Abstract
This study was designed to test the hypothesis that prenatal exposure of guinea pigs to the organophosphorus (OP) pesticide chlorpyrifos (CPF) disrupts the structural and functional integrity of the brain. Pregnant guinea pigs were injected with chlorpyrifos (20 mg/kg, s.c.) or vehicle (peanut oil) once per day for ten consecutive days, starting approximately on the 50th day of gestation. Cognitive behavior of female offspring was examined starting at 40–45 post-natal days (PND) using the Morris Water Maze (MWM), and brain structural integrity was analyzed at PND 70 using magnetic resonance imaging (MRI) methods, including T2-weighted anatomical scans and Diffusion Kurtosis Imaging (DKI). The offspring of exposed mothers had significantly decreased body weight and brain volume, particularly in the frontal regions of the brain including the striatum. Furthermore, the offspring demonstrated significant spatial learning deficits in MWM recall compared to the vehicle group. Diffusion measures revealed reduced white matter integrity within the striatum and amygdala that correlated with spatial learning performance. These findings reveal the lasting effect of pre-natal exposure to CPF as well as the danger of mother to child transmission of CPF in the environment.
Keywords: Chlorpyrifos, MRI, diffusion tensor imaging, prenatal exposure, neurodevelopment
1. Introduction
Prenatal exposure to organophosphorus (OP) pesticides such as chlorpyrifos (CPF) is a major public health concern (Crinnion 2010). The developing fetus is particularly vulnerable to OP toxicants by infiltration of the maternal blood supply (Whyatt and Perera 1995; Lassiter et al. 1998; Bradman et al. 2003), and absorbs an approximately four-fold greater amount than the exposed mother due to accumulation in fetal tissues (Hunter et al. 1999; Akhtar et al. 2006). For populations that live or work in agricultural areas, this exposure is notoriously difficult to avoid and harmful to the health and proper development of growing children (Hanke and Jurewicz 2004). Epidemiological studies have reported that children exposed to CPF in the womb suffer serious neurodevelopmental outcomes such as decreased intelligence quotient (Bouchard et al. 2011; Engel et al. 2011; Rauh et al. 2011), neurodevelopmental delays (Lovasi et al. 2011), a high prevalence of attention deficit disorders (Rauh et al. 2006; Marks et al. 2010), and even overt reductions in brain cortical thickness (Rauh et al. 2012).
Organophosphorus (OP) pesticides account for more than 50% of the pest-control agents used worldwide. Chlorpyrifos in particular is popular for its low cost and broad effectiveness against most forage insects and soil pests. Though banned for residential use since 2001 (U.S. EPA 2006), it remains to be the most common OP insecticide in the United States, where use has been used in the range of 3.2 to 4.1 thousand tons/year since 2008 (Solomon et al. 2014) and still accounts for hundreds of cases of acute intoxication per year (US EPA 2013). Residential use of CPF is still prevalent in the European Union and in many developing countries (Saunders et al. 2012), where exposure is also a major concern.
The acute toxicity of CPF and related OP compounds is primarily due to their irreversible inhibition of acetylcholinesterase (AChE), an enzyme that hydrolyzes the neurotransmitter acetylcholine in the central and peripheral nervous system. The chronic neurodevelopmental effects of CPF of interest to this study are believed to arise by a different mechanism, as they occur even at dosages well below the threshold for signs of acute intoxication (Monnet-Tschudi et al. 2000; Slotkin et al. 2006). Prenatal development depends on the proper balance and timing of neurotropic factors. As such, an excess of cholinergic stimulation during a critical stage is very likely to inflict developmental abnormalities (Rice and Barone 2000; Slotkin 2004). Preclinical studies show a multitude of effects that are more complex than simple cholinergic toxicity (reviewed in Li et al., 2012). For example, CPF exposure results in altered serotonin and dopamine signaling in rats exposed during the neonatal and prenatal periods (Aldridge et al. 2005), disruption of the adenylyl cyclase cascade in neonatally exposed rats (Song et al. 1997), altered gene expression in the brain of postnatally exposed rats (Betancourt et al. 2006), perturbed axonal and dendritic growth in rat neuronal cell culture (Howard et al. 2005), and impaired performance in rats and mice on behavioral tests such as the passive avoidance task (Vatanparast et al. 2013), Morris water maze (Turgeman et al. 2011), a novel foraging behavior maze (Haviland et al. 2010), and in the 16-arm radial maze (Icenogle et al. 2004).
The present study was designed to test the hypothesis that in utero exposure of guinea pigs to CPF causes cognitive deficits that are accompanied by disruption of the structural integrity of cerebral cortical structures. Guinea pigs (Caviaporcellus) were selected as the animal model for two primary reasons: their similarities to humans in terms of relative brain development prior to birth (Dobbing& Sands, 1970), and the sensitivity of their cholinergic system to OP compounds (Inns and Leadbeater 1983; Fonnum et al. 1985). We exposed guinea pigs to sub-acute doses of CPF or to peanut oil during a critical prenatal period of brain development. Upon adulthood, we assessed their spatial learning performance in the Morris water maze and the structural integrity of their brains in in vivo magnetic resonance imaging (MRI). The MRI portion of the study focused on three types of analyses. From conventional T2-weighted images, we obtained accurate in vivo volumetric measurements of the forebrain. The prenatal period of interest to this study is a peak time of brain growth in both guinea pigs and humans, particularly in the frontal and striatal areas of the brain (Dobbing and Sands 1970; Kalaria and Prince 1988). From diffusional kurtosis imaging (DKI) analyses, we obtained measurements of fractional anisotropy (FA) and diffusivity, which are informative of the structural integrity of white matter (Jellison et al. 2004; Hüppi and Dubois 2006) as well as kurtosis metrics that have been strongly linked to the integrity of cellular microstructure (Cheung et al. 2009; Zhuo et al. 2012).
The results presented here demonstrate that following prenatal exposure to CPF, female guinea pigs present with spatial learning deficits that are accompanied by disruption of the structural integrity of the corpus callosum, striatum, and amygdala – brain regions known to play key roles in processing cognitive functions. These findings are far reaching as they provide timely input for a comprehensive assessment of the developmental toxicity of CPF. In addition, they provide evidence to support the concept that the guinea pig is a translationally relevant model of prenatal exposure to OP pesticides. Finally, these results help guide future evaluation of therapeutic approaches aimed at treating neurological disorders resulting from in utero pesticide exposure.
2. Materials and Methods
2.1 Animal model
Pregnant female Hartley guinea pigs were purchased from Charles River Laboratories (Wilmington, MA). On arrival, guinea pigs were at gestation day (GD) 33–38. Animals were kept in a light and temperature-controlled animal care facility, with food and water provided ad libitum. Starting on approximately the 50th day of gestation, dams were given a subcutaneous injection between the shoulder blades consisting of either CPF dissolved in peanut oil (25 mg/kg) o peanut oil (0.5 ml/kg) as a vehicle control. The treatments were repeated every 24 h consecutively for a total of ten days. CPF and peanut oil were purchased from Sigma Aldrich (St. Louis, MO). The oral LD50 of chlorpyrifos in guinea pigs is 504 mg/kg, and the oral and subcutaneous LD50s of OP compounds are generally very similar (McCollister et al. 1974). The cumulative dose of OP used here is below the 1.6 x LD50 threshold for reliable organophosporus-induced seizures in rats (Shih and McDonough 1997). The 50th day of gestation was chosen as it coincides with the timing for the brain growth spurt (GD ~ 50) and the period of rapid myelination (GD ~ 60) in guinea pigs (Kapoor and Matthews 2008). Both humans and guinea pigs are precocial species, in which the majority of brain development occurs prior to birth. By comparison, altricial species such as rats and mice instead exhibit a postnatal brain growth spurt (Dobbing and Sands 1970). Guinea pigs and humans also share lower levels of circulating carboxylesterases that hydrolyze OP compounds when compared to the high levels found in rats and mice (Inns and Leadbeater 1983; Fonnum et al. 1985).
Animals were dosed with CPF subcutaneously rather than orally because the soft palate of the guinea pig is continuous with the base of the tongue, and the opening through which to pass a feeding tube is very small. Oral gavage is also very stressful to guinea pigs and interferes with their normal feeding behavior. Animals were monitored every 15 minutes after treatment for the first 2 hours, and then every hour for the next 8 hours. No animals displayed signs of overt toxicity. All experiments were carried out in accordance with the rules and regulations set forth by the University of Maryland School of Medicine Institutional Animal Care and Use Committee regarding the care and use of animals under a protocol approved by the committee, and complied with the principles of the ‘1996 Guide for the Care and Use of Laboratory Animals.
Guinea pigs were born on gestation day 67–72 and weaned on postnatal day (PND) 15–20. After weaning, offspring were housed according to sex in groups of two to four animals/cage. Starting on PND 40–45, the cognitive behavior of the female guinea pigs was assessed by their ability to find a hidden platform in the Morris water maze as described below. The present study focused exclusively on female offspring because previous studies have provided evidence that the cognitive deficits that develop following prenatal exposure of rodents to CPF are sex selective, being more pronounced among females than males (Levin et al. 2002; Haviland et al. 2010).
Magnetic resonance imaging (MRI) of humans and rodents has shown that short-term learning tasks trigger transient, albeit significant structural changes in white and grey matter of specific brain regions, including the hippocampus (Driemeyer et al. 2008; Sagi et al. 2012; Hofstetter et al. 2013). Therefore, in the present study, animals were subjected to MRI no earlier than 10 days after the last training day in the Morris water maze.
