Abstract
There are many different sexually transmitted infections that can cause proctitis. Recognition of the common symptoms with anoscopic examination is crucial in accurate diagnosis of the pathogen. Clinicians should have a high index of suspicion of more than one inciting pathogen. Treatment should be prompt and extended to sexual partners who have been exposed to the disease. Effective treatment can alleviate the discomfort and potentially serious complications associated with sexually transmitted proctitides. This article illustrates and discusses the clinical presentations, diagnostic pearls, and treatments of sexually transmitted proctitides.
Keywords: sexually transmitted proctitis, proctitides, anorectal receptive intercourse, anal infections
Sexually acquired proctitis with inflammation of the rectal mucosa (the distal 15 cm) results from direct rectal inoculation of pathogens. Acute proctitis in persons who have practiced receptive anorectal intercourse is usually sexually acquired. However, the diagnosis cannot be definitively ruled out in patients who do not report a history of anal intercourse. A thorough history and physical examination can alert the physician to the possibility of a sexually transmitted infection.
Chlamydia, gonorrhea, herpes simplex virus (HSV), and syphilis are among the most common causes of sexually transmitted proctitis. Recently, sexually transmitted infections (STIs) from lymphogranuloma venereum (LGV) have been reported in men who have sex with men (MSM) and the diagnosis and treatment are being reviewed.1 The AIDS era brought in a shift of the clinical and etiologic spectrum of intestinal infections. Opportunistic infections from cytomegalovirus (CMV) may cause ulcerative anorectal lesions in this population.2 HIV is important to address because so many of the complications and manifestations of the disease affect the anus, rectum, and colon. This review examines the causative organisms, clinical features, diagnosis, and treatment of sexually transmitted proctitides.
Neisseria Gonorrhea
Epidemiology
Gonorrhea is the second most commonly reported sexually transmitted bacterial infection in the United States. It is a disease of the young, sexually active patient. In 2012, persons in the age group of 15 to 44 years accounted for 95% of the reported gonorrhea cases with known age. Following a 74% decline in the rate of reported gonorrhea during the period 1975 to 1997, gonorrhea rates have seen a plateau in the past 10 years. Gonorrhea rates decreased further to 98.1 cases per 100,000 population in 2009. This is the lowest published rate since the recording of gonorrhea rates began. Since 2009, the gonorrhea rate has increased slightly each year to 107.5 cases per 100,000 population in 2012. This translates to 334,826 cases of gonorrhea being reported in the United States in 2012.3 Gonorrhea rates among women have been slightly higher since 2001, but recently the rates among men have increased. At least 50% of the male patients and up to 95% of the female patients with rectal gonorrhea are asymptomatic, accounting for a large pool of disease carriers in the community. A significant percentage of women diagnosed with gynecologic gonorrhea will also have gonorrhea in the anorectum. This is believed to be due to the contiguous spread from genital infection. Only 4% of the women with gonorrhea will have the anorectum as the only site of involvement, whereas 40 to 50% of the men who practice anoreceptive intercourse will have the anorectum as the exclusive site of infection.
Signs and Symptoms
Neisseria gonorrhea (N. gonorrhea) is a gram-negative intracellular diplococcus. It is one of the most common causes of proctocolitis. The typical symptoms include mucopurulent discharge with tenesmus and pruritus. Bleeding may be present on occasions. Commonly, examination with anoscopy will reveal the yellow mucous discharge from the anal crypts with gentle pressure on the external perianal area. Proctoscopic examination will show the characteristic mucopurulent discharge extending less than 10 cm from the anal verge and occasionally nonspecific proctitis, such as erythema and irritation as demonstrated in Fig. 1. It is important to remember that patients may be asymptomatic or simply complain of mild discharge.
Fig. 1.
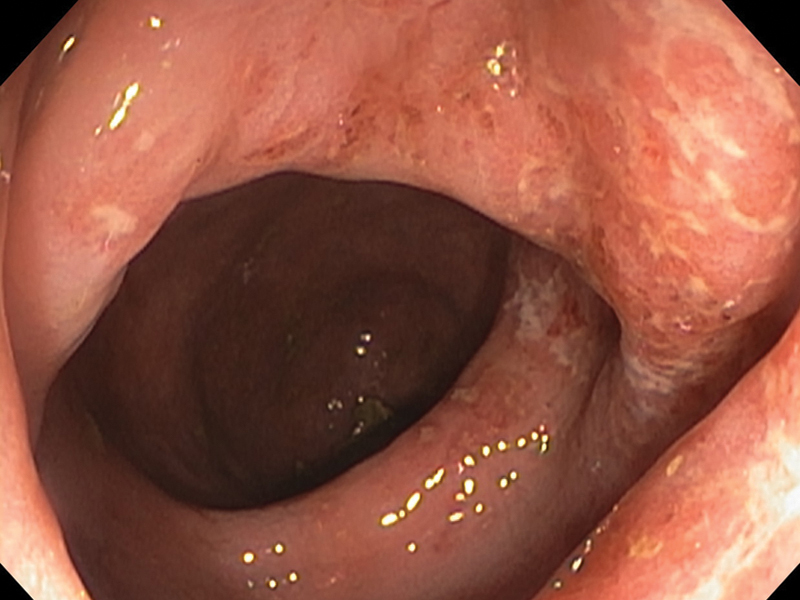
Endoscopic picture of gonorrheal proctitis, showing mucosal edema, erythema, with whitish plaques.
