Abstract
The synthetic rodenticide, tetramethylenedisulfotetramine (TMDT), is a persistent and highly lethal GABA-gated Cl− channel blocker. TMDT is clandestinely produced, remains popular in mainland China, and causes numerous unintentional and deliberate poisonings worldwide. TMDT is odorless, tasteless, and easy to manufacture, features that make it a potential weapon of terrorism. There is no effective treatment. We previously characterized the effects of TMDT in C57BL/6 mice and surveyed efficacies of GABAergic and glutamatergic anticonvulsant treatments. At 0.4 mg/kg i.p., TMDT produced neurotoxic symptomatology consisting of twitches, clonic and tonic-clonic seizures, often progressing to status epilepticus and death. If administered immediately after the occurrence of the first clonic seizure, the benzodiazepine diazepam (DZP) effectively prevented all subsequent seizure symptoms, whereas the NMDA receptor antagonist dizocilpine (MK-801) primarily prevented tonic-clonic seizures. The latter agent, however, appeared to be more effective at preventing delayed death. The present study further explored these phenomena, and characterized the therapeutic actions of DZP and MK-801 as combinations. Joint treatment with both DZP and MK-801 displayed synergistic protection against tonic-clonic seizures and 24 hour lethality as determined by isobolographic analysis. Clonic seizures, however, remained poorly controlled. A modification of the treatment regimen, where DZP was followed 10 min later by MK-801, yielded a reduction in both types of seizures and improved overall outcome. Simultaneous monitoring of subjects via EEG and videography confirmed effectiveness of this sequential regimen. We conclude that TMDT blockage at GABAA receptors involves early activation of NMDA receptors, which contribute to persistent ictogenic activity. Our data predict that a sequential combination treatment with DZP followed by MK-801 will be superior to either individual therapy with, or simultaneous administration of, these two agents in treating TMDT poisoning.
Keywords: tetramethylenedisulfotetramine, GABA, NMDA, seizures, neurotoxicity, isobologram
1. INTRODUCTION
Tetramethylenedisulfotetramine (TMDT) is a potent synthetic neurotoxic chemical which has been used worldwide as a rodenticide (Chen and Lu, 2004, Croddy, 2004). Although its production, sale, and use are now banned due to its persistence in the environment and lack of selective toxicity, it is widely available, particularly in mainland China. A survey by China’s National Poison Control Center found 50% of 116 rodenticides sold in year 2000 contained TMDT (Croddy, 2004). Between 1991 and 2010, there were as many as 14,000 documented cases of TMDT poisonings, with nearly 1,000 resulting in death (Casida, 2011). Numerous poisonings have occurred worldwide (Intusoma and Sornsrivichai, 2009), including in Europe and the U.S (Zhang et al., 2011). An incident that raised awareness of public health authorities in the U.S. was that of a 15-month-old girl in New York City, NY, who ingested a rodenticide powder containing TMDT (2003). She suffered severe seizures and, despite 6 months of anti-seizure therapy, was developmentally delayed. A 2013 incident in Hebei Province caught worldwide attention when two young girls died from TMDT-tainted yogurt drink as a result of a rivalry between kindergartens (Moore, 2013).
Exposure to TMDT is not only possible via ingestion but also via inhalation and entry through wounds on the skin. In humans, the symptoms are nausea, vomiting, and twitching which can give way to clonic and tonic-clonic seizures. Exposure to higher doses leads to status epilepticus and death (Lu et al., 2008). A standardized treatment for TMDT poisoning has not been established (Flomenbaum et al., 2006), and better treatment modalities are clearly needed (Poon et al., 2005, Lu et al., 2008).
TMDT is a noncompetitive antagonist at the GABAA receptor as demonstrated in vitro by its ability to displace the selective GABAA receptor-chloride channel ligand, [3H]-ethynylbicycloorthobenzoate (3H-EBOB) and to block Cl− flow through the GABA-operated ionophore, diminishing neuronal inhibitory drive (Ratra and Casida, 2001). Likewise, prototypical noncompetititve GABAA receptor antagonists can displace 14C-TMDT (Zhao et al., 2014). Therefore, efforts to restore GABAA receptor function or dampen the resulting hyperexcitability should be therapeutic for instances of TMDT exposure.
We and others have recently characterized a mouse model of TMDT poisoning.(Shakarjian et al., 2012, Zolkowska et al., 2012). Using this model, our laboratory has evaluated the efficacies of the GABAA receptor positive modulator diazepam (DZP) and noncompetitive NMDA receptor (NMDAR) antagonists ketamine and dizocilpine maleate(MK-801) administered singly (Shakarjian et al., 2012). These counteractive single drug-based treatments dose-dependently reduced both the number of TMDT-induced tonic-clonic seizures and the probability of lethal outcome. Importantly, despite the effectiveness of a high dose of DZP in modifying all acute convulsant TMDT actions, mice still often experienced delayed seizures and died within 24 hours of exposure to the agent. In contrast, the NMDA receptor antagonists, although intrinsically less effective in ameliorating TMDT-induced clonic seizures, protected animals from delayed death. In this study, we analyzed the effectiveness of dosing protocols using combined DZP and MK-801 treatment, to test the hypothesis that co-administration of drugs from GABAA and NMDA receptor classes will provide significantly better outcomes for the TMDT syndrome than single drug treatment from either class. We constructed dose-response curves for the agents administered singly, or as fixed-ratio combinations and used the isobolographic method to quantitatively analyze the value of such treatments in preventing seizures and death provoked by TMDT exposure.
2. MATERIALS AND METHODS
2.1 Chemicals
Dimethylsulfoxide (DMSO), DZP and MK-801 were obtained from Sigma-Aldrich (St. Louis, MO). DZP and MK-801 were administered singly in a volume of 10 ml/kg. Normal saline for injection was the diluent. Combinations of DZP and MK-801 were administered as a fixed ratio (3:1 or 11:1) in volumes ranging from 5 to 12 ml/kg (See Table 3 for individual drug concentrations). TMDT of ≥98% purity (CAS 80-12-6, MW=240.26), was kindly provided by Dr. Lowri S. De Jager of the Center for Food Safety and Applied Nutrition, U.S. Food and Drug Administration. TMDT was stored in a secure location at room temperature. Multiple small aliquots (1–2 mg) were weighed out in one session to minimize hazards of compound handling, by individuals wearing nitrile gloves, facemask and lab coat. Stocks of TMDT were prepared in DMSO at a concentration of 10 mg/ml. Prior to use, they were diluted in normal saline for injection to a final concentration of 0.04 mg/ml and administered to mice in a volume of 10 ml/kg. Both stocks and injection solutions remained stable (based on activity) for 30 days when stored at 4°C.
