indicators include slower, steady breathing rates (~ 1 cycle per second) coupled with the absence of toe-pinch reflex; physical tests should be administered after the animal is positioned in the stereotax.
CAUTION. Operations involving the use of isoflurane should be conducted in a well-ventilated area with active scavenging to prevent the accumulation of anesthetic vapors.
6| Transferring the mouse from the induction chamber to the stereotax Once the mouse is fully sedated, turn off the isoflurane and oxygen, then use the flush valve to flush the induction chamber with oxygen to prevent experimenter inhalation of isoflurane.
7| Redirect the airflow of the anesthesia system to the stereotax.
8| Carefully remove the mouse from the induction chamber, placing the mouse abdomen-down on the heating pad, which should be pre-warmed and set to 37.5 ±1 °C.
9| Administer maintenance levels of anesthetic for the remainder of the surgery; 1.5% isoflurane (range: 0.9-1.8%) in 100% oxygen (0.5 l/min). Breathing should be regular and shallow. Sparse heaving/gasping (once every 3-4 s) is an indication that a lower isoflurane level is required or that the height of the base supporting the body needs to be adjusted to minimize strain on the diaphragm and neck. Conversely, irregular rapid breathing and/or a palpable response to the toe-pinch reflex indicates that the isoflurane level should be increased. Once the user has become proficient in monitoring levels of anesthesia and postural adjustment, rates of successful recovery from surgical anesthesia should exceed 90%. If rates remain low, consider using non-invasive blood pressure monitoring (and/or blood gas monitoring) to determine the anesthetic level (and body posture; see below) that ensures stable physiological parameters within the normal range throughout the surgery.
10| Loading the mouse into the stereotax With the abdomen still resting on the heating pad, use your dominant hand to gently hold the mouse's head, while using the index finger on your free hand to gently drop the lower jaw to expose the incisors.
11| Insert the top, central incisors into the hole located on the inside base of the mouse anesthesia adapter of the stereotax (the nosecone shield must be retracted behind this hole).
12| Once the incisors are in place, advance the nosecone over the snout (Fig. 3a), but not so far that motion of the mouse's head is restricted, as this may hinder placement of the ear bars in correct position.
Figure 3. Placement in the stereotax and initial skin incision.
(a) Image of an anesthetized mouse after proper placement in the stereotax (PROCEDURE Steps 1-18). Blue arrows indicate the inflow and outflow of gas anesthesia (isoflurane and oxygen), with the nosecone advanced over the snout (PROCEDURE Step 12). The animal's body is resting on a feedback-regulated heating pad and has been covered with an unfolded surgical sponge (PROCEDURE Step 14; for thermal insulation). Ophthalmic ointment has been applied to the eyes (to prevent cataracts) and the earbars have been engaged into the external auditory meatus to secure the skull (PROCEDURE Steps 15 and 17). (b-e) Hair removal and preparation of the surgical site (PROCEDURE Steps 19-22). Hair is trimmed (b; PROCEDURE Step 19), and removed by depilation (c; PROCEDURE Steps 20, 21), followed by 3 alternating washes with 70% isopropyl alcohol (d) and betadine (e) to prepare the site for incision (PROCEDURE Step 22). (f-i) Making an incision in the skin. (f) A pair of #5/45 forceps pulls the skin taught while an incision (~12-15 mm) is made (PROCEDURE Steps 22-24). (g-h) Excision of the epidermis from the skull using forceps and push-scissors (PROCEDURE Step 25). (i) Image of the skull after full excision and prior to the removal of the periosteum (PROCEDURE Step 26). These surgical procedures (Figs. 3-8) were approved by the IACUC at BIDMC.
TROUBLESHOOTING
13| Ensure that the temperature probe remains properly positioned to measure body temperature accurately.
14| Place the unfolded surgical gauze on the dorsal surface of the mouse's body for additional insulation (Fig. 3a).
15| Cover the eyes generously with Puralube ophthalmic ointment to prevent drying and cataract formation (Fig. 3a).
16| Administer atropine (0.216 mg/kg atropine sulfate and 3.6 mg/kg sterile saline; ~0.1 cc of 10X-diluted atropine) intraperitoneally (IP). Atropine is a competitive antagonist of muscarinic cholinergic receptors and helps prevent obstruction of the airways by acting on the parasympathetic nervous system to inhibit the salivary and mucus glands. Check the level of anesthesia and positioning of the mouse's body before continuing – the body should be positioned so that ear bars elevate the head by only a few millimeters with respect to the trunk. Excessive head elevation can impair breathing.
17| Engaging the ear bars to secure the head To secure the head of the mouse in the stereotax, position each non-puncture ear bar in the external auditory meatus within the ear canal (Fig. 3a).
CRITICAL STEP. It is important that ear bar placement is accurate and that the pressure applied is sufficient to prevent any movement, while preserving moderate intra-ocular pressure (to prevent cataract formation). Failure to ensure correct placement and pressure may result in respiratory problems, skull fracture and/or puncturing of the eardrum.
18| Advance the nose cone cover over the snout so that it is approximately 2-3 mm past the tip of the nostrils. This step will push the whiskers towards the eyes of the mouse. To avoid cutting the whiskers (which represents an additional source of stress upon recovery), use Paralube to temporarily affix them to the snout (Fig. 3a).
19| Hair removal Trim the hair on the scalp with a pair of fine scissors (Fig. 3b).
CAUTION Be careful not to pierce the skin.
20| Apply a depilatory agent (e.g. Nair) and massage into the scalp for a maximum of 30 s using a sterile cotton-tipped applicator (Fig. 3c).
21| Remove the depilatory agent using sterile cotton-tipped applicators. This should result in a hair-free area on the scalp that extends 1-3 mm beyond the perimeter of skin to be excised, ensuring asepsis by eliminating the presence of hair under any subsequent application of dental cement. Avoid application on the skin within 2 mm of the eyes. Alternatively, shaving may be acceptable if care is taken to avoid small cuts and to remove debris.
22| Scalp incision Remove any remaining hair using isopropyl alcohol swabs (Fig. 3d) and apply betadine solution using sterile cotton-tipped applicators (Fig. 3e). Repeat this washing procedure three times, alternating between alcohol and betadine, leaving the final application of betadine on the skin (Fig. 3e).
23| Hold a pair of #5/45 forceps in your non-dominant hand and use them to lightly grasp approximately 2-3 mm of skin between the eyes. Carefully pull this skin forward until the epidermis over the medial aspect of the skull is pulled taut to allow for an easier and more precise incision.
24| Hold the scalpel handle attached to a sterile #10 blade in your dominant hand and start the incision at the midline, approximately 3 mm posterior of the eyes. The length of the incision should be approximately 12-15 mm long (Fig. 3f,g).
CRITICAL STEP. When cutting in PROCEDURE Steps 24 and 25, it is crucial to maintain a 1-2 mm strip of bare epidermis along the perimeter of the hairline to maintain asepsis (Fig. 3h,i).
25| To excise the epidermis from the scalp, continue holding the #5/45 forceps in your non-dominant hand and use them to pull the skin taut at the top left side of the incision; using a pair of push scissors to excise the skin over the left hemisphere of the skull (Fig. 3g-h). Use the same technique to remove the remaining skin on the right hemisphere (Fig. 3i).
CAUTION. Take extra precaution when cutting near the eyes and ears to prevent disruption of normal skin tension and/or severing any nerves or blood vessels that are part of these sensory organs (as either of these outcomes could delay healing or cause a disruption of vision/hearing).
26| Use a pair of #5/45 forceps to remove any remaining periosteum. If this connective tissue has already dried to the surface of the skull, use a #10 scalpel blade to scrape it off.
27| Finish cleaning any remnants from the skull using sterile cotton-tipped applicators moistened with sterile saline.
CRITICAL STEP. PROCEDURE Steps 26 and 27 will help ensure permanent adhesion of headpost to skull. This procedure requires that the mouse skull is secured properly in the ear bars to prevent skull movement during cleaning.
28| Marking the center coordinates of the craniotomy Measure the desired coordinates with a pair of calipers and use a scalpel blade to lightly mark each axis. The points at which these lines intersect will designate the center of the craniotomy (Supplementary Fig. 3a). Please note, the steps we describe below involve a 5 mm cranial window implant in the left hemisphere, centered over mouse primary visual cortex (V1). The center coordinates we use for this particular cranial window implant are 3.10 mm lateral from lambda, and 1.64 mm anterior of the lambdoid suture.
29| With a pneumatic dental drill, use a FG¼ dental carbide to lightly etch this point for better visibility, which is necessary for accurate headpost placement (Supplementary Fig. 3a).
