Abstract
Objective
The current study compared growth parameters of girls’ and boys’ BMI trajectories from infancy to middle childhood, and evaluated these parameters as predictors of cardiovascular disease (CVD) risk in adolescence.
Methods
Using 657 children from the NICHD Study of Early Child Care and Youth Development (SECCYD), quadratic growth curve analyses were conducted to establish growth parameters (intercept, slope, quadratic term) for girls and boys from 15 months to age 10 ½. Parameters were compared across gender and evaluated as predictors of a CVD risk index at age 15, controlling for characteristics of the adiposity rebound (AR) including age at which it occurred and children’s BMI at the rebound.
Results
Boys had more extreme trajectories of growth compared to girls with higher initial BMI at 15 months (intercept), more rapid declines in BMI before the AR (slope), and sharper rebound growth in BMI after the rebound (quadratic term). For boys and girls, higher intercept, slope, and quadratic term values predicted higher CVD risk at age 15, controlling for characteristics of the AR.
Conclusions
Findings suggest that individuals at risk for developing CVD later in life may be identified before the AR by elevated BMI at 15 months and slow BMI declines. Due to the importance of early intervention in altering lifelong health trajectories, consistent BMI monitoring is essential in identifying high-risk children.
Keywords: adiposity rebound, adolescence, body mass index, cardiovascular disease
Current estimates suggest that 37% of normal weight, 49% of overweight, and 61% of obese U.S. adolescents have at least one cardiovascular disease (CVD) risk factor.1 Because CVD risk tracks from adolescence to adulthood,2 understanding CVD risk antecedents can help identify individuals vulnerable to disease. Childhood physical growth trajectories may predict problematic health during the transition into adolescence and beyond.3,4
Norm-referenced growth charts, stratified by body mass index (BMI) percentile, depict age-related BMI change. BMI sharply increases from birth to 6 months due to accelerated fat gain, plateaus in late infancy, then begins to decline due to body lengthening and slowed fat cell production in toddlerhood.5,6 Around ages 5–6, children experience the adiposity rebound (AR), a surge of fat cell production and corresponding BMI increases.7 Children in higher BMI percentiles tend to experience sharper initial declines and earlier, more rapid rebound growth than those in lower percentiles.6 Although children with an early AR are at higher risk for overweight or obesity in adolescence and early adulthood,3 past research focuses on AR timing and weight at single time points rather than patterns of BMI change across time to predict later health. Because the development of CVD involves pathological processes beginning early in life,2 considering childhood growth trajectories provides a more complete picture of the emergence of health risk during the critical period of adolescence.
Childhood BMI growth is best described quadratically with three parameters: an intercept representing BMI at the beginning of observation, a linear slope to describe BMI declines before the AR, and a quadratic term to describe rebound growth rate. Because WHO and CDC growth charts are stratified by BMI percentile, they do not represent individual children’s growth over time. The growth model under consideration will add to the literature by analyzing individual children’s BMI growth and describing average characteristics of trajectories in a given sample. Additionally, associations among children’s growth parameters and gender differences in these parameters will be evaluated. Using BMI change across childhood to predict adolescent CVD risk conceptualizes risk emergence as a process unfolding over time.2
In addition to children who experience an early AR, those who are persistently at or above the 75th BMI percentile, or who cross upward into higher percentiles, are at heightened risk for obesity and metabolic disruptions in adolescence and adulthood.8,9 Thus, there are many growth trajectories associated with later health risk.10 However, little is known about whether these varying trajectories predict differences in CVD risk.
Although increases in BMI are related to increases in CVD risk,11 BMI does not account for body fat distribution, which predicts CVD risk independent from BMI.12 Anthropomorphic measurements, specifically waist-hip-ratio (WHR) and skinfold thickness (SKF), indicate central adiposity, which is associated with CVD risk above and beyond being overweight.12 The current study combines anthropomorphic data with BMI and blood pressure (BP) measurements, creating a more complete picture of CVD risk than is possible using only BMI.
Gender may also play a role in how childhood growth trajectories influence subsequent obesity. Some evidence suggests that that girls tend to experience the AR earlier than boys,13 particularly in the highest BMI percentiles (≥ 97th).7 Other studies have found negligible or nonexistent gender differences in age14,15 and BMI at the AR.16 There are, however, gender differences in CVD risk; boys tend to have higher BP17 and are more likely to be overweight throughout adolescence and young adulthood compared to girls.18,19 To our knowledge, no studies to date have evaluated whether childhood growth trajectories differentially predict CVD risk in adolescent girls versus boys.
The aim of the current study is to use multiple group latent growth curve modeling (LGCM) to examine gender differences in the shapes of children’s BMI trajectories from 15 months to age 10 ½, and to determine the predictive value of trajectory shape on adolescent boys’ versus girls’ CVD risk over and above characteristics of the AR.