2.2 MRI experiments
All experiments were performed on a Bruker Biospec 7.0 Tesla 30 cm horizontal bore scanner equipped with a BGA20S gradient system interfaced to a Bruker Paravision 5.1 console. A Bruker four-element 1H surface array coil was used as the receiver and a Bruker 154 mm birdcage coil as the transmitter.
Anesthesia was induced by placing each guinea pigs in an induction chamber with a gas mixture of O2 (1 L/min) and isoflurane (4%; IsoFlo, Abbot Laboratories, North Chicago, IL). Following an induction period lasting approximately 5–10 minutes, each animal was placed prone in an animal holder and the four-element surface array coil was fixed over the brain of the animal. The animal holder was moved to the center of the magnet and the isoflurane level was set to 3%. The level of isoflurane was further adjusted based on the respiration rate changes of the animal for the remainder of the experiment. An MR-compatible small-animal monitoring and gating system (SA Instruments, Inc., New York, USA) was used to monitor respiration rate, and body temperature was maintained at 36–37°C using a silicone pad with warm water circulation. Body weight was measured using a digital scale at the end of the MR scan session.
A scout image consisting of three slices (axial, mid-sagittal, and coronal) was used to localize the guinea pig brain for later scans. A FASTMAP rapid shimming protocol (Gruetter 1993) was used to reduce the external magnetic field inhomogeneity in the region of interest. Total acquisition time was 12 s.
For anatomical reference, fast spin echo based T2-weighted MR images were obtained with a repetition time/effective echo time (TR/TEeff) of 6197/60 ms, 8 echo trains, a 35×35 mm2 field of view (FOV), and a matrix size of 256×256. A total of 20 slices at 1 mm slice thickness and two averages were obtained in the coronal plane for a total acquisition time of 7 min, 21 s.
DKI images were acquired at the same coronal slice locations as the matching T2-weighted images using a single shot spin echo EPI sequence with a TR/Teeff of 8500/45 ms, a matrix size of 96×96, 30 gradient directions, and two b-values (1000 s/mm2 and 2000 s/mm2). Five averages of b = 0 were also obtained. A total of 20 coronal slices at 1 mm slice thickness and two averages were obtained for a total acquisition time of 18 min, 25 s.
2.3 Morris Water Maze Test
Guinea pigs were trained to find a hidden platform in the Morris water maze using a procedure similar to that described in Mamczarz et al., (2011). This test was selected for its sensitivity to spatial behavior, procedural learning and memory deficits in guinea pigs (Byrnes et al., 2004; de Groot et al., 2001; Dringenberg et al., 2001; Filliat et al., 2002; Lewejohann et al., 2010). Briefly, guinea pigs were subjected to five days of training in a galvanized pool (198-cm diameter) filled to a 40-cm depth with tap water made opaque with tempera paint. Each day, guinea pigs were trained to find the platform (20-cm diameter) in four 90-s trials interspaced by 15-s inter-trial intervals. Escape latency was the primary output variable of interest, defined as the time in seconds until the animal was detected in the center of the platform. The starting quadrant was assigned pseudo-randomly for each trial, while the submerged platform was kept in a constant position. A video camera coupled with the video tracking Any-Maze software (Stoelting Co, Wood Dale IL) was used to record and track the swimming of the animals during the experiment.
2.4 MR Image Analysis
All imaging data were transferred to an off-line server where the image analysis was performed. The T2-weighted anatomical images were used to manually identify brain regions including the hippocampus, forebrain, parenchyma (white + gray matter), and cerebrospinal fluid (CSF). These specific regions of interest (ROIs) were drawn within the Medical Image Processing, Analysis, and Visualization software (MIPAV v5.3.1, CIT, NIH, Bethesda, MD; McAuliffe et al., 2001). A guinea pig brain atlas (Rapisarda and Bacchelli, 1977) was used as a reference to aid the delineation of the individual ROIs.
Images obtained from the DKI acquisition were motion-corrected prior to reconstruction using in house-developed software in MATLAB (Mathworks, Natick, MA) according to a previously published method (Zhuo et al. 2012). Maps of the various diffusion parameters including fractional anisotropy (FA), mean diffusivity (MD), radial (RD) and axial (AD) diffusivity, radial kurtosis (RK) and mean kurtosis (MK) were generated. These parameters provide measures of the linearity or directional restriction of water diffusion (FA), diffusion or kurtosis in all directions (MD, MK) and diffusion or kurtosis in the axial or radial directions (AD, AK, RD, RK). ROIs queried from diffusion images included the whole forebrain, medial corpus callosum, medial cerebral cortex, hippocampi, thalamus, striatum, and the amygdalae. An example of the aforementioned set of ROIs is displayed for reference in figure 1, below.
Figure 1.
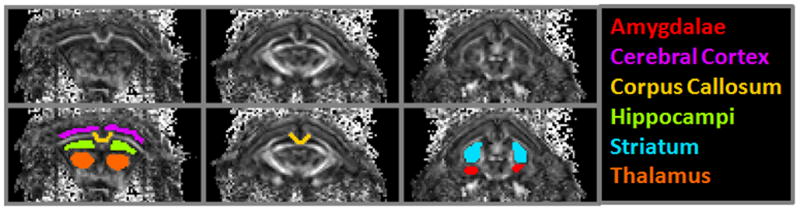
Representative ROIs sampled for DKI analysis, using select cropped slices of an FA image as a grayscale reference background. ROIs for each slice in the upper panel are shown overlaid in the lower panel. These include the cerebral cortex, hippocampi, striatum, thalamus, amygdalae, and corpus callosum. Whole forebrain ROIs were also drawn for analysis (not shown).
Luxol Fast Blue Staining
After the completion of MRI and behavioral studies, guinea Ppgs were placed under isoflurane anesthesia and their brains were fixed via transcardial perfusion using a volume of 120 ml 1% paraformaldehyde in phosphate buffered saline (PBS, pH 7.4) until blood was cleared. This procedure was followed immediately by 200 ml 4% paraformaldehyde in PBS. Brains were then removed and stored at 4°C in 4% paraformaldehyde in PBS for 4 days, after which time the brains were transferred to cold PBS for 24 h, followed by storage in 30% sucrose for 4 days at 4°C for cryoprotection. Brains were then frozen under crushed dry ice for 2 min, wrapped in labeled foil, and stored at −80°C until sectioned. Coronal brain sections were cut at 20-μm thickness using a cryostat and placed on gelatin-coated slides.
Slides were chosen corresponding to plates #760 to #800 of the guinea pig brain atlas provided by University of Wisconsin, Madison Brain Collection from 14 female guinea pigs. Luxolfast blue staining was used for myelin visualization using a Luxol Fast blue kit (IHC World LLC, Woodstock, MD). Slides were allowed to come to room temperature and sequentially washed in 100% ethanol and 95% ethanol before being placed in Luxol Fast Blue solution overnight at 57°C. Slides were then washed in 95% ethanol and differentiated twice in lithium carbonate solution(IHC World LLC, Woodstock, MD) for 30 s followed by 70% ethanol for 30 s followed by a wash in distilled water. Slides were then allowed to air dry for 1 h before being put into 95% ethanol for 1 min and 100% ethanol in 3 min followed by a xylene bath until being coverslipped. Slides were mounted using DPX mounting medium (Sigma-Aldrich, St. Louis, MO), coverslipped and stored at room temperature. Images of tissue were acquired using Nikon E1000 Microscope Eclipse/video-based Image 1 analysis system (Nikon Instruments Inc., Melville, NY). Images in the fields of the lateral nucleus of the amygdala were captured using a 10X objective. Images were blindly subjected to intensity analysis using Nikon NIS-Elements Advanced Research image software (Nikon Instruments Inc, Melville, NY). Mean intensity measurements were obtained from four circular (radius 150 μm) regions of interest interspaced by 75 ± 10 μm in the lateral nucleus of the amygdala of each animal.
2.5 Statistical Analysis
Results in the text are presented as means ± standard deviation. Statistical comparisons were made between the chlorpyrifos and vehicle (peanut oil) groups using the multivariate General Linear Model (GLM) function in SPSS (SPSS Statistics for Windows, Version 21, Armonk, NY: IBM Corp.). Dependent values were compared using group (CPF or vehicle) as the factor, and the GLM least squares corrected model was used to correct for correlated measures within each modality (behavioral, volumetric, diffusion). Pearson’s r bivariate correlations were used to assess the relationship between MWM, volumetric measures, and diffusion parameters.
3. Results
3.1 Morris Water Maze Performance
Learning to escape onto the hidden platform in the Morris water maze was significantly impaired among guinea pigs that had been prenatally exposed to CPF. A repeated measures ANOVA with training day as a repeated measure factor revealed a significant main effect of training day [F (4, 72) = 18.768; p < 0.001] and treatment [F (1, 18) = 4.435; p = 0.05) on escape latency (Figure 2A), with guinea pigs that had been exposed prenatally to CPF taking longer to escape onto the hidden platform than animals that had been exposed to PO. The swim speeds of the guinea pigs were comparable regardless of whether they had been prenatally exposed to CPF or PO (inset figure 2A). A learning index was defined as the mean escape latency of the five days of training for the guinea pigs in each treatment group, as described in Mamczarz et al. (2011). As shown in Figure 2B, the learning index of CPF-exposed guinea pigs was significantly longer than that of PO-exposed animals, a finding that indicates that prenatal exposure to CPF leads to cognitive deficits in young adult guinea pigs.
