Abstract
Clostridium difficile infection (CDI) is the most frequent cause of nosocomial diarrhea. It has become a significant dilemma in the treatment of patients, and causes increasing morbidity that, in extreme cases, may result in death. Persistent and recurrent disease hamper attempts at eradication of this infection. Escalating levels of treatment and novel therapeutics are being utilized and developed to treat CDI. Further trials are warranted to definitively determine what protocols can be used to treat persistent and recurrent disease.
Keywords: recurrent Clostridium difficile, BI/NAP1/027, fecal microbiota therapy
Clostridium difficile infection (CDI) is a burdensome problem that has plagued the medical field for years. An anaerobic, gram-positive, spore-forming bacillus, infection with Clostridium difficile can be cured with oral and/or intravenous antibiotics. However, increased incidence and development of more virulent strains has led to more cases of persistent or recurrent disease.1 These indolent infections lead to escalating health-care costs, significant morbidity, and can proceed to death.
Transmission
CDI is the most prevalent cause of antibiotic-associated nosocomial diarrhea with close to 3,000,000 episodes of CDI occurring each year.2 The potential for spreading the disease is great as people can be carriers without the presence of symptoms. Transmission occurs by the fecal–oral route or by contact with fomites, as Clostridium difficile spores are readily encountered on contaminated hospital surfaces and carried by health-care workers. In fact, these spores are so hardy that alcohol-based antiseptics are not enough. Hands must be sufficiently washed with chlorhexidine soap and water to eradicate the risk of transmission.3 In addition, gowns and gloves should be worn by health-care workers and visitors entering the rooms of infected persons or those in whom infection is suspected.4 Patients may also pass the infection to each other, making it necessary to identify and isolate infected patients. Lending to the difficulty of controlling this often indolent infection, colonization of C. difficile is seen in 20 to 50% of the adults in hospitals and long-term care facilities.1 Furthermore, there has been an increase in community-acquired CDI that may not be associated with antibiotic use or recent hospitalization.5
Pathogenesis
Following exposure, the pathogenesis of CDI typically begins with antibiotic treatment or chemotherapy disrupting normal colonic flora, allowing C. difficile to flourish, leading to the elaboration of toxin A (enterotoxin) and toxin B (cytotoxin) which causes mucosal inflammation and injury.1 Mild CDI can manifest as watery diarrhea (up to 10–15 times a day), abdominal pain, cramping, fever, and leukocytosis. Symptoms can progress in moderate to severe cases with the development of sepsis, pseudomembranous colitis or fulminant colitis with bowel perforation, toxic megacolon, and death.6
Epidemiology
The most significant change in the epidemiology of CDI has been the evolution of hypervirulent strains, most notably BI/NAP1/027. This strain yields dramatically higher levels of toxins A and B and confers fluoroquinolone resistance.5 Further, it elaborates binary toxin, which has an unclear role, but may act synergistically with toxins A and B to contribute to more severe disease.5 The trajectory of BI/NAP1/027 recognition mirrors the increased incidence and severity of CDI seen in the early 2000s, as more patients were proceeding to fulminant disease necessitating colectomy, which increased mortality.1
Risk Factors
Primary risk factors for the development of CDI include advanced age (greater than 65 years), antibiotic use, severe illness, and hospitalization.1 2 Secondary factors that also increase the risk include gastric acid suppression (with proton pump inhibitors or histamine-2 receptor antagonists), gastrointestinal procedures, chemotherapy, residence at a long-term care facility, inflammatory bowel disease, and immunosuppression.1 2 Furthermore, in those infected with C. difficile, low levels of vitamin D have been shown to be an independent predictor of poor outcome and are associated with higher recurrence.7
Diagnosis
Patients with clinically significant watery diarrhea should prompt testing for C. difficile. Though no best standard test has been established, diagnosis can be confirmed with a stool test positive for C. difficile or by endoscopic examination.3 Laboratory tests for stool testing include: cell culture, polymerase chain reaction (PCR) to identify DNA coding for toxins, enzyme immunoassay (EIA) for C. difficile toxins A and B, enzyme immunoassay (EIA) for C. difficile glutamate dehydrogenase, and cell culture cytotoxicity neutralization assay (Table 1).2 6 Cell culture is the gold standard with nearly 100% sensitivity and specificity; however, it is labor intensive and has a long turnaround for results. PCR testing is typically the preferred testing method used as results can be made available in an hour and its sensitivity is greater than EIA.3 6 There is no role for repeat testing to assess for the eradication of C. difficile once the clinical symptoms have resolved. Further, asymptomatic patients and health-care workers should not be routinely tested.4
Table 1. Diagnostic testing for Clostridium difficile .