Methods
Participants
Participants were enrolled in the NICHD Study of Early Child Care and Youth Development (SECCYD), a longitudinal investigation at 10 U.S. research sites beginning in 1991. During predetermined 24-hour intervals, all women who gave birth were screened for eligibility. Families were excluded if the mother was under 18, planned to move, had a multiple birth, acknowledged substance abuse, did not speak English, or lived more than an hour from the site; or if the newborn had a disability or was hospitalized for over 7 days. A conditionally random sample of 1,525 families was eligible, and 1,364 of these families entered the study upon completion of a home interview when infants were one month old. This sample consisted of 52% boys, 24% children of color, 45% first-born children, 11% mothers without high school diplomas, and 14% single parents. See study website for additional recruitment details: https://www.nichd.nih.gov/research/supported/seccyd/Pages/seccyd.aspx. Each site received Institutional Review Board (IRB) approval for data collection, and The University of Texas at Dallas IRB approved these secondary analyses.
Children with height and weight data available at the 15-month assessment were included in the current study. This criterion resulted in a subsample of 657 families, with slightly more males (57%) than females. Seventy-seven percent of children were Caucasian, 12% were African American, 6% were Hispanic, and 5% were of mixed or other ethnicities. The sample was economically diverse, with 34.5% low-income (income-to-needs ratios <2), 42.3% middle-income, and 23.2% high-income families (income-to-needs ratios >5).
Procedure
Families reported demographic information during a home visit when the child was 1 month old. At 15, 24, 36, and 54 months, age 7, age 9, and age 10 ½, research assistants measured children’s height and weight in the laboratory. At age 15, adolescents completed a health and physical development assessment (HPDA).
Measures
Body mass index
Research assistants measured children’s height and weight using a scale and a wall-mounted yardstick. Body mass index (BMI, kg/m2) and z-scores were calculated using a CDC program.
Child age and BMI at the AR
Visual inspection method criteria were used to select the age (months) and BMI (kg/m2) at each child’s lowest BMI value, with the requirement that BMI increased by at least .1 kg/m2 at each subsequent measurement.7,20
Adolescent CVD risk
Waist and hip circumferences were measured by a physician or nurse practitioner to the nearest millimeter. WHR was calculated by dividing waist by hip circumference, with higher values indicating greater central adiposity.12 Triceps and subscapular SKF measurements to the nearest millimeter were summed to comprise total SKF, with higher values indicating greater central adiposity.12 Systolic BP (mmHg) to the nearest millimeter was taken while seated after two minutes of rest from the non-dominant arm. BMI was calculated as described above. Elevated BMI and central body fat (WHR, SKF) independently and synergistically contribute to CVD risk,11 and elevated BP is an early marker of cardiovascular pathology that tracks into adulthood.21 Because these risk factors reinforce one another in their contributions to CVD morbidity and mortality,22 the adolescent risk composite was created by standardizing and summing WHR, SKF, systolic BP, and BMI (rs=.25–.80, ps<.01).
Results
Analysis Plan
Latent growth curve modeling (LGCM) measures change within individuals on a given variable across three or more time points and creates latent variables corresponding to growth parameters. Comparisons of model fit indicate which type of growth (e.g., linear vs. quadratic) best fits the data. The current study utilizes LCGM to describe children’s BMI change from 15 months to age 10 ½. First, we will use the entire sample to establish the pattern of change, which we expect to be quadratic based on CDC growth standards.6 Quadratic growth parameters include an intercept, describing children’s 15-month BMI, a slope, describing initial BMI declines before the AR, and a quadratic term, indicating the sharpness of rebound BMI growth following the AR.
LGCM computes growth parameters at the individual level, then reports means and variances of the intercept, slope, and quadratic term across individuals. Significance values for estimated means indicate whether the parameter is significantly different than zero. Significance values for estimated variances indicate variability between individuals on the growth parameter; significant variance in BMI trajectory parameters would indicate significant heterogeneity in the shapes of children’s growth over time.
Multiple group modeling allows one to test whether an observed categorical variable moderates the growth model. We will evaluate gender differences by analyzing boys’ and girls’ BMI growth curves separately, using chi-square (χ2) difference tests comparing an unconstrained model freely estimating boys’ and girls’ growth to a series of constrained models with growth parameters set to be equal across gender. Then, we will use intercept, slope, and quadratic terms as predictors of boys’ and girls’ adolescent CVD risk. We will conceptualize predictor-outcome relations as regressions, reported as standardized regression coefficients (β) indicating change in standard deviation (SD) units in the outcome that occurs as a result of each SD unit change in the predictor. To determine whether relations between growth parameters and CVD risk are independent of AR age and BMI, we will enter these AR covariates in models as correlations with each growth parameter, reported as r values. Analyses will account for missing data using full information maximum likelihood (FIML) in Mplus 6.11.23
Researchers may use raw BMI values or BMI z-scores to conduct LGCM describing children’s growth over time. BMI z-scores are standardized using a nationally representative external reference group of same-age, same-sex children,6 and indicate a child’s position relative to peers.24 Thus, BMI z-score trajectories indicate how children’s BMI relative to peers changes over time. Raw BMI growth trajectories describe children’s BMI change over time and have been recommended for use in longitudinal analyses over z-scores.25,26 Because our research tests gender differences in the shapes of BMI growth, raw values are most appropriate for the substantive analyses; BMI z-scores are presented for descriptive purposes only (Table 1).