Diagnosis
Gram stains of endocervical specimens, pharyngeal or rectal specimens are not sufficient to detect infection, and, therefore, are not recommended.4 Despite this, when gonorrhea is suspected, highly sensitive and specific testing is recommended because it will enhance partner notification. Culture, nucleic acid hybridization tests, and nucleic acid amplification tests (NAAT) are available for the detection of infection with N. gonorrhea and when obtaining a rectal specimen, careful attention to obtaining the specimen directly from the anorectum will increase the positive yield.5 NAAT tests are not recommended by Food and Drug Administration (FDA) for use in the rectum, pharynx, and conjunctiva; however, some public and private laboratories have established performance specifications for using NAAT with rectal and pharyngeal swab specimens, thereby allowing results to be used for clinical management.6
Treatment
Neisseria gonorrhea has developed resistance to each of the antibiotics used for treatment of gonorrhea. In the last decade, the development of fluoroquinolone resistance has resulted in the availability of only a single class of antibiotics that meets the efficacy standards set by the Center for Disease Control and Prevention (CDC): the cephalosporins.4 7 Most recently, dual therapy (to cover Chlamydia concurrently) with ceftriaxone and either azithromycin or doxycycline is now the only CDC-recommended treatment regimen for gonorrhea.4 In patients with penicillin allergies, it is important to remember that only 5 to 10% of the patients will have reactions to first-generation cephalosporins and even fewer will react to third-generation cephalosporins. For this reason, the CDC recommends only those with severe reactions to penicillin to avoid ceftriaxone. However, in this case, consultation with an infectious disease specialist is recommended and azithromycin 2 g orally as monotherapy may be used.
Chlamydia and Lymphogranuloma Venereum
Epidemiology
C. trachomatis infection is the most commonly reported sexually transmitted bacterial infection in the United States. It is among the most prevalent of all STIs, and since 1994 comprised the largest proportion of all STIs reported to the CDC. In 2012, a total of 1,422,976 chlamydial infections were reported to CDC in 50 states and the District of Columbia.3 The majority of rectal chlamydial infections occur in MSM. There are 15 known serotypes, with the G, D, and J types being the most predominant among isolates of MSM.8
LGV is a sexually transmitted disease (STD) caused by serovars L1–L3 of Chlamydia trachomatis. Transmission of disease is through anoreceptive intercourse, with an incubation period ranging from 5 days to 2 weeks; secondary involvement can occur as a late manifestation of genital infection. Before 2003, it was thought to be a “tropical” disease that was rare in Western industrialized societies, but a large outbreak in 2003 in the Netherlands has led to the discovery of multiple cases throughout the Western world. The majority of cases are caused by L2 in MSM as a distinct proctitis.
Signs and Symptoms
Non-LGV Chlamydia trachomatis infections of the anorectum are usually asymptomatic. When symptoms are present, they manifest as proctitis/colitis symptoms namely, tenesmus, pain, and fever. Proctoscopy shows signs of nonspecific inflammation, erythema, and injected mucosa, but no ulcerations or abscesses.
Unlike other chlamydial urogenital infections that are generally restricted to epithelial surfaces, L serovars are invasive, can cause severe inflammation with systemic symptoms, and have a preference for lymphatic tissues. LGV will typically present with one or more genital ulcers or papules. The second stage then consists of unilateral or bilateral, fluctuant buboes (lymphadenopathy) and anorectal ulcers. The third and final stages can comprise abscesses, granulomas, fistulas, genital elephantiasis, stricturing, and sterility. Proctoscopic examination during the first and second stages will demonstrate a more pronounced inflammation with hematochezia, ulceration, and friable mucosa. LGV can be distinguished from other proctitides by the characteristic lymphadenopathy.
Diagnosis
Diagnosis is based on clinical suspicion, epidemiological information, and exclusion of other etiologies for proctocolitis, inguinal adenopathy, or genital/rectal ulcers. C. trachomatis testing should be performed on specimens obtained from lesion swabs or bubo aspirates. These specimens can be sent for culture, nucleic acid detection, or direct immunofluorescence. Polymerase chain reaction-based genotyping can be used to distinguish non-LGV from LGV serovars, but these are not widely available.4 However, patients with a clinical syndrome of proctocolitis, lymphadenopathy, and/or genital ulceration should be treated empirically for LGV.
Treatment
If an anorectal exudate is detected on examination or if polymorphonuclear leukocytes are detected on a gram-stained smear of anorectal secretions, the patient should be treated for N. gonorrhea and C. trachomatis while awaiting additional laboratory tests. This involves ceftriaxone 250 mg intramuscular × 1 dose and doxycycline 100 mg orally twice daily for 7 days. For LGV, the CDC recommends treatment with doxycycline 100 mg orally twice daily for 21 days. Alternatively, Azithromycin 1 g orally once a week for 3 weeks is probably as effective.4
Granuloma Inguinale (Donovanosis)
Epidemiology
Granuloma inguinale (Donovanosis) is a slowly progressive ulcerative disease of the skin and lymphatics of the genital and perianal areas caused by infection due to Calymmatobacterium granulomatis. It is endemic in tropical and developing areas, including India, Guyana, New Guinea, central Australia, and southern Africa and is rare in the United States. It can be caused by both sexual and nonsexual contact. Symptoms most commonly present 1 to 12 weeks after the infection and transmission occurs through contact with the open sores.