30| Retraction of lateral muscles Use a pair of #5/45 forceps (with the tips closed together) to tease away the lateral muscles connected to the temporal suture (Supplementary Fig. 3b). Gently insert the closed tips directly at the point where muscle attaches to the suture connecting the temporal and parietal plates, beginning approximately 2-3 mm behind the eye. Once an initial separation of bone and muscle has been achieved, gently run the closed tips of the forceps in the posterior direction—separating the muscle from the skull until the temporal and parietal plates meet the lambdoid suture. Then use Vetbond to fix the retracted muscle in place to maintain a low profile (Fig. 4a). This step should result in the exposure of approximately 2-3 mm of the temporal plate.
Figure 4. Implanting the headpost.
(a) The retracted muscle is fixed in place ~2-3 mm below the temporal bone suture using Vetbond (blue arrow; PROCEDURE Step 30). The perimeter of the 5 mm diameter craniotomy is traced using a sterile surgical skin marker (left; PROCEDURE Step 31). (b) The titanium headpost is centered on the skull and secured in place using a small amount of cyanoacrylate (PROCEDURE Step 39). Dotted white line (left) indicates plane of cross-sectional viewing the associated schematic (right). Black arrows indicate headpost. (c) C&B Metabond (white arrows) is applied to all areas surrounding the headpost for permanent attachment to the skull (PROCEDURE Step 40).
CRITICAL STEP. PROCEDURE Step 30 is essential for a well-centered headpost implant with respect to the desired coordinates of the craniotomy (in this case, lateral V1). Muscle retraction will also increase the surface area of exposed bone tissue that will be available as a contact point for C&B Metabond, thus ensuring structural integrity of the implant.
CRITICAL STEP. Be extremely cautious when working near muscles and connective tissue located near the lateral edge of the lambdoid suture (where it meets the temporal and parietal plates) to avoid damage to superficial auditory nerves. As muscle is retracted closer to the posterior regions, there is higher likelihood of bleeding due to underlying vasculature. If bleeding does occur, irrigate the affected area with sterile saline and apply gel foam if bleeding persists.
31| Tracing the craniotomy Using a pair of Castroviejo surgical calipers set to the radius of the craniotomy, place one of the two teeth in the center coordinate that was etched in PROCEDURE Step 29, and use the other tooth to trace the circumference of the craniotomy using a surgical skin marker (Fig. 4a).
32| Implanting EEG wires Secure the EEG mounting holder to a movable stage (see Equipment Setup and Supplementary Fig. 6a). CRITICAL STEP. Implanting wires for the EEG (PROCEDURE Steps 32-37) can be useful for long-term monitoring of brain states, but is not recommended during initial training on basic cranial window implant methods.
33| Use an FG¼ dental carbide to drill small holes in the locations where the EEG wires are to be implanted (note that the active and reference electrodes are positioned in one hemisphere, while the ground is anterior to Bregma in the opposite hemisphere; Supplementary Fig. 6b).
34| Load the EEG into the mounting holder (Supplementary Fig. 6a) and position the wires in close proximity to the surface of the skull. Then carefully position each wire over its respective implant site (PROCEDURE Step 32); the tips of the wires should be bent at 90° (see Equipment Setup) and positioned to rest flat on the skull directly adjacent to their respective implant site (Supplementary Fig. 6c).
CRITICAL STEP. PROCEDURE Step 32 is designed to enable proper placement of the wires onto the surface of the dura (Supplementary Fig. 6). Move each of the wires into its implant site (Supplementary Fig. 6d) and secure it in place using a small amount of Vetbond or cyanoacrylate (Supplementary Fig. 6e). Ensure that the glue has fully hardened before implanting the next wire.
36| Repeat PROCEDURE Step 35 to finish implanting the other two wires of the EEG.
37| Once all of the wires have been implanted, carefully remove the EEG port from its holder and position of the wire leads well outside of the range of the craniotomy (Supplementary Fig. 6f). If necessary, use Vetbond to secure the wires in place and prevent movement during the headpost implant (PROCEDURE Steps 38 – 40).
CRITICAL STEP. Substantial clearance of the wires from the craniotomy is necessary to prevent damage, because subsequent steps involve drilling in this area.
38| Installing the titanium headpost Clean the skull of any dried blood or debris using sterile cotton-tipped applicators moistened with sterile saline.
39| Position the headpost over the center of the craniotomy so that it rests roughly in the same plane as that expected for the cranial window implant. The headpost should make solid contact with two locations on the skull. Once the headpost is correctly positioned, add two small drops of cyanoacrylate to secure an initial attachment to the skull. Apply light pressure until the cyanoacrylate has completely dried (Supplementary Fig. 6f; see also Fig. 4b).
CRITICAL STEP. For more lateral headpost implants, varying degrees of medial head tilt are required in order for the headpost to be co-planar with the cranial window implant (which simplifies chronic imaging). In addition, the ipsilateral ear bar may interfere with placement of the headpost. If this occurs, loosen the ipsilateral ear bar and retract it slightly from the external auditory meatus. Then carefully rotate the skull towards the contralateral side (so that the center coordinate of the craniotomy is facing upwards), and advance the ear bar back into place to support the skull.
CAUTION. Ensure that the repositioned ear bar is not applying pressure below the mandible to avoid obstruction of breathing.
TROUBLESHOOTING
40| After the headpost has been secured in place, apply a generous amount of C&B Metabond to the outside perimeter, covering any exposed bone to ensure a complete seal with headpost, thus minimizing the chance of leakage when imaging using water-immersion objectives (Fig. 4c). Allow the C&B Metabond to fully harden before proceeding.
PAUSE POINT
In some cases, it may be useful to recover the animal, in which case headpost habituation and subsequent awake transcranial intrinsic imaging (following full recovery from surgery) can provide delineation of cortical brain regions (i.e. for subsequent targeted implants or injections). In this case, the exposed skull inside the headpost should be protected with Kwik-Cast and post-operative recovery steps should be followed (PROCEDURE Steps 76-78). Prior to subsequent surgery, PROCEDURE Steps 1-16 and 18 should be repeated.
41| Placing the mouse into the headpost clamps Disengage the ear bars from contact with the mouse's head and slightly retract the nose cone shield in order to allow elevation or depression of the mouse's head so that it can be safely secured in the headpost clamps (see Equipment Setup). CRITICAL STEP. PROCEDURE Steps 41-48 improve stability during drilling, and ensure that the craniotomy region is coplanar with the microscope plane of focus, and that the window implant is roughly coplanar with the headpost (facilitating functional imaging).
42| Place the headpost clamps into the clamping forks on the breadboards that have been secured to the base of the stereotax (see Equipment Setup) and position them near the arms of the headpost. Then secure the clamping fork using a M6 cap screw.
43| Place one arm of the headpost into the secured headpost clamp, keeping the mouse's incisors in the holes of the nose cone (the secured headpost clamp may need to be repositioned to keep the incisors in place). Before tightening the screw on the Altos clamp, make sure that the arm of the headpost is resting flush with the bottom jaw of the clamp (Supplementary Fig. 2f).
CAUTION. If the headpost clamps are not properly aligned, excessive torque may be applied to the headpost and can result in dislocation from the skull.
44| Tighten the screw on the Altos clamp to secure headpost.
45| Position the second headpost clamp over the free arm of the headpost and secure the clamping fork. Finish securing the headpost by tightening the screw on the Altos clamp. Supplementary Fig. 2b illustrates the mouse supported in the headpost clamps after the cement has dried.
TROUBLESHOOTING
46| Once both arms of the headpost have been clamped, advance the nose-cone shield over the snout (relative to anatomical position) and slightly elevated. Note that the mouse's nose will now be slightly elevated and rotated about the body, which will likely prevent the nose-cone shield from fully advancing over the snout (Supplementary Fig. 2b).
TROUBLESHOOTING
47| Adjust the height of the base supporting the body to correct for any height differences of the head that may have resulted from transferring the mouse from the ear bars to the headpost clamps.
48| Monitor the level of anesthesia to ensure it is sufficient before continuing with PROCEDURE Step 49.
49| Etching the craniotomy Use an FG¼ carbide attached to lightly etch the circumference of the craniotomy that was traced in PROCEDURE Step 31 (Fig. 4a).
CAUTION. When drilling the craniotomy, be careful to avoid drilling the same area for more than 2 s in order to prevent overheating due to friction (which may cause bleeding underneath the skull).
CAUTION. Be careful not to drill too deep in PROCEDURE Step 49; the goal is to create a physical boundary that can be used in the following steps that involve thinning the skull.