| Test | Sensitivity | Specificity | Cost |
|---|---|---|---|
| Toxigenic culture | High | High | $10–30 |
| Cell culture neutralization assay | High | High | $15–25 |
| GDH | High | Low | $5–15 |
| Toxin EIA tests | Low | High | $5–15 |
| PCR | High | High | $20–50 |
Abbreviations: EIA, enzyme immunoassay; GDH, glutamate dehydrogenase; PCR, polymerase chain reaction.
Plain abdominal X-rays, computed tomography of the abdomen and pelvis, and endoscopy can serve as adjuncts in diagnosing CDI (Fig. 1). Imaging studies may reveal colonic thickening, free perforation, colon distension or megacolon, whereas endoscopy may demonstrate pseudomembranes, which are nearly pathognomonic for CDI (Fig. 2).2 6 During colonoscopy, care must be taken not to overinflate the bowel and cause perforation.
Fig. 1.
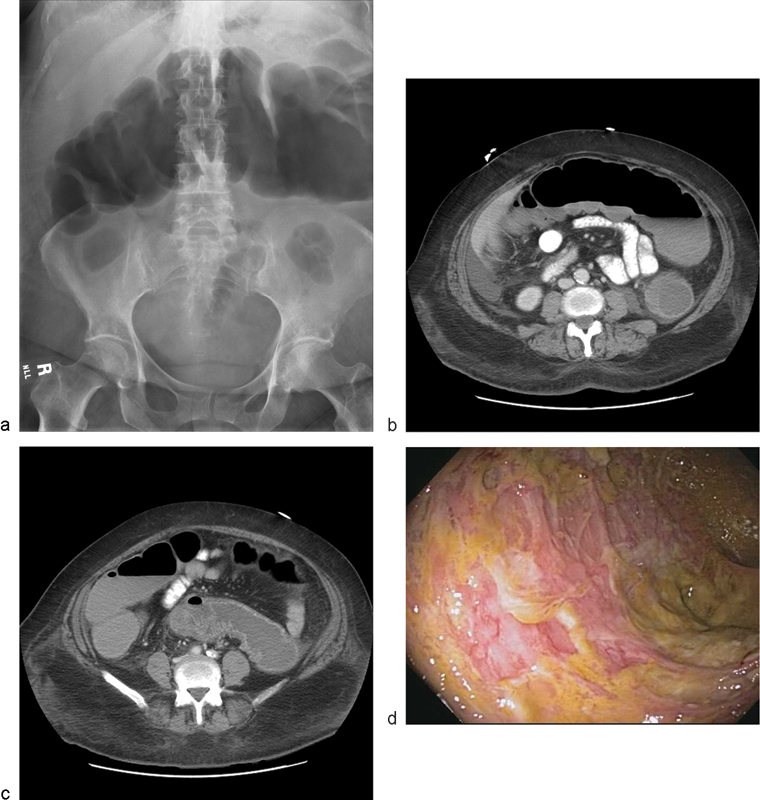
A 67-year-old female with toxic megacolon, pseudomembrane development, and mucosal sloughing: (a) X-ray showing dilated transverse colon, (b) computed tomographic scan of dilated colon and thickened mucosa, (c) computed tomographic scan demonstrating mucosal thickening, and (d) colonoscopic image of pseudomembranes sloughing. (Image courtesy Anjali Kumar, MD, MedStar Washington Hospital Center.)
Fig. 2.