Table 3. Incidence of TMDT-induced tonic-clonic seizures and lethality in mice post-treated with Diazepam-MK-801 binary mixtures.
Fractions (n/N) under “Clonic Seizures,” “Tonic-clonic Seizures” and “Death” indicate the number of mice (n) presenting with the phenomenon out of the total number of mice in the group (N); fractions under # indicate the total number of episodes (n) out of the total number of mice in the group (N). TMDT and treatments were administered ip.
| Treatment (mg/kg) | Clonic Seizures | Tonic-clonic Seizures | Cumulative Death (n/N) | |||
|---|---|---|---|---|---|---|
| n/N | # | n/N | # | one hour | 24 hour | |
| Control (TMDT alone) | 19/19 | 49/19 | 19/19 | 27/19 | 19/19 | 19/19 |
| DZP 0.36/MK-801 0.12 | 5/5 | 31/5 | 4/5 | 16/5 | 3/5 | 4/5 |
| DZP 0.48/MK-801 0.18 | 5/5 | 45/5 | 5/5 | 13/5 | 0/5 | 1/5 |
| DZP 0.72/MK-801 0.24 | 7/7 | 43/7 | 3/7 | 4/7 | 0/7 | 5/7 |
| DZP 1.05/MK-801 0.36 | 7/7 | 25/7 | 2/7 | 2/7 | 1/7 | 4/7 |
| DZP 1.2/MK-801 0.4 | 9/9 | 38/9 | 1/9 | 1/9 | 0/9 | 3/9 |
| DZP 1.44/MK-801 0.48 | 7/7 | 8/7 | 0/7 | 0/7 | 0/7 | 1/7 |
| DZP 1.2; MK-801 0.4 after 10 min | 9/9 | 13/9 | 0/9 | 0/9 | 0/9 | 0/9 |
| DZP 1.8/MK-801 0.16 (11:1) | 7/7 | 7/7 | 0/7 | 0/7 | 0/7 | 6/7 |
2.2 Animals
All experiments were approved by the Institutional Animal Care and Use Committee of the New York Medical College and conformed to the Revised Guide for the Care and Use of Laboratory Animals (2011). Adult male C57BL/6 mice (~25 g) were obtained from Charles River Laboratories (Wilmington, MA). Mice were kept in our AAALAC-accredited animal facilities, housed four to five per standard plexiglass cage containing bedding, a cotton nestlet, and covered with a filter top. Animals were given food and water ad libitum on a regular light cycle (on at 07:00 and off at 19:00), and were allowed to acclimate at least 48 h prior to experimentation. All efforts were made to minimize pain and the number of animals used, while maintaining sufficient statistical power using pre-hoc power analyses to determine group size.
2.3. Testing TMDT syndrome and treatments
Testing took place between 1100 hours and 1600 hours. All treatments were administered intraperitoneally (i.p.). On the day of an experiment, mice were randomly split into testing and control groups and placed individually in clear plexiglass cages. Sample size power analysis ((Faul et al., 2009) http://www.gpower.hhu.de) was performed using data from our previous experiments (Shakarjian et al., 2012). The analysis, with six groups with α preset to 0.05 and power (1− β) = 0.8, yielded an effect size of 0.878 and a total sample size for the dose-response of 24, i.e., four per group, and an actual power of 0.83. To err on the conservative side, minimum n was set at five per subgroup. Mice were observed during the course of one hour following TMDT administration for latency of onset and number of twitches, clonic and tonic-clonic seizures, and incidence and latency to death. There were additional observation points at 2, 3, 6 and 24 hours following the TMDT administration. Anticonvulsant treatments were delivered immediately after the occurrence of the first clonic seizure, which represents the first decisive symptom of the TMDT syndrome. As we have reported previously (Shakarjian et al., 2012), mice administered TMDT initially exhibited twitches of the body and an erect upright tail (Straub tail). Twitches were followed by unilateral or bilateral clonic seizures without loss of righting reflex. One or more clonic seizures preceded single or multiple bouts of tonic-clonic seizures, characterized by wild running followed by loss of righting reflex, and tonic flexion and extension of all limbs. Tonic-clonic seizures were often, but not always, followed by death (Shakarjian et al., 2012). Mice that survived the one hour observation period were checked the following day for 24 hour survival. Afterwards, they were scored on a scale of 1 to 5 for seizure progression of their responses to TMDT. The score was based upon detailed behavioral analysis of TMDT toxicity and on score systems developed for primarily generalized seizures (Velíšková, 2006). A score of 1 was applied to mice exhibiting a single clonic seizure (i.e. the seizure prior to treatment – this indicated 100% effectiveness of treatment), 2 for multiple clonic seizures, 3 for tonic-clonic seizures, 4 for lethality within 24 hours, and 5 for lethality within one hour. Since only three animals displayed multiple tonic-clonic seizures as their most severe reaction, we did not assign a unique score for this outcome.
2.4 EEG recordings
Combined EEG and videorecording was performed as previously described (Shakarjian et al., 2012). Cortical electrodes were implanted under combined ketamine/xylazine anesthesia (100/10 mg/kg, ip). Four silver ball electrodes were placed epidurally in a bilateral arrangement above the frontal and occipital cortices, while one screw placed in the nasal bone, and another positioned behind lambda, served as reference electrode and ground, respectively. Electrodes and screws were covered with dental acrylic. Mice were returned to their home cages for an approximately one week recovery following surgery.
Baseline EEG was recorded for 5 minutes prior to ip injection of mice with 0.4 mg/kg TMDT. EEG was continuously recorded until the first clonic seizure occurred. Mice were then injected ip with a mixture of DZP 1.2 mg/kg and MK-801 0.4 mg/kg or DZP 1.2 mg/kg followed 10 min later by MK-801 0.4 mg/kg. EEG recording continued for 24 h under infrared illumination. Simultaneous and synchronized video were captured to correlate EEG with behavioral signs using a Pinnacle Solutions, Inc. (Kansas City, MO, U.S.A.) 3-channel EEG/video system.
