Abstract
Alzheimer’s disease (AD) is defined by presence of two pathological hallmarks, the intraneuronal neurofibrillary tangle (NFT) formed by abnormally processed tau, and the extracellular amyloid plaques formed primarily by the amyloid beta peptide (Aβ). In AD it is likely that these two proteins act in concert to impair neuronal function, and there is evidence to suggest that one of the key targets on which they converge is the mitochondria. For example, overexpression of a pathologic form of tau in rat primary cortical neurons exacerbates Aβ-induced mitochondrial membrane potential (ΔΨm) loss due to impairment of the calcium (Ca2+) buffering capability of mitochondria. However the role of physiological levels of tau in mediating Aβ-induced mitochondrial dysfunction was not examined. Therefore in this present study we used primary neurons from wild type (WT) and tau knockout (tau−/−) mice to investigate whether endogenous tau facilitates Aβ–induced ΔΨm loss and alterations in cytosolic calcium (Ca2+cyt). Knocking out tau significantly protected mouse primary cortical neurons from loss of ΔΨm caused by low concentrations of Aβ42, which supports our previous findings. However, the absence of tau resulted in significantly greater increases in Ca2+cyt in response to Aβ treatment when compared to those observed in WT mouse primary cortical neurons. This unexpected outcome may be explained by findings that suggest tau−/− neurons display certain phenotypic abnormalities associated with alterations in Ca2+cyt. Overall, data indicate that tau facilitates Aβ-induced mitochondrial dysfunction and this effect is independent of Aβ-induced alterations in Ca2+cyt.1
Keywords: Alzheimer’s disease, tau, amyloid beta, mitochondrial dysfunction, calcium dyshomeostasis
Introduction
Tau and Aβ are both essential elements in the pathogenesis of AD. Initially Aβ was considered to be the primary effector of AD, with tau playing a minor secondary role [14]. However, it is now clear that tau is an essential player in the progression of AD that can have effects independent of, parallel to, or in concert with Aβ [34]. This first became apparent when it was demonstrated that tau−/− mouse primary neurons were protected from Aβ-induced neurotoxicity compared to neurons derived from WT mice [28]. There are now numerous studies indicating that tau and Aβ act in concert to elicit damage to neurons [6, 7, 25, 29]. Further, studies in mouse models have shown that reduction or absence of endogenous murine tau attenuates spatial memory deficits in mice overexpressing a familial AD form of human amyloid precursor protein (hAPP), as well as preventing excitotoxin induced epileptic activity on a hAPP or non-transgenic background [31]. These studies indicate that expression of tau is required for Aβ to elicit certain negative effects in neurons supporting the idea that tau and Aβ work cooperatively. Evidence for this has also been shown in humans. In a longitudinal study, cerebral spinal fluid (CSF) from cognitively intact elderly adults was analyzed for a pathological form of tau phosphorylated at threonine 181 (ptau-181) and Aβ42 upon entry into the study. Participants who tested positive for Aβ42 were more likely to experience cognitive decline over the course of the study, but only if ptau-181 was also present in their CSF [6].
The conceptual framework for tau and Aβ acting cooperatively to cause neuronal dysfunction originated with a study that compared patterns of protein deregulation in mouse models that develop neurofibrillary tangles of tau and/or plaques of Aβ. Rhein, et al. [29], conducted a proteomic analysis of vesicular preparations from non-transgenic mice, mice carrying familial AD mutations in APP and presenilin 2 (PS2) that develop Aβ pathology (APPswPS2N141I ), pR5 mice that express tau with the P301L frontotemporal dementia with parkinsonism (FTDP) mutation causing tangle formation, and triple transgenic (3xTgAD) mice that carry genes for mutant APP, mutant PS1 and P301L tau that exhibit both Aβ and tau pathology. One third of the deregulated proteins in these mice were mitochondrial proteins involved in oxidative phosphorylation (OxPHOS). Mice expressing mutant tau showed deregulation of OxPHOS complex I proteins , while mice expressing genes with Aβ-forming mutations showed deregulation of complex IV proteins. The 3xTgAD mice showed deregulation of proteins related to both complex I and IV and had the earliest and worst mitochondrial dysfunction compared to mice exhibiting either Aβ or tau pathology alone. Therefore, tau and Aβ can damage mitochondria separately, but are able to cooperate when both are present and enhance the pathological defects that they each cause [8, 29].