Table 1.
Descriptive Information for Study Variables.
| Overall
|
Boys
|
Girls
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| n | M (SD) | Range | n | M (SD) | Range | n | M (SD) | Range | |
| BMI, 15 mos. | 657 | 18.12 (1.27) | 16.72 – 33.03 | 376 | 18.26 (1.45) | 18.26 – 33.03 | 281 | 17.95 (.97) | 16.72 – 20.81 |
| .33 (.82) | −2.25 – 2.85 | .21 (.81) | −2.02 – 2.58 | .48 (.80) | −2.25 – 2.85 | ||||
|
| |||||||||
| BMI, 24 mos. | 538 | 17.31 (1.22) | 14.33 – 24.24 | 303 | 17.41 (1.15) | 14.90 – 24.24 | 235 | 17.17 (1.29) | 14.33 – 22.22 |
| .55 (.76) | −1.67 – 3.59 | .57 (.69) | −1.42 – 3.58 | .51 (.82) | −1.67 – 3.08 | ||||
|
| |||||||||
| BMI, 36 mos. | 575 | 16.69 (1.29) | 13.86 – 28.03 | 326 | 16.68 (1.31) | 14.02 – 28.03 | 249 | 16.70 (1.26) | 13.86 – 23.26 |
| .57 (.85) | −1.92 – 5.33 | .50 (.89) | −1.92 – 5.35 | .67 (.80) | −1.80 – 3.40 | ||||
|
| |||||||||
| BMI, 54 mos. | 545 | 16.49 (1.41) | 12.78 – 23.33 | 306 | 16.48 (1.34) | 13.84 – 23.33 | 239 | 16.51 (1.50) | 12.77 – 22.04 |
| .70 (.84) | −2.84 – 3.62 | .68 (.85) | −1.71 – 3.62 | .74 (.83) | −2.84 – 2.65 | ||||
|
| |||||||||
| BMI, age 7 | 528 | 17.29 (2.53) | 12.93 – 29.43 | 294 | 17.27 (2.56) | 13.40 – 29.43 | 234 | 17.32 (2.49) | 12.93 – 26.94 |
| .71 (.86) | −2.22 – 2.88 | .71 (.87) | −2.06 – 2.88 | .71 (.85) | −2.22 – 2.63 | ||||
|
| |||||||||
| BMI, age 9 | 486 | 19.10 (3.88) | 13.47 – 39.38 | 270 | 19.19 (4.01) | 13.78 – 39.38 | 216 | 18.99 (3.71) | 13.47 – 33.67 |
| .79 (.92) | −1.84 – 2.76 | .86 (.89) | −1.75 – 2.76 | .71 (.95) | −1.84 – 2.73 | ||||
|
| |||||||||
| BMI, age 10 ½ | 481 | 20.77 (4.90) | 14.32 – 53.12 | 271 | 20.90 (5.11) | 14.32 – 53.12 | 210 | 20.61 (4.61) | 14.35 – 35.53 |
| .74 (1.00) | −1.77 – 2.89 | .81 (.99) | −1.77 – 2.89 | .66 (1.02) | −1.65 – 2.59 | ||||
|
| |||||||||
| BMI at AR | 384 | 16.06 (1.17) | 12.78 – 21.27 | 213 | 16.08 (1.07) | 13.40 – 21.27 | 171 | 16.06 (1.29) | 12.77 – 20.52 |
| .26 (.78) | −2.84 – 2.61 | .22 (.76) | −2.06 – 2.09 | .32 (.82) | −2.84 – 2.61 | ||||
|
| |||||||||
| Age at AR (mo.) | 384 | 63.17 (23.14) | 24 – 108 | 213 | 63.18 (23.82) | 24 – 108 | 171 | 63.16 (22.33) | 24 – 108 |
|
| |||||||||
| CVD Risk, age 15 | 476 | .02 (.81) | −1.44 – 4.28 | 268 | .13 (.87) | −1.44 – 4.28 | 208 | −.11 (.71) | −1.30 – 2.06 |
Note. BMI z-score descriptive information listed in italics. A zBMI of 0 indicates a BMI value equivalent to the national average for a same-sex, same-age child. BMI units: kg/m2. AR: Adiposity Rebound. CVD: cardiovascular disease.