50| Take the cranial window (see EQUIPMENT SETUP) out of 70% ethanol and allow it to dry on the sterile surgical drape, then place the window above the skull and verify the precision of the circle etched in PROCEDURE Step 49. Make any necessary adjustments in the template so that the cranial window will fit snug inside the subsequent craniotomy.
51| After the template has been completed, use the top layer of the cranial window to determine if any C&B Metabond needs to be drilled away from the margins outside of the craniotomy to ensure direct, consistent contact with this part of the cranial window on top of the skull, then place the window back into 70% ethanol.
CAUTION. Handle with care to avoid scratching the cranial window.
52| Drill away excess C&B Metabond in these areas using a FG4 carbide; stop drilling once the skull is exposed (Fig. 5b).
Figure 5. Placing the mouse into the headpost clamps and drilling the craniotomy.
(a) The mouse is removed from the ear bars and loaded into the headpost clamps for craniotomy (PROCEDURE Steps 41-48; this improves stability for drilling and, in some cases, facilitates imaging by positioning the cranial window in precisely the same plane as that spanned by the headpost). Dotted white line (left) indicates plane of cross-sectional view in the associated schematic (right). (b) The craniotomy is etched using an FG¼ carbide drill bit (left, unmarked; right, blue) and excess C&B Metabond is drilled away using an FG4 carbide (left, unmarked; right, grey) to expose the skull surrounding the craniotomy (PROCEDURE Steps 49-52). Previously exposed muscle tissue that was previously secured using Vetbond (PROCEDURE Step 30) may become exposed (red arrows). (c) The skull is cleared of debris and the craniotomy perimeter is thinned using an FG4 carbide, which involves intermittent irrigation with saline to avoid overheating of the underlying brain (PROCEDURE Steps 53-55). Craniotomy drilling is then completed using an EF4 carbide (PROCEDURE Step 56).
CAUTION. If the headpost has been implanted at a very lateral location on the skull, it will be necessary to drill away a larger volume of C&B Metabond above the temporal aspect of the skull. This will likely also involve drilling through muscle tissue (Fig. 5b) that was previously secured with Vetbond (PROCEDURE Step 30).
TROUBLESHOOTING
53| Clear the skull of any debris by using the air gun on the dental delivery system. Wipe away any remaining debris away using sterile cotton-tipped applicators moistened with sterile saline.
54| Thinning the skull Thin down the skull around the outside perimeter of the craniotomy using an FG4 carbide (Fig. 5c). The main goal to be accomplished with thinning down the skull is to create a thin, yet homogenous, level plane for the uppermost layer of the cranial window to rest on.
CAUTION. When thinning the skull, irrigate with sterile saline intermittently to prevent overheating of the superficial brain mater. The thinned skull should not have any breaks in it due to excessive drilling. Once this step is complete, the skull should then be slightly pliable when soaked in sterile saline, yet rigid when the bone tissue is dry.
CAUTION. Thinning the skull may result in bleeding underneath the skull due to overheating. To avoid this, do not allow the drill bit to remain in one spot for more than 2 s while thinning. It is important to note that there is vasculature embedded in the skull itself, as thinning the bone tissue will likely result in occasional spurts of skull bleeding. When this occurs, stop drilling and irrigate with sterile saline.
TROUBLESHOOTING
55| Once thinning has been completed, irrigate the skull with sterile saline, using sterile surgical sponges to soak up the fluid.
56| Complete drilling of the craniotomy using an EF4 carbide drill bit (Fig. 5c; Note: EF4 provides more precision than FG¼). Stop drilling periodically to irrigate the skull with sterile saline. While drilling, small cracks between the bone flap and the surrounding skull will begin to separate and become slightly moistened by cerebrospinal fluid, giving the bone tissue a wet appearance. This is a hallmark indicator of having successfully drilled through the skull.
CAUTION. Be careful to not pierce through bone and dura when performing the craniotomy.
CAUTION. If any portion of the craniotomy rests over a sinus, drill over these areas with extreme caution.
TROUBLESHOOTING
57| Submerge the skull in sterile saline and gently probe the circular, non-drilled region of skull within the craniotomy (bone flap) to confirm that this region is completely separated from the surrounding skull. If any solid connections between the bone flap and the surrounding skull remain, use a Bonn micro probe to loosen or break any connections. If more drilling is required, soak up the sterile saline and complete drilling until the bone flap is loose around the entire craniotomy and re-submerge in saline.
58| Remove the bone flap with a pair of #5/45 forceps (Fig. 6a).
Figure 6. Cranial window placement.
(a) The skull is submerged in saline (indicated by blue water drop symbol at top right) and the bone flap is removed using forceps (PROCEDURE Steps 57-59). (b) The exposed brain remains submerged in sterile saline and the cranial window is implanted into the craniotomy and held in place using a fashioned cotton-tipped applicator loaded into a stereotaxic micromanipulator (PROCEDURE Steps 60-67). (c) Saline is soaked up using surgical sponges and Kim-wipes (black arrow), except for directly under the cranial window (PROCEDURE Steps 68-69).
CAUTION. Craniotomies larger than 3 mm have increased risk of damaging the brain upon removal of the bone flap. In these instances, a cantilever-like downward motion of the bone flap at the opposite end the craniotomy can be prevented using a Bonn micro probe.
TROUBLESHOOTING
59| Irrigate the exposed brain with sterile saline. If bleeding occurs and is difficult to stop, apply gel foam and continue to irrigate. Once bleeding has ceased, keep the brain submerged in sterile saline (Fig. 6a, left).
60| Implanting the cranial window Load and secure the fashioned cotton-tipped applicator into the stereotax micromanipulator (Fig 6b, see also Equipment Setup; Supplementary Fig. 5).
61| Remove the cranial window from the 70% ethanol bath and allow it to dry on the sterile drape.
62| Use a pair of #5/45 forceps to hold the cranial window and add a few drops of sterile saline to the top layer; this will disrupt the surface tension of the saline and allow the cranial window to sink below the surface. Once the cranial window is fully submerged, use #5/45 forceps to position it over the craniotomy.
63| Position the micromanipulator so that the fashioned cotton-tipped applicator is above the cranial window as it rests on the dura (which remains submerged in sterile saline).
CAUTION. Depending on the working distance of the objective being used in the surgical scope, the tilt and/or height of stereoscope itself may need to be adjusted in order to position micromanipulator arm vertically over the center of the cranial window.
64| Tighten the screw lock of the micromanipulator to disable rotational movement in the horizontal plane.
65| Manually lower the fashioned cotton-tipped applicator using the dorsal/ventral arm until it is resting directly on top of the cranial window.
CAUTION. Little to no pressure should be applied at this point.
66| If necessary, use #5/45 forceps to make any fine adjustments in the position of the cranial window so that the bottom cover glass in the window assembly will fit correctly in the craniotomy.
TROUBLESHOOTING
67| Carefully lower the stereotaxic arm until the fashioned cotton-tipped applicator begins to bow slightly. If the skull has been properly thinned (PROCEDURE Steps 54-69), the saline-soaked bone tissue will become slightly pliable and bend downward as the cotton-tipped applicator continues to increase the amount of pressure being applied to the surface of the cranial window (Fig. 6b).
CAUTION. Stop applying pressure when the cotton-tipped applicator begins to bow (Supplementary Fig. 5b), or if cortical blood flow is obstructed.
TROUBLESHOOTING
68| Soak up the sterile saline using sterile surgical sponges.
69| Use sterile Kim-wipes to remove any fluid beneath the top layer of the cranial window (the portion resting on the surface of the skull, see Fig. 6c).
CAUTION. Ensure that no fluid is removed from beneath the portion of the cranial window that is resting on the surface of the brain.
TROUBLESHOOTING
70| Once the inside of the well is dry, apply a small amount of Vetbond to create an initial seal between the top layer of the cranial window and the skull (Fig. 7a).
Figure 7. Cranial window implant and placement of imaging well.
(a) Vetbond is carefully and conservatively applied to create a thin seal between the skull and the sides of the top layer of the cranial window (blue arrows). Excess Vetbond is wicked away using a sterile Kim-wipe (PROCEDURE Steps 70-71). (b) C&B Metabond is applied to all areas inside of the headpost, including any exposed skull as well as the portion of the cranial window resting on the skull (white arrows; PROCEDURE Step 72). (c) The C&B Metabond has fully hardened and the fashioned cotton-tipped applicator is retracted (PROCEDURE Steps 73). The imaging well has been glued to the top of the headpost (black arrows) using cyanoacrylate. For imaging during visual stimulus presentation, the imaging well and C&B Metabond is coated with a low profile layer of black dental acrylic for light blocking purposes (gray arrows; PROCEDURE Steps 74-75).