The role of tau and Aβ in mitochondrial dysfunction is particularly interesting due to evidence suggesting mitochondrial damage occurs early in AD and is central to its etiology [3, 11, 35]. Evidence of impaired tricarboxylic acid (TCA) cycle protein activity and deregulation of mitochondrial genes encoding OxPHOS proteins has been shown in postmortem brains of AD patients, which likely compromised metabolism [1, 19]. Female 3xTgAD mice that develop both Aβ and tau pathologies mimick these disease characteristics very early. At 3 months of age, these mice display decreased mitochondrial respiration and protein levels of pyruvate dehydrogenase, a key TCA cycle protein [37]. Thy-1 APPSW/L mice also show decreased ATP production, ΔΨm, and OxPHOS complex IV activity, with increased reactive oxygen species (ROS) production at 3 months of age. This coincides with detection of intracellular Aβ, but occurs several months prior to extracellular Aβ deposition suggesting mitochondrial deficits precede Aβ pathology and are a key pathogenic element in AD [15].
Calcium deregulation also contributes to AD etiology and has been associated with mitochondrial dysfunction [2, 30, 32]. Cybrids produced by transforming AD patients’ platelet mitochondria into SH-SY5Y human neuroblastoma cells show increased basal Ca2+cyt, and inositol triphosphate (IP3)-induced Ca2+ release from the endoplasmic reticulum (ER), compared with cybrids from healthy controls [33]. There is also evidence that total cellular Ca2+ levels are elevated in cultured fibroblasts from AD patients as compared to aged and young controls [23]. Elevated Ca2+cyt is known to induce rapid Ca2+ uptake by mitochondria in microdomains of high Ca2+cyt that exist near Ca2+ channels on the plasma membrane and ryanodine receptors on the endoplasmic reticulum (ER) [22, 30]. The resultant mitochondrial Ca2+ (Ca2+mito) overload can cause depolarization of ΔΨm, increased ROS production, opening of the mitochondrial permeability transition pore (mPTP), and mitochondrial swelling - especially in synaptic mitochondria, which are more likely to exist in high Ca2+cyt microdomains [13, 38]. Furthermore, a recent study found Aβ42 oligomers cause Ca2+ influx into primary rat neurons from cortex, hippocampus, and cerebellum, which leads to Ca2+mito overload, mPTP opening, and cell death [32]. In contrast, Ma et. al., argue that mitochondrial malfunction underlies Ca2+ dyshomeostasis as dissipation of ΔΨm and mPTP opening in N2A cells stably expressing hAPP695 leads to impairment of store-operated Ca2+ entry [18]. It is unclear whether mitochondrial dysfunction precedes Ca2+cyt disruption or vice versa.
Recently we showed that treatment of primary neurons with sub-lethal concentrations of Aβ40 fibrils caused ΔΨm in the presence of endogenous tau. Overexpression of a pathological form of tau truncated at D421 also caused mitochondrial dysfunction on its own and exacerbated mitochondrial dysfunction induced by Aβ40 fibrils in rat primary neurons and in a cortical neuronal cell line [25, 26]. We also showed that tau phosphorylated at Ser396/404 (PHF-1 epitope) enhances Aβ-induced mitochondrial injury [27]. This suggested that tau and Aβ act in cooperation to cause mitochondrial damage. However, these previous studies in primary neurons were carried out using exogenously expressed tau. To more clearly define the role of tau mediating the effect of Aβ on mitochondrial function in this current study we examined the effects of Aβ on ΔΨm in neurons from tau−/− mice. As expected, we found that neurons from tau−/− mice were resistant to Aβ42-induced ΔΨm loss compared to neurons from WT mice. However we unexpectedly found that tau−/− neurons showed a more pronounced Ca2+cyt elevation in response to Aβ42, suggesting that tau facilitates Aβ induced ΔΨm loss in a manner that is independent from Ca2+cyt dyshomeostasis. The fact that Aβ42 induced a greater increase in Ca2+cyt in tau−/− neurons may indicate that the lack of tau causes neuronal functional changes that are not universally beneficial, a factor that needs to be considered when using these mice to understand AD pathogenesis [4, 5].
Materials and Methods
Aβ Oligomer Formation
Synthetic human Aβ42 peptide was obtained from Calbiochem (PP69, NJ, USA) in 250µg aliquots. Entire aliquots were reconstituted to 250µM in sterile PBS and incubated at 37°C without agitation for 3 days. The presence of fibrils and oligomers was confirmed via electron microscopy. Working solutions of 0.5µM Aβ42 were diluted from the 250µM stock in sterile PBS.
Animals
Animals were housed and euthanized in accordance with guidelines of the University of Rochester committee on animal resources. Wild type C57Bl/6 (000664) and tau−/− (B6.129X1-Mapttm1Hnd/J, 007251) mice used in this study were obtained from Jackson Laboratories. Tau−/− mice were originally made and characterized by Dawson et al. [5]. These mice carry a homozygous deletion of the Mapt gene and have been backcrossed at least ten times onto on a C57BL/6 background.