2.5 Data Analysis
Isobolographic analysis was used for quantitative evaluation of drug interactions (Tallarida et al., 1989). Dose–response curves were first constructed for DZP and MK-801 administered singly. This included both new observations and the individual agent data from our previous studies (Shakarjian et al., 2012). The ED50 (dose calculated to reduce toxic endpoint by 50%) and its associated variances and 95% confidence intervals (CI) in terms of total dose were then calculated from the curves. For drug combinations, dose was expressed as the total dose of the binary mixture, i.e., the doses of DZP and MK-801 added together. Prior to isobolographic analysis, dose-response curves were tested for parallelism to one another, to which all conformed. Theoretical additive ED50 values were then calculated for combinations of DZP and MK-801. These values were used for comparison with experimentally derived ED50 values for the binary mixture by application of Student’s two-tailed t-test. If the actual ED50 value were significantly (p<0.05) smaller than the theoretical value, the interaction would be considered synergistic (supra-additive). Conversely, an actual ED50 value significantly (p<0.05) greater than the theoretical value would indicate the interaction to be infra-additive (Gessner and Shakarjian, 1985). Finally, a strictly additive interaction would be indicated when the theoretical and experimental ED50 values were not significantly different from each other. Also constructed were dose reduction index tables. These display the theoretical and actual fold reductions in drug dose produced by administering a binary mixture rather than a monotherapy (Chou, 2010). All dose-response and isobolographic analyses were performed with the J-FlashCalc pharmacological statistics software package publicly available through the generosity of Dr. Michael Ossipov (Department of Pharmacology, University of Arizona College of Medicine, Tucson, AZ; (http://www.u.arizona.edu/~michaelo/jflashcalc.html, Last updated, Nov 1, 2013). Regression analyses were performed using SigmaPlot v. 11 (Systat Software Inc., Chicago, IL), and non-parametric statistical analyses (Mann-Whitney U Test for group by group comparisons) using StatView 5.0.1 (Abacus, SAS).
3. RESULTS
3.1 Dose-Response of Individual Treatments
We constructed full dose-response relations of the individual drugs for each endpoint of interest to determine their ED50s. C57BL/6 mice were treated i.p. with 0.4 mg/kg TDMT, a dose we previously found consistently produces, within one hour, twitches, clonic seizures, tonic-clonic seizures and death that define the characteristic syndrome following exposure to this agent. DZP and MK-801, administered immediately after observing the first decisive symptom of TMDT poisoning (clonic seizure), were individually tested for their abilities to attenuate the syndrome over the course of this observation period as a function of dose. Each agent dose-dependently reduced occurrence of tonic-clonic seizures and death (Table 1). MK-801 was the more potent of the two agents in inhibiting these actions of TMDT. While one-hour lethality was reduced in the groups receiving the lowest doses of DZP and MK-801 administered, progressively higher drug doses were required to detect a preventative effect upon tonic-clonic seizures, and twenty-four hour lethality, respectively (Table 1).
Table 1. Incidence of TMDT-induced tonic-clonic seizures and lethality after post-treatment with Diazepam (DZP) or MK-801 monotherapy.
Fractions under “Clonic Seizures (n/N),” “Tonic Clonic Seizures,” and “Death.” indicate the number of mice (n) presenting with the phenomenon out of the total number of mice in the group (N); fractions under # indicate the total number of episodes (n) out of the total number of mice in the group (N). TMDT and treatments were administered ip.
| Group | Clonic Seizures | Tonic-clonic Seizures | One hour lethality | 24 hour lethality (cumulative) | |
|---|---|---|---|---|---|