CAUTION. Vetbond has a very low viscosity and tends to clog the applicators used to apply it. Check to make sure that the spout of the applicator is not clogged to avoid accidental overflow above the top layer of the cranial window. Do not allow Vetbond to spread over the top surface of the cranial window. As a further precaution, small drops of Vetbond can be applied to a sterile surface and dabbed onto the sides of the cranial window using an applicator.
TROUBLESHOOTING
71| Absorb any excess Vetbond using a sterile Kim wipe.
72| Apply C&B Metabond over any exposed skull and the portion of the cranial window that is resting on the skull (being careful to prevent any obstruction of visibility of the brain surface). Allow the C&B Metabond to fully cure before continuing to PROCEDURE Step 73 (Fig. 7b).
73| Raise the vertical arm to lift the fashioned cotton-tipped applicator away from the surface of the cranial window.
74| Use cyanoacrylate to glue the imaging well (see Equipment Setup) to the surface of the titanium headpost.
75| Apply black dental cement to the inside and outside of the imaging well and to all regions of C&B Metabond for light-blocking purposes (see Drug and Reagent Setup; Fig 7c). Ensure that additional dental cement does not rise appreciably above the cranial window margins to avoid damage to short working distance (e.g. 2 mm) imaging objectives.
76| Post-operative care At the end of the surgery, but before discontinuing anesthetic, administer analgesia (inject 0.50 mg/kg meloxicam, a non-steroidal anti-inflammatory (NSAID), subcutaneously, SC; alternative NSAIDs to meloxicam may be used, such as carprofen). Wait 10 minutes.
77| Discontinue anesthesia and allow the animal to wake. The mouse should be kept on the heating pad until ambulant.
78| Administer prophylactic antibiotics (cefazolin, 500 mg/kg injection, IM; and sulfatrim, 1:32 in H2O) and allow the animal to recover.
CAUTION. All animal experiments must be performed in accordance with the guidelines and regulations held by the institution at which they are conducted in.
Applications involving removal and replacement of the cranial window
As described in the flowchart in Fig. 1, at a later date following the initial window implant,performing the following optional component procedures can enable various extensions of the initial cranial implant. Procedures for cranial window removal (OPTION A, required for subsequent options) and additional procedures prior to subsequent replacement of the chronic cranial window allow for (i) cleaning of the imaging area, including durotomy / bone growth removal (OPTION B), (ii) functionally-targeted intracerebral injection (Supplementary Text), and (iii) microprism implant for deep imaging across cortical layers (OPTION C). An example of bone regrowth that will impair two-photon imaging in vivo is described in Fig. 8a-c. Conducting OPTIONS A and B together allows extraction of this bone regrowth. The window removal/replacement strategies also allow for replacement with cranial windows containing drilled or laser-cut holes of various shapes and sizes allows stable cell-attached recordings (A. Kerlin and S. Chatterjee, unpublished observations) or pharmacological studies (G. Goldey and M. Andermann, unpublished observations) during anesthetized or awake imaging of pressurized, adjacent cortical regions.
Figure 8. Cranial window removal and cleaning.
(a) Top: image of the same mouse as in Figs. 3-7, 77 days after initial window implant. The mouse is anesthetized and secured in the headpost clamps prior to cranial window removal and local removal of skull regrowth and thickened dura. Note that the surgical rig has been covered with a sterile drape to protect the stereotax from debris created from drilling through dental cement. Bottom: zoomed-in view of the imaging well and cranial window as they appear through the surgical microscope. (b) Zoom-in of blue box region in a. The white arrow indicates the outer edge of the 5 mm diameter cranial window. (c) Zoomed-in view of the green box in b, comparing healthy brain tissue (left of dotted black line) to an area of bone-growth (semi-opaque appearance to the right of dotted black outline). (d) Image of the cranial window after the black dental cement (see a,b) has been drilled away from the inside of the imaging well (OPTION A, Step vi). The white arrow points to the edge (white circular outline, 5 mm diameter) of the portion of the window resting on the brain. The orange arrow indicates the edge of the top layer of coverglass (8 mm diameter orange circle), whose outer 1.5 mm annulus is resting on the skull; note that all dental cement has been cleared from the top surface of this outer annulus. (e) Image of the cranial window as the outer annulus of the top coverglass is being drilled away using an FG4 carbide and a pair of #5/45 forceps. This procedure allows the inner glass ‘plug’ to temporarily remain in place, thus maintaining the integrity and cleanliness of the craniotomy (OPTION A, Step vii). The white arrow indicates the edge (white outline) of the inner plug, and the orange arrow and line outline the remaining perimeter of the top coverglass; note that the white C&B Metabond in d (previously beneath the top coverglass annulus) has been drilled away, and the underlying skull has been adequately and evenly thinned (OPTION A, Step viii). (f) Image of the brain after the well has been cleared of debris from drilling (OPTION A, Step vix), followed by removal of the cranial window plug (OPTION A, Step x). The white arrow indicates a Bonn micro probe being used to pierce through the dura and pry the bone growth (dotted-black line) from the surface of the brain (OPTION B, Step ii). (g) An image of the brain after removal of the bone-growth and most of the dura. White arrow indicates the dura as it is being removed by gentle circumferential tugging near the craniotomy margin (OPTION B, Step i). The black arrow indicates the transverse sinus, and the water drop (bottom right corner) indicates that the brain is submerged in sterile saline. (h) Image of the brain after the cleaning procedure (durotomy and bone-growth removal) has been performed and a new, 5 mm cranial window has been implanted (OPTION B, Step v). All scale bars are 1 mm.
OPTION A) CRANIAL WINDOW REMOVAL (15-30 MINUTES)
i| Repeat PROCEDURE Steps 1-16 and 18.
ii| Repeat PROCEDURE Steps 42-47.
iii| Fill the headpost well with 70% ethanol and wait at least 90 s.
CRITICAL STEP The goal of this and the next step is to disinfect and clean the headpost well of any debris.
iv| Remove the ethanol using sterile surgical sponges and/or sterile cotton-tipped applicators.
v| Use an FG4 carbide to shave down any cement above of the top layer of the cranial window (Fig. 8d). If the drill bit is kept completely orthogonal to the transverse plane, the cement can be drilled away without cracking the window.
vi| Use an FG4 drill bit to drill away the dental cement directly surrounding the cranial window, then clear the headpost well of any debris caused by drilling (i.e. by irrigation with sterile saline). This should result in exposure of the underlying skull (Fig. 8e).
vii| With a pair of #5/45 forceps in one hand, close the tips together and gently place them on top of the cranial window, and use a FG4 drill bit to drill away the portion of the cranial window that is resting on the surface of the skull (Fig. 8e). Continue to hold the forceps in place to prevent the center portion of the window from being ejected out of the craniotomy. If done cautiously, the center portion of the window should remain snug in the craniotomy, with no brain insult or bleeding (Fig. 8e).
TROUBLESHOOTING
CAUTION. If the cranial window's center portion (or ‘plug’) is not kept within the craniotomy upon completion of this step, two problems will likely occur: First, debris resulting from drilling will stick to the dura, which may irritate the brain and/or lead to infection and/or decreased optical clarity. Second, thinning down the skull surrounding the perimeter of the craniotomy (OPTION A, Step viii) will result in an inward expansion of bone-tissue, and in turn, a slightly smaller craniotomy (since the plug was not in place to resist this inwards expansion of bone). If the latter occurs, the new cranial window will not fit into the craniotomy and the surgery will have to be terminated. It is plausible that success may otherwise be achieved by not thinning down the skull, and instead replacing the current cranial window with a new window containing an extra inner coverglass for added thickness. This has been less fruitful of an approach since drilling away the top layer of the cranial window often results in slight drilling into the skull, and thus inward expansion of bone tissue.
viii| Uniformly thin down the underlying skull as described in PROCEDURE Steps 54-55 (Fig. 8e) so that adequate pressure can be applied when the new cranial window is implanted. This step is necessary in order ensure that the skull surrounding the craniotomy is as thin as it was following the original craniotomy (PROCEDURE Steps 54-57), despite subsequent increases in thickness due to Vetbond and C&B Metabond application (PROCEDURE Step 70) and skull regrowth and thickening during aging.
ix| Irrigate the inside of the headpost well using sterile saline to remove any debris.
x| Fill the inside of the headpost well with sterile saline and carefully remove the remainder of the cranial window from the inside of the craniotomy using a pair of #5/45 forceps.
OPTION B) DUROTOMY AND/OR BONE GROWTH REMOVAL (30-60 MINUTES)
i| Remove the cranial window as described in Option A.