Western Blotting
Lysates were prepared from WT and tau−/− mouse cerebral cortex, protein concentrations determined and 15µg of protein were separated by electrophoresis and immunoblotted with a polyclonal antibody to tau (Dako, CA, USA) and a monoclonal antibody to mouse β-actin (Millipore, MA, USA).
Mouse Primary Cortical Neuron Culture
Multi-well tissue culture plates containing 15mm #1 thickness glass coverslips were coated with 0.2% polyethylenimine (PEI) in borate buffer (pH 8.5) at room temperature in the dark for 6 hours. Plates were then rinsed with sterile distilled water and dried overnight in the dark. Mouse primary cortical neurons were isolated from C57Bl/6 or tau−/− mouse embryos at embryonic day (E18). Pregnant dams were euthanized with CO2 followed by decapitation and embryos were extracted immediately. Cortices were aseptically dissected from embryos and were chemically dissociated in pre-warmed 0.25% trypsin/EDTA for 20 minutes at room temperature. Cortices were rinsed with warm DMEM before mechanical dissociation via trituration in neurobasal media supplemented with 0.5mM L-glutamine, 25uM glutamate and B27 supplement. Neurons were then plated at a density of 8x105 cells/mL and were maintained at 37°C in a 5% CO2 atmosphere. Every 4 days, the neurons received a half volume media change.
Mitochondrial Membrane Potential and Cytosolic Calcium Imaging
Between DIV7 and DIV9, neurons were loaded with 100nM Mitotracker Red CMXRos (M-7521, Life Technologies, NY, USA) and 1µM Fluo4 AM (F-14201, Life Technologies, NY, USA) in KREBS ringer buffer (136mM NaCl, 10mM HEPES, 4.7mM KCl, 1.25 mM MgSO4, 1.25mM CaCl2, and 25mM glucose, pH 7.4) for 30 min at 37°C to track ΔΨm and Ca2+cyt, respectively. After dye loading, neurons were rinsed with KREBS ringer buffer and imaged on a heated stage with a 40x oil immersion objective on a Zeiss Axio Observer D1 inverted fluorescent microscope. Using AxioVision software, time-lapse images were collected once a minute for thirty minutes at 595nm (Mitotracker Red CMXRos) and 488nm (fluo4 AM). Fluorescent excitation was attenuated to 25% with a Colibri light-emitting diode system. Aβ42 was added at 5 min, after a stable baseline was established. Data is presented as an average change in fluorescence (ΔF) from the baseline reading and total difference in ΔF between the first image and the last.
Statistical Analysis
A linear mixed model generalized estimating equation was used to study the effects of Aβ42 concentration and time on ΔΨm and Ca2+cyt outcomes by comparing the slopes of the full time-lapse traces. Non-parametric ANOVA was used to compare the endpoints (mean change from baseline at the thirty-minute time point) between genotypes and Aβ42 concentrations for ΔΨm and Ca2+cyt experiments. Non-parametric tests were used in this case due to the non-normal data distribution.