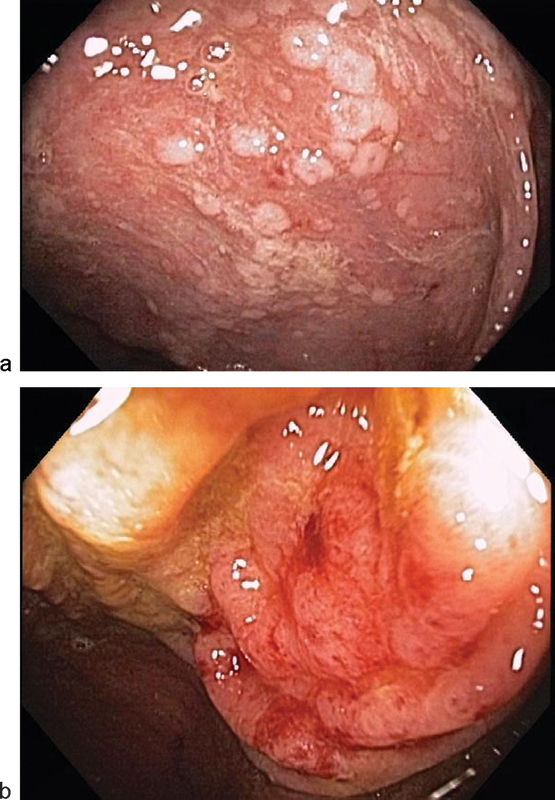
Classic endoscopic findings of: (a) pseudomembranes and (b) thickened, hyperemic, and edematous mucosa. (Image courtesy Anjali Kumar, MD, MedStar Washington Hospital Center.)
Treatment
Table 2 describes strategies for initial treatment, and treatment of primary and recurrent infections. Primary treatment of CDI is a discontinuation of the antibiotic that initially disrupted the normal colonic flora. Continuous usage of antibiotics other than those to treat C. difficile significantly decreases the cure rate and increases the time to resolution of diarrhea.3 8 In cases where the patients' symptoms are mild with watery diarrhea, only minimal abdominal pain, and cramping, metronidazole administered orally or intravenously is the current standard of care. For patients with moderate to severe infections—which may be manifested with increasing fever, leukocytosis, or signs of end-organ damage—oral vancomycin is preferred, and may also be instilled as an enema. In those with mild disease that fail to improve or worsen on metronidazole after 5 to 7 days, conversion to oral vancomycin treatment is recommended.3 Current recommendations state that antiperistaltic agents to treat diarrhea should be avoided because they may precipitate worsening disease.2 Colonoscopy may also be helpful in treatment if vancomycin irrigation can be administered in the proximal colon (Fig. 3) through the scope's irrigation channel, or if a long colonic tube can be placed at the time of endoscopy for antibiotic enemas. This tube can be placed endoscopically with or without the use of fluoroscopy by piggybacking or dragging a long catheter in with the colonoscope as described by Stephenson et al and Groff et al.9 10
Table 2. Clostridium difficile treatment strategies.
| Patient presentation | Treatment options |
|---|---|
| Asymptomatic carrier | No treatment |
| Initial episode or first recurrence | Discontinue inciting antibiosis |
| Mild disease | Metronidazole 500 mg PO q 8 hr × 10–14 d |
| Moderate–severe disease | Vancomycin 125 mg PO q 6 hr × 10–14 d |
| Severe or complicated disease | Vancomycin 500 mg PO q 6 hr and metronidazole 500 mg IV q 8 hr, and vancomycin enema q 6 hr |
| Second recurrence | Tapered/pulsed vancomycin: Vancomycin 125 mg PO q 6 hrs × 10–14 d, then q 12 hr × 7 d, then qd × 7 d, then qod × 8 d, then once every 3 × 15 d |
| Third recurrence | Fecal microbiota therapy or |
| Fidaxomicin 200 mg PO q 12 hr × 10 d or | |
| IVIg |
Abbreviations: d, day; hr, hours; PO, per os (oral); q, every; qd, daily; qod, every other day.
Fig. 3.