Preliminary Analyses
The current sample is not significantly different from the full SECCYD sample in terms of income, t(1271)=.371, p=.71, or child ethnicity, χ2(5)=2.09, p=.837, but children in the subsample are more likely to be male than children in the full sample, χ2(1)=16.01, p<.01. The retention rate from 15 months to 15 years was 68% in the subsample. Children who attrited were not significantly different from children who remained in the study with regard to income, t(380.69) = −.14, p = .886, ethnicity, χ2(6)=6.07, p=.415, or gender, χ2(1)=.59, p=.441.
Descriptive information and correlations between study variables are included in Tables 1 and 2. The average AR age was 63.17 months and the average AR BMI was 16.06 kg/m2. These values did not significantly differ between genders, tage(416.15)=1.02, p=.309, tBMI(330.29)=.18, p=.860. On average, boys had higher CVD risk indices than girls, t(474)=3.25, p<.01. Positive BMI z-score means at each age suggest that the current sample tends to be higher in BMI (i.e., above the 50th percentile) compared to age and gender referenced norms.
Table 2.
Correlations among Study Variables.
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|
| 1. BMI, 15 mos. | .62** | .38** | .23** | .15** | .12** | .10* | .01 | .36** | .06 |
| 2. BMI, 24 mos. | .80** | .61** | .45** | .40** | .36** | −.08 | .70** | .27** | |
| 3. BMI, 36 mos. | .73** | .57** | .52** | .50** | −.08 | .81** | .38** | ||
| 4. BMI, 54 mos. | .81** | .75** | .69** | −.29** | .85** | .52** | |||
| 5. BMI, age 7 | .92** | .87** | −.52** | .70** | .68** | ||||
| 6. BMI, age 9 | .95** | −.61** | .60** | .80** | |||||
| 7. BMI, age 10 ½ | −.60** | .57** | .78** | ||||||
| 8. Age at AR (mo) | −.18** | −.46** | |||||||
| 9. BMI at AR (kg/m2) | .42** | ||||||||
| 10. CVD Risk, age 15 |
Note. AR: Adiposity Rebound. CVD: Cardiovascular Disease.
p < .01.
p < .05.
Growth Curve Analyses
First, we identified the growth model (linear versus quadratic) for children’s BMI change from 15 months through 10 ½ years, using chi-square difference tests to select the best-fitting model. The first model assumed linear change from 15 months to age 10 ½, fixing each time point to decimal values in accordance with time intervals between assessments. The second model assumed quadratic change across this time period, and provided a significantly better fit to the data than the linear model, χ2D(8)=1,703.74, p<.01, consistent with WHO and CDC growth charts.6 On average, BMI declined from 15 months to 54 months, followed by a quadratic rebound and subsequent BMI increases.
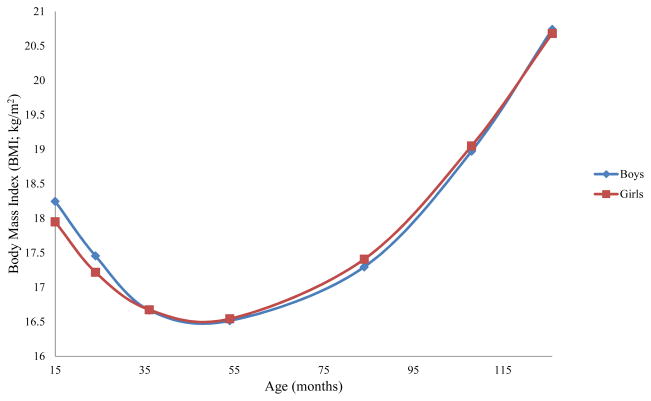
Next, we used gender as a grouping variable to test whether growth patterns differed for boys and girls. Chi-square difference tests revealed that allowing the intercept, χ2D(1)=11.09, p<.01, slope, χ2D(1)=9.36, p<.01, and quadratic term, χ2D(1)=5.41, p<.03, to differ for girls and boys resulted in better model fit than constraining them to be equal across groups. Figure 1 displays boys’ and girls’ trajectories. Boys had higher 15-month BMIs, more rapid declines before the AR, and more rapid quadratic rebounds compared to girls (see Table 3). For boys, but not girls, higher 15-month BMI was associated with steeper initial BMI declines, and this association was significantly different between genders, χ2D(1)=12.39, p<.01. Boys’ higher 15-month BMI was associated with faster rebound growth. The same association was marginal for girls, and was marginally different between genders, χ2D(1)=2.66, p<.10. For both genders, faster initial BMI declines were associated with more rapid rebound growth.
Figure 1.
Boys’ and Girls’ Body Mass Index Trajectories from 15 months through age 10 ½.
Table 3.