Results
Tau−/− cortical neurons are protected from Aβ42-induced ΔΨm loss
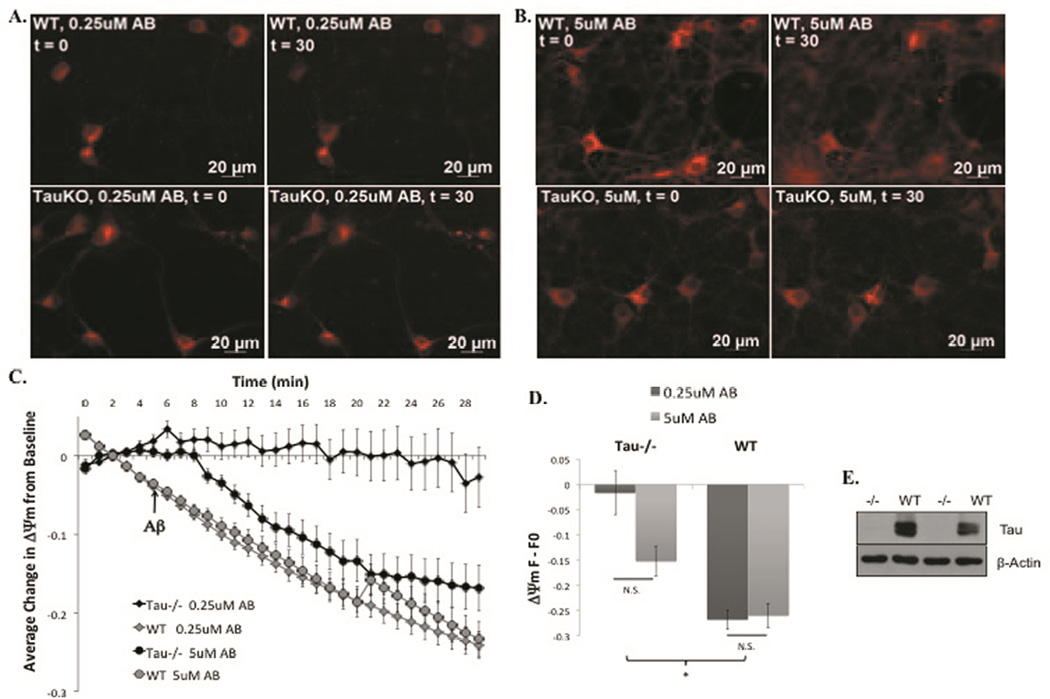
Time-lapse imaging of tau−/− and WT mouse primary cortical neurons was performed using the ΔΨm indicator MitoTracker Red CMXRos. WT primary cortical neurons showed large, decreases in ΔΨm in response to acute treatment with both 0.25µM (Fig 1, A, top) and 5µM Aβ42 (Fig 1, B, top). In contrast, tau−/− neurons maintained baseline ΔΨm in response to 0.25µM Aβ42 (Fig 1 A, bottom) and exhibited ΔΨm loss in response to 5µM Aβ42 that was significantly less severe than that of WT (Fig 1, B, bottom) according to linear mixed model statistical analysis comparing the slopes of the quantified 30-minute time-lapse experiments (Fig 1, C). Quantitation of the total change in ΔΨm between baseline and the last imaging time point also showed that tau−/− neurons were significantly protected at both concentrations of Aβ42 compared to WT mouse neurons (Fig 1 D). We also confirmed the absence of tau expression in the tau−/− model (Fig 1 E). These data suggest that tau plays a role in facilitating mitochondrial damage induced by low levels of Aβ42. However, sufficiently high concentrations of Aβ42 overcome the necessity of tau’s facilitation.
Figure 1. Tau−/− cortical neurons are protected from ΔΨm loss induced by low concentrations of Aβ42.
Representative images from t=0 (left) and t=30 (right) of WT (top) and tau−/− (bottom) primary cortical neurons loaded with ΔΨm indicator, MitoRed, and treated with 0.25µM (A) or 5µM Aβ42 (B). Quantification of full time-lapse experiments (C) and the change from t=0 to t=30 (D). Ablation of tau was confirmed via western blot analysis of cortical lysates from tau−/− and WT mouse brains using a tau polyclonal antibody and β-actin monoclonal antibody (E). *=P<0.05
Tau−/− cortical neurons are more susceptible to Aβ42-induced Ca2+cyt increases than WT neurons
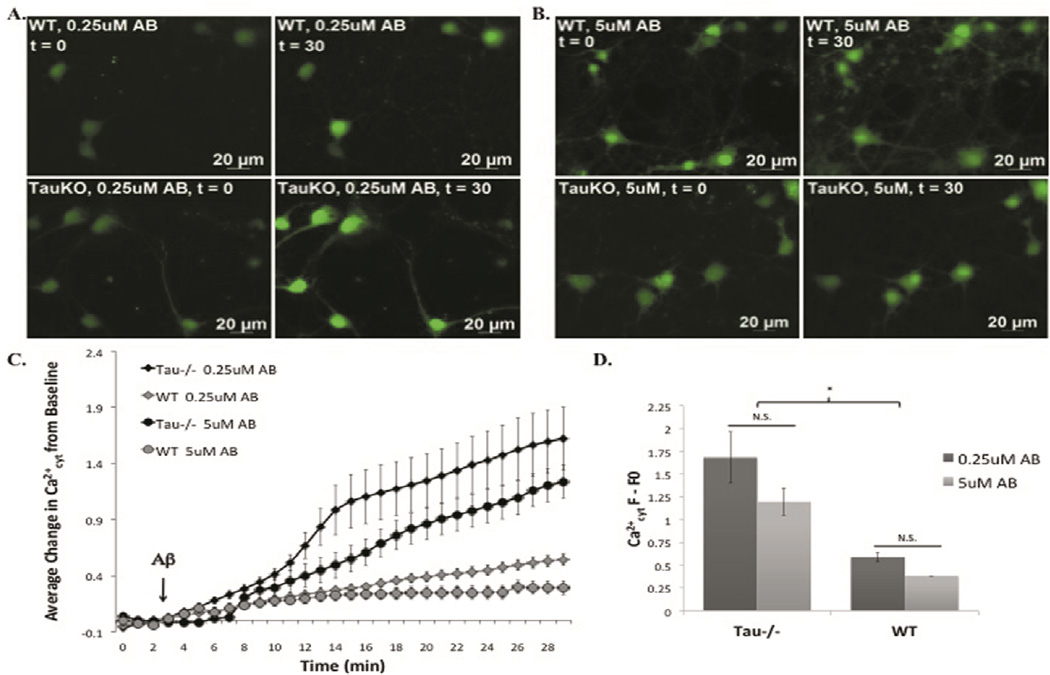
Tau−/− and WT primary cortical neurons were loaded with the Ca2+cyt indicator, Fluo4, prior to treatment with a range of Aβ42 concentrations during time-lapse imaging. Contrary to our expectations, tau−/− neurons were more susceptible to Aβ42-induced Ca2+cyt increase than WT neurons (Fig 2). Comparison of the slope of time-lapse traces revealed that both 0.25µM and 5µM Aβ42 induced a greater increase in Ca2+cyt in tau−/− neurons than in WT neurons (Fig 2, C). In addition, the total change in Ca2+cyt over the entire experiment (comparing fluo4 fluorescence at baseline to that at the last time point) was also significantly higher in tau−/− neurons than in WT, but no significant difference was found between Aβ concentrations within genotypes suggesting the effect of Aβ42 on Ca2+cyt increase is not dose-dependent (Fig 2, D).