Signs and Symptoms
The infections typically start as a painless nodule that breaks down into a sharply demarcated ulcer with a beefy-red friable base of granulation tissue. They may appear on the skin, genitalia, perianal area or migrate to the upper buttocks and lower legs. Lesions around the anus may cause stenosis.9 There is an increased risk of superinfection and coinfection by other pathogenic microbes, including HIV.
Diagnosis
Culture of C. granulomatis is difficult and not routinely available. Diagnosis requires visualization of Donovan bodies (numerous bacilli in the cytoplasm of macrophages demonstrated with Giemsa or Wright stain) in smears of scrapings from the ulcer base or histologic sections.
Treatment
Treatment of donovanosis is doxycycline 100 mg orally twice daily for 1 week or trimethoprim 800 mg-sulfamethoxazole 160 mg orally twice daily for at least 3 weeks. HIV-associated granuloma inguinale may take longer to heal, and the addition of a parenteral aminoglycoside to the regimen is highly recommended (gentamicin at 1 mg/kg given intravenous every 8 hours).4 Due to the fibrotic nature of the ulcerations and resultant scars, surgical correction may be needed for disfiguring lesions or cicatricial/stenotic lesions.
Syphilis
Epidemiology
Syphilis is a systemic disease caused by the spirochete Treponema pallidum. Transmission is thought to occur when mucocutaneous syphilitic lesions are present. If left untreated, it can cause significant complications and can facilitate the transmission of HIV. Although the rate of primary and secondary syphilis in the United States declined to 89.7% during 1990 to 2000, the rate increased annually during 2001 to 2009 before decreasing in 2010, and remained unchanged during 2011. After persistent declines during 1992 to 2003, the rate among women increased from 0.8 cases (in 2004) to 1.5 cases (in 2008) per 100,000 population, declining to 0.9 cases per 100,000 population in 2011 and 2012.
Although syphilis rates in the United States are low, there has been a relative recent increase due to high-risk behavior in the HIV-positive MSM population. The estimated proportion of primary and secondary syphilis cases attributable to MSM increased from 7% in 2000 to 64% in 2004.3 In 2012, among cases of primary and secondary syphilis for whom sex of partner was known, MSM accounted for 75% of the primary and secondary syphilis cases.3
Signs and Symptoms
Syphilis has been divided into progressive stages to assist with treatment and follow-up. Most persons will present with primary or secondary syphilis. Primary syphilis in the anorectum presents as a painful chancre (Fig. 2). The organisms introduced into the anorectum will have an incubation period of 2 to 6 weeks, after which an ulcer will appear. Unlike the genital ulcers, the anorectal ulcers will be painful and are commonly mistaken for anal fissures. The ulcer of syphilis will typically be broad based, present between the anal verge and dentate line, and be located off the midline. In addition, it may be associated with discharge, urgency of defecation, tenesmus or multiple in number. These characteristics can help distinguish between common anal fissure and the chancre of syphilis.10
Fig. 2.
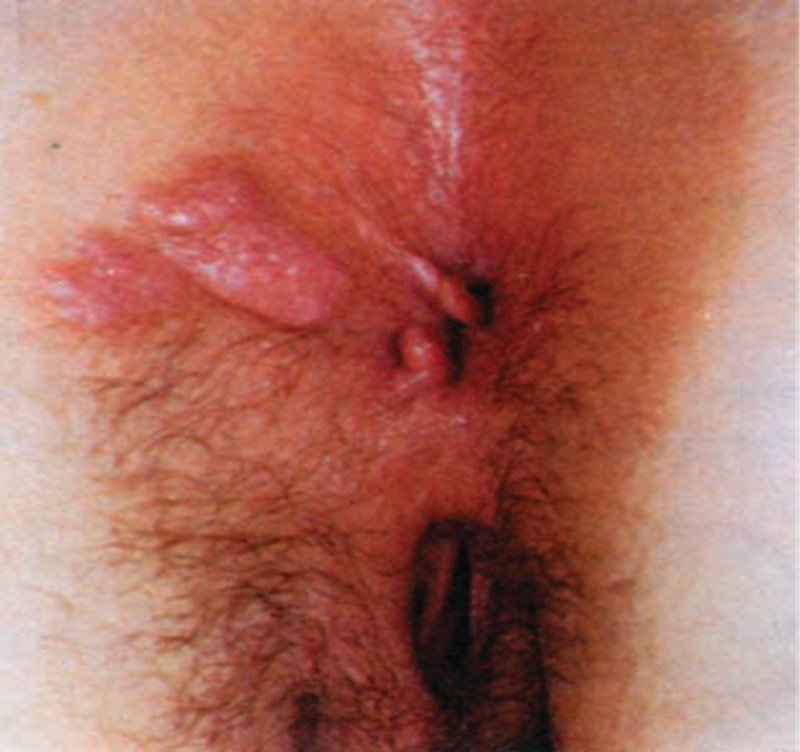
Syphilitic ulcer in the perianal area. (Reproduced with permission from Gordon, PH. Principles and Practice of Surgery for the Colon, Rectum and Anus, 3rd ed. New York: Informa Healthcare; 2007:253.)
If left untreated, primary syphilis will progress to secondary syphilis. Secondary syphilis is characterized by a maculopapular rash seen in the hands and feet. Systemic symptoms may include fever, arthralgias, malaise, weight loss, sore throat, and headache. Condyloma lata may appear at this stage and is often mistaken for human papilloma virus as demonstrated in Fig. 3.