| n/N | # | ||||
| Control (TMDT alone) | 19/19 | 49/19 | 19/19 | 19/19 | 19/19 |
| DZP 1 mg/kg | 4/5 | 27/5 | 5/5 | 3/5 | NA |
| DZP 2 mg/kg | 5/5 | 29/5 | 3/5 | 1/5 | 5/5 |
| DZP 3 mg/kg | 6/6 | 9/6 | 1/6 | 1/6 | 5/6 |
| DZP 5 mg/kg | 16/16 | 29/16 | 3/16 | 0/16 | 4/9 |
| DZP 7 mg/kg | 9/9 | 11/9 | 1/9 | 1/9 | 6/9 |
| DZP 9 mg/kg | 7/7 | 7/7 | 0/7 | 0/7 | 0/7 |
| DZP ED50 | 2.42±0.758 | 0.848±0.834 | 5.48±1.5 | ||
| MK801 0.2 mg/kg | 6/6 | 39/6 | 6/6 | 2/6 | 6/6 |
| MK801 0.3 mg/kg | 7/7 | 40/7 | 6/7 | 4/7 | 7/7 |
| MK801 0.5 mg/kg | 12/12 | 243/12 | 8/12 | 1/12 | 2/6 |
| MK801 0.7 mg/kg | 7/7 | 62/7 | 4/7 | 1/7 | 3/7 |
| MK801 1 mg/kg | 15/15 | 69/15 | 2/15 | 0/15 | 0/9 |
| MK-801 ED50 | 0.597±0.130 | 0.187 ± 0.145 | 0.504±0.085 | ||
3.2 Dose-Response and Isobolographic Analysis of Combination Treatments
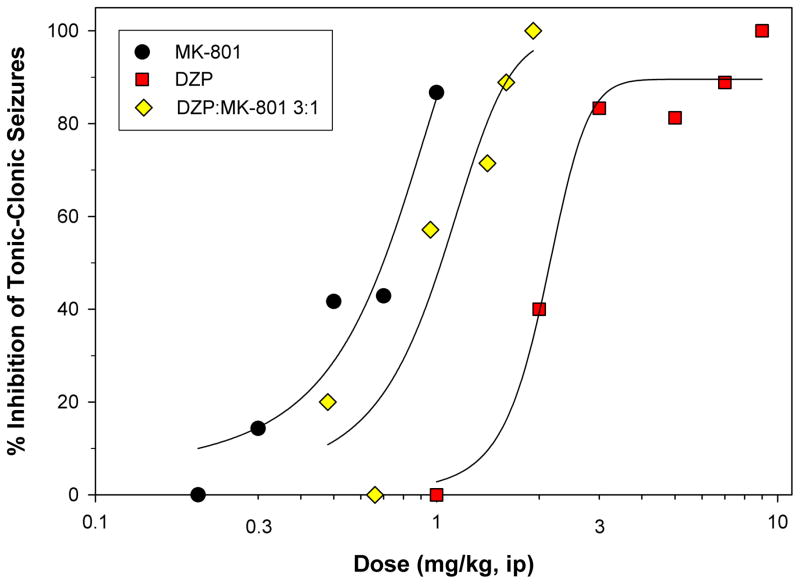
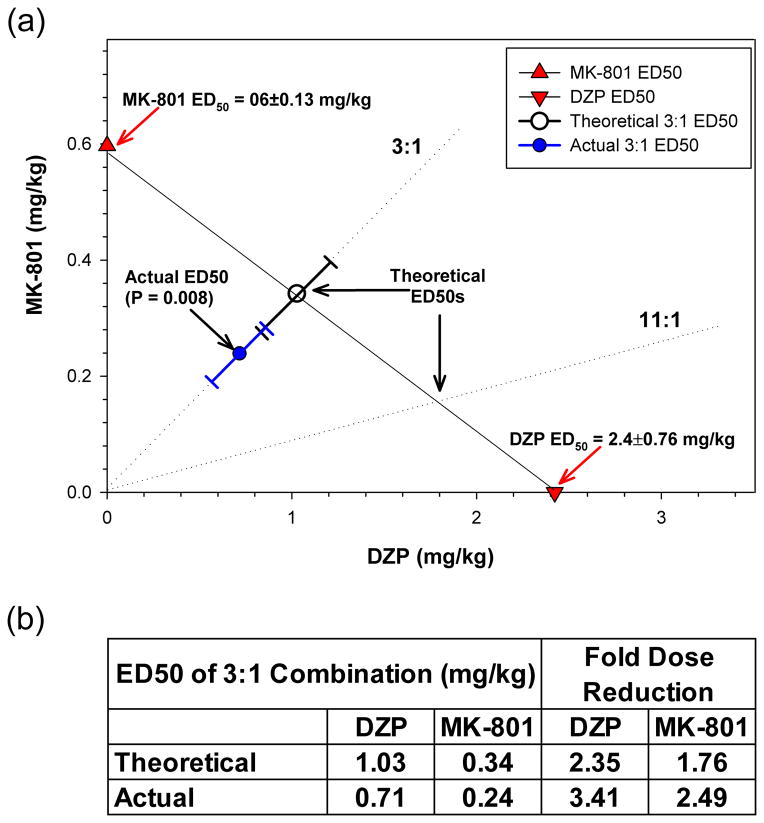
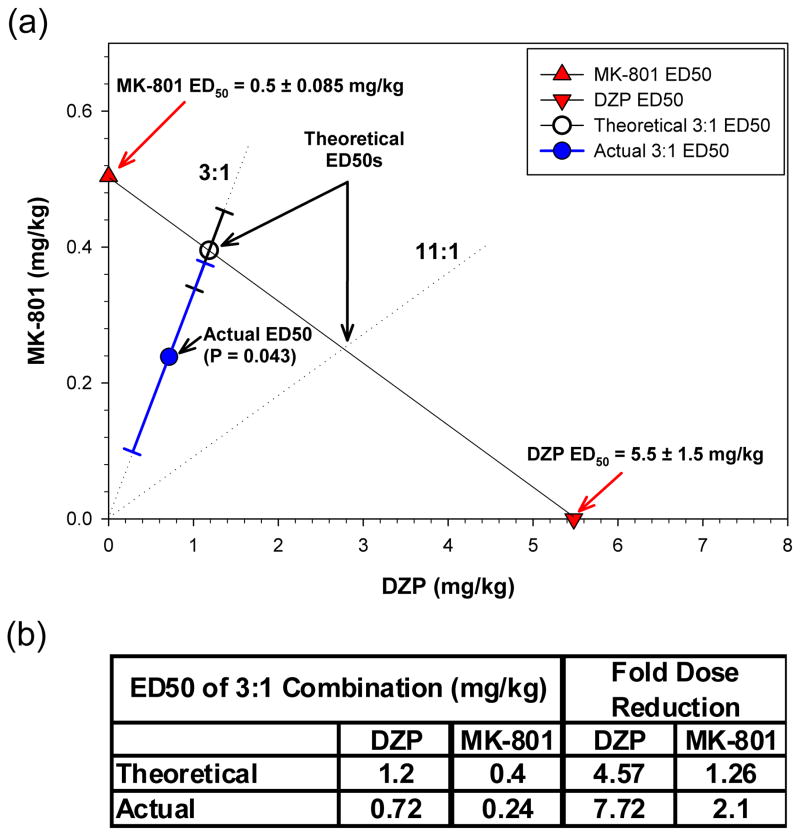
Experiments were then performed to determine the efficacy of combinations of DZP and MK-801. A dose ratio of DZP:MK-801 3:1 was selected because it combines roughly equipotent doses of each individual agent for inhibition of tonic-clonic seizures. A range of this fixed-ratio dose mixture was administered to mice immediately after the first clonic seizure to determine ED50 values of the combination for inhibition of the appearance of tonic-clonic seizures and lethality. Post-treatment was selected for clinical relevance in an instance of actual human poisoning. As shown in Figure 1, the 3:1 fixed ratio produced a dose-response situated between the MK-801 and DZP dose-response curves. When these data were subjected to isobolographic analysis (Figure 2a), the ED50 for the drug combination fell below the isoadditive ED50 line. Thus, the 3:1 fixed ratio data show that significantly lower doses of DZP and MK-801 in combination were required to produce half-maximal protection from TMDT-induced tonic-clonic seizures than predicted by strict additivity (Student’s t-test, p<0.008). This is reflected in the dose reduction index table (Figure 2b) which shows that whereas an additive interaction would be predicted to lead to a 2.35- and 1.76-fold reduction in the doses of DZP and MK-801, the actual dose reduction was 3.41- and 2.49-fold, for the two agents, respectively. By this measure, the DZP:MK-801 3:1 combination was synergistically superior to its individual components for preventing this symptom. While the ED50 of the 3:1 binary mixture for protection against one-hour lethality fell below the isoadditive line, suggesting synergism, variability for this endpoint was such that statistical significance was not reached. Accordingly, we analyzed the effectiveness of this combined treatment in preventing lethality 24 hours post TMDT exposure. In this case, we did find a significant supra-additive effect of the binary mixture, compared to each agent administered individually (Student’s t-test, p<0.05, Figure 3).
Figure 1. Dose-response curves for MK-801 and DZP.
administered individually or as a 3:1 fixed ratio for the post-treatment of TMDT-induced tonic-clonic seizures. Circles: MK-801; Squares: DZP; Diamonds: DZP:MK-801 3:1
Figure 2. Isobologram for the interaction DZP and MK-801 in suppressing TMDT-induced tonic-clonic seizures.
(a) ED50s ± 95% CI of DZP (downwards triangle) and MK-801 (upwards triangle) administered singly were plotted on the x and y axes, respectively. The line of additivity connects these ED50s. Open circles: Theoretical ED50 ± 95% CI for inhibition of tonic-clonic seizures by DZP:MK-801 3:1. Closed circles: Actual ED50 ± 95% CI of the DZP:MK-801 3:1 combination. This dose was significantly below the theoretical point of additivity (p<0.006). Dotted lines: depict the 3:1 and 11:1 DZP:MK-801 fixed ratios. Treatment with the predicted ED50 dose for the DZP:MK-801 11:1 combination yielded greater inhibitory activity than expected, suggesting that synergy observed by these two agents is not isolated to their 3:1 combination.