Figure 2. Tau−/− cortical neurons are more susceptible to Aβ42 induced Ca2+cyt increase than WT neurons.
Representative images of t=0 (left) and t=30 (right) for WT (top) and tau−/− (bottom) mouse primary cortical neurons loaded with the Ca2+cyt indicator, fluo4, and treated with 0.25µM (A) and 5µM Aβ42 (B). Full time-lapse experiments (C) and the change from t=0 to t=30 (D) were quantified. *=P<0.05
Discussion
Tau and Aβ can cause mitochondrial dysfunction independently, but recent studies suggest they may act cooperatively to amplify their effects [7, 8, 26, 29, 37]. Our lab has previously shown that overexpression of pathologic forms of tau exacerbates Aβ-induced mitochondrial dysfunction in primary rat neurons and immortalized cortical neurons derived from mouse embryos [25, 27]. This result was intriguing, but came with the caveat that overexpression of proteins may cause off-target effects in cellular models. Therefore, we used neurons from tau−/− mice to verify that endogenous tau facilitates Aβ42-induced mitochondrial dysfunction.
The results of this study clearly demonstrate that endogenous tau facilitates Aβ42-induced ΔΨm. Low concentrations of Aβ42 caused significantly greater loss of ΔΨm in primary mouse neurons that expressed endogenous tau compared to those in which tau expression was ablated. It is worth noting that the ΔΨm loss we observed in this study occurred within a much smaller scale than what was observed in our overexpression model [25]. Nonetheless, these findings clearly support the conclusion that tau and Aβ42 act cooperatively to cause mitochondrial damage in AD.
Previous studies suggest Ca2+ homeostasis is impaired in AD based on evidence of enhanced total cellular Ca2+ concentrations and IP3-mediated mobilization of Ca2+ stores in cultured fibroblasts from AD patients [16, 23]. More recent reports have implied that Ca2+cyt dyshomeostasis underlies Aβ toxicity in neurons [32] and neural cell lines [17, 18, 32]. The mechanism by which Aβ causes enhanced Ca2+ levels in these cells is a matter of some debate. For example it has been suggested that Aβ may enhance activity of N-type voltage gated Ca2+ channels in rat cerebellar granule neurons [24], prolong depolarization of rat hippocampal neurons by physically blocking fast-inactivating potassium channels [12] or inhibiting the sodium/potassium-ATPase that usually maintains resting membrane potential [20, 21], or by creating Ca2+ permeable pores in the plasma membrane of GT1-7 cells [17].
Other studies have suggested that mitochondria play an integral role in Aβ-induced Ca2+ dyshomeostasis and toxicity via a mechanism involving Ca2+mito overload, dissipation of ΔΨm, mPTP opening, and impairment of store-operated Ca2+ entry to replenish depleted internal Ca2+ stores [18, 23, 32, 33, 38]. Since approximately half of all mitochondria in cells are localized to microdomains of high Ca2+cyt, they are highly susceptible to Ca2+-induced injury [22, 30, 38]. Because mitochondrial dysfunction and Ca2+ dyshomeostasis are inextricably intertwined in AD, we investigated the contribution of tau and Aβ to these pathologic phenomena.