Fig. 3.
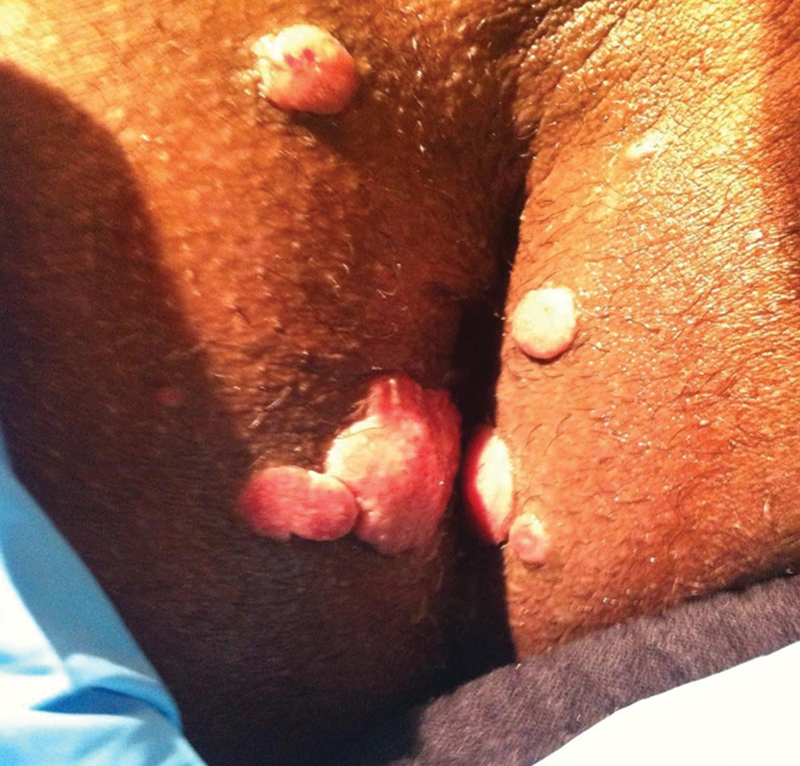
Condyloma lata of syphilis seen in gluteal cleft.
Diagnosis
Darkfield examination (showing corkscrew-shaped yellow-green spirochetes) and tests to detect Treponema pallidum in lesion exudates or tissues are the definitive methods for detecting early syphilis. A presumptive diagnosis of syphilis is possible with the use of two types of serologic tests: (1) nontreponemal tests (e.g., Venereal Disease Research Laboratory [VDRL] and Rapid Plasma Reagin [RPR]) and (2) treponemal tests (e.g., fluorescent treponemal antibody absorbed [FTA-ABS] tests, the T. pallidum passive particle agglutination [TP-PA] assay, various EIAs [enzyme immunoassay], and chemiluminescence immunoassays). The use of only one type of serologic test is insufficient for diagnosis because each type of test has limitations, including the possibility of false-positive test results in persons without syphilis.4
Treatment
Penicillin G, administered parenterally, is the primary treatment for all stages of syphilis.4 Treatment of the early stages of syphilis is with a single dose of 2.4 million units of long-acting benzathine penicillin G given intramuscularly. In the later stages (or if the patient is HIV-positive), three consecutive doses given at 2-week intervals is recommended. Follow-up testing with VDRL or RPR should be repeated at 6-month intervals (HIV-negative patients) or 3-month intervals (HIV-positive patients) for 1 year. For patients with a penicillin allergy, doxycycline 100 mg orally twice daily for 14 days or tetracycline 400 mg four times daily for 2 weeks may be used.
All patients with syphilis in any stage should be checked for HIV. In addition, partners potentially exposed within 90 days should be treated presumptively.
Herpes Simplex Virus
Epidemiology
The HSV is most commonly associated with vesicular eruptions near mucocutaneous junctions. It was estimated that 20% of Americans are seropositive for HSV-2. National trend data on the seroprevalence of HSV-2 among those in the age group of 14 to 49 years were compared with National Health and Nutrition Examination Survey (NHANES). Seroprevalence decreased from 21% in the period 1994 to 1998 to 17% in 1999 to 2004 and 16.2% after 2004.3 Although seroprevalence is decreasing, most persons with HSV-2 have not received a diagnosis.
Diagnosis
Traditionally, HSV type 1 (HSV-1) caused fever blisters or cold sores around the mouth and was not sexually acquired in contrast to the type 2 form (HSV-2) which caused painful blisters in the genital area from sexual contact. However, with the increased frequency of oral–genital sex, both types may be found in each location. HSV proctitis is thought to progress from the perianal skin into the anal canal and then into the rectum. It occurs commonly in patient populations that are immunosuppressed from bone marrow or solid organ transplantation or from HIV/AIDS and in individuals who engage in anoreceptive intercourse. HSV may cause an ulcerative type of proctitis in patients with AIDS.11 Prophylaxis with antivirals has reduced the incidence of HSV proctitis in patients with solid organ transplants by 73%.12
Patients with HSV proctitis may complain of anorectal pain, discharge, tenesmus, or rectal bleeding. During the acute phase, difficulty with urination, sacral paresthesias, and temporary fecal incontinence may occur.13 Examination of the perianal area is critical to the diagnosis of HSV. The most severe cases present with perianal erythema and ulcerations (Fig. 4) and can be confused with a Candidal infection. Patients have exquisite pain and sensitivity during digital rectal and anoscopic examination. Sigmoidoscopic examination reveals an acute proctitis with mucosal edema and ulcerations. In the immunocompetent patient, HSV proctitis rarely extends beyond 15 cm.14
Fig. 4.