(b) Dose Reduction Table compares the ED50 doses of DZP and MK-801 predicted for an additive interaction and the actual doses for the 3:1 combination. The reduction in dose required to obtain a 50% reduction in tonic-clonic seizures was much greater than predicted, reflecting a synergistic interaction of the combination.
Figure 3. Isobologram depicting prevention of TMDT-induced 24 h lethality by DZP, MK-801 and their combinations.
(a) As in Figure 2, ED50s ± 95% CI of DZP (downwards triangles) and MK-801 (upwards triangles) administered singly were plotted on the x and y axes, respectively. The line of additivity connects these ED50s. Open circles: Theoretical ED50 ± 95% CI for inhibition of 24 h lethality by DZP:MK-801 3:1. Closed circles: Actual ED50 ± 95% CI of the DZP:MK-801 3:1 combination. This dose was significantly below the theoretical point of additivity (p<0.0299). Dotted lines: depict the 3:1 and 11:1 DZP:MK-801 fixed ratios.
(b) Dose Reduction Table compares the ED50 doses of DZP and MK-801 predicted for an additive interaction and the actual doses for the 3:1 combination. The reduction in dose required to obtain a 50% reduction in 24 h lethality was much greater than predicted, reflecting a synergistic interaction of the combination.
Table 2 summarizes the ED50 results for inhibition of tonic-clonic seizures, lethality over one hour, and lethality at 24 hours for the combination studies. Closer examination of ED50 values derived from these experiments revealed that approximately 30 percent less total drug was required to suppress tonic-clonic seizures, and 40 percent less to inhibit 24 hour lethality, than predicted by a strictly additive interaction. Significantly, the actual ED50 doses for these endpoints were approximately the same, i.e., 0.71 mg/kg DZP combined with 0.24 mg/kg MK-801. Comparing with the actual ED50s for 24 hour lethality of the individual agents, this represents 13 percent of the DZP ED50 dose combined with 48 percent of the MK-801 ED50 dose.
Table 2. ED50 Values for Inhibition of TMDT-induced Tonic-Clonic Seizures and Lethality by Diazepam, MK-801, Administered Singly, or as a Binary Mixture.
Actual and theoretical values were determined using the J Flashcalc program as noted in Methods. Statistical analyses were performed using two-tailed Student’s t-test. TMDT and treatments were administered ip.
| Treatment | Tonic-Clonic Seizures (mg/kg) | 1h Lethality (mg/kg) | 24h Lethality (mg/kg) |
|---|---|---|---|
| DZP | 2.42 ± 0.758 | 0.848 ± 0.834 | 5.48 ± 1.5 |
| MK-801 | 0.597±0.130 | 0.187 ± 0.145 | 0.504±0.085 |
| DZP:MK 3:1 Theoretical | 1.37 ± 0.251 (DZP 1.03/MK 0.34) | 0.451 ± 0.274 (DZP 0.34/MK 0.11) | 1.58 ±0.229 (DZP 1.2/MK 0.40) |
| DZP:MK 3:1 Actual | 0.947 ± 0.174 (DZP 0.71/MK 0.24) | 0.263 ± 0.274 (DZP 0.2/MK 0.066) | 0.953 ±0.553 (DZP 0.71/MK 0.24) |
| Theoretical vs Actual | P-value = 0.0077 | P-value = 0.3455 | P-value = 0.043 |
| DZP:MK 11:1 Theoretical | 1.931± 0.597 (DZP 1.8/MK 0.16) | 0.656± 0.630 (DZP 671/MK 0.061) | 3.2± 0.680 (DZP 2.9/MK 0.27) |
3.3 Protection of C57BL/6 Mice from TMDT-induced Clonic Seizures
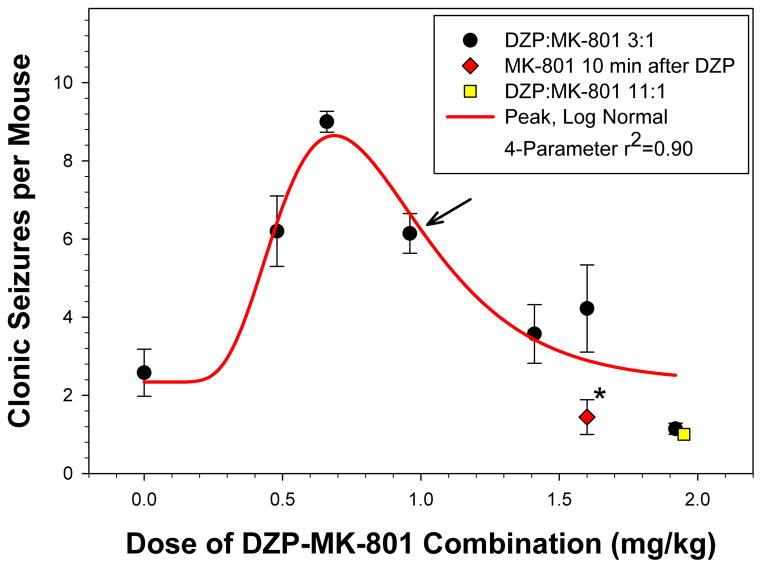
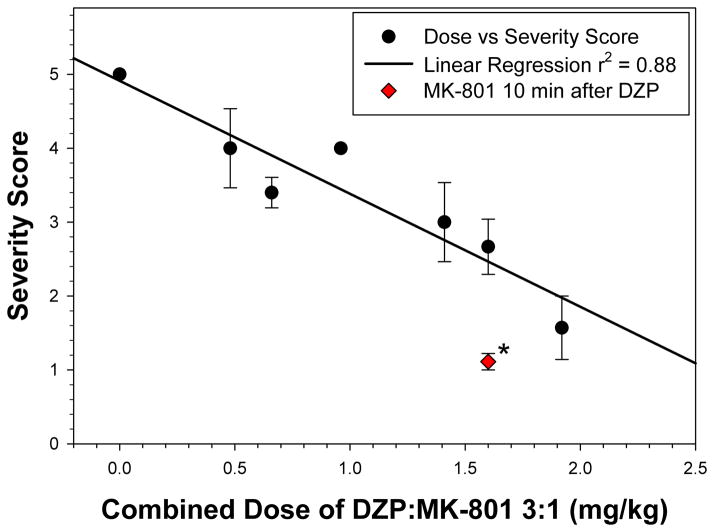
Additional experiments were performed to further characterize the combined effect of DZP and MK-801 on the TMDT syndrome. Although control of the most severe components of the TMDT syndrome, i.e., tonic-clonic seizures and lethality, was observed in the above experiments, control of clonic seizures was still lacking. In fact, the dose-response for the combination followed a bell-shaped function with clonic seizure frequency increasing approximately four-fold before returning to baseline at the highest doses tested (Figure 4). We therefore examined the effectiveness of a DZP:MK-801 3:1 ratio (1.2/0.4 mg/kg) treatment when MK-801 was administered 10 min after DZP, comparing it to simultaneous administration of the anticonvulsant drugs. Delaying treatment with MK-801 by 10 min greatly improved clonic seizure control by significantly reducing the number of clonic seizures observed (p<0.02, Figure 4). This was accomplished without lowering the effectiveness of the combination for inhibition of tonic-clonic seizures or lethality (Table 3). Rather, as shown in Figure 5, the overall effectiveness, based upon severity score, was greatly increased by separation of DZP and MK-801 administration times by a 10 min interval (p<0.002).