Figure 2.
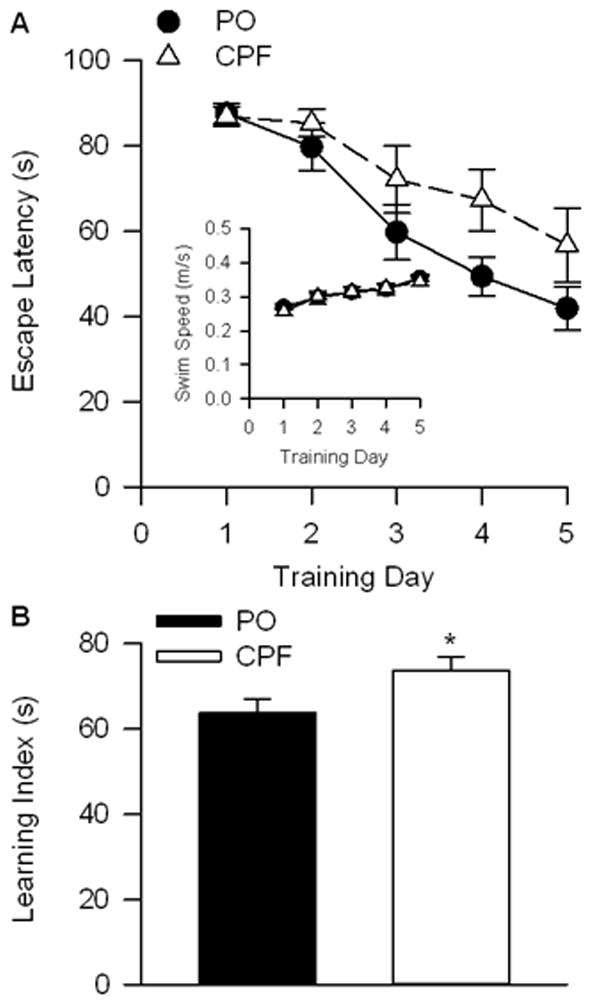
Morris water maze (MWM) results. A)mean escape latency in seconds for each of five training days (1–5). Error bars represent SEM. B) Learning index as the mean and SEM for all five training days.
3.2 Body Weight and Forebrain Volume
The present study reports results obtained from twenty female offspring born from seven pregnantguinea pigs. Three of these dams were injected with CPF, bearing 10 (n = 2, 4, 4) female offspring, and four were injected with PO, bearing 10 (n = 1, 2, 2, 5) female offspring. At the time the animals were imaged (i.e., on PND 65–76), guinea pigs that had been prenatally exposed to CPF had approximately 11.0% lower body weight (n = 10, 441.7 ± 63.4 g) than those exposed only to peanut oil (n = 10, 496.7 ± 47.4 g), p = .041.
Analysis of in vivo MRI revealed that the overall volume of the forebrain including parenchyma & internal CSF space was ~4.9% lower among CPF exposed guinea pigs (n = 10, 2690.0± 34.0 mm3) than among the control, age-matched animals (n = 10, 2827.4 ± 23.9 mm3, p = .004). Likewise, forebrain parenchymal volume was ~4.8% lower among CPF-exposed guinea pigs (n = 10, 2603.0 ± 102.1 mm3) than among control animals (n = 10, 2734.0 ± 80.9 mm3, p = 0.005). Body weight was uncorrelated with forebrain or parenchymal volume, indicating that these differences were not simply dependent on the overall body weight. As shown in Figure 3, significant reductions of volume by prenatal exposure to CPF were confined to the frontal areas of the brain.
Figure 3.
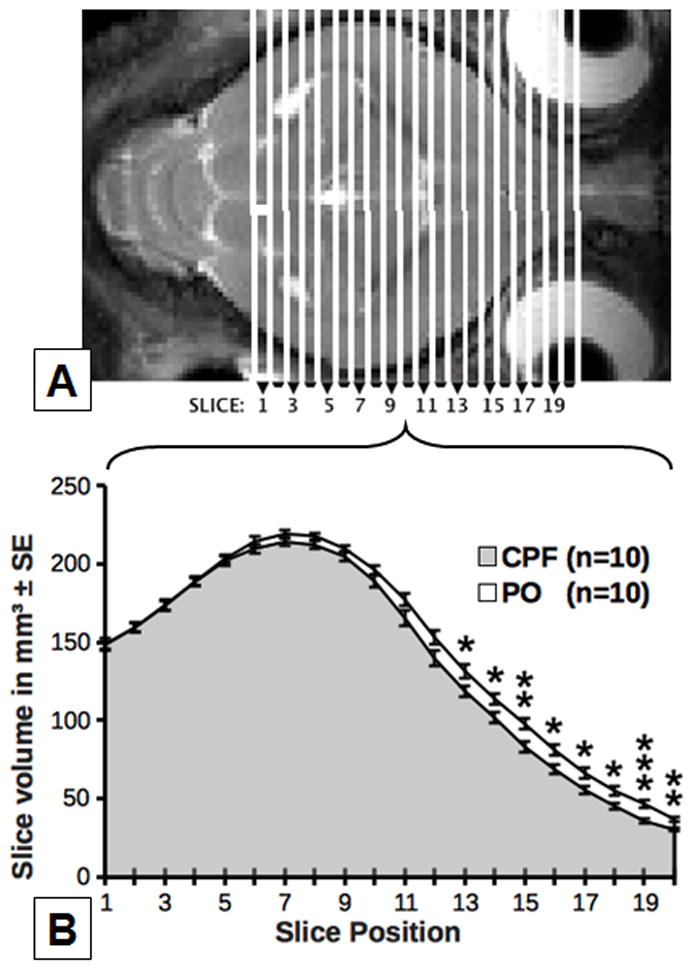
Effects of CPF on brain volume on a slice-by-slice basis in comparison with vehicle-injected (PO) animals. The axial T2-weighted image above (A) displays the anatomical slice location for the volumes shown on the line graph below (B).* within slice indicates p < 0.05; ** indicates p <0.01, *** indicates p < 0.001.
In agreement with the anterior volume reductions found above, the CPF group had an ~8.3% reduction in striatal volume (n = 10, 102.3 ± 5.9 mm3) compared to the PO group (n = 10, 111.5 ± 6.8 mm3, p = .005). Striatal volume was very strongly correlated with both forebrain volume (r = .834, p < .001) and parenchymal volume (r = .837, p = .000). While the volume of the amygdalae was also of interest, their borders could not be clearly delineated on T2 images, precluding an accurate volumetric analysis.
3.3 Diffusion Measures
As in other animal species, fractional anisotropy (FA) and axial diffusivity (AD) were higher in the corpus callosum, the largest white matter structure in the brain, than in the amygdala, cortex, hippocampus, striatum, and thalamus of all guinea pigs (Figure 4). While prenatal exposure to CPF caused a significant reduction of FA in the corpus callosum (p = .014), no effect was detected by the other diffusivity measures (MD, AD, RD). Analysis of the DKI parameters, which are strongly linked to the complexity of the cellular microstructure, provided additional evidence of the impact that prenatal exposure to CPF has on the structural integrity of the corpus callosum. As shown in Figure 5, all three kurtosis measures; MK (p = .009), AK (p = .008), and RK (p= .023) were significantly lower in the corpus callosum of offspring that had been prenatally exposed to CPF compared to control animals.
Figure 4.
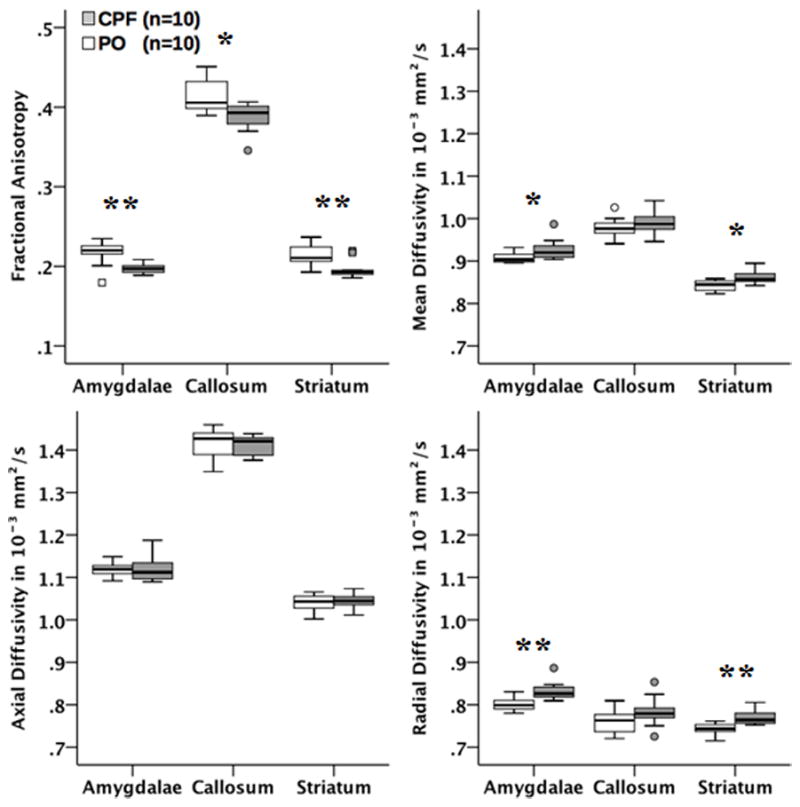
Boxplots of the effects of CPF on diffusivity changes as determined by DKI parameters (FA, MD, AD, RD) in several brain areas. Significant changes between the two groups were observed. * indicates p < 0.05; ** indicates p <0.01.