Intercept, Slope, and Quadratic Term Means, Variances, and Correlations for Boys and Girls.
| Boys
|
Girls
|
|
|||||
|---|---|---|---|---|---|---|---|
| Mean (SE) | Variance (SE) | Mean (SE) | Variance (SE) | Correlations | |||
| 1. | 2. | 3. | |||||
| 1. Intercept | 18.02 (.07)** | 1.26 (.14)** | 17.65 (.08)** | .64 (.09)** | – | −.42** | .32** |
| 2. Slope | −8.02 (.34)** | 19.03 (2.93)** | −6.48 (.38)** | 12.73 (2.17)** | .04 | – | −.44** |
| 3. Quadratic term | 10.88 (.35)** | 22.05 (3.22)** | 9.59 (.40)** | 21.50 (2.85)** | .17* | −.47** | – |
Note: Significant mean values indicate that the pattern of change indicated by the given parameter is significantly different from zero. Significant variances indicate significant inter-individual variability in the given parameter. Correlations above the diagonal are for boys, correlations below diagonal are for girls.
p < .01.
p < .05.
BMI Trajectories and Adolescent Cardiovascular Risk
After evaluating the growth pattern and gender differences, we added children’s AR age and BMI as covariates, and adolescent CVD risk as an outcome variable predicted by BMI growth parameters. We evaluated model fit using the root mean square error of approximation (RMSEA), comparative fit index (CFI), and Tucker-Lewis index (TLI). Model fit was acceptable, RMSEA=.08, 90% C.I. (.069–.097); CFI=.978; TLI=.961.
Both 15-month BMI and initial BMI declines were unrelated to AR age for boys and girls (Table 4). Earlier AR was associated with more rapid quadratic rebounds for boys and girls. For both genders, higher 15-month BMI and slower initial declines were related to higher AR BMI. BMI at the AR was not associated with quadratic rebound for either gender.
Table 4.
BMI Growth Parameters Predicting Adolescent Cardiovascular Risk.
| Boys
|
Girls
|
|||
|---|---|---|---|---|
| r (SE) | β (SE) | r (SE) | β (SE) | |
| Covariates | ||||
| Age at AR | .05 | – | .02 | – |
| BMI at AR | .00 | – | .00 | – |
| Predictors | ||||
| Intercept | – | .15 (.04)** | – | .16 (.04)** |
| Slope | – | .84 (.08)** | – | .74 (.08)** |
| Quadratic term | – | .86 (.07)** | – | .89 (.06)** |
p <.01.
p < .05.
Growth parameters were associated with adolescent CVD risk, controlling for children’s AR age and BMI (Table 4). For both genders, higher 15-month BMI, slower declines before the AR, and faster quadratic BMI rebounds following the AR predicted higher adolescent CVD risk. There were no significant gender differences in these associations. For boys and girls, associations between AR age, AR BMI, and adolescent CVD risk were nonsignificant when including growth parameters as predictors. Because the inclusion of family income and child ethnicity in analyses did not alter findings, these potential confounds were not included.
Discussion
This study evaluated gender differences in childhood growth trajectories, adding to the literature by comparing boys’ and girls’ BMI growth patterns. Using BMI growth across childhood to predict adolescent CVD risk addresses the reality that disease risk is a developmental process with foundations in childhood. Examining adolescent risk is particularly important because CVD risk factors have become alarmingly prevalent in U.S. adolescents,1 and adolescent CVD risk tracks to adulthood.27 Discovering antecedents to adolescent risk can inform interventions that may alter problematic trajectories associated with adult chronic disease.
Boys and girls did not differ on AR age or BMI. This contributes to mixed findings regarding AR timing, and is consistent with evidence that gender differences may only be found in specific cases, such as the highest BMI percentiles7 or low-income samples.28,29 The current sample contains mostly middle-income Caucasian children, and findings suggest that gender differences in AR timing may not occur in children with these demographic characteristics.16
Boys’ BMI trajectories tended to start higher, decline faster, and rebound faster than girls’, and boys’ growth parameters were all related. For boys only, higher 15-month BMI was associated with faster BMI declines and faster rebounds, suggesting that boys with higher 15-month BMIs undergo the most intense changes in physical growth during this developmental period. This is consistent with CDC and WHO growth charts, which depict faster declines and more rapid rebounds for boys in higher BMI percentiles.6 Despite similar growth standards for high-percentile girls,6 relationships between girls’ 15-month BMI and their BMI declines and rebounds were either nonsignificant or marginal. Thus, while the shape of boys’ childhood growth trajectories could be predicted by 15-month BMI, this was not the case for girls. It is unclear whether biological (e.g., sex-based hormonal changes) or environmental factors account for these differences, but future research should consider whether parental attitudes about boys’ and girls’ body sizes or differential feeding practices contribute to gender differences in BMI trajectory shapes.