CAUTION. Before proceeding to OPTION B, Step ii, any bleeding that resulted from the cranial window removal should be under control.
ii| Use a Bonn micro probe to pierce the dura in an area that is devoid of cortical vasculature, or in an area where bone regrowth is present (Fig. 8f).
CAUTION. Two important variables should be considered before dura or bone tissue is removed from the craniotomy. First, as the length of time that the cranial window has been implanted prior to the durotomy increases, the thickness of the dura will typically increase (as well as the density and/or thickness of dural vasculature). Although this makes the dura much easier to differentiate from cortex, far more bleeding will occur during the durotomy. In this case, the surgeon should remain calm while performing the durotomy. The quality of the original cranial window implant (e.g., the degree of consistent pressure on the brain throughout the window) will determine the rate of dural thickening and whether osseous tissue beneath the cranial window also begins to emerge, rendering the cleaning procedure more challenging. For best results, it is recommended that high quality optics are used to analyze the state of the brain beneath the cranial window prior to this procedure. A skilled observer can easily differentiate the different categories of regrowth discussed above, and thus better plan for the steps required in the surgery.
iii| Use a pair of #5/45 (or #5SF) forceps to grab a hold of the dura where it was just previously punctured.
iv| With the dura in the grip of the forceps, gently peel it from the brain and tear it radially from the edge of the craniotomy. Then continue to tear the dura in a circumferential fashion until it has been completely removed from the cortex. Immediately irrigate with sterile saline and apply gel foam.
CAUTION. If the durotomy is being performed near a sinus, a pair of fine push-scissors should be used to cut the dura in order to prevent excessive bleeding from the sinus (Fig. 8g).
TROUBLESHOOTING
v| Complete PROCEDURE Steps 60-75 to implant the cranial window (Fig. 8h). Make sure to have properly evaluated the cranial window prior to implant (see Equipment Setup for details).
TROUBLESHOOTING
vi| Complete PROCEDURE Steps 76-78 for post-operative care.
OPTION C) MICROPRISM IMPLANT FOR FUNCTIONAL IMAGING ACROSS ALL CORTICAL LAYERS (60-90 MINUTES)
CRITICAL. Below we describe, in greater detail, the protocol used for chronic calcium imaging in Andermann et al., 2013.
i| Remove the cranial window as described in OPTION A.
ii| Perform a large durotomy as described in OPTION B, Steps ii-iv.
iii| Load the straight-edge dissecting knife into the vertical stereotaxic arm and position the tip of the blade over the posterior aspect of cortex that is going to be incised. The blade of the knife should be facing the anterior aspect of the cortex, since the incision will be made in a posterior-to-anterior fashion. For incisions not made along this axis, try to choose an axis on incision that can be made parallel to the horizontal arm of the micromanipulator.
CRITICAL STEP. OPTION C, Step iii requires prior planning for successful results. Prior to implanting optical devices such as a microprism assembly, high-resolution pictures of the brain surface (after cranial window implant) must be taken and scaled for use as a template. This template will then be used to (1) construct the microprism assembly (a microprism glued below a standard cranial window) given the geometric specifications of the current cranial window implant (to allow precise anatomical implantation of the microprism) and (2) perform the cortical incision at a location that will allow proper placement of the implant (see Equipment Setup; Supplementary Fig. 4).
TROUBLESHOOTING
iv| Digitally zero the micromanipulator location once the blade touches the cortex, then slowly lower the blade to make an incision that is 10% deeper than the vertical height of the microprism. Irrigate with sterile saline periodically when performing the vertical component of the incision. Using a micromanipulator minimizes damage to cortex adjacent to the microprism insertion, and to underlying white mater. It also allows a slower, more precise incision.
v| Continue making the incision by driving the blade in a posterior-to-anterior fashion along the target incision—again, exceeding the total length of the incision by 10% of the length of the microprism. Irrigate with sterile saline periodically while performing the incision. Minimal, controlled back-and-forth motion (<100 μm) of the blade may further prevent brain compression or an excessively long incision.
vi| Carefully retract the dissecting knife from the brain, then immediately irrigate the cortex with sterile saline and apply gel foam.
vii| Remove the dissecting knife from the micromanipulator and replace it with the vacuum line.
viii| To hold the microprism assembly from above (OPTION C, Step ix), first place an 18G Monoject blunt cannula (Kendall) on the tip of the vacuum line, then turn on the vacuum (standard wall vacuum pressure is sufficient).
ix| Remove the microprism assembly from the 70% ethanol and allow it to dry, then grip the assembly from above using suction (i.e. using the blunted cannula attached to a vacuum line).
x| Position the microprism assembly above the gel foam that is covering the incision.
xi| Remove the gelfoam from above the incision; if bleeding occurs irrigate with sterile saline reapply gel foam until bleeding subsides. Then adjust the rotational alignment of the microprism assembly so that the bottom edge of the microprism is in-line with the incision.
xii| Lower the microprism assembly and digitally zero its position when the lower edge first contacts the brain.
TROUBLESHOOTING
xiii| Insert the microprism assembly into the brain, lowering the vertical arm of the stereotax slowly until the top layer of the cranial window is resting on the surface of the skull. The implant should be lowered slightly (~100-200 μm) beyond the length of the microprism to ensure adequate pressure of the cranial window on the brain surface (as previously described).
xiv| If possible, advance the microprism assembly slightly (~200-400 μm) so that the imaging face can apply more pressure to the brain mater directly in front of it.
CRITICAL STEP. Failure to achieve an adequate amount of pressure between the imaging face and the brain mater in front of it may result in the accumulation of blood and/or dural growth, either of which will obstruct imaging through the microprism.
xv| Soak up the sterile saline using sterile surgical sponges.
xvi| Use sterile Kim-wipes to remove any fluid beneath the annulus of the top layer of the microprism assembly (the portion resting on the surface of the skull).
CAUTION. Ensure that no fluid is removed from beneath the portion of the microprism assembly that is resting on the surface of the brain.
xvii| Once the inside of the well is dry, apply a small amount of Vetbond to create an initial seal between the microprism assembly and the skull.
CAUTION. Vetbond has a very low viscosity and tends to clog the applicators used to apply it. Check to make sure that the applicator is not clogged to avoid excess application. Do not allow Vetbond to be applied to the top surface of the cranial window.
xviii| Absorb any excess Vetbond using a sterile cotton-tipped applicator
xix| Apply C&B Metabond over any exposed skull and the portion of the microprism assembly that is resting on the skull (being careful to prevent any obstruction of visibility of the brain surface. Allow the C&B Metabond to fully cure before continuing to OPTION C, Step xx.
xx| Turn the vacuum line off, then raise the vertical arm to lift the cannula away from the surface of the microprism assembly.
xxi| Apply black dental cement to the inside and outside of the imaging well; make sure to cover all regions of C&B Metabond for light-blocking purposes (see Equipment Setup). Ensure that additional dental cement does not rise appreciably above the cranial window margins to avoid damage to short working distance (~2 mm) imaging objectives.
xxii| Repeat PROCEDURE Steps 76 – 78 for post-operative care.
TIMING. For approximate durations of each procedure, see Fig. 1. Note that while accuracy and adherence to protocol and sterility should always be favored relative to speed (particularly when long-term brain health is paramount), surgery times will often decrease substantially with practice.
TROUBLESHOOTING
See Table 1 for troubleshooting guidance.
Table 1.