Figure 4. Protection of C57BL/6 Mice from TMDT-induced Clonic Seizures.
Figure depicts seizures per mouse as a function of dose of the DZP:MK-801 combination treatment. Circles: DZP:MK-801 3:1 fixed ratio doses, and Solid line: Peak, log normal four parameter nonlinear regression of these points (r2 = 0.90). Diamond: A 3:1 dose (DZP:MK-801 1.2:0.4 mg/kg), where MK-801 was administered 10 min after DZP administration. This regimen provided a significantly greater suppression of clonic seizures (p= 0.018, Mann-Whitney U Test). Square: An 11:1 dose (DZP:MK-801 1.8:0.16 mg/kg) providing effective suppression of clonic seizures.
Figure 5. Reduction in severity of TMDT symptoms by combined DZP and MK-801 treatment.
Figure depicts severity score as a function of dose of the DZP:MK-801 combination treatment. Circles: DZP:MK-801 3:1 fixed ratio doses, and Solid line: linear regression of these points (r2 = 0.88). Diamond: A 3:1 dose (DZP:MK-801 1.2:0.4 mg/kg), where MK-801 was administered 10 min after DZP administration. This latter regimen provided a significantly improved severity score relative to simultaneous administration of DZP and MK-801 (p= 0.0013, Mann-Whitney U Test)).
3.4 EEG Recordings from Mice Treated by Simultaneous or Sequential DZP:MK-801 Regimens after TMDT Poisoning
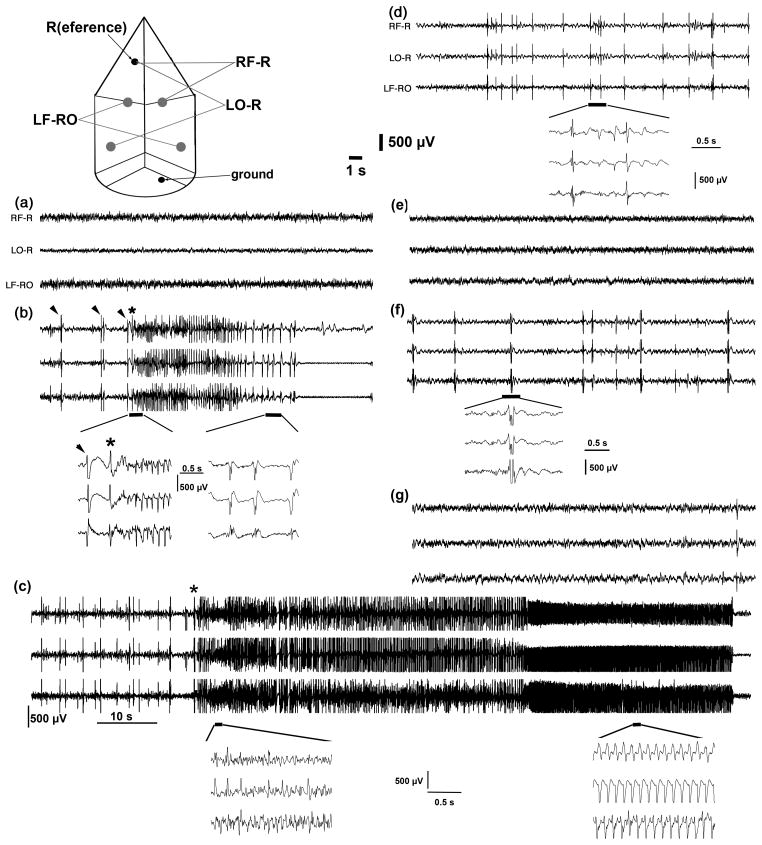
To further confirm that treatment of the TMDT syndrome with a sequential DZP/MK-801 regimen is more effective than the same treatment delivered simultaneously, we followed these treatments in mice using simultaneous EEG and video recordings. For this experiment two mice were devoted to sequential treatment and two to simultaneous delivery of both drugs, after the initial clonic seizure (Figure 6). We observed marked differences between these regimens. First, the two mice receiving simultaneous DZP and MK-801 experienced 6 and 14 clonic seizures before perishing at 7 and 10 hours post-TMDT exposure, respectively. Out of the 20 clonic seizures in these mice, nine were longer than 30 s. On the other hand, we recorded only a few (3 and 5) brief clonic seizures (under 20 s) in the mice receiving sequential DZP and MK-801, during the entire 24 hour recording period. Instead, these mice experienced many brief interictal discharges associated with whole body twitches. Secondly, both mice administered the simultaneous DZP/MK801 treatment perished after experiencing status epilepticus during the 24-hour recording period (7 and 10 hours post-TMDT exposure as indicated above). In contrast, both mice treated with DZP followed 10 min afterwards with MK-801 survived the entire duration. All observed behavioral seizures were accompanied by corresponding EEG and vice versa except for interictal discharges in mice treated with concurrent DZP and MK-801 just before their demise.
Figure 6.
EEG recordings from mice treated by simultaneous (a–d) or sequential (e–g) DZP:MK-801 3:1 regimens after TMDT poisoning Top - scheme of cortical electrodes and montages. There were two active electrodes over the frontal (sensorimotor) cortex and two electrodes over the occipital (visual) cortex. Reference electrode was represented by a screw in the nasal bone, a screw above the cerebellum served for the purpose of grounding. RF-R = right frontal vs. reference; LO-R = left occipital vs. reference. LF-RO = left frontal vs. right occipital. Time mark 1 s and calibration 500 μV for all traces except if noted otherwise.
(a) Baseline (pre-TMDT) EEG recording in a mouse later injected with TMDT (0.4 mg/kg i.p.) and after the first clonic seizure with simultaneous diazepam (1.2 mg/kg ip) + dizocilpine maleate (0.4 mg/kg i.p.; DZP+MK-801).