Figure 5.
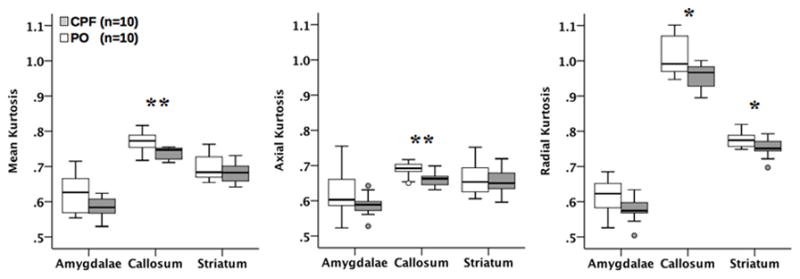
Boxplots of the effects of CPF on microstructural complexity changes as determined by DKI parameters (MK, AK, RK) in several brain areas. Significant changes between the two groups were observed. * indicates p < 0.05; ** indicates p <0.01.
DKI parameters also differed strongly within the striatum and amygdalae (see Figures 4&5). In the striatum there was a decrease in FA (p = .006) and an increase in MD, RD, and RK (p = .012, p = .002, = .048, respectively) in the CPF group. The amygdalae displayed the same pattern, with a decrease in FA (p = .002) and increase in MD and RD (p = .024, p = .002, respectively). At the forebrain level, only RK was reduced in the CPF group (p = .038). This consistent pattern of diffusion parameter changes (decreased FA & MK, increased MD & RD) in the striatum and amygdalae strongly suggests compromised axonal integrity or demyelination among the CPF treated animals. To confirm imaging findings, LuxolFast Blue staining was used to assess myelination in the lateral nucleus of the amygdala in the guinea pigs exposed to CPF and PO. As shown in Figure 6, the intensity of LuxolFast Blue staining was significantly lower (p<0.05) in the lateral nucleus of the amygdala of female guinea pigs that had been prenatally exposed to CPF compared to the same nucleus of age-matched female guinea pigs that had been prenatally exposed to PO vehicle. This histological finding supports the notion that prenatal exposure of guinea pigs to chlorpyrifos results in significant demyelination in the amygdala of young adult female guinea pigs. The observed DKI alterations were specific to the corpus callosum, striatum, and amygdalae and were not evident in the other regions examined, including the medial cerebral cortex, hippocampus, and thalamus.
Figure 6.
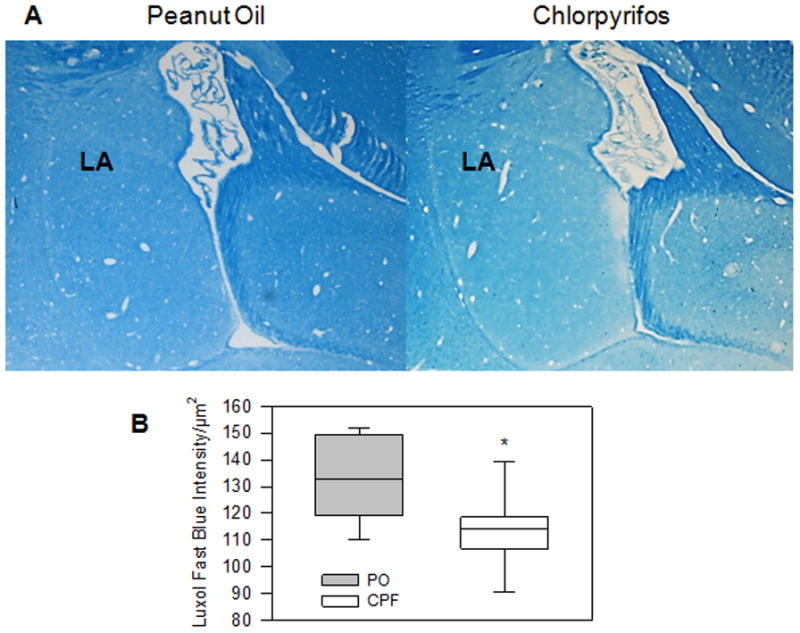
Myelination in the lateral nucleus of the amygdala of guinea pigs prenatally exposed to chlorpyrifos or peanut oil vehicle. A. Representative photomicrographs of Luxol Blue staining of slices from a guinea pig that was prenatally exposed to peanut oil (left) and from an age-matched guinea pig that was prenatally exposed to chlorpyrifos (right). B. Quantification of the intensity of Luxol fast blue staining in the lateral nucleus (LA) of the amygdala of guinea pigs that had been prenatally exposed to peanut oil (PO, n = 6) or chlorpyrifos (CPF, n = 7) was done using slices that were not counterstained. *, p < 0.05 according to the unpaired t-test.
3.4 Relationship between forebrain volume and diffusion measures
The correlation between forebrain volume and diffusion measures was examined to determine if the microstructural integrity of the affected areas was related to overall forebrain volume. Forebrain volume was closely related to diffusion measures in the striatum, being negatively correlated with striatal MD (r = −.496, p = .026) and RD (r = −.507, p = .022) and positively correlated with striatal MK (r = .551, p = .012), AK (r = .463, p = .040), and RK (r = .580, p = .007) as shown by the scatter plots in Figure 7. Significant positive correlations were also found between forebrain volume and measures of FA (r = .462, p = .041), MK (r = .451, p = .046), and AK (r = .475, p = .034) in the corpus callosum, as shown by the scatter plots in Figure 8. These findings are consistent with expectations of higher axonal integrity being associated with a more normal or “healthy” brain volume. All other ROIs used in the DKI measures were not significantly correlated with forebrain volume. Striatal volume and striatal diffusion measures were not significantly correlated (data not shown), and amygdala volume was not calculated as the borders of these structures could not be accurately delineated on T2 images
Figure 7.
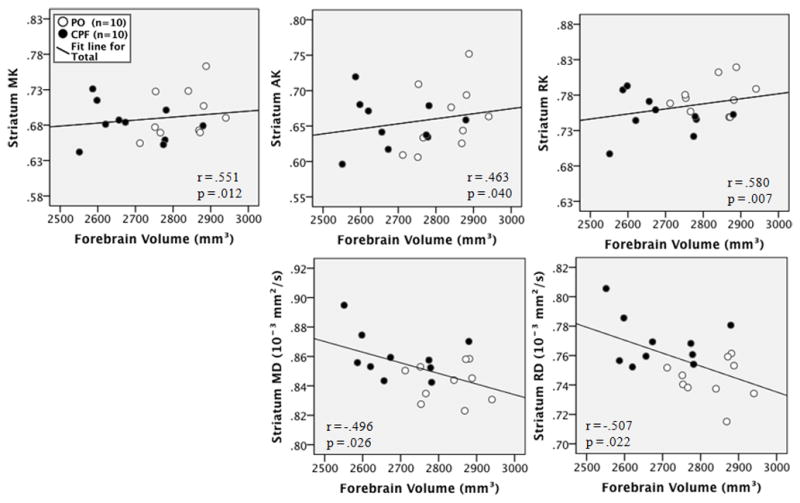
Scatterplots of the correlation between forebrain volume and striatal DKI measures for each group. Black dots indicate the CPF group, white indicates the PO (vehicle) group.
Figure 8.
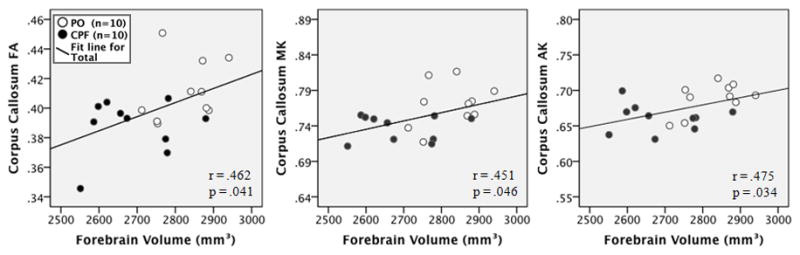
Scatterplots of the correlation between forebrain volume and corpus callosum DKI measures for each group. Black dots indicate the CPF group, white indicates the PO (vehicle) group.
3.5 Relationship between Water Maze Performance and Diffusion Measures
The mean escape latency was positively correlated with striatal MD (r = .535, p = .015) and RD (r = .497, p = .026, as shown in the scatterplots in Figure 9. In the amygdalae, MWM escape latency was positively correlated with MD (r = .494, p = .027) and RD (r = .560, p = .010) and negatively correlated with FA (r = −.520, p = .019), MK (r = −.473, p = .035), and AK (r = −.552, p = .012), as shown in the scatterplots in Figure 10. In the corpus callosum, negative correlations were observed between MWM latency and callosal FA (−.462, r = .041) MK (r = −.460, p = .041), and AK (r = −.472, p = .036), as shown in the scatterplots in Figure 11. All other ROIs used in the DKI measures were not significantly correlated with MWM escape latency. These results demonstrate that spatial learning performance in the Morris water maze is related to the structural integrity of the corpus callosum, striatum, and amygdalae.
Figure 9.