Troubleshooting table.
| Step | Problem | Possible Reason | Solution |
|---|---|---|---|
| 12 | Abnormal breathing | The body of the mouse is not properly positioned or supported | Reposition mouse by raising or lowering body and heating pad |
| 39 | Abnormal breathing | The skull has been rotated too much and the left (ipsilateral) ear bar is obstructing airflow through the trachea | Reposition the head so that the left ear bar makes contact with solid skull |
| 45 | Bottom jaw of one (or both) of the Altos clamps does not line up with the headpost | There is a height difference between the two altos clamps | Recalibrate alignment of headpost clamps (see Surgical Equipment Setup & Supplementary Fig. 2e) |
| 45 | Headpost detaches from the skull when the Altos clamps are tightened | There is a rotational difference between the two headpost clamps | Recalibrate alignment of headpost clamps (see Surgical Equipment Setup & Supplementary Fig. 2e) |
| 46 | The nose-cone shield does not cover the snout | There is a substantial degree of tilt in the headpost implant | Adjust the height, level of rotation, and/or level of elevation in the mouse anesthesia gas head |
| 52 | Drilling away the cement has resulted in bleeding of the underlying muscle | The drill bit has severed underlying muscle tissue that was not properly treated with Vetbond in Step 25 | Irrigate with sterile saline and reapply Vetbond |
| 54, 56 | Subdural bleeding occurs when thinning the skull | Overheating due to drilling for too long or at high speed without intermittent sterile saline rinse | Continue only if bleeding is minor or localized (see also Caution Statements in Step 54) |
| Skull has been thinned down too much | No solution; next time, drill slower and irrigate more frequently | ||
| 58 | Blood emerges from the site of drilling | A blood vessel was punctured | Irrigate with sterile saline until bleeding stops |
| A sinus was punctured | Irrigate with sterile saline until bleeding stops; if bleeding persists apply gel foam to sinus and continue to irrigate until bleeding stops | ||
| 58 | Dura is torn | The drill bit punctured the dura when drilling the craniotomy | Perform a durotomy only if the dura has been torn over the area to be imaged, otherwise proceed as usual; if brain or vasculature appears damaged, terminate surgery |
| 58 | Dura is torn and excessive bleeding occurs | The dura was pulled with too much tension over an area with an underlying sinus | Apply gel foam immediately followed by light irrigation of sterile saline (while the gel foam rests on the affected area). Expect fatality rates to exceed 50%. Success rates can occasionally be improved by proceeding with the cranial window implant promptly after the sinus has be torn (as the pressure from the cranial window can prevent sinusoidal blood loss). In such cases, thicker dura and/or bone growth underneath the cranial window may result, thus requiring additional subsequent procedures to improve optical clarity |
| 58 | Subdural bleeding | A cortical blood vessel has been pierced or has ruptured due to excessive pressure buildup in the brain | Perform a durotomy (OPTION B). If successful (cortical vasculature is intact edema is minimal, and bleeding is controlled), start at Step 50. Otherwise, terminate surgery |
| 58 | Bleeding occurs upon removal of the bone flap | A blood vessel has been ripped or punctured | Irrigate with sterile saline until bleeding stops; if bleeding persists, apply gel foam and continue to irrigate until bleeding stops |
| A sinus has be ripped or punctured | Apply gel foam and continue to irrigate until bleeding stops. If excessive bleeding occurs, the surgery may result in fatality | ||
| 58 | Excessive edema upon removal of the bone flap | Rough drilling | No solution; refine motor skills and be more delicate to avoid drilling past the skull in future |
| Dexamethasone was not administered early enough prior to surgery | No solution; wait at least 4 hours after administration, prior to surgery | ||
| 59 | Cortical edema occurs after performing a craniotomy | The craniotomy is too large (ie. >3 mm) and is positioned too far laterally from the midline | If possible, use a 3 mm cranial window |
| 66 | The cranial window does not fit into the craniotomy | The craniotomy is too small | Remove the cranial window and try to expand the craniotomy using a micro probe (angled 45°). Expect lower success rates |
| Excessive amount of optical glue was used during fabrication and has resulted in overflow–making the cranial window too large to fit | This can be avoided by evaluating the quality and size of cranial windows prior to surgical use | ||
| 67 | The cranial window breaks | Too much pressure was applied to the window by the fashioned cotton-tipped applicator | Terminate surgery, as the underlying brain will likely be damaged |
| The skull margins surrounding the craniotomy were not thinned consistently or sufficiently | No solution; terminate surgery. Next time, allow the skull to soak in saline and probe for pliability around the skull margins | ||
| 67 | The different layers of the cranial window become separated | The cranial window was not properly glued together, likely due to too little optical glue | Evaluate cranial windows under the surgical scope prior to use. Lack of optical glue will result in air spaces or bubbles between the coverglasses. In this case, or if overflow of excessive optical glue is observed, discard window |
| 69 | Fluid wicking leads to air space in one or more portions of the interface between cranial window and brain surface | Inadequate pressure applied to the brain surface prior to wicking | Slightly reduce the vertical pressure being applied by the micromanipulator and add more sterile saline to fill any air spaces between the brain and the cranial window. Reapply pressure, and remove saline until the bone is dry, but avoid removal from beneath the portion of the cranial window resting on the skull |
| The skull margins surrounding the craniotomy were not thinned consistently or sufficiently and therefore are preventing both adequate pressure and uniform contact between the brain and the cranial window | No solution; this will likely result in thickening of the dura and/or bone regrowth. It is likely that a follow up surgery will be required to fix this problem | ||
| 70 | Vetbond overflows and attaches the cotton-tipped applicator to the cranial window | Excessive pressure was applied through the spout of the Vetbond applicator | See ‘CAUTION’ in PROCEDURE Step 71 |
| 70 | Vetbond comes in contact with the brain surface | Not enough pressure was applied to applicator | No solution, terminate surgery |
| Bone has not been thinned uniformly and/or sufficiently | No solution, terminate surgery | ||
| OPTION A, Step vii | The center portion of the cranial window (the part resting on the brain) has become loose (i.e. is not snug within the craniotomy) | Irrigation must be performed to remove any debris remaining in the headpost well prior to removal of the plug | |
| OPTION A, Step vii | Bleeding has resulted underneath the center portion of the cranial window (the part resting on the brain) | Drilling away the top layer of the cranial window resting on the skull has aggravated underlying vasculature, resulting in a bleed | If bleed is minimal and does not obstruct viewing of the surgical site, continue to drill off the top layer and complete thinning the surrounding skull; if not, terminate surgery |
| Excessive force on applicator caused the window to buckle | No solution, terminate surgery | ||
| OPTION B, Step iv | Cortical vascular is removed from the brain upon removal of the dura | If this occurs on the same day that the craniotomy has been performed, then it is likely to be a result of human error | No solution; refine motor skills and be more delicate. Terminate surgery |
| If this occurs on a separate day after the brain has already been exposed via craniotomy, then it is likely that the first craniotomy has resulted in cortical scarring and adhesion to the dura | No solution; terminate surgery | ||
| OPTION B, Step iv | A sinus is punctured while performing the durotomy | The dura was pulled with too much tension over an area with an underlying sinus | Try to control any bleeding by applying gel foam, then lightly irrigate with sterile saline as the gel foam rests over the punctured sinus. If bleeding persists, terminate surgery |
| OPTION C, Step xii | The microprism assembly doesn't line up with the incision | Human error; line of incision in brain or positioning of microprism on cranial window was imprecise | No solution; terminate surgery |
| General problem related to intracerebral injections | An air bubble is observed inside the micropipette | Human error; the Hamilton syringe was not loaded correctly | Re-load injection syringe |
ANTICIPATED RESULTS
The ability to separate these procedures into multiple, distinct surgeries over time helps ensure optimal cranial window optical clarity, high success rates, and increased accuracy of functionally targeted injections. With sufficient practice, a careful surgeon can achieve success rates upwards of 80-90%. Similarly high success rates can be achieved for window removal/replacement and associated procedures, including functionally targeted focal cortical injections and/or insertion of a chronic microprism. Following these procedures, we consistently found neural response properties in mouse primary visual cortex to be in the normal range (see refs. 12-15 for details). Below we describe some typical applications that outline the current and future value of these methods, and factors to consider when interpreting results.
Figure 9 illustrates the window implant and removal procedures described above, in an application to targeted two-photon calcium imaging of long-range axonal boutons. Following initial window implant, we use widefield intrinsic autofluorescence imaging in awake mice to identify visual cortical areas, as illustrated in Fig. 9a-b (65 days after initial implant; adapted from ref. 15). We then remove the cranial window for targeted AAV-GCaMP3 calcium expression to within a cortical area (67 days after initial implant). We verified that GCaMP3 expression was restricted within a single visual cortical area (12 days after AAV-GCaMP3 injection) by confirming that widefield GCaMP3 imaging of visually-evoked responses did not demonstrate mirror reversals of the retinotopic map (Fig. 9c-d). Subsequently, two-photon calcium imaging was performed from long-range axonal boutons of the infected neurons, imaged in a downstream cortical area (Fig. 9e-g; 28 days after AAV-GCaMP3 injection). The clarity of the window is important for maintaining the ability to visualize individual axonal boutons in vivo (see also ref. 20). A similar workflow was used for imaging of cell bodies in other experiments (e.g. refs. 13, 15), including cell bodies as deep as cortical layer 515
Figure 9. Targeted GCaMP3 expression and subcellular imaging in awake mice.