Given that tau−/− mouse primary neurons were protected from Aβ42-induced ΔΨm, we predicted their mitochondrial Ca2+ handling mechanisms would be intact, allowing them to effectively buffer Aβ42-induced Ca2+cyt fluxes. Surprisingly, we observed just the opposite. Tau−/− neurons showed significantly larger increases in Ca2+cyt produced by both low and high concentrations of Aβ42 compared to WT neurons. The exacerbation of Aβ42-induced Ca2+cyt increases in tau−/− neurons may be due to alterations in neuronal function due to the absence of tau. While the tau−/− mouse is a well-established model [28, 31], neurons from these mice show abnormal rates of neurite outgrowth [5, 28, 31] and the mice are more susceptible to Aβ-induced neurodegeneration at 12 months of age [4]. Axon outgrowth is governed by Ca2+ transients and large, frequent L-type Ca2+ channel transients cause growth cones to pause, effectively slowing neurite outgrowth [36]. Given that tau−/− mice exhibit slowed neurite outgrowth, they likely exhibit larger Ca2+ transients than their WT counterparts. A study by Furukawa et. al. [10], suggests that this may happen in any model in which tau’s role in microtubule homeostasis is impaired. They found that expression of a FTDP mutant form of tau whose microtubule binding function is impaired causes increased L-type Ca2+ channel activity in a manner that requires MT depolymerization [9]. This suggests that the tau−/− mouse neuron cultures may have aberrant Ca2+ signaling mediated by microtubule destabilization, which makes them more susceptible to secondary insults, such as Aβ.
Despite previous work linking mitochondrial damage to Ca2+ deregulation, the results of the current study suggest that there may be a disconnect between Aβ42 induced mitochondrial damage and Ca2+cyt dyshomeostasis. In t tau−/− primary cortical neurons large Ca2+cyt increases did not coincide with loss of ΔΨm suggesting that impairment of mitochondrial Ca2+ buffering was not the cause of the Ca2+cyt increase and that high Ca2+cyt does not always lead to mitochondrial damage.
Conclusion
Tau facilitates loss of ΔΨm induced by low concentrations of Aβ42 independent of Ca2+cyt deregulation. In contrast, the absence of tau in primary cortical neurons facilitated Aβ42-induced Ca2+cyt increases, which may indicate Ca2+ dyshomeostasis previously linked to microtubule destabilization [9] and developmental abnormalities in tau−/− neurons [5, 36]. Taken together, these outcomes suggest that decreasing tau - but not ablating it - may be beneficial to ameliorate AD-related mitochondrial dysfunction.
Highlights.
Tau−/− mouse neurons are protected from Aβ42-induced ΔΨm loss
Tau−/− mouse neurons are sensitized to Aβ42-induced Ca2+cyt increases
Tau facilitates Aβ-induced mitochondrial dysfunction independent of Ca2+ alterations
Reduction of tau may prevent AD-related mitochondrial dysfunction
Complete ablation of tau may impair Ca2+ homeostasis
Acknowledgements
The authors thank Rodrigo Quintanilla, Ph.D. for instruction in the basic imaging techniques used herein. Statistical analyses were performed in part by Changyong Feng, Ph.D. This work was supported by NIH grant NS076789.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Aβ – amyloid beta, AD – Alzheimer’s disease, APP – amyloid precursor protein, Ca2+cyt – cytosolic calcium, Ca2+mito – mitochondrial calcium, ΔΨm – mitochondrial membrane potential, ER – endoplasmic reticulum, FTDP – frontotemporal dementia with parkinsonism, hAPP – human amyloid precursor protein, mPTP – mitochondrial permeability transition pore, OxPHOS – oxidative phosphorylation, PS2 – presenilin 2, ROS – reactive oxygen species, TCA – tricarboxylic acid, WT – wild type, 3xTgAD – triple transgenic AD mouse model
References
- 1.Bubber P, Haroutunian V, Fisch G, Blass JP, Gibson GE. Mitochondrial abnormalities in Alzheimer brain: mechanistic implications. Ann Neurol. 2005;57:695–703. doi: 10.1002/ana.20474. [DOI] [PubMed] [Google Scholar]
- 2.Csordas G, Renken C, Varnai P, Walter L, Weaver D, Buttle KF, Balla T, Mannella CA, Hajnoczky G. Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol. 2006;174:915–921. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis JN, Hunnicutt EJ, Jr, Chisholm JC. A mitochondrial bottleneck hypothesis of Alzheimer's disease. Molecular medicine today. 1995;1:240–247. doi: 10.1016/s1357-4310(95)91532-x. [DOI] [PubMed] [Google Scholar]