Scatterplots of the correlation between MWM escape latency and striatal DKI measures for each group. Black dots indicate the CPF group, white indicates the PO (vehicle) group.
Figure 10.
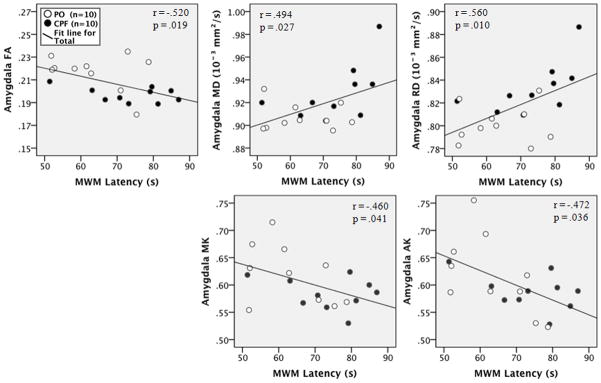
Scatterplots of the correlation between MWM escape latency and amygdalar DKI measures for each group. Black dots indicate the CPF group, white indicates the PO (vehicle) group.
Figure 11.
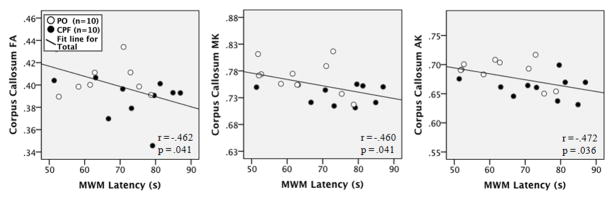
Scatterplots of the correlation between MWM escape latency and corpus callosum DKI measures for each group. Black dots indicate the CPF group, white indicates the PO (vehicle) group.
Discussion
The present study demonstrates that exposure of guinea pigs to a sub-acute dose of the OP pesticide CPF during a critical period of prenatal development causes significant spatial learning deficits later in life. These striking deficits are accompanied by a significant reduction of forebrain volume and disruption of the structural integrity of the corpus callosum, amygdala, and striatum – brain regions known to have a critical role in cognitive processing. These results reveal the crippling effect of CPF on neurodevelopment and highlight the dangers associated with maternal CPF exposure.
Analyses of T2-weighted images provide detailed volumetric measurements of whole brain and specific ROIs without the need for destructive histology. In humans, gross brain volumetric changes measurable by MR anatomical scans have been observed as a result of prenatal exposure to sub-acute doses of CPF (Rauh et al. 2012). Earlier histological studies on prenatal exposure of guinea pigs to the related OP compound trichlorfon revealed a severe reduction in brain weight and hypoplasia of the cerebellar cortex in the affected animals(Mehl et al. 1994, 2007). The prenatal period examined in the present study is a peak time for brain growth in guinea pigs, particularly in the frontal and striatal areas (Dobbing and Sands 1970; Kalaria and Prince 1988). Accordingly, the volumetric reductions in the CPF group consisted of an attenuation of the anterior portion of the forebrain, leaving the posterior areas relatively intact. This is likely due to the sensitive developmental stage the fetal brain was engaged in at the time of exposure. In terms of neurodevelopmental timing, Guinea pigs and humans are both precocial species and thus comparable in the timing of neurodevelopment, with the “brain growth spurt” occuring prenatally (Altman and Das 1967; Mehl et al. 2007). It is during this rapid period of prenatal growth that the developing brain tissue is most vulnerable to disruption through interaction with an external agent (Eriksson 1997). Much like humans, a guinea pig also has an effectively mature striatum at birth, while the striatum of a mouse or rat is only approximately 50% mature at that time (Kalaria and Prince 1988). This links the timing of the CPF exposure to that of striatal development, and further emphasizes the importance of using guinea pigs in studies of early neurodevelopment. It is apparent from these results that prenatal exposure to CPF has stifled the development of the striatum during an extremely sensitive period of its growth.
In the current study we also probed the microstructural properties of brain tissue using advanced imaging techniques. DKI provides information about subtle microstructural changes such as white matter integrity and reactive astrogliosis that are not observable by other structural MR imaging methods (Zhuo et al. 2012). The values derived from DKI scans include information about the extent, constraint, and direction of water diffusion as well as its deviation from a Gaussian distribution. In addition to the standard diffusion tensor parameters such as FA, MD, RD, and AD, the DKI acquisition is also able to provide parameters such as MK, RK, and AK. While FA and diffusivity values have been proven to be effective in characterizing white matter, the use of kurtosis is relatively new. The kurtosis values describe the integrity of water-impeding microstructures such as cell membranes, axons, and myelin in that voxel, with higher kurtosis indicating an increase in the amount and complexity of these barriers to diffusion. Kurtosis measures also are deemed more sensitive than standard diffusion measures for the purpose of detecting microstructural changes within gray matter (Hui et al. 2008; Cheung et al. 2009).
Prenatal exposure of guinea pigs to CPF resulted in significant reduction in FA and in kurtosis measures (MK, AK, and RK) within the corpus callosum. In the amygdala of CPF-exposed animals, kurtosis measures remained unchanged. However, FA was lower and both MD and RD were higher in the amygdala of CPF-exposed guinea pigs than in control animals. Quantification of Luxol fast blue has been used previously (Deshmukh et al., 2013; Ihara et al., 2010) to understand the role myelination plays in various disease states. In this study we examined the effect of prenatal exposure to CPF on the staining intensity of Luxol fast blue in the amygdala of guinea pigs. Guinea pigs exposed prenatally to chlorpyrifos show marked reduction in myelination within the lateral nucleus of the amygdala compared to those prenatally exposed to PO vehicle. This is in line with the imaging findings in this study showing that prenatal exposure to CPF leads to increases in MD and reduction of FA, which are suggestive of increased extracellular space and potentially reduced myelination. Finally, the prenatal exposure to CPF caused a reduction in FA and RK with a concomitant increase in MD and RD in the striatum. The concomitant reductions in FA and kurtosis measures in both the corpus callosum and the striatum of CPF-exposed guinea pigs are suggestive of a decrease in the degree of water diffusion restriction that can be accounted for by a number of factors including reduced packing of axons, increased membrane permeability, disruption of internal axonal structure, increased tissue water content, and decreased myelination (Papadakis et al. 1999). In this respect, it is noteworthy that a previous study demonstrated that the expression of myelin-associated glycoprotein, a transmembrane glycoprotein that is essential for the maintenance of the mature myelinated unit (Quarles et al. 1973), was decreased in the brain of neonatal rats following their exposure between postnatal days 1 and 6 to different doses of CPF (Betancourt et al. 2006). In vitro studies have also provided evidence that CPF-oxon, one of the major metabolites of CPF, can bind directly to and decrease the polymerization of tubulin, the protein that makes the microtubules involved in maintaining the structural and functional integrity of axons (Grigoryan and Lockridge 2009; Terry et al. 2012). The increase in MD, a measure of the average distance that a water molecule travels within a period of time, in the amygdala and striatum of CPF-exposed guinea pigs is also suggestive of increased extracellular space, most likely due to cellular membrane disruption, cell death, and/or vasogenic edema (see Zhuo et al., 2012 and references therein). Of interest, in vivo studies have demonstrated that early postnatal exposure of rats to CPF induces a dose-dependent reduction in the expression of nerve growth factor in the forebrain (Betancourt et al., 2006), an effect that can contribute to increased oxidative stress and cell death.
The performance of the guinea pigs in the Morris water maze correlated well with DKI measures obtained from the amygdala, striatum, and corpus callosum. While mean escape latency was inversely correlated to FA, MK, and AK in the amygdala, corpus callosum, and striatum, it was directly correlated to MD and RD in the amygdala and striatum. Although a cause-consequence relationship cannot be traced between the structural damage and the behavioral impairment presented by animals exposed prenatally to CPF, the present results suggested that impairment of the spatial learning performance in the Morris water maze, manifested as a prolongation of the escape latency, increases as the disruption of the structural integrity of the striatum, amygdala, and corpus callosum becomes more pronounced. The integrity of the striatum is vital for performance of the MWM and is heavily involved in spatial and procedural learning (McDonald and White, 1994; Packard and Knowlton, 2002; Packard and McGaugh, 1992). Given the well-known role of the Striatum in movement disorders such as Parkinson’s and Huntington’s disease, as well as motivation and reward (Worbe et al., 2009), this is a finding that translates clearly in regard to human neurodevelopment and welfare. The likely contribution of the amygdala to these results is that it modulates the spatial and procedural learning normally associated with the striatum and hippocampus (Packard et al., 1994). The corpus callosum connects these affected regions between the hemispheres, and impaired transmission by these fibres may account for some of the behavioral deficit noticed.
These findings are consistent with studies demonstrating that laboratory animals and humans with lesions in the striatum, amygdala, and corpus callosum present cognitive deficits (Block et al. 1993; Sauerwein and Lassonde 1994; Galliot et al. 2010; Chida et al. 2011). A recent MRI study demonstrated that children exposed prenatally to CPF presented overt changes in the brain cortical structure, including reduced cortical thickness, which correlated with their cognitive deficits (Rauh et al., 2012). An earlier case report also provided evidence that children presented brain defects in the corpus callosum following prenatal exposure to CPF (Sherman 1996).