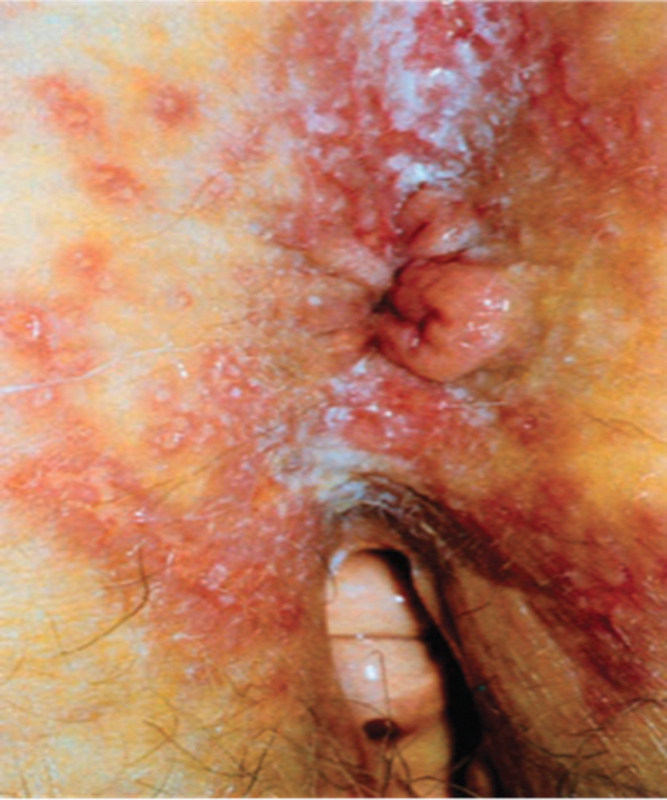
Herpes lesions seen surrounding the anus. (Reproduced with permission from Gordon, PH. Principles and Practice of Surgery for the Colon, Rectum and Anus, 3rd ed. New York: Informa Healthcare; 2007:251.)
The gold standard for diagnosing HSV is the viral culture with Tzanck preparation. However, the treatment of HSV proctitis is based on a high index of suspicion rather than a positive laboratory test. Also, there are several HSV type specific antibody tests that are available to confirm the diagnosis (Table 1).
Table 1. Treatment of patients presenting with herpes simplex virus.
| Medication | Dose | Duration |
|---|---|---|
| First clinical episode of moderate HSV (can use any of the following medications) | ||
| Acyclovir | 400 mg orally TID | 10 d |
| Valacyclovir | 1 g orally BID | 7–10 d |
| Famciclovir | 250 mg orally TID | 7–10 d |
| Treatment of AIDS patients with severe HSV | ||
| Acyclovir | 5–10 mg/kg intravenous q8 h | Continue until clinical resolution followed by oral antiviral suppressive therapy |
| Suppressive therapy for HSV in AIDS patients (any of the following) | ||
| Acyclovir | 400–800 mg orally TID | Indefinitely |
| Valacyclovir | 500 mg orally BID | Indefinitely |
| Famciclovir | 500 mg orally BID | Indefinitely |
| Suppressive therapy for HSV in the immunocompetent patient | ||
| Acyclovir | 400 mg orally BID | 12 m |
Abbreviations: AIDS, acquired immune deficiency syndrome; BID, two times a day; HSV, herpes simplex virus; q8, every 8 hours; TID, three times a day.
Treatment
The management of HSV proctitis primarily focuses on symptomatic relief with the use of oral analgesics as well as sitz baths. Antiviral medications, such as acyclovir, valacyclovir, and famciclovir, have been shown to decrease viral shedding and shorten the symptomatic period.11 The symptoms of HSV usually resolve spontaneously within 2 to 3 weeks, but the virus remains in the body for life and recurrences are common.
The CDC recommendations for the treatment of HSV proctitis are the same as those for genital HSV (Table 2). Severe HSV infection in patients with AIDS should be treated with intravenous acyclovir (Table 2). HIV-positive patients or transplant patients should be considered for suppressive therapy (Table 1). Continuous or episodic oral acyclovir treatment has been demonstrated to be safe and relatively effective and most effective in patients who are subject to frequent exacerbations.11 15 Counseling for HSV treatment should include education regarding its transmission and safe sex practices.