Children with higher 15-month BMIs tended to have higher AR BMIs and higher adolescent CVD risk, suggesting that for both genders, higher 15-month BMI increases the likelihood of remaining high in BMI throughout childhood. These findings are consistent with the idea of the persistence of obesity,30 rather than the notion that CVD risk is highest in individuals experiencing rapid catch-up growth following low BMI in infancy.7,31 Because newborns hospitalized for over one week were excluded from the SECCYD, these findings may not represent catch-up growth in premature or low birth weight children.5
Slower BMI declines were associated with higher AR BMI for both genders; children experiencing slower declines likely reach a nadir at a greater BMI value than more rapidly declining children. Slower declines were also strongly associated with higher adolescent CVD risk for both genders, consistent with evidence of persistence of obesity rather than catch-up growth in this sample. The fact that slower BMI declines early in life predicted adolescent CVD risk highlights the importance of closely monitoring growth in late infancy and toddlerhood; beliefs that children should be chubby during this period and that they will outgrow their overweight status likely contribute to a lack of recognition of problematic growth.5 Interestingly, sharper quadratic BMI rebounds were not related to higher AR BMI, indicating that there may be children in this sample who are not persistently high in BMI, but nonetheless experience sharp rebounds along with the associated increases in CVD risk. On average, these individuals were younger at the AR, which may have given them more time to accumulate adiposity than individuals rebounding later.19
Children’s BMI intercepts, slopes, and quadratic terms predicted adolescent CVD risk over and above AR age and BMI. Despite interrelations among growth parameters and AR characteristics, neither age nor BMI at AR were associated with adolescent CVD risk when growth parameters were included as predictors. This suggests that examining childhood growth longitudinally and considering CVD risk as a developmental process unfolding over time provide important predictive information for adolescent health risk.
The current study offers several contributions to the literature. First, the SECCYD contains children growing up in the 1990s during the emergence of the childhood obesity epidemic, providing an excellent opportunity to examine developmental antecedents of obesity and related CVD risk among adolescents. Additionally, this study is among the first to examine gender differences in the shapes of BMI growth during the critical period of the AR. Finally, the model predicting adolescent CVD risk controls for AR age and BMI, establishing childhood growth patterns as important predictors above and beyond the single time point of the AR.
Despite these strengths, the present study is not without limitations. First, although economically diverse, the sample is predominantly Caucasian; there were not enough ethnic minority individuals to make gender by ethnicity comparisons. Because ethnic minority individuals are disproportionately affected by obesity and CVD,32 future work should investigate gender by ethnicity comparisons of childhood BMI trajectories to inform interventions for at-risk populations. However, significant variability in boys’ and girls’ growth parameters suggests a variety of BMI trajectories present within this ethnically homogeneous sample. Techniques like growth mixture modeling (GMM) allow researchers to group children based on trajectory parameters, and use group membership to predict subsequent risk.33 Although overall gender differences found in the current study represent an important first step, future research should use GMM to determine whether distinct trajectory groups exist within genders, and how group membership predicts boys’ versus girls’ CVD risk. Second, time spacing between data collection points was inconsistent; waves from 15 months to age 10 ½ were between 9 and 30 months apart. Although more frequent assessments are ideal for estimating the AR, the benefits of the SECCYD remain, including a large, national sample that provided ample power to conduct quadratic growth curve analyses.
The present study suggests that childhood growth trajectories provide important information for examining the emergence of adolescent CVD risk as a developmental process, as well as a predictive utility for informing early interventions to prevent CVD. Overall, findings suggest that boys and girls with elevated 15-month BMIs that remain persistently high throughout toddlerhood, early childhood, and middle childhood tend to have heightened CVD risk in adolescence. Although children’s intercept, slope, and quadratic growth were interrelated, each parameter independently predicted adolescent CVD risk, suggesting the importance of growth during each developmental period.
Monitoring, charting, and evaluating children’s BMI trajectories before the AR may be particularly useful for detecting problematic growth trajectories before children have already experienced sharp rebound growth. For example, parents of infants and toddlers might consider this a period of watchful waiting, recognizing that genes contribute to children’s weight status,34 but that parental feeding decisions (e.g., breast versus bottle, demand versus scheduled feeding) impact children’s developing self-regulatory abilities, which may ultimately impact weight status.35 Parents of preschoolers and grade-schoolers should encourage physical activity and healthy eating behaviors, not necessarily to promote weight loss in their children, but to build long-term healthy habits that have the potential to ameliorate CVD risk, regardless of children’s weight-related genetic predispositions. Intervening during this critical time period has the potential to prevent the development of CVD risk in adolescence, which has implications for reducing the incidence of adult chronic illness.36,37
Implications and Contribution.
The current study establishes patterns of BMI growth from 15 months to age 10 ½ as important predictors of girls’ and boys’ cardiovascular risk at age 15, over and above the timing of and children’s BMI at the adiposity rebound (AR).
Acknowledgments
The NICHD Study of Early Child Care is a study directed by a Steering Committee and supported by NICHD through a cooperative agreement (U10) that calls for scientific collaboration between the grantees and the NICHD staff.