(a) To target injections and find boundaries in the weeks or months after initial window implant, we first obtained images of changes in intrinsic autofluorescence in response to visual stimuli placed at three different positions within the visual field (darker region = stronger visual response; Upper scalebar inset: percent change in fluorescence). For this mouse, mapping occurred 65 days after initial window implant and 2 days prior to AAV-GCaMP3 injection and window replacement. Colored circles mark the center of the activated region in each visual cortical area: V1 (purple), LM (green), AL (red), RL (pink), AM (dark blue), and PM (light blue). Lower insets: relative position of three visual stimuli (40° diameter, square-wave drifting grating). Note that as the visual stimulus moves towards lateral visual field (bottom panel), the regions of activation become further apart. (b) Raw fluorescence image from the experiment in a. Circles are from positions marked in a: 1st and 3rd positions are closed circles, 2nd position is an open circle. Arrows indicate direction of retinotopic organization from nasal to temporal visual space. (c) Raw fluorescence image from same mouse as in a, 15 days after window removal and injection of AAV-GCaMP3 targeted to within area V1. Locations of each brain area are labeled using vasculature landmarks from b. Scale bars in a-c: 500 μm. (d) Pixel-based map of the stimulus location that evoked the strongest GCaMP3 response (see inset for position color code; 25° diameter, square-wave drifting grating). Map brightness is scaled to maximum evoked response strength. Note the lack of reversals of retinotopy suggesting that the injection was contained entirely within V1. Same imaging session as in c. (e) Left, in vivo image of visual cortex. V1 was covered to prevent saturation and inset was taken with lower illumination. Middle and right, average change in fluorescence (ΔF/F; 24 trials) of V1 axons arriving in area PM, in response to two different visual stimuli. Imaging depth: 90 μm. Inset: schematic of visual stimuli. Scale bar: 500 and 50 μm. (f) Left, maximum projection of all single condition responses. Middle, expansion of maximum projection. Right, identification of significantly responsive boutons. Scale bar: 50 and 15 μm. (g) Average visual response timecourses of the colored boutons from f. Shaded regions are ± SEM; blue lines represent duration of stimulus (5 s; 24 repetitions per stimulus type). Data in e-g were obtained 28 days after AAV-GCaMP3 injection and window replacement. Adapted from ref. 14. Experiments were approved by the IACUC at HMS.
The above example highlights the utility of sustained window clarity both prior to and following removal/replacement of the cranial window. Fig. 2d-i provides a second example of sustained window clarity at 4 months after initial window implant, as well as 2 months after window subsequent window removal/replacement. Two additional examples of five-month timelines of window clarity and GCaMP3 expression, from initial implant through window removal/replacement and AAV-GCaMP3 injection, are shown in Fig. 10.
Figure 10. Long-term clarity of cranial windows and window replacements.
Left and right pairs of columns illustrate timeline (gray vertical line), in days following initial window implant, through cranial window removal and AAV expression of GCaMP6, in two example adult mice. Green dots: sites of 100 nl injection of AAV.syn.GCaMP6f.WPRE.SV40. Top row: brightfield images prior to (left) and following (right) cranial window removal and AAV-GCaMP6 injection. Both surgeries occurred 34 days after initial window implant. Bottom three rows: brightfield (left) and blue-green epifluorescence (right; all exposure times: 0.5 s) images illustrate window clarity and emergence of GCaMP6 expression across months. Scalebar: 1 mm. Experiments were approved by the IACUC at BIDMC.
The protocols described above also facilitate targeted insertion of a microprism into cortex for long-term functional imaging. We could routinely record the same large set of cell bodies across all cortical layers across days and weeks in awake mice using calcium imaging through a microprism14 (Fig. 11a). This method allows both epifluorescence and two-photon calcium imaging across all 6 cortical layers using a standard two-photon microscope (Fig. 11b). When imaging in primary visual cortex, neurons in all layers were driven by visual stimuli, allowing generation of visual response tuning maps throughout a cortical column (Fig. 11c; 21 days after microprism insertion). Visual response tuning of the same neurons imaged through the prism was stable across days (Fig. 11d; 21 and 23 days after prism implant).
Figure 11. Chronic functional imaging across cortical layers using a microprism.
Illustration of laser beam path through a prism implanted into neocortex. The reflective hypotenuse of the prism converts the horizontal imaging plane into a vertical plane. (b) Left: Widefield epifluorescence image of a 1 mm prism implanted in visual cortex, focused 1 mm below the cortical surface. Fluorescent expression of AAV-GCaMP3 can be seen through the cranial window (green arrow), and through the prism. Right: Two-photon image taken through the same prism (maximum intensity projection across 15 minutes of recording) at 205 μm lateral to the microprism imaging face, 21 days after prism implant. In this same mouse, cellular responses to visual stimuli varying in location, orientation, and temporal and spatial frequency were then imaged simultaneously across all cortical layers of mouse area V1 through a microprism. Scale, 1 mm (left) and 100 μm (right). (c) Maximum intensity projection of fractional change in GCaMP3 fluorescence (ΔF/F), computed across a stack of average single-condition visual response maps for each of 96 stimulus conditions. Data were collected 23 days after prism implant, 180 μm from the prism face. Visually responsive neurons (white spots) were evident in all cortical layers. Scale, 100 μm. (d) Left: These data were used to generate orientation preference maps across cortical layers. Right: Neurons across all layers (numbered in blue in c) showed sharp orientation tuning that was consistent at days 21 and 23 following prism implant. Orientation tuning curves are averaged across stimulus locations, spatial and temporal frequencies, and normalized by peak response. Colored lines and colored inset in polar plots indicate estimates of orientation preference. These five neurons had strong responses in both sessions, exceeding 30% ΔF/F for the preferred orientation. (e) Chronic microprism imaging also enabled functional imaging of long-range axonal boutons in deeper cortical layers. To image long-range cortico-cortical axonal arbors in awake mice through a prism (see ref. 15), AAV-GCaMP3 was injected into area V1 and a microprism was subsequently inserted at the site of V1 innervation of posteromedial secondary visual area PM (cf. Fig. 9e). Robust visually evoked activity was observed in V1 axonal boutons innervating deeper layers of area PM (480-510 μm below the cortical surface; 1 day post-implant). (f) Left: Maximum-intensity projection of average single-condition response maps to upwards drifting stimuli varying in spatial and temporal frequency. Active boutons appear as white spots. Scale, 20 μm. Right: Time courses of three individual active boutons (see arrows in left panel) demonstrated robust single-trial responses to visual stimulation (gray bars), no fluorescence cross-talk, and little photobleaching (not shown). These data were adapted from ref. 15. Experiments were approved by the IACUC at HMS.
Because in vivo functional imaging of individual axons and synaptic boutons has been restricted to superficial depths in cortex15,20 (~0-150 μm deep), we previously asked whether use of a microprism could enable monitoring of long-range axonal activity deep within the cortex (Fig. 11e). As in Fig. 9, we made a small injection of AAV-GCaMP3 into area V1, and inserted the prism into the posteromedial secondary visual cortical area (PM), an area densely innervated by V1 axons, with the prism oriented to face area V1. We observed robust single-trial visual responses of individual boutons at depths of 480-510 mm below the cortical surface (putative layer 5) during presentation of stimuli at multiple temporal frequencies and spatial frequencies at 1 day post-implant (Fig. 11f). Thus, this method extends in vivo functional imaging of axonal arbors15,20 to arbors that innervate deeper cortical layers. Further, the study of long-range projection axons via a microprism represents a less invasive application of this method with fewer caveats than for imaging of cell bodies near the prism face: while damage to long-range axonal boutons near the prism face cannot be ruled out (see below), these boutons report the activity of neurons whose dendrites are safely located millimeters from the prism implant.
In future, chronic two-photon imaging through a microprism may also be combined with voltage-sensitive indicators to monitor the flow of information through a cortical column in real-time. As discussed in detail elsewhere14, the chronic insertion of a 1 mm microprism into cortex clearly is accompanied by damage to local neural processes and to the neurovascular unit. Care must be taken in interpreting neural data and validating results against known benchmarks14. Vascular remodeling immediately adjacent to the prism face can also occur over the course of weeks (see Supplementary Fig. 2 in ref. 14), and should be considered if studying neurovascular processes. Our protocol is designed to minimize cortical damage, in part by performing the procedure after window removal (i.e. with significantly less baseline cortical edema than when performed together with a craniotomy), by carefully limiting the extent of incision prior to prism insertion, and by positioning the prism and incision to avoid major blood vessels. Nevertheless, lasting changes in glial cells were limited to <100 μm from the prism face (see Fig. 1 in ref. 14), and visual response properties of the same neurons imaged prior to and several days following prism insertion remained largely unchanged (see Fig. 2 in ref. 14).