(b) Synchronous EEG discharges of spike-and-wave character (marked by arrowheads and associated behaviorally with whole body twitches) and an EEG seizure (asterisk at onset; poly-spike, later spike-and-wave) associated with clonic behavioral seizure after TMDT in the same mouse. This clonic seizure was the first distinct feature of TMDT poisoning and treatment (DZP + MK-801) has been initiated immediately afterwards (within 10 s). First inset shows onset of clonic seizure with longer time base. Please note the first spike followed by a slow wave (marked by an arrowhead) followed after the second spike-wave complex (*) by a burst of spikes (polyspikes). Second inset shows polyspike-and-wave EEG pattern at the end of this clonic seizure.
(c) Prolonged EEG discharges corresponding to a clonic seizure (onset marked with an asterisk; duration 90 s) in the same mouse now about 2 hours after the combined (simultaneous) DZP and MK-801 treatment. Clonic seizures longer than 30 s regularly occurred in the mice treated simultaneously. Time mark 10 s, calibration 500 μV. First inset (with own time mark and calibration) shows a period from the onset of this clonic seizure characterized by frequent spikes, although not clearly developed in all traces. Second inset demonstrates typical rhythmical spike-wave complexes from the terminal part of this prolonged seizure.
(d) Profound attenuation of background EEG activity with interictal discharges in the same mouse about 15 min prior to demise at 7 hours after TDMT poisoning. Inset shows several of these discharges characterized by very short polyspikes, sharp wave-slow wave complexes, sharp waves, and spike-wave complexes.
(e) Baseline (pre-TMDT) EEG recording in a mouse later injected with TMDT (0.4 mg/kg i.p.) and DZP followed in 10 min with MK-801 (sequential treatment). Baseline EEG activity was analogous in all mice, please compare with (a).
(f) Frequent interictal EEG discharges accompanied by whole body twitches represented a hallmark of TMDT toxicity in the mice treated with sequential DZP (1.2 mg/kg i.p.) and MK-801 (0.4 mg/kg). Inset reveals the character of interictal discharges as polyspike-wave complexes.
(g) Almost complete recovery of EEG activity (cf. to (e)) in the mouse at 24 hours after TMDT injection and sequential treatment with DZP and MK-801. Please note a single interictal discharge at the end of the recording.
3.5 DZP:MK-801 11:1 Treatment after TMDT poisoning
One final experiment was performed to provide an indication as to whether the observed efficacy of the DZP:MK-801 combination was limited to the dose ratio tested, or present in other regions of the isobologram. We administered 0.4 mg/kg TMDT to mice, followed by an 11:1 dose ratio of DZP:MK-801, at a dose (1.8:0.16 mg/kg) that matches the theoretically-derived ED50 for tonic-clonic seizures at this ratio (Table 2). Protection from TMDT-induced tonic-clonic seizures greatly exceeded that predicted for this parameter, providing 100% prevention of the occurrence of tonic-clonic seizures and ensuring 100% one-hour survival. While this dose was insufficient to protect mice from lethality within 24 hours, it should be noted that the ED50 for this parameter was predicted by the isobologram to be over 60% higher (DZP 2.9:MK-801 0.27 mg/kg).
4. DISCUSSION
In a previous study (Shakarjian et al., 2012), we demonstrated the value of two distinct classes of agents, GABAA receptor positive modulators and noncompetitive NMDAR blockers, in controlling the proconvulsant and lethal actions of TMDT. In the present study, we demonstrate how combining diazepam and MK-801, representative agents from these disparate pharmacological classes, effectively inhibits the TMDT syndrome. Moreover, we provide quantitative evidence that these two agents (likely at different fixed ratios) interact in a synergistic manner to reduce TMDT neurotoxic symptomatology and improve survival much more effectively than either administered individually. We describe how one particular symptom of TMDT poisoning, clonic seizures, is resistant to (or is even exacerbated by) MK-801 administered singly or as a binary mixture with diazepam. Furthermore, we demonstrate how sequential, versus simultaneous administration of agents from each class, is more efficacious in inhibiting effects of prior exposure to TMDT. Finally, we Illustrate how cortical EEG activity reflects the improvement in control of the TMDT syndrome observed by sequential treatment with diazepam and MK-801.
There has been a practice of using drug combinations to manage difficult to treat seizures and epilepsy (Deckers et al., 2000, Deckers et al., 2001, St Louis, 2009), with acceptance of this approach even greater in cases of emergent status epilepticus (Wasterlain et al., 2011). Certain combinations provide improvement in seizure control, with or without accompanying changes in adverse effect profile. Such “rational polytherapy” posits that the combination of two agents with differing mechanisms of action will be most likely to produce a truly complementary result (St Louis, 2009). In the case of treatment of acute poisoning with TMDT, we applied this concept by administering DZP and MK-801 in combination. DZP was the first choice as it is a GABAA receptor positive modulator, promoting Cl− conductance in the same channel that TMDT blocks, and it is frequently resorted to in cases of acute seizures requiring emergency management (Sirven and Waterhouse, 2003). MK-801, which is a highly selective activity-dependent open channel blocker of NMDAR (Wong et al., 1986, Woodruff et al., 1987, Aram et al., 1989), was chosen because of our previous demonstration of its efficacy against TMDT-induced seizures (Shakarjian et al., 2012) and ability to reverse TMDT-induced inward Ca2+ fluxes in cultured hippocampal neurons (Cao et al., 2012). In our previous study, we found that each individual treatment had distinct advantages and disadvantages when administered as monotherapies against TMDT poisoning. DZP controlled both acute clonic and tonic-clonic seizures, but much less reliably protected against TMDT lethality at 24 hours. On the other hand, MK-801 treatment protected mice against TMDT-induced tonic-clonic seizures and delayed lethality, but was less effective against clonic seizures and, at a lower dose, even exacerbated this type of seizures (Shakarjian et al., 2012).
In the present study, we observed some notable differences in the dose-responses of DZP and MK-801 for inhibition of the endpoints monitored. Half-maximal inhibition of one-hour lethality required the lowest dose of MK-801, whereas respective inhibition of tonic-clonic seizures and 24-hour mortality required a 2- to 2.5-fold greater dose. Interestingly, the spread of the DZP ED50s was broader. While the ED50 for prevention of one-hour mortality was 0.85 ± 0.834 mg/kg, the ED50s for inhibition of tonic-clonic seizures and 24-hour mortality were approximately 3-fold and 6.5-fold higher, respectively. The much greater dose required for half-maximal suppression of 24-hour compared to one-hour mortality suggests that DZP, at least in a single-dose regimen, is less suited than MK-801 to ensuring extended survival after TMDT exposure. Current literature suggests that the pharmacokinetics of DZP and MK-801 are comparable in rodents (Vezzani et al., 1989, Walker et al., 1998, Musteata et al., 2008, Wegener et al., 2011).(Shakarjian et al., 2012). Rather, the difference may be due to seizure-induced alterations in GABAA and NMDA receptor dynamics in the brain, favoring sustained activation of NMDA-dependent pathways, and the need to suppress these pathways in order to achieve survival (Mazarati and Sankar, 2006).