Abbreviations
- AR
adiposity rebound
- BMI
body mass index
- BP
blood pressure
- CDC
Centers for Disease Control
- CVD
cardiovascular disease
- LCGM
latent growth curve modeling
- SKF
skinfold thickness
- WHO
World Health Organization
- WHR
waist –hip ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.May AL, Kuklina EV, Yoon PW. Prevalence of cardiovascular disease risk factors among US adolescents, 1999–2008. Pediatrics. 2012;129:1035–41. doi: 10.1542/peds.2011-1082. [DOI] [PubMed] [Google Scholar]
- 2.Lobelo F, Pate RR, Dowda M, Liese AD, Daniels SR. Cardiorespiratory fitness and clustered cardiovascular disease risk in US adolescents. J Adolesc Health. 2010;47:352–9. doi: 10.1016/j.jadohealth.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 3.Rolland-Cachera MF, Deheeger M, Maillot M, Bellisle F. Early adiposity rebound: Causes and consequences or obesity in children and adults. Intl J Obesity. 2006;30:S11–S17. doi: 10.1038/sj.ijo.0803514. [DOI] [PubMed] [Google Scholar]
- 4.Nonnemaker JM, Morgan-Lopez AA, Pais JM, et al. Youth BMI trajectories: evidence from the NLSY97. Obesity. 2009;17:1274–80. doi: 10.1038/oby.2009.5. [DOI] [PubMed] [Google Scholar]
- 5.Adair LS. Child and adolescent obesity: Epidemiology and developmental perspectives. Physiol Behav. 2008;94:8–16. doi: 10.1016/j.physbeh.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 6.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC growth charts for the United States: Methods and Development. Hyattsville, MD: National Center for Health Statistics, US Dept of Health and Human Services; 2002. [PubMed] [Google Scholar]
- 7.Rolland-Cachera MF, Deheeger M, Bellisle F, Sempé M, Guilloud-Bataille M, Pataois E. Adiposity rebound in children: A simple indicator for predicting obesity. Am J Clin Nutr. 1984;39:129–35. doi: 10.1093/ajcn/39.1.129. [DOI] [PubMed] [Google Scholar]
- 8.Nader PR, O’Brien M, Houts R, Bradley R, Belsky J, Crosnoe R, et al. Identifying risk for obesity in early childhood. Pediatrics. 2006;118:e594–601. doi: 10.1542/peds.2005-2801. [DOI] [PubMed] [Google Scholar]
- 9.Pryor LE, Tremblay RE, Boivin M, et al. Developmental trajectory of body mass index in early childhood and their risk factors: an 8-year longitudinal study. Arch Pediatr Adolesc Med. 2011;165:906–12. doi: 10.1001/archpediatrics.2011.153. [DOI] [PubMed] [Google Scholar]
- 10.Magee CA, Caputi P, Iverson DC. Identification of distinct body mass index trajectories in Australian children. Pediatr Obes. 2013;8:189–98. doi: 10.1111/j.2047-6310.2012.00112.x. [DOI] [PubMed] [Google Scholar]
- 11.Pi-Sunyer X. Obesity: criteria and classification. Proc Nut Soc. 2000;59:505–9. doi: 10.1017/s0029665100000732. [DOI] [PubMed] [Google Scholar]
- 12.Power C, Lake JK, Cole TJ. Measurement and long-term health risks of child and adolescent fatness. Intl J Obesity. 1997;21:507–26. doi: 10.1038/sj.ijo.0800454. [DOI] [PubMed] [Google Scholar]
- 13.Boonpleng W, Park CG, Gallo AM. Timing of adiposity rebound: A step toward preventing obesity. Pediatr Nurs. 2012;38:37–42. [PubMed] [Google Scholar]
- 14.Eriksson JG, Forsén T, Tuomilehto J, Osmond C, Barker DJP. Early adiposity rebound in childhood and risk of type 2 diabetes in adult life. Diabetologia. 2003;46:190–4. doi: 10.1007/s00125-002-1012-5. [DOI] [PubMed] [Google Scholar]
- 15.Skinner JD, Bounds W, Carruth BR, Morris M, Ziegler P. Predictors of children’s body mass index: A longitudinal study of diet and growth in children aged 2–8. Intl J Obesity. 2004;28:476–82. doi: 10.1038/sj.ijo.0802405. [DOI] [PubMed] [Google Scholar]
- 16.Whitaker RC, Pepe MS, Wright JA, Seidel KD, Dietz WH. Early adiposity rebound and the risk of adult obesity. Pediatrics. 1998;101:e5–e10. doi: 10.1542/peds.101.3.e5. [DOI] [PubMed] [Google Scholar]
- 17.Ojeda NB, Intapad S, Alexander BT. Sex differences in the developmental programming of hypertension. Acta Psychologica. 2014;210:307–16. doi: 10.1111/apha.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Govindan M, Gurm R, Mohan S, Kline-Rogers E, Corriveau N, Goldberg C, et al. Gender differences in physiological markers and health behaviors associated with childhood obesity. Pediatrics. 2013;132:468–74. doi: 10.1542/peds.2012-2994. [DOI] [PubMed] [Google Scholar]