Table 2. Sexually transmitted proctitis: symptoms, diagnostic pearls, and treatment.
| Pathogen | Symptoms | Findings | Diagnosis pathology |
Treatment | Other |
|---|---|---|---|---|---|
| Neisseria gonorrhea | Itching, painful defecation, tenesmus, thick yellow mucopurulent discharge, urethritis | Pus when push on crypts | Intracellular gram-negative diplococci Thayer–Martin plate |
Ceftriaxone 250 intramuscular × 1 + (treat for Chlamydia also) azithromycin 1 g orally or doxycycline 100 mg orally × 7 d |
Most common cause in HIV-infected patients Reportable Most patients are asymptomatic |
| Chlamydia trachomatis |
Proctitis, rectal pain, tenesmus, fever |
Erythematous rectal mucosa | Cell culture Direct immunofluorescent |
Doxycycline 100 mg twice daily × 7 d Azithromycin 1 g x 1 d |
Most common strains D and K |
| Lymphogranuloma venereum Chlamydia trachomatis |
As above (Chlamydia trachomatis, but worse), perianal ulcers (foul smelling), severe proctitis including mucoid and hemorrhagic discharge, unilateral inguinal lymphadenopathy (bubo) | Endoscopic inflammation and ulceration. Late stages may show fistulae and strictures | Aspirate from bubo Crypt abscess and granuloma |
Same, prolonged therapy | Strains L1, 2, 3 Elephantiasis Strictures/fissure |
| Treponema pallidum (syphilis) | Proctitis chancre—painful or painless fissure like, unusual location; rectal masses; lymphadenopathy |
Spirochete, mobile Dark field examination Fluorescent treponemal antibody absorption test or microhemagglutination assay VRDL/RPR |
Penicillin 2.4 M units intramuscularly for 2 wk | ||
| CMV | Proctocolitis, diarrhea, fever, hemorrhage, perforation |
Submucosal hemorrhage Mucosal ulcerations |
Intranuclear viral inclusion bodies Cytoplasmic Inclusion bodies |
Ganciclovir Foscarnet | 94% homosexual males positive Perforation |
| HSV | Fever, tenesmus, constipation, radiculopathy |
Associated with coalesced vesicles and pustules Distal 10 cm |
Viral isolation Monoclonal antibody |
Acyclovir 400 mg 5 times/day × 5 d (increased dose for proctitis) Foscarnet or valacyclovir |
Abbreviations: CMV, cytomegalovirus; HSV, herpes simplex virus; RPR, rapid plasma reagin; VRDL, Venereal Disease Research Laboratory.
HIV/AIDS-Related Proctocolitis
Nonspecific proctitis and colitis have been associated with HIV infection. In addition, mucosal inflammation is associated with an increased risk of HIV transmission; therefore, the appropriate management of proctitis in HIV patients can reduce the amount of HIV shedding.14
Diarrhea has been reported to affect up to 50% of the patients with AIDS in North America. It can be a significant cause of morbidity resulting in a decreased quality of life.16 HIV infection itself is thought to cause minor alterations in the architecture of the villi, which may lead to mild malabsorption of carbohydrates in the small bowel.4 Increased permeability resulting from cytokine activation by foreign antigens contributes to diarrhea. In addition, HIV has amino acid sequences similar to those of vasoactive intestinal peptide (VIP) and may induce diarrhea by upregulation of VIP receptors, which may cause pathogen-negative diarrhea.17
Patients presenting with diarrhea and abdominal pain should be assessed with a detailed history paying careful attention to the sexual history, travel, diet, and medications. Recent changes in medications and exposure to antibiotics should be inquired. The use of antiretrovirals (particularly nelfinavir and ritonavir) may lead to medication-induced diarrhea. Implicated agents mainly include protease inhibitors, that is, lopinavir, fosamprenavir, and atazanavir.18
It is also important to ascertain the most recent CD4 cell count, which will have a direct impact on diagnostic testing. Patients with CD4 cell counts < 100 cells/µL are at risk for opportunistic infections, such as cryptosporidium, CMV, or microsporidium. Any patient who has had recent exposure to antibiotics, Clostridium difficile is an important diagnostic consideration. In a retrospective study of more than 11,000 episodes of diarrheal illness among HIV-infected patients, C. difficile was the most commonly identified pathogen, accounting for 54% of all the bacterial infections.19
The American Gastroenterological Association created guidelines for the evaluation of chronic diarrhea in HIV-infected patients.20 Recommendations include stool samples for bacteria and parasites and checking for C. difficile. For those in whom stool studies fail to identify an infectious cause, endoscopy and mucosal biopsy are recommended. Flexible sigmoidoscopy with mucosal biopsies for microscopic examination for bacterial and mycobacterial culture may be adequate. However, if CMV infection is suspected, colonoscopy is recommended due to its involvement of the proximal colon.21 Upper endoscopy with duodenal biopsies should be performed when other investigations have failed to reveal a source. This may aid in identifying Giardia, microsporidia, or mycobacterium avium complex (MAC). Among identified causes of diarrhea in patients with AIDS, the most common opportunistic pathogens are CMV, MAC, and protozoans.20 Treatment of pathogen-negative diarrhea consists of rehydration, antimotility agents, and somatostatin analogues.
Cytomegalovirus
CMV is a double-stranded DNA virus from the herpes virus family. It is present in feces, urine, semen, and saliva but does not usually produce symptoms in the immune competent patient. However, it has been described in the immune competent host after anal intercourse.22 23 CMV infection in adults typically causes a mononucleosis-like syndrome with fever, malaise, lymphocytosis, and abnormal liver function tests. Sexually transmitted CMV proctitis presents as a triad of mononucleosis-like illness with rectal bleeding shortly after anal intercourse. Typical endoscopic findings include rectal mucositis and ulcerations, although polypoid lesions have been described in several cases. Although the role of antiviral therapy in primary CMV proctitis has not been defined, it is probably not essential in all cases, as most of the reported cases were resolved spontaneously without complications. Treatment with antiviral therapy is recommended in the immunosuppressed. Before the availability of highly active antiretroviral therapy (HAART), CMV gastrointestinal disease occurred in up to 5% of the patients with AIDS, primarily in those with advanced immunosuppression. Happily, the incidence of CMV gastrointestinal disease has decreased substantially since HAART became available.24
Gastrointestinal manifestations of CMV can occur along the entire intestinal tract causing ulceration, colitis/proctitis, and hemorrhage. The colon is the main target in the intestinal tract, and ileocolitis is the major colorectal finding.25 The diagnosis of CMV colitis should be considered in patients with AIDS who develop an acute abdomen. The symptoms of colonic CMV include watery diarrhea, abdominal pain, and bloody stools.