- 4.Dawson HN, Cantillana V, Jansen M, Wang H, Vitek MP, Wilcock DM, Lynch JR, Laskowitz DT. Loss of tau elicits axonal degeneration in a mouse model of Alzheimer's disease. Neuroscience. 2010;169:516–531. doi: 10.1016/j.neuroscience.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dawson HN, Ferreira A, Eyster MV, Ghoshal N, Binder LI, Vitek MP. Inhibition of neuronal maturation in primary hippocampal neurons from tau deficient mice. J Cell Sci. 2001;114:1179–1187. doi: 10.1242/jcs.114.6.1179. [DOI] [PubMed] [Google Scholar]
- 6.Desikan RS, McEvoy LK, Thompson WK, Holland D, Brewer JB, Aisen PS, Sperling RA, Dale AM. I. Alzheimer's Disease Neuroimaging, Amyloid-beta--associated clinical decline occurs only in the presence of elevated P-tau. Archives of neurology. 2012;69:709–713. doi: 10.1001/archneurol.2011.3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eckert A, Schmitt K, Gotz J. Mitochondrial dysfunction - the beginning of the end in Alzheimer's disease? Separate and synergistic modes of tau and amyloid-beta toxicity. Alzheimer's research & therapy. 2011;3:15. doi: 10.1186/alzrt74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eckert A, Schulz KL, Rhein V, Gotz J. Convergence of Amyloid-beta and Tau Pathologies on Mitochondria In Vivo. Mol Neurobiol. 2010 doi: 10.1007/s12035-010-8109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furukawa K, Wang Y, Yao PJ, Fu W, Mattson MP, Itoyama Y, Onodera H, D'Souza I, Poorkaj PH, Bird TD, Schellenberg GD. Alteration in calcium channel properties is responsible for the neurotoxic action of a familial frontotemporal dementia tau mutation. J Neurochem. 2003;87:427–436. doi: 10.1046/j.1471-4159.2003.02020.x. [DOI] [PubMed] [Google Scholar]
- 10.Furukawa R, Maselli A, Thomson SA, Lim RW, Stokes JV, Fechheimer M. Calcium regulation of actin crosslinking is important for function of the actin cytoskeleton in Dictyostelium. J Cell Sci. 2003;116:187–196. doi: 10.1242/jcs.00220. [DOI] [PubMed] [Google Scholar]
- 11.Gibson GE, Shi Q. A mitocentric view of Alzheimer's disease suggests multi-faceted treatments. J Alzheimers Dis. 2010;20(Suppl 2):S591–607. doi: 10.3233/JAD-2010-100336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Good TA, Smith DO, Murphy RM. Beta-amyloid peptide blocks the fast-inactivating K+ current in rat hippocampal neurons. Biophysical journal. 1996;70:296–304. doi: 10.1016/S0006-3495(96)79570-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gunter TE, Pfeiffer DR. Mechanisms by which mitochondria transport calcium. The American journal of physiology. 1990;258:C755–C786. doi: 10.1152/ajpcell.1990.258.5.C755. [DOI] [PubMed] [Google Scholar]
- 14.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 15.Hauptmann S, Scherping I, Drose S, Brandt U, Schulz KL, Jendrach M, Leuner K, Eckert A, Muller WE. Mitochondrial dysfunction: an early event in Alzheimer pathology accumulates with age in AD transgenic mice. Neurobiol Aging. 2009;30:1574–1586. doi: 10.1016/j.neurobiolaging.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Ito E, Oka K, Etcheberrigaray R, Nelson TJ, McPhie DL, Tofel-Grehl B, Gibson GE, Alkon DL. Internal Ca2+ mobilization is altered in fibroblasts from patients with Alzheimer disease. Proc Natl Acad Sci U S A. 1994;91:534–538. doi: 10.1073/pnas.91.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawahara M, Kuroda Y. Molecular mechanism of neurodegeneration induced by Alzheimer's betaamyloid protein: channel formation and disruption of calcium homeostasis. Brain research bulletin. 2000;53:389–397. doi: 10.1016/s0361-9230(00)00370-1. [DOI] [PubMed] [Google Scholar]
- 18.Ma T, Gong K, Yan Y, Song B, Zhang X, Gong Y. Mitochondrial modulation of store-operated Ca(2+) entry in model cells of Alzheimer's disease. Biochemical and biophysical research communications. 2012;426:196–202. doi: 10.1016/j.bbrc.2012.08.062. [DOI] [PubMed] [Google Scholar]
- 19.Manczak M, Park BS, Jung Y, Reddy PH. Differential expression of oxidative phosphorylation genes in patients with Alzheimer's disease: implications for early mitochondrial dysfunction and oxidative damage. Neuromolecular medicine. 2004;5:147–162. doi: 10.1385/NMM:5:2:147. [DOI] [PubMed] [Google Scholar]
- 20.Mark RJ, Hensley K, Butterfield DA, Mattson MP. Amyloid beta-peptide impairs ion-motive ATPase activities: evidence for a role in loss of neuronal Ca2+ homeostasis and cell death. J Neurosci. 1995;15:6239–6249. doi: 10.1523/JNEUROSCI.15-09-06239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mattson MP, Cheng B, Davis D, Bryant K, Lieberburg I, Rydel RE. beta-Amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. J Neurosci. 1992;12:376–389. doi: 10.1523/JNEUROSCI.12-02-00376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montero M, Alonso MT, Carnicero E, Cuchillo-Ibanez I, Albillos A, Garcia AG, Garcia-Sancho J, Alvarez J. Chromaffin-cell stimulation triggers fast millimolar mitochondrial Ca2+ transients that modulate secretion. Nature cell biology. 2000;2:57–61. doi: 10.1038/35000001. [DOI] [PubMed] [Google Scholar]