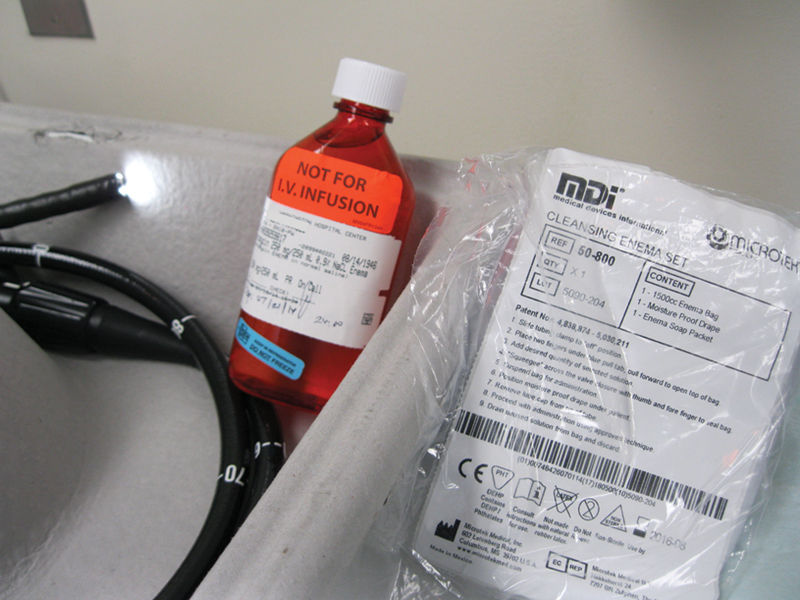
Vancomycin solution enema (250 mg in 250 mL) can be administered through the rectum via a standard enema set (pictured on the right), or into the proximal colon via the colonoscope (pictured on the left) irrigation channel. (Image courtesy Anjali Kumar, MD, MedStar Washington Hospital Center.)
Fidaxomicin, a macrolide antibiotic, was Food and Drug Administration-approved (FDA) for the treatment of CDI in 2011.9 In industry-funded Phase III clinical trials, it was shown to be comparable to vancomycin and led to significantly lower rates of recurrence of CDI.10 Its narrow spectrum, active against C. difficile and most staphylococci and enterococci, proves useful as it has minimal activity against gram-negatives and may help support gut flora in rebuffing recurrence.11 12
Recurrence
Most patients respond well to treatment and have discontinuation of symptoms with standard metronidazole and/or vancomycin antibiotic therapy for 10 to 14 days. However, some patients may have symptoms that persist or recur following satisfactory treatment. Recurrent CDI occurs in 20 to 30% of the patients, with increasing rates of recurrence with each subsequent episode.5 In clinical settings, it is impossible to distinguish a recurrence that develops as a relapse of CDI with the same strain of C. difficile versus a reinfection that is the result of a new strain.13 Surprisingly, recurrent CDI does not seem to be the result of resistance. Therefore, when it does recur, it can be treated in the same fashion as already discussed.
When subsequent recurrences occur, treatment recommendations become less straightforward. Metronidazole is not recommended as treatment beyond a first recurrence of mild disease as prolonged use may lead to neurotoxicity.4 For second recurrences, tapered plans with pulsed doses of vancomycin over 6 weeks have been suggested (Table 2). In recent years, therapy with fidaxomicin has been offered as an adjunct, particularly in patients on a concomitant antibiotic for systemic infection.8 However, standard guidelines for usage have not been established, and the current high cost of fidaxomicin (> $2,800 for a full course) inhibits wider use.14
Therapies with nonantibiotic treatments, such as probiotics, intravenous immunoglobulins, and fecal microbiota transplant (FMT), have also garnered attention.15 There are no significant data supporting the use of probiotics, such as Saccharomyces boulardii or Lactobacillus plantarum, as has been suggested and examined in the past.3 Further, lack of quality control and regulation makes probiotics unreliable adjuncts to traditional therapies.3 Immunoglobulins have been suggested to be helpful for severe or recurrent C. difficile in several case reports, but a significant series has yet to show its definitive benefit.16
FMT has shown to be very promising particularly in the population of persistent or recurrent CDI.17 In a review of 317 patients, it was shown to lead to resolution in 92% of the persistent and recurrent disease cases.18 It is clear that restoration of gut flora is paramount in the struggle against recurrent CDI.