Therefore, it appears that the prenatal toxicity of CPF in humans can be faithfully reproduced in guinea pigs. Prenatal and/or early neonatal exposure of rats and mice to CPF affects their brain development and causes neurocognitive deficits later in life that also resemble those seen in humans exposed to the pesticide in utero(Levin et al. 2001, 2002; Turgeman et al. 2011). However, striking differences exist between the CNS development of mice or rats and that of humans, making it difficult to extrapolate sensitive gestational periods from rats and mice to humans. In short-gestation species such as the rat and mouse, the majority of CNS growth spurt occurs postnatally, generally within the first three weeks after birth. In long-gestation species, including guinea pigs, non-human primates, and humans, this growth spurt starts mid-gestation in the uterus, peaks during the last third part of pregnancy, and ends shortly after birth (Dobbing and Sands 1970, 1973; Byrnes et al. 2001). Thus, the guinea pig emerges as a more appropriate rodent model system than rats and mice to assess the detrimental effects of developmental exposure to OP pesticides and the effectiveness of various treatments in countering the toxicity of these pesticides.
5. Conclusion
The current study demonstrated that the structural integrity of the adult guinea pig brain is disrupted following the prenatal exposure to sub-acute doses of the OP pesticide CPF. Volumetric analysis of T2-weighted images revealed that most of the damage imparted by the prenatal exposure to CPF was confined to the anterior forebrain, and DKI analyses provided evidence that the primary forebrain structures affected by this exposure were the striatum, the amygdala, and the corpus callosum, all of which are known to be involved in cognitive processing. Furthermore histological examination confirmed reduction in myelination in CPF treated guinea pigs in the amygdale. Subsequently, correlational analyses between DKI measures and the mean time the animals took to escape onto the hidden platform in the Morris water maze demonstrated that the structural integrity of these brain regions is associated with adequate spatial learning performance of the guinea pigs. To our knowledge this is the first study to use complementary non-invasive methods, specifically MRI and behavioral assays, to assess the developmental toxicity of CPF in a precocious small animal model. Since prenatal exposure to CPF induces behavioral deficits and disruption of guinea pig brain structure that resemble those seen in humans, the present findings introduce the guinea pig as a translationally relevant small animal model of the neurodevelopmental toxicity of this pesticide. This is of major significance for future evaluations of the effectiveness of therapeutic approaches aimed at treating and/or preventing neurological disorders that result from prenatal exposure to CPF, and potentially other OP pesticides.
Table 1.
Summary of significant group differences in behavioral, anatomical, and DKI measures. Data is presented as mean ± standard deviation. MD, AD, and RD are expressed in units of 10−3mm2/s. FA, MK, AK, & RK are dimensionless ratios.
| BEHAVIORAL: | ||||
|---|---|---|---|---|
| measure | PO (n=10) | CPF (n=10) | p | |
| - MWM Escape latency | s | 63.7 ± 10.2 | 73.7 ± 11.0 | 0.050 |
| ANATOMICAL: | ||||
|---|---|---|---|---|
| measure | PO (n=10) | CPF (n=10) | p | |
| - Body weight | g | 496.7 ± 47.4 | 441.7 ± 63.4 | 0.041 |
| - Forebrain volume | mm3 | 2827.4 ± 23.9 | 2690.0 ± 34.0 | 0.005 |
| - Striatal volume | mm3 | 111.5 ± 6.8 | 102.3 ± 5.9 | 0.005 |
| DIFFUSION: | ||||
|---|---|---|---|---|
| measure | PO (n=10) | CPF (n=10) | p | |
| - Corpus Callosum | FA | 0.41 ± 0.02 | 0.39 ± 0.02 | 0.014 |
| MK | 0.77 ± 0.03 | 0.74 ± 0.02 | 0.009 | |
| AK | 0.61 ± 0.02 | 0.60 ± 0.05 | 0.008 | |
| RK | 1.01 ± 0.06 | 0.96 ± 0.04 | 0.023 | |
|
| ||||
| - Striatum | FA | 0.21 ± 0.01 | 0.20 ± 0.01 | 0.006 |
| MD | 0.84 ± 0.01 | 0.86 ± 0.02 | 0.012 | |
| RD | 0.74 ± 0.01 | 0.77 ± 0.01 | 0.002 | |
| RK | 0.78 ± 0.02 | 0.75 ± 0.03 | 0.048 | |
|
| ||||
| - Amygdalae | FA | 0.22 ± 0.02 | 0.20 ± 0.01 | 0.002 |
| MD | 0.91 ± 0.01 | 0.92 ± 0.03 | 0.024 | |
| RD | 0.80 ± 0.02 | 0.83 ± 0.02 | 0.002 | |
|
| ||||
| - Forebrain | RK | 0.86 ± 0.06 | 0.81 ± 0.03 | 0.038 |
Table 2.
Summary of significant Pearson correlations of DKI measures with forebrain volume and MWM escape latency
| FOREBRAIN VOLUME by DKI: | |||
|---|---|---|---|
| ROI (n = 20) | measure | r | p |
| - Striatum | MK | 0.551 | 0.012 |
| AK | 0.463 | 0.040 | |
| RK | 0.580 | 0.007 | |
| MD | −0.496 | 0.026 | |
| RD | −0.507 | 0.022 | |
|
| |||
| - Corpus Callosum | FA | 0.462 | 0.041 |
| MK | 0.451 | 0.046 | |
| AK | 0.475 | 0.034 | |
| MWM ESCAPE LATENCY by DKI: | |||
|---|---|---|---|
| ROI (n = 20) | measure | r | p |
| - Striatum | MD | 0.535 | 0.015 |
| RD | 0.497 | 0.026 | |
|
| |||
| - Amygdalae | MD | 0.494 | 0.027 |
| FA | −0.520 | 0.019 | |
| RD | 0.560 | 0.010 | |
| MK | −0.460 | 0.041 | |
| AK | −0.472 | 0.036 | |
|
| |||
| - Corpus Callosum | FA | −0.462 | 0.041 |
| MK | −0.460 | 0.041 | |
| AK | −0.472 | 0.036 | |
Highlights.
Guinea pigs exposed to chlorpyrifos (CPF) during prenatal period leads to learning deficits.
Learning deficits are accompanied by significant reduction of forebrain volume.
Diffusion kurtosis imaging reveals disruption of structural integrity in various brain regions.
Changes in diffusion kurtosis imaging metrics correlate with spatial learning deficits.
Sub-acute maternal exposure to CPF can be crippling to the normal neurodevelopment of the child.
Acknowledgments
This study was partly supported by a grant 5R01ES019282 from the National Institute of Environmental Health Sciences. RJM is supported by a Training grant in Molecular and Mechanistic Toxicology (T32 ES00726; PI: Albuquerque).
Footnotes
Conflict of interest statement
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akhtar N, Srivastava MK, Raizada RB. Transplacental disposition and teratogenic effects of chlorpyrifos in rats. J Toxicol Sci. 2006 Dec;31(5):521–7. doi: 10.2131/jts.31.521. [DOI] [PubMed] [Google Scholar]
- Aldridge JE, Meyer A, Seidler FJ, Slotkin TA. Alterations in Central Nervous System Serotonergic and Dopaminergic Synaptic Activity in Adulthood after Prenatal or Neonatal Chlorpyrifos Exposure. Environ Health Perspect. 2005 Apr 28;113(8):1027–31. doi: 10.1289/ehp.7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J, Das GD. Postnatal neurogenesis in the guinea-pig. Nature. 1967 Jun 10;214(5093):1098–101. doi: 10.1038/2141098a0. [DOI] [PubMed] [Google Scholar]
- Betancourt AM, Burgess SC, Carr RL. Effect of developmental exposure to chlorpyrifos on the expression of neurotrophin growth factors and cell-specific markers in neonatal rat brain. Toxicol Sci. 2006 Aug;92(2):500–6. doi: 10.1093/toxsci/kfl004. [DOI] [PubMed] [Google Scholar]
- Block F, Kunkel M, Schwarz M. Quinolinic acid lesion of the striatum induces impairment in spatial learning and motor performance in rats. Neurosci Lett. 1993 Jan;149(2):126–8. doi: 10.1016/0304-3940(93)90752-7. [DOI] [PubMed] [Google Scholar]
- Bouchard MF, Chevrier J, Harley KG, Kogut K, Vedar M, Calderon N, et al. Prenatal exposure to organophosphate pesticides and IQ in 7-year-old children. Environ Health Perspect. 2011 Aug;119(8):1189–95. doi: 10.1289/ehp.1003185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradman A, Barr DB, Henn BGC, Drumheller T, Curry C, Eskenazi B. Measurement of Pesticides and Other Toxicants in Amniotic Fluid as a Potential Biomarker of Prenatal Exposure: A Validation Study. Environ Health Perspect. 2003 Aug 7;111(14):1779–82. doi: 10.1289/ehp.6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes ML, Reynolds JN, Brien JF. Effect of prenatal ethanol exposure during the brain growth spurt of the guinea pig. Neurotoxicol Teratol. 2001;23(4):355–64. doi: 10.1016/s0892-0362(01)00150-7. [DOI] [PubMed] [Google Scholar]