- 23.Peterson C, Goldman JE. Alterations in calcium content and biochemical processes in cultured skin fibroblasts from aged and Alzheimer donors. Proc Natl Acad Sci U S A. 1986;83:2758–2762. doi: 10.1073/pnas.83.8.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Price SA, Held B, Pearson HA. Amyloid beta protein increases Ca2+ currents in rat cerebellar granule neurones. Neuroreport. 1998;9:539–545. [PubMed] [Google Scholar]
- 25.Quintanilla RA, Dolan PJ, Jin YN, Johnson GV. Truncated tau and Abeta cooperatively impair mitochondria in primary neurons. Neurobiol Aging. 2012;33:619, e625–e635. doi: 10.1016/j.neurobiolaging.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quintanilla RA, Matthews-Roberson TA, Dolan PJ, Johnson GV. Caspase-cleaved tau expression induces mitochondrial dysfunction in immortalized cortical neurons: implications for the pathogenesis of Alzheimer disease. J Biol Chem. 2009;284:18754–18766. doi: 10.1074/jbc.M808908200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quintanilla RA, von Bernhardi R, Godoy JA, Inestrosa NC, Johnson GV. Phosphorylated tau potentiates Abeta-induced mitochondrial damage in mature neurons. Neurobiol Dis. 2014;71:260–269. doi: 10.1016/j.nbd.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 28.Rapoport M, Dawson HN, Binder LI, Vitek MP, Ferreira A. Tau is essential to beta -amyloidinduced neurotoxicity. Proc Natl Acad Sci U S A. 2002;99:6364–6369. doi: 10.1073/pnas.092136199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rhein V, Song X, Wiesner A, Ittner LM, Baysang G, Meier F, Ozmen L, Bluethmann H, Drose S, Brandt U, Savaskan E, Czech C, Gotz J, Eckert A. Amyloid-beta and tau synergistically impair the oxidative phosphorylation system in triple transgenic Alzheimer's disease mice. Proc Natl Acad Sci U S A. 2009;106:20057–20062. doi: 10.1073/pnas.0905529106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rizzuto R, Brini M, Murgia M, Pozzan T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science. 1993;262:744–747. doi: 10.1126/science.8235595. [DOI] [PubMed] [Google Scholar]
- 31.Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, Gerstein H, Yu GQ, Mucke L. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer's disease mouse model. Science. 2007;316:750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- 32.Sanz-Blasco S, Valero RA, Rodriguez-Crespo I, Villalobos C, Nunez L. Mitochondrial Ca2+ overload underlies Abeta oligomers neurotoxicity providing an unexpected mechanism of neuroprotection by NSAIDs. PloS one. 2008;3:e2718. doi: 10.1371/journal.pone.0002718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheehan JP, Swerdlow RH, Miller SW, Davis RE, Parks JK, Parker WD, Tuttle JB. Calcium homeostasis and reactive oxygen species production in cells transformed by mitochondria from individuals with sporadic Alzheimer's disease. J Neurosci. 1997;17:4612–4622. doi: 10.1523/JNEUROSCI.17-12-04612.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Small SA, Duff K. Linking Abeta and tau in late-onset Alzheimer's disease: a dual pathway hypothesis. Neuron. 2008;60:534–542. doi: 10.1016/j.neuron.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swerdlow RH, Khan SM. A "mitochondrial cascade hypothesis" for sporadic Alzheimer's disease. Med Hypotheses. 2004;63:8–20. doi: 10.1016/j.mehy.2003.12.045. [DOI] [PubMed] [Google Scholar]
- 36.Tang F, Dent EW, Kalil K. Spontaneous calcium transients in developing cortical neurons regulate axon outgrowth. J Neurosci. 2003;23:927–936. doi: 10.1523/JNEUROSCI.23-03-00927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yao J, Irwin RW, Zhao L, Nilsen J, Hamilton RT, Brinton RD. Mitochondrial bioenergetic deficit precedes Alzheimer's pathology in female mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2009;106:14670–14675. doi: 10.1073/pnas.0903563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yarana C, Sanit J, Chattipakorn N, Chattipakorn S. Synaptic and nonsynaptic mitochondria demonstrate a different degree of calcium-induced mitochondrial dysfunction. Life sciences. 2012;90:808–814. doi: 10.1016/j.lfs.2012.04.004. [DOI] [PubMed] [Google Scholar]