An accurate diagnosis can sometimes be difficult to obtain. The appearance of CMV proctocolitis can range from punctate and superficial erosions to deep ulcerations and hemorrhage. Biopsies should be taken to make a definitive diagnosis. Histologically, CMV is characterized by mucosal inflammation, tissue necrosis, vascular endothelial cell damage, and the presence of intranuclear and intracytoplasmic inclusions (Fig. 5).26
Fig. 5.
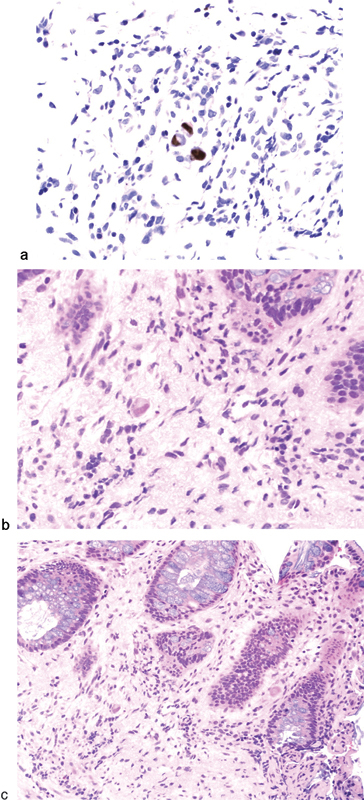
(a) Cytomegalovirus immunostained section; (b) Rectal biopsy showing proctitis and intranuclear and intracytoplasmic inclusions by hematoxylin and eosin-stained section under high power; and (c) under low power. (Courtesy John Liang MD, MedStar Washington Hospital Center, Washington, DC).
Ganciclovir is the preferred agent for the treatment of CMV-related proctocolitis.27 Surgical intervention for CMV is reserved for perforation, massive bleeding, and toxic megacolon. However, a patient with mild peritoneal signs, without free air, may be treated with ganciclovir in addition to other appropriate antibiotics for aerobic and anaerobic organisms. When emergency surgery is required, resection of the involved segment of colon and end stoma is performed.
Conclusion
Taking a sexual history is important in the diagnosis and it may predict whether a patient is at risk for sexually transmitted proctitis. Condom use does not guarantee protection from STIs, which are often spread by skin to skin contact without penetration. Table 1 summarizes the treatment of proctitides described in this review. Recognition of the common symptoms with anoscopic examination is crucial in accurate diagnosis of the pathogen. In addition, clinicians should have a high index of suspicion of more than one inciting pathogen. Treatment should be prompt and extended to sexual partners who have been exposed to the disease. Effective treatment can alleviate the discomfort and potentially serious complications associated with sexually transmitted proctitides.
Acknowledgments
The authors are grateful for the invaluable contributions and critiques from Dr. Lee E. Smith, who acted as the senior mentor for this work. We also thank Dr. Philip Gordon and Dr. John Liang for contributing photos. Finally, acknowledgments are given to Allison Estep of Georgetown University Medical School for editorial assistance and to Alexandra Dubinskaya, MD for assistance with literature review. The ASCRS CREST Committee has permitted us to share our work in this manner.