- 19.Guo SS, Huang C, Maynard LM, Demerath E, Towne B, Chumlea WC, et al. Body mass index during childhood, adolescence and young adulthood in relation to adult overweight and adiposity: The Fels Longitudinal Study. Intl J Obesity. 2000;24:1628–35. doi: 10.1038/sj.ijo.0801461. [DOI] [PubMed] [Google Scholar]
- 20.Kroke A, Hahn S, Buyken AE, Liese AD. A comparative evaluation of two different approaches to estimating age at adiposity rebound. Intl J Obesity. 2006;30:261–266. doi: 10.1038/sj.ijo.0803143. [DOI] [PubMed] [Google Scholar]
- 21.Falkner B. Hypertension in children and adolescents: epidemiology and natural history. Pediatr Nephrol. 2010;25:1219–24. doi: 10.1007/s00467-009-1200-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berenson GS, Srinivasan SR, Bao W, Newman WP, Travy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. N Engl J Med. 1998;338:1650–6. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- 23.Muthén L, Muthén B. Mplus User’s Guide. Los Angeles, CA: Muthén & Muthén; 2012. [Google Scholar]
- 24.Must A, Anderson SE. Body mass index in children and adolescents: considerations for population-based applications. Int J Obesity. 2006;30:590–4. doi: 10.1038/sj.ijo.0803300. [DOI] [PubMed] [Google Scholar]
- 25.Berkley CS, Colditz GA. Adiposity in adolescents: change in actual BMI works better than change in BMI z-score for longitudinal studies. Ann Epidemiol. 2007;17:14–50. doi: 10.1016/j.annepidem.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 26.Cole TJ, Faith MS, Pietrobello A, Heo M. What is the best measure of adiposity change in growing children: BMI, BMI %, BMI z-score or BMI centile? Eur J Clin Nutr. 2005;59:419–25. doi: 10.1038/sj.ejcn.1602090. [DOI] [PubMed] [Google Scholar]
- 27.Mulye TP, Park MJ, Nelson CD, Adams SH, Irwin CE, Brindis CD. Trends in adolescent and young adult health in the United States. J Adolesc Health. 2009;45:8–24. doi: 10.1016/j.jadohealth.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 28.Irigoyen M, Glassman ME, Chen S, Findley SE. Early onset of overweight and obesity among low-income 1- to 5-year olds in New York City. J Urban Health. 2008;85:545–54. doi: 10.1007/s11524-008-9285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kain J, Corvalan C, Lera L, Galván M, Uauy R. Accelerated growth in early life and obesity in preschool Chilean children. Obesity. 2009;17:1603–8. doi: 10.1038/oby.2009.37. [DOI] [PubMed] [Google Scholar]
- 30.Cole TJ. Children grow and horses race: Is the adiposity rebound a critical period for later obesity? BMC Pediatrics. 2004;4 doi: 10.1186/1471-2431-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dulloo AG, Jacquet J, Montani J. Pathways from weight fluctuations to metabolic diseases: Focus on maladaptive thermogenesis during catch-up fat. Intl J Obesity. 2002;26:S46–S57. doi: 10.1038/sj.ijo.0802127. [DOI] [PubMed] [Google Scholar]
- 32.Pi-Sunyer FX, Becker DM, Bouchard C, Carleton RA, Colditz GA, Dietz WH, et al. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. National Heart, Lung, and Blood Institute, National Institutes of Health, US Dept of Health and Human Services; 1998. [Google Scholar]
- 33.Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol. 2010;6:109–38. doi: 10.1146/annurev.clinpsy.121208.131413. [DOI] [PubMed] [Google Scholar]
- 34.Ventura AK, Loken E, Birch LL. Developmental trajectories of girls’ BMI across childhood and adolescence. Obesity. 2009;17:2067–74. doi: 10.1038/oby.2009.123. [DOI] [PubMed] [Google Scholar]
- 35.Birch LL, Fisher JO. Development of eating behaviors among children and adolescents. Pediatrics. 1998;101:539–49. [PubMed] [Google Scholar]
- 36.Eckberg J, Angbratt M, Valter L, Nordvall M, Timpka T. History matters: Childhood weight trajectories as a basis for planning community-based obesity prevention to adolescents. Intl J Obesity. 2012;36:524–8. doi: 10.1038/ijo.2011.263. [DOI] [PubMed] [Google Scholar]
- 37.Sobol-Goldberg S, Rabinotitz J, Gross R. School-based obesity prevention programs: A meta- analysis of randomized controlled trials. Obesity. 2013;21:2422–28. doi: 10.1002/oby.20515. [DOI] [PubMed] [Google Scholar]