- Byrnes ML, Richardson DP, Brien JF, Reynolds JN, Dringenberg HC. Spatial acquisition in the Morris water maze and hippocampal long-term potentiation in the adult guinea pig following brain growth spurt--prenatal ethanol exposure. Neurotoxicol Teratol. 2004;26:543–51. doi: 10.1016/j.ntt.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Cheung MM, Hui ES, Chan KC, Helpern JA, Qi L, Wu EX. Does diffusion kurtosis imaging lead to better neural tissue characterization? A rodent brain maturation study. Neuroimage. 2009 Apr 1;45(2):386–92. doi: 10.1016/j.neuroimage.2008.12.018. [DOI] [PubMed] [Google Scholar]
- Chida Y, Kokubo Y, Sato S, Kuge A, Takemura S, Kondo R, et al. The alterations of oligodendrocyte, myelin in corpus callosum, and cognitive dysfunction following chronic cerebral ischemia in rats. Brain Res. 2011 Sep;1414:22–31. doi: 10.1016/j.brainres.2011.07.026. [DOI] [PubMed] [Google Scholar]
- Crinnion WJ. The CDC fourth national report on human exposure to environmental chemicals: what it tells us about our toxic burden and how it assist environmental medicine physicians. Altern Med Rev. 2010 Jul;15(2):101–9. [PubMed] [Google Scholar]
- de Groot DM, Bierman EP, Bruijnzeel PL, Carpentier P, Kulig BM, Lallement G, Melchers BP, Philippens IH, van Huygevoort AH. Beneficial effects of TCP on soman intoxication in guinea pigs: seizures, brain damage and learning behaviour. J Appl Toxicol. 2001;21:S57–65. doi: 10.1002/jat.812. [DOI] [PubMed] [Google Scholar]
- Deshmukh VA, Tardif V, Lyssiotis CA, Green CC, Kerman B, Kim HJ, Padmanabhan K, Swoboda JG, Ahmad I, Kondo T, Gage FH, Theofilopoulos AN, Lawson BR, Schultz PG, Lairson LL. A regenerative approach to the treatment of multiple sclerosis. Nature. 2013;502:327–332. doi: 10.1038/nature12647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Growth and development of the brain and spinal cord of the guinea pig. Brain Res. 1970 Jan 6;17(1):115–23. doi: 10.1016/0006-8993(70)90311-2. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Quantitative growth and development of human brain. Arch Dis Child. 1973 Oct;48(10):757–67. doi: 10.1136/adc.48.10.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driemeyer J, Boyke J, Gaser C, Büchel C, May A. Changes in gray matter induced by learning--revisited. PLoS One. 2008 Jan;3(7):e2669. doi: 10.1371/journal.pone.0002669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dringenberg HC, Richardson DP, Brien JF, Reynolds JN. Spatial learning in the guinea pig: cued versus non-cued learning, sex differences, and comparison with rats. Behav Brain Res. 2001;124:97–101. doi: 10.1016/s0166-4328(01)00188-7. [DOI] [PubMed] [Google Scholar]
- Engel SM, Wetmur J, Chen J, Zhu C, Barr DB, Canfield RL, et al. Prenatal exposure to organophosphates, paraoxonase 1, and cognitive development in childhood. Environ Health Perspect. 2011 Aug;119(8):1182–8. doi: 10.1289/ehp.1003183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson P. Developmental neurotoxicity of environmental agents in the neonate. Neurotoxicology. 1997 Jan;18(3):719–26. [PubMed] [Google Scholar]
- Filliat P, Foquin A, Lallement G. Effects of chronic administration of huperzine A on memory in guinea pigs. Drug Chem Toxicol. 2002;25:9–24. doi: 10.1081/dct-100108469. [DOI] [PubMed] [Google Scholar]
- Fonnum F, Sterri SH, Aas P, Johnsen H. Carboxylesterases, importance for detoxification of organophosphorus anticholinesterases and trichothecenes. Fundam Appl Toxicol. 1985 Dec;5(6 Pt 2):S29–38. doi: 10.1016/0272-0590(85)90112-5. [DOI] [PubMed] [Google Scholar]
- Galliot E, Levaillant M, Beard E, Millot J-L, Pourié G. Enhancement of spatial learning by predator odor in mice: involvement of amygdala and hippocampus. Neurobiol Learn Mem. 2010 Feb;93(2):196–202. doi: 10.1016/j.nlm.2009.09.011. [DOI] [PubMed] [Google Scholar]
- Grigoryan H, Lockridge O. Nanoimages show disruption of tubulin polymerization by chlorpyrifos oxon: implications for neurotoxicity. Toxicol Appl Pharmacol. 2009 Oct;240(2):143–8. doi: 10.1016/j.taap.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruetter R. Automatic, localizedin Vivo adjustment of all first-and second-order shim coils. Magn Reson Med. 1993 Jun;29(6):804–11. doi: 10.1002/mrm.1910290613. [DOI] [PubMed] [Google Scholar]
- Hanke W, Jurewicz J. The risk of adverse reproductive and developmental disorders due to occupational pesticide exposure: an overview of current epidemiological evidence. Int J Occup Med Environ Health. 2004 Jan;17(2):223–43. [PubMed] [Google Scholar]
- Haviland JA, Butz DE, Porter WP. Long-term sex selective hormonal and behavior alterations in mice exposed to low doses of chlorpyrifos in utero. Reprod Toxicol. 2010 Jan;29(1):74–9. doi: 10.1016/j.reprotox.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Hofstetter S, Tavor I, TzurMoryosef S, Assaf Y. Short-term learning induces white matter plasticity in the fornix. J Neurosci. 2013 Jul;33(31):12844–50. doi: 10.1523/JNEUROSCI.4520-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard AS, Bucelli R, Jett DA, Bruun D, Yang D, Lein PJ. Chlorpyrifos exerts opposing effects on axonal and dendritic growth in primary neuronal cultures. Toxicol Appl Pharmacol. 2005 Sep 1;207(2):112–24. doi: 10.1016/j.taap.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Hui ES, Cheung MM, Qi L, Wu EX. Towards better MR characterization of neural tissues using directional diffusion kurtosis analysis. Neuroimage. 2008 Aug 1;42(1):122–34. doi: 10.1016/j.neuroimage.2008.04.237. [DOI] [PubMed] [Google Scholar]
- Hunter DL, Lassiter TL, Padilla S. Gestational exposure to chlorpyrifos: comparative distribution of trichloropyridinol in the fetus and dam. Toxicol Appl Pharmacol. 1999 Jul 1;158(1):16–23. doi: 10.1006/taap.1999.8689. [DOI] [PubMed] [Google Scholar]
- Hüppi PS, Dubois J. Diffusion tensor imaging of brain development. Semin Fetal Neonatal Med. 2006 Dec;11(6):489–97. doi: 10.1016/j.siny.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Icenogle LM, Christopher NC, Blackwelder WP, Caldwell DP, Qiao D, Seidler FJ, et al. Behavioral alterations in adolescent and adult rats caused by a brief subtoxic exposure to chlorpyrifos during neurulation. Neurotoxicol Teratol. 2004;26(1):95–101. doi: 10.1016/j.ntt.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Ihara M, Polvikoski TM, Hall R, Slade JY, Perry RH, Oakley AE, Englund E, O’Brien JT, Ince PG, Kalaria RN. Quantification of myelin loss in frontal lobe white matter in vascular dementia, Alzheimer’s disease, and dementia with Lewy bodies. Acta Neuropathol. 2010;119:579–589. doi: 10.1007/s00401-009-0635-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inns RH, Leadbeater L. The efficacy of bispyridinium derivatives in the treatment of organophosphonate poisoning in the guinea-pig. J Pharm Pharmacol. 1983 Jul;35(7):427–33. doi: 10.1111/j.2042-7158.1983.tb04316.x. [DOI] [PubMed] [Google Scholar]
- Jellison BJ, Field AS, Medow J, Lazar M, Salamat MS, Alexander AL. Diffusion tensor imaging of cerebral white matter: a pictorial review of physics, fiber tract anatomy, and tumor imaging patterns. AJNR Am J Neuroradiol. 2004 Mar;25(3):356–69. [PMC free article] [PubMed] [Google Scholar]
- Kalaria RN, Prince AK. Neurochemical development of the striatum in a precocial (guinea pig) and an altricial (rat) species. Int J Dev Neurosci Off J Int Soc Dev Neurosci. 1988;6(2):161–6. doi: 10.1016/0736-5748(88)90041-x. [DOI] [PubMed] [Google Scholar]
- Kapoor A, Matthews SG. Prenatal stress modifies behavior and hypothalamic-pituitary-adrenal function in female guinea pig offspring: effects of timing of prenatal stress and stage of reproductive cycle. Endocrinology. 2008 Dec;149(12):6406–15. doi: 10.1210/en.2008-0347. [DOI] [PubMed] [Google Scholar]
- Lassiter TL, Padilla S, Mortensen SR, Chanda SM, Moser VC, Barone S. Gestational exposure to chlorpyrifos: apparent protection of the fetus? Toxicol Appl Pharmacol. 1998 Sep;152(1):56–65. doi: 10.1006/taap.1998.8514. [DOI] [PubMed] [Google Scholar]
- Levin ED, Addy N, Baruah A, Elias A, Christopher NC, Seidler FJ, et al. Prenatal chlorpyrifos exposure in rats causes persistent behavioral alterations. Neurotoxicol Teratol. 2002;24(6):733–41. doi: 10.1016/s0892-0362(02)00272-6. [DOI] [PubMed] [Google Scholar]
- Levin ED, Addy N, Nakajima A, Christopher NC, Seidler FJ, Slotkin TA. Persistent behavioral consequences of neonatal chlorpyrifos exposure in rats. Brain Res Dev Brain Res. 2001 Sep;130(1):83–9. doi: 10.1016/s0165-3806(01)00215-2. [DOI] [PubMed] [Google Scholar]