It is important to note that, following both standard craniotomies and prism insertions, some surgeries can result in window implants with residual patches of blood above the brain (e.g. Fig. 7c; blood patches may also accrue at the prism imaging face). In these cases (but not in the case of intracortical bruising due to excessive drilling), clarity will typically improve substantially as blood is absorbed over the span of days to weeks (see e.g. Supplementary Fig. 2 in ref. 14), enabling high-resolution two-photon imaging. Despite the fact that our cranial window is composed of multiple coverslips, subcellular spatial resolution is routinely achieved, as demonstrated by our ability to image neural activity of individual putative long-range axonal boutons in superficial cortical layers15 (Fig. 9) and in deeper layers via a microprism14 (Fig. 11).
Concluding remarks
We have outlined a detailed set of versatile protocols that employ removable chronic cranial window implants for imaging in posterior cortex of awake, behaving mice. In the future, these methods can likely be modified for imaging in more lateral (cf. Figs. 2d-i) or anterior cortical regions. In addition, temporary replacement with a cranial window with a 120 μm – 1000 μm hole at a targeted location should enable pipette access for stable two-photon imaging of a nearby, pressurized cortical area during cell-attached recordings (A. Kerlin and S. Chatterjee, unpublished data), and application and washout of various drugs during simultaneous imaging in awake mouse cortex (G. Goldey and M. Andermann, unpublished data). We look forward to working with other groups to further develop and standardize surgical procedures for use in mice as well as other model systems, such as rats, carnivores and non-human primates. Such collaboration is a key step towards effectively assessing results across a growing number of laboratories using chronic imaging to understand cortical structure, function, and plasticity.
Supplementary Material
Online summary.
This protocol describes how to implant a removable cranial window enabling manipulations such as targeted virus injections and microprism insertion, for one- and two-photon calcium imaging of the same cortical brain regions for several months.
ACKNOWLEDGEMENTS
The authors would like to thank W.-C. Lee, M. Histed, D. Smith, K. Kuchibhotla and M. Henry for assistance developing the initial window implant and headpost design, A. Vagodny, J. Curry, C. Holland, K. Levandowski, and D. Woloszyn for technical assistance, and J. Maunsell for sharing his surgical rig during initial stages of development of these procedures. M. Kirk performed the window implant in Fig. 9. We also thank members of the Reid and Andermann labs for useful discussions. We thank Loren L. Looger, Janelia Farm Research Campus, Howard Hughes Medical Institute for provision of AAV-GCaMP321 via the U. Penn. Vector Core. We thank Vivek Jayaraman, Ph.D., Rex A. Kerr, Ph.D., Douglas S. Kim, Ph.D., Loren L. Looger, Ph.D., and Karel Svoboda, Ph.D. from the GENIE Project, Janelia Farm Research Campus, Howard Hughes Medical Institute, for provision of AAV-GCaMP622 via the U. Penn. Vector Core. The writing of this protocol was supported by grants from the Smith Family Foundation, the Pew Scholars Program in the Biomedical Sciences, the Klarman Family Foundation, the Boston Nutrition and Obesity Research Center, the Association for Aging Research New Investigator Award in Alzheimer's Disease, and the Harvard-MIT Joint Research Grants Program (M.A.).
Footnotes
AUTHOR CONTRIBUTIONS
The procedures were developed by G.G., D.R., M.A. and A.K., and refined by L.G. and V.B., together with assistance from R.C.R. The figures, schematics were done by G.G. and M.A. The associated surgeries were done by G.G. (except for the surgery in Fig. 9). Analyses of glial activation were carried out by D.S. Lastly, G.G. and M.A. wrote the manuscript with assistance from D.R., A.K., L.G., V.B. and R.C.R.
COMPETING FINANCIAL INTERESTS
The authors declare that they have no competing financial interests.
REFERENCES
- 1.Yang G, Pan F, Parkhurst CN, Grutzendler J, Gan W-B. Thinned-skull cranial window technique for long-term imaging of the cortex in live mice. Nat. Protoc. 2010;5:201–208. doi: 10.1038/nprot.2009.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marker DF, Tremblay M-E, Lu S-M, Majewska AK, Gelbard HA. A Thin-skull Window Technique for Chronic Two-photon Imaging of Murine Microglia in Models of Neuroinflammation. J. Vis. Exp. 2010 doi: 10.3791/2059. doi:10.3791/2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shih AY, Mateo C, Drew PJ, Tsai PS, Kleinfeld D. A Polished and Reinforced Thinned-skull Window for Long-term Imaging of the Mouse Brain. J. Vis. Exp. 2012 doi: 10.3791/3742. doi:10.3791/3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelly EA, Majewska AK. Chronic Imaging of Mouse Visual Cortex Using a Thinned- skull Preparation. J. Vis. Exp. 2010 doi: 10.3791/2060. doi:10.3791/2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dombeck DA, Khabbaz AN, Collman F, Adelman TL, Tank DW. Imaging Large- Scale Neural Activity with Cellular Resolution in Awake, Mobile Mice. Neuron. 2007;56:43–57. doi: 10.1016/j.neuron.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holtmaat A, et al. Long-term, high-resolution imaging in the mouse neocortex through a chronic cranial window. Nat. Protoc. 2009;4:1128–1144. doi: 10.1038/nprot.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao VY, Ye Y, Mastwal SS, Lovinger DM, Costa RM, Wang KH. In vivo two- photon imaging of experience-dependent molecular changes In cortical ceurons. J. Vis. Exp. 2013;(71):e50148. doi: 10.3791/50148. doi:10.3791/50148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mostany R, Portera-Cailliau C. A Craniotomy Surgery Procedure for Chronic Brain Imaging. J. Vis. Exp. 2008 doi: 10.3791/680. doi:10.3791/680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu H-T, Pan F, Yang G, Gan W-B. Choice of cranial window type for in vivo imaging affects dendritic spine turnover in the cortex. Nat. Neurosci. 2007;10:549–551. doi: 10.1038/nn1883. [DOI] [PubMed] [Google Scholar]
- 10.Kerlin AM, Andermann ML, Berezovskii VK, Reid RC. Broadly tuned response properties of diverse inhibitory neuron subtypes in mouse visual cortex. Neuron. 2010;67:858–871. doi: 10.1016/j.neuron.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonin V, Histed MH, Yurgenson S, Reid RC. Local diversity and fine-scale organization of receptive fields in mouse visual cortex. J. Neurosci. 2011;31:18506–18521. doi: 10.1523/JNEUROSCI.2974-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andermann ML, Kerlin AM, Reid RC. Chronic cellular imaging of mouse visual cortex during operant behavior and passive viewing. Front. Cell. Neurosci. 2010;4 doi: 10.3389/fncel.2010.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andermann ML, Kerlin AM, Roumis DK, Glickfeld LL, Reid RC. Functional specialization of mouse higher visual cortical areas. Neuron. 2011;72:1025–1039. doi: 10.1016/j.neuron.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andermann ML, Gilfoy N, Goldey GG, Sachdev R, Wölfel M, McCormick DA, Reid RC, Levene MJ. Chronic cellular imaging of entire cortical columns in awake mice using microprisms. Neuron. 2013;80:900–913. doi: 10.1016/j.neuron.2013.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glickfeld LL, Andermann ML, Bonin V, Reid RC. Cortico-cortical projections in mouse visual cortex are functionally target specific. Nat. Neurosci. 2013;16:219–226. doi: 10.1038/nn.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.William CM, Andermann ML, Goldey GG, et al. Synaptic plasticity defect following visual deprivation in alzheimer's disease model transgenic mice. J. Neurosci. 2012;32:8004–8011. doi: 10.1523/JNEUROSCI.5369-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arieli A, Grinvald A, Slovin H. Dural substitute for long-term imaging of cortical activity in behaving monkeys and its clinical implications. J. Neurosci. Methods. 2002;114:119–133. doi: 10.1016/s0165-0270(01)00507-6. [DOI] [PubMed] [Google Scholar]
- 18.Breitbard A, Ablaza V. Chapter 7: Implant Materials. In: Thorne C, editor. Grabb and Smith's Plastic Surgery. 6th edition Williams and Wilkins, Publishers; Lippincott: 2007. [Google Scholar]
- 19.Manivasagam G, Dhinasekaran D, Rajamanickam A. Biomedical Implants: Corrosion and its Prevention - A Review. Recent Patents on Corrosion Science. 2010;2:40–54. [Google Scholar]
- 20.Petreanu L, Gutnisky DA, Huber D, Xu NL, O'Connor DH, Tian L, Looger L, Svoboda K. Activity in motor-sensory projections reveals distributed coding in somatosensation. Nature. 2012;489:299–303. doi: 10.1038/nature11321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tian L, et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nature Methods. 2009;6:875–881. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen T-W, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.