Role for Surgery
Though surgical consultation should be obtained when symptoms progress beyond moderate disease, no validated staging system exists for CDI. The development of severe CDI is usually defined as clinical signs of end-organ dysfunction, mental status change, profound leukocytosis, increasing lactate, vasopressor requirement, or worsening abdominal examination warranting an urgent evaluation.2 In these cases, consideration for surgery is imperative. It is difficult to identify the critical window of time when the disease process is severe enough to necessitate an operation.
Currently, the most agreed upon procedure is total abdominal colectomy with end ileostomy (Fig. 4). There is no role for anastomosis or proctectomy in these severely ill patients; given the imperative to the expedite the patient's time under general anesthesia which increases the potential for hypotension and ischemia. However, the creation of a loop ileostomy with colonic lavage of 8 L of warmed polyethylene glycol 3,350/electrolyte solution and subsequent antegrade colonic treatment with vancomycin (500 mg/500 mL Lactated Ringers every 8 hours for 10 days) is gaining interest as an alternative method for aggressive early surgical treatment or in those for which the morbidity of total colectomy may already be too high.19 Neal et al reported that this procedure could be performed laparoscopically in 83% of the patients at the University of Pittsburgh. Their results showed a significant decrease (p = 0.006) in mortality rates, 9 versus 50% in the traditional total colectomy with ileostomy group.19
Fig. 4.
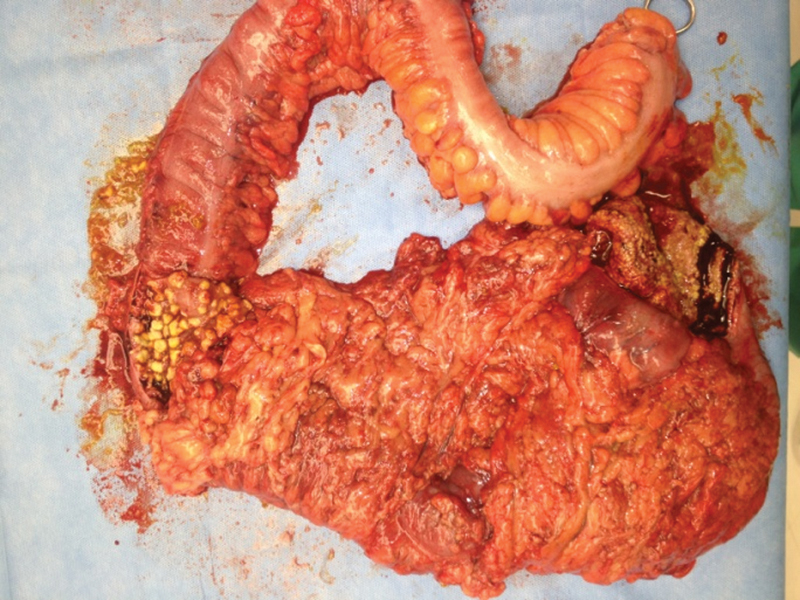
Surgical intervention (with total colectomy) is indicated for severe Clostridium difficile infection which causes toxic colitis. Specimen photos show severity of this patient's disease. (Image courtesy James FitzGerald, MD, MedStar Washington Hospital Center).
Conclusion
CDI continues to cause a huge strain on health care, and treatment of persistent and recurrent disease can be vexing. Avoiding the use of antibiotics for unsubstantiated etiologies, as well as preventing the spread of organisms with universal protocol techniques and maintaining a high level of suspicion in diagnosing and treating CDI may help decrease the burden of this disease. Further trials are warranted to definitively determine what protocols can be used to treat persistent and recurrent disease.
Acknowledgments
The ASCRS CREST committee has permitted us to share our work in this manner. The authors thank Alexandra Dubinskaya, MD for assistance with references.
References
- 1.Lamont J T Up to Date: Clostridium difficile in adults: Epidemiology, microbiology, and pathophysiology Available at: http://www.uptodate.com/contents/clostridium-difficile-in-adults-epidemiology-microbiology-and-pathophysiology. Last updated June 24, 2014. Accessed on June 28, 2014.