- Lewejohann L, Pickel T, Sachser N, Kaiser S. Wild genius - domestic fool? Spatial learning abilities of wild and domestic guinea pigs. Front Zool. 2010;7:9. doi: 10.1186/1742-9994-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li AA, Lowe KA, McIntosh LJ, Mink PJ. Evaluation of epidemiology and animal data for risk assessment: chlorpyrifos developmental neurobehavioral outcomes. J Toxicol Environ Health B Crit Rev. 2012 Jan;15(2):109–84. doi: 10.1080/10937404.2012.645142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovasi GS, Quinn JW, Rauh VA, Perera FP, Andrews HF, Garfinkel R, et al. Chlorpyrifos exposure and urban residential environment characteristics as determinants of early childhood neurodevelopment. Am J Public Health. 2011 Jan;101(1):63–70. doi: 10.2105/AJPH.2009.168419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamczarz J, Kulkarni GS, Pereira EFR, Albuquerque EX. Galantamine counteracts development of learning impairment in guinea pigs exposed to the organophosphorus poison soman: clinical significance. Neurotoxicology. 2011 Dec;32(6):785–98. doi: 10.1016/j.neuro.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks AR, Harley K, Bradman A, Kogut K, Barr DB, Johnson C, et al. Organophosphate pesticide exposure and attention in young Mexican-American children: the CHAMACOS study. Environ Health Perspect. 2010 Dec;118(12):1768–74. doi: 10.1289/ehp.1002056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCollister SB, Kociba RJ, Humiston CG, McCollister DD, Gehring PJ. Studies of the acute and long-term oral toxicity of chlorpyrifos (O,O-diethyl-O-(3,5,6-trichloro-2-pyridyl) phosphorothioate) Food Cosmet Toxicol. 1974;12(1):45–61. doi: 10.1016/0015-6264(74)90321-6. [DOI] [PubMed] [Google Scholar]
- Mehl A, Schanke TM, Johnsen BA, Fonnum F. The effect of trichlorfon and other organophosphates on prenatal brain development in the guinea pig. Neurochem Res. 1994 May;19(5):569–74. doi: 10.1007/BF00971332. [DOI] [PubMed] [Google Scholar]
- Mehl A, Schanke TM, Torvik A, Fonnum F. The effect of trichlorfon and methylazoxymethanol on the development of guinea pig cerebellum. Toxicol Appl Pharmacol. 2007 Mar;219(2–3):128–35. doi: 10.1016/j.taap.2006.10.024. [DOI] [PubMed] [Google Scholar]
- Monnet-Tschudi F, Zurich MG, Schilter B, Costa LG, Honegger P. Maturation-dependent effects of chlorpyrifos and parathion and their oxygen analogs on acetylcholinesterase and neuronal and glial markers in aggregating brain cell cultures. Toxicol Appl Pharmacol. 2000 Jun 15;165(3):175–83. doi: 10.1006/taap.2000.8934. [DOI] [PubMed] [Google Scholar]
- Mullins RJ, Xu S, Pereira EFR, Mamczarz J, Albuquerque EX, Gullapalli RP. Delayed hippocampal effects from a single exposure of prepubertal guinea pigs to sub-lethal dose of chlorpyrifos: A magnetic resonance imaging and spectroscopy study. Neurotoxicology. 2013 May;36:42–8. doi: 10.1016/j.neuro.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadakis NG, Xing D, Houston GC, Smith JM, Smith MI, James MF, et al. A study of rotationally invariant and symmetric indices of diffusion anisotropy. Magn Reson Imaging. 1999 Jul;17(6):881–92. doi: 10.1016/s0730-725x(99)00029-6. [DOI] [PubMed] [Google Scholar]
- Quarles RH, Everly JL, Brady RO. Evidence for the close association of a glycoprotein with myelin in rat brain. J Neurochem. 1973 Nov;21(5):1177–91. doi: 10.1111/j.1471-4159.1973.tb07573.x. [DOI] [PubMed] [Google Scholar]
- Rauh V, Arunajadai S, Horton M, Perera F, Hoepner L, Barr DB, et al. Seven-year neurodevelopmental scores and prenatal exposure to chlorpyrifos, a common agricultural pesticide. Environ Health Perspect. 2011 Aug;119(8):1196–201. doi: 10.1289/ehp.1003160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh VA, Garfinkel R, Perera FP, Andrews HF, Hoepner L, Barr DB, et al. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics. 2006 Dec;118(6):e1845–59. doi: 10.1542/peds.2006-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh VA, Perera FP, Horton MK, Whyatt RM, Bansal R, Hao X, et al. Brain anomalies in children exposed prenatally to a common organophosphate pesticide. Proc Natl Acad Sci U S A. 2012 May 15;109(20):7871–6. doi: 10.1073/pnas.1203396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D, Barone S. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000 Jun;108(Suppl):511–33. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagi Y, Tavor I, Hofstetter S, Tzur-Moryosef S, Blumenfeld-Katzir T, Assaf Y. Learning in the fast lane: new insights into neuroplasticity. Neuron. 2012 Mar;73(6):1195–203. doi: 10.1016/j.neuron.2012.01.025. [DOI] [PubMed] [Google Scholar]
- Sauerwein HC, Lassonde M. Cognitive and sensori-motor functioning in the absence of the corpus callosum: neuropsychological studies in callosal agenesis and callosotomized patients. Behav Brain Res. 1994 Oct;64(1–2):229–40. doi: 10.1016/0166-4328(94)90135-x. [DOI] [PubMed] [Google Scholar]
- Saunders M, Magnanti BL, CorreiaCarreira S, Yang A, Alamo-Hernández U, Riojas-Rodriguez H, et al. Chlorpyrifos and neurodevelopmental effects: a literature review and expert elicitation on research and policy. Environ Health. 2012 Jan;11(Suppl 1):S5. doi: 10.1186/1476-069X-11-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman JD. Chlorpyrifos (Dursban)-associated birth defects: report of four cases. Arch Environ Health. 1996;51(1):5–8. doi: 10.1080/00039896.1996.9935986. [DOI] [PubMed] [Google Scholar]
- Shih TM, McDonough JH. Neurochemical mechanisms in soman-induced seizures. J Appl Toxicol. 1997;17(4):255–64. doi: 10.1002/(sici)1099-1263(199707)17:4<255::aid-jat441>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol Appl Pharmacol. 2004 Jul 15;198(2):132–51. doi: 10.1016/j.taap.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Levin ED, Seidler FJ. Comparative developmental neurotoxicity of organophosphate insecticides: effects on brain development are separable from systemic toxicity. Environ Health Perspect. 2006 May;114(5):746–51. doi: 10.1289/ehp.8828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon KR, Williams WM, Mackay D, Purdy J, Giddings JM, Giesy JP. Properties and uses of chlorpyrifos in the United States. Rev Environ Contam Toxicol. 2014 Jan;231:13–34. doi: 10.1007/978-3-319-03865-0_2. [DOI] [PubMed] [Google Scholar]
- Song X, Seidler FJ, Saleh JL, Zhang J, Padilla S, Slotkin TA. Cellular mechanisms for developmental toxicity of chlorpyrifos: targeting the adenylyl cyclase signaling cascade. Toxicol Appl Pharmacol. 1997 Jul;145(1):158–74. doi: 10.1006/taap.1997.8171. [DOI] [PubMed] [Google Scholar]
- Terry AV, Beck WD, Warner S, Vandenhuerk L, Callahan PM. Chronic impairments in spatial learning and memory in rats previously exposed to chlorpyrfos or diisopropylfluorophosphate. Neurotoxicol Teratol. 2012;34(1):1–8. doi: 10.1016/j.ntt.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeman G, Pinkas A, Slotkin TA, Tfilin M, Langford R, Yanai J. Reversal of chlorpyrifos neurobehavioral teratogenicity in mice by allographic transplantation of adult subventricular zone-derived neural stem cells. J Neurosci Res. 2011 Aug;89(8):1185–93. doi: 10.1002/jnr.22631. [DOI] [PubMed] [Google Scholar]
- U.S. EPA. Interim registration eligibility decision for chlorpyrifos. 2006 Available from: http://www.epa.gov/oppsrrd1/REDs/chlorpyrifos_ired.pdf.
- US EPA. Chlorpyrifos: Preliminary Evaluation of the Potential Risks from Volatilization. Washington, DC: 2013. [Google Scholar]
- Vatanparast J, Naseh M, Baniasadi M, Haghdoost-Yazdi H. Developmental exposure to chlorpyrifos and diazinon differentially affect passive avoidance performance and nitric oxide synthase-containing neurons in the basolateral complex of the amygdala. Brain Res. 2013 Feb;1494:17–27. doi: 10.1016/j.brainres.2012.11.049. [DOI] [PubMed] [Google Scholar]
- Whyatt RM, Perera FP. Application of biologic markers to studies of environmental risks in children and the developing fetus. Environ Health Perspect. 1995 Sep;103(Suppl):105–10. doi: 10.1289/ehp.95103s6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo J, Xu S, Proctor JL, Mullins RJ, Simon JZ, Fiskum G, et al. Diffusion kurtosis as an in vivo imaging marker for reactive astrogliosis in traumatic brain injury. Neuroimage. 2012 Jan;59(1):467–77. doi: 10.1016/j.neuroimage.2011.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]