References
- 1.Davis T W, Goldstone S E. Sexually transmitted infections as a cause of proctitis in men who have sex with men. Dis Colon Rectum. 2009;52(3):507–512. doi: 10.1007/DCR.0b013e31819ad537. [DOI] [PubMed] [Google Scholar]
- 2.Puy-Montbrun T, Ganansia R, Lemarchand N, Delechenault P, Denis J. Anal ulcerations due to cytomegalovirus in patients with AIDS. Report of six cases. Dis Colon Rectum. 1990;33(12):1041–1043. doi: 10.1007/BF02139221. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention . Atlanta: U.S. Department of Health and Human Services; 2013. Sexually Transmitted Disease Surveillance 2012. [Google Scholar]
- 4.Workowski K A Berman S; Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010 MMWR Recomm Rep 201059(RR-12):1–110. [PubMed] [Google Scholar]
- 5.Deheragoda P. Diagnosis of rectal gonorrhoea by blind anorectal swabs compared with direct vision swabs taken via a proctoscope. Br J Vener Dis. 1977;53(5):311–313. doi: 10.1136/sti.53.5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC) . Update to CDC's sexually transmitted diseases treatment guidelines, 2006: fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep. 2007;56(14):332–336. [PubMed] [Google Scholar]
- 7.Geisler W M, Whittington W L, Suchland R J, Stamm W E. Epidemiology of anorectal chlamydial and gonococcal infections among men having sex with men in Seattle: utilizing serovar and auxotype strain typing. Sex Transm Dis. 2002;29(4):189–195. doi: 10.1097/00007435-200204000-00001. [DOI] [PubMed] [Google Scholar]
- 8.O'Farrell N. Donovanosis. Sex Transm Infect. 2002;78(6):452–457. doi: 10.1136/sti.78.6.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maron D Sexually Transmitted Diseases American Society of Colon and Rectal Surgeons; 2011 Core Subjects. [Google Scholar]
- 10.Cha J M, Choi S I, Lee J I. Rectal syphilis mimicking rectal cancer. Yonsei Med J. 2010;51(2):276–278. doi: 10.3349/ymj.2010.51.2.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cone L A, Woodard D R, Potts B E, Byrd R G, Alexander R M, Last M D. An update on the acquired immunodeficiency syndrome (AIDS). Associated disorders of the alimentary tract. Dis Colon Rectum. 1986;29(1):60–64. doi: 10.1007/BF02555292. [DOI] [PubMed] [Google Scholar]
- 12.Lavery E A, Coyle W J. Herpes simplex virus and the alimentary tract. Curr Gastroenterol Rep. 2008;10(4):417–423. doi: 10.1007/s11894-008-0078-8. [DOI] [PubMed] [Google Scholar]
- 13.Joseph D, Jin H, Ryan C, Chey W Y. Resolution of anorectal incontinence in herpes proctitis confirmed by anorectal manometry. Gastrointest Endosc. 1997;45(5):429–432. doi: 10.1016/s0016-5107(97)70159-7. [DOI] [PubMed] [Google Scholar]
- 14.Rompalo A M. Diagnosis and treatment of sexually acquired proctitis and proctocolitis: an update. Clin Infect Dis. 1999;28 01:S84–S90. doi: 10.1086/514721. [DOI] [PubMed] [Google Scholar]
- 15.Voth M L, Akbari R P. Sexually transmitted proctitides. Clin Colon Rectal Surg. 2007;20(1):58–63. doi: 10.1055/s-2007-970201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaplowitz L G, Baker D, Gelb L. et al. Prolonged continuous acyclovir treatment of normal adults with frequently recurring genital herpes simplex virus infection. JAMA. 1991;265(6):747–751. [PubMed] [Google Scholar]
- 17.Smith P D, Quinn T C, Strober W, Janoff E N, Masur H. NIH conference. Gastrointestinal infections in AIDS. Ann Intern Med. 1992;116(1):63–77. doi: 10.7326/0003-4819-116-1-63. [DOI] [PubMed] [Google Scholar]
- 18.Keating J, Bjarnason I, Somasundaram S. et al. Intestinal absorptive capacity, intestinal permeability and jejunal histology in HIV and their relation to diarrhoea. Gut. 1995;37(5):623–629. doi: 10.1136/gut.37.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veljkovic V Metlas R Raspopovic J Pongor S Spectral and sequence similarity between vasoactive intestinal peptide and the second conserved region of human immunodeficiency virus type 1 envelope glycoprotein (gp120): possible consequences on prevention and therapy of AIDS Biochem Biophys Res Commun 1992. 15;1892705–710. [DOI] [PubMed] [Google Scholar]
- 20.U.S. Department of Health and Human Services Health Resources and Services Administration Guide for HIV/AIDS Clinical Care. Rockville, MD: HIV/AIDS Bureau; January 2011 Available at: http://hab.hrsa.gov/deliverhivaidscare/clinicalguide11/cg-501_diarrhea.html. Accessed on May 30, 2014.
- 21.Sanchez T H, Brooks J T, Sullivan P S. et al. Bacterial diarrhea in persons with HIV infection, United States, 1992-2002. Clin Infect Dis. 2005;41(11):1621–1627. doi: 10.1086/498027. [DOI] [PubMed] [Google Scholar]
- 22.Wilcox C M, Rabeneck L, Friedman S. AGA technical review: malnutrition and cachexia, chronic diarrhea, and hepatobiliary disease in patients with human immunodeficiency virus infection. Gastroenterology. 1996;111(6):1724–1752. doi: 10.1016/s0016-5085(96)70040-9. [DOI] [PubMed] [Google Scholar]
- 23.Kram H B, Shoemaker W C. Intestinal perforation due to cytomegalovirus infection in patients with AIDS. Dis Colon Rectum. 1990;33(12):1037–1040. doi: 10.1007/BF02139220. [DOI] [PubMed] [Google Scholar]
- 24.Rabinowitz M, Bassan I, Robinson M J. Sexually transmitted cytomegalovirus proctitis in a woman. Am J Gastroenterol. 1988;83(8):885–887. [PubMed] [Google Scholar]
- 25.Studemeister A. Cytomegalovirus proctitis: a rare and disregarded sexually transmitted disease. Sex Transm Dis. 2011;38(9):876–878. doi: 10.1097/OLQ.0b013e31821a5a90. [DOI] [PubMed] [Google Scholar]
- 26.Bini E J, Gorelick S M, Weinshel E H. Outcome of AIDS-associated cytomegalovirus colitis in the era of potent antiretroviral therapy. J Clin Gastroenterol. 2000;30(4):414–419. doi: 10.1097/00004836-200006000-00012. [DOI] [PubMed] [Google Scholar]
- 27.Bizer L S, Pettorino R, Ashikari A. Emergency abdominal operations in the patient with acquired immunodeficiency syndrome. J Am Coll Surg. 1995;180(2):205–209. [PubMed] [Google Scholar]