- 2.Stanley JD(1), Bartlett J G Dart B W 4th Ashcraft J H Clostridium difficile infection Curr Probl Surg 2013507302–337. [DOI] [PubMed] [Google Scholar]
- 3.Surawicz C M Brandt L J Binion D G et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections Am J Gastroenterol 20131084478–498., quiz 499 [DOI] [PubMed] [Google Scholar]
- 4.Cohen S H, Gerding D N, Johnson S. et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31(5):431–455. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 5.Kelly C P, LaMont J T. Clostridium difficile—more difficult than ever. N Engl J Med. 2008;359(18):1932–1940. doi: 10.1056/NEJMra0707500. [DOI] [PubMed] [Google Scholar]
- 6.LaMont J T Up to Date: Clostridium difficile in adults: Clinical manifestations and diagnosis Available at: http://www.uptodate.com/contents/clostridium-difficile-in-adults-clinical-manifestations-and-diagnosis. Last updated Feb 24, 2014. Accessed on May 8, 2014.
- 7.Wang W J, Gray S, Sison C. et al. Low vitamin D level is an independent predictor of poor outcomes in Clostridium difficile-associated diarrhea. Therap Adv Gastroenterol. 2014;7(1):14–19. doi: 10.1177/1756283X13502838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mullane K M, Miller M A, Weiss K. et al. Efficacy of fidaxomicin versus vancomycin as therapy for Clostridium difficile infection in individuals taking concomitant antibiotics for other concurrent infections. Clin Infect Dis. 2011;53(5):440–447. doi: 10.1093/cid/cir404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stephenson K R, Rodriguez-Bigas M A. Decompression of the large intestine in Ogilvie's syndrome by a colonoscopically placed long intestinal tube. Surg Endosc. 1994;8(2):116–117. doi: 10.1007/BF00316622. [DOI] [PubMed] [Google Scholar]
- 10.Groff W. Colonoscopic decompression and intubation of the cecum for Ogilvie's syndrome. Dis Colon Rectum. 1983;26(8):503–506. doi: 10.1007/BF02563739. [DOI] [PubMed] [Google Scholar]
- 11.Golan Y, Epstein L. Safety and efficacy of fidaxomicin in the treatment of Clostridium difficile-associated diarrhea. Therap Adv Gastroenterol. 2012;5(6):395–402. doi: 10.1177/1756283X12461294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Louie T J, Miller M A, Mullane K M. et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364(5):422–431. doi: 10.1056/NEJMoa0910812. [DOI] [PubMed] [Google Scholar]
- 13.Figueroa I, Johnson S, Sambol S P, Goldstein E J, Citron D M, Gerding D N. Relapse versus reinfection: recurrent Clostridium difficile infection following treatment with fidaxomicin or vancomycin. Clin Infect Dis. 2012;55 02:S104–S109. doi: 10.1093/cid/cis357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fidaxomicin Available at: https://online.epocrates.com/noFrame/showPage.do?method=drugs&MonographId=6128&ActiveSectionId=10. Accessed on June 7, 2014.
- 15.Cocanour C S. Best strategies in recurrent or persistent Clostridium difficile infection. Surg Infect (Larchmt) 2011;12(3):235–239. doi: 10.1089/sur.2010.080. [DOI] [PubMed] [Google Scholar]
- 16.O'Horo J, Safdar N. The role of immunoglobulin for the treatment of Clostridium difficile infection: a systematic review. Int J Infect Dis. 2009;13(6):663–667. doi: 10.1016/j.ijid.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 17.Borody T J Leis S Pang G Wettstein A R Up to Date: Fecal microbiota transplantation in the treatment of recurrent Clostridium difficile infection Available at: http://www.uptodate.com/contents/fecal-microbiota-transplantation-in-the-treatment-of-recurrent-clostridium-difficile-infection Last updated Aug 29, 2013. Accessed on May 8, 2014.
- 18.Gough E, Shaikh H, Manges A R. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis. 2011;53(10):994–1002. doi: 10.1093/cid/cir632. [DOI] [PubMed] [Google Scholar]
- 19.Neal M D Alverdy J C Hall D E Simmons R L Zuckerbraun B S Diverting loop ileostomy and colonic lavage: an alternative to total abdominal colectomy for the treatment of severe, complicated Clostridium difficile associated disease Ann Surg 20112543423–427., discussion 427–429 [DOI] [PubMed] [Google Scholar]