Upon establishment of ED50s for the individual agents, we tested whether combined DZP/MK-801 administration could provide more effective treatment of both near-term clonic and tonic-clonic seizures and longer-term survival from TMDT poisoning at tolerable doses. Efficacy of these agents as a binary mixture would provide a proof of principle that combined activation of GABAA receptor transmission and suppression of NMDAR transmission is superior to targeting only one of these receptor-regulated processes in TMDT poisoning. Our data revealed the efficacy of this combination in a mouse model and support the use of an NMDAR antagonist in combination with a benzodiazepine for treatment of human TMDT intoxication. The data also indicate that sequential administration with a benzodiazepine first, and an NMDAR antagonist to follow, is likely to yield superior results than administration of a cocktail of these agents at once. A recent case report corroborates our observations, including the sequential treatment with a GABAA agonist followed by an NMDA antagonist (Chau et al., 2005). This report described treatment of a patient who presented with status epilepticus after ingesting food-containing TMDT. The seizures persisted despite treatment with benzodiazepines, thiopental, and phenytoin, but were finally controlled by subsequent infusion of the NMDAR antagonist, ketamine.
Isobolographic analysis is a useful method for quantitatively determining the outcome of agent combinations upon a biological endpoint (Tallarida, 2011). The method takes into account the sigmoid dose-response relationships of single agents and their combinations interacting with biological systems (Gessner, 1995, Greco et al., 1995). It can be demonstrated graphically by Cartesian plotting of ED50s of the individual drugs for a particular endpoint (in our case, inhibition of seizures or lethality). A line (the isobol, (Tallarida, 2011)), drawn between the two ED50s represents the line of additivity, i.e., the concentrations of combinations of the two drugs that would be expected to produce a half-maximal effect, assuming that their effects are strictly additive. Our finding, using a DZP:MK-801 3:1 dose ratio, that ED50s for inhibition of TMDT-induced tonic-clonic seizures (Figure 2) and 24 hour mortality (Figure 3) fell significantly below the isobole demonstrates that these two agents greatly potentiate each other’s actions, producing a therapy superior to that of either individual agent. A simple examination of another region of the isobologram using DZP and MK-801 in an 11:1 ratio suggested that this synergistic behavior extends beyond the centerpoint of the isobole for tonic-clonic seizure suppression. Given that DZP and MK-801 have disparate side-effect profiles (Troupin et al., 1986, McNamara, 2011) we predict that a reduction in the individual agents of this magnitude will produce a treatment with lower overall toxicity and more opportunity for dose escalation in an emergency setting to control severe TMDT poisoning.
One undesirable feature of seizure treatment with NMDAR antagonists is increased frequency of clonic seizures (Velíšek and Mareš, 1992, Velíšková and Velíšek, 1992, Hashimoto et al., 2003). In our previous study of monotherapies for TMDT poisoning, this phenomenon was apparent during a one-hour observation period when treating TMDT-exposed mice with either MK-801 or ketamine (Shakarjian et al., 2012). The present study verified this phenomenon and showed that the number of clonic seizures per mouse was also high in subjects receiving the DZP/MK-801 3:1 combination. One explanation is that treatment with MK-801 at any dose that prolongs survival of TMDT-exposed mice simply extends the time-period when the clonic seizures can occur. Second, it may be that the suppression of tonic-clonic seizures by MK-801 treatment unmasks existing clonic seizures. Finally, MK-801 may be directly promoting clonic seizure activity in the presence of the excitatory actions of TMDT. Based upon our observation of mice treated with 0.2 to 1 mg/kg MK-801 (Table 1), we believe that prolonged survival plays a role at lower doses (0.2–0.3 mg/kg). At the moderate doses (0.5, and 0.7 mg/kg), higher frequency of clonic seizures occurred accompanied by only a little change in the frequency of tonic-clonic seizures (or one hour survival) suggesting direct stimulation of clonic seizures by MK-801. Finally, at the high dose (1 mg/kg), tonic-clonic seizures were eliminated, while clonic seizures persisted, albeit at a reduced frequency, likely due to a more general seizure suppressing action of this dose.
In our experiments with the 3:1 dose ratio of DZP:MK-801, we found that a change of regimen from simultaneous to sequential administration of the agents significantly reduced the incidence of clonic seizures (Table 3 and Figure 4). Importantly, our sequential treatment regimen was at least as effective at suppressing 24-hour lethality as simultaneous treatment, and closer examination of toxic symptomatology via severity scoring indicated that sequential treatment provided an overall better outcome (Figure 5). Simultaneous EEG and videorecording revealed superior control of both electrographic and corresponding behavioral seizure signs by the sequential regimen (Figure 6). We suggest that sequential administration allows DZP (and its active metabolites) to attain relevant brain levels and prevail at early time points, thus preventing the MK-801 elicited clonic seizure exacerbation.
The experimental literature contains positive reports of the use of GABAA receptor agonist and NMDAR antagonist combinations in several different rodent seizure models suggesting a general applicability of such treatment for seizures with unknown origin (Rice and DeLorenzo, 1999, Martin and Kapur, 2008, Wasterlain et al., 2011). However, our work is significant in that there is a surprising dearth of information available regarding combined treatment with GABAA receptor modulators and NMDAR antagonists in GABA network-initiated seizure models (notable example: (Kulkarni and Ticku, 1989). Although a logical treatment for exposure to TMDT might be a GABAA receptor modulator, based upon proximity of action, our results and clinical reports of TMDT poisonings clearly indicate weaknesses of this as a singular approach. Further, we demonstrate that significant improvements in therapeutic response can be obtained by altering the relative time of administration of the individual agents. Finally, a critical aspect we provide is our approach to quantify the interaction, using isobolographic analysis of the full dose ranges for the agents of interest and their binary mixtures, thus providing a more unambiguous determination of the value of a drug combination.
While many investigators have examined the value of combination therapy in clinical and non-clinical situations, approaches to this problem have varied, both in terms of choosing agents with similar or disparate mechanisms of action to combine, and in terms of study design. It is currently difficult to predict what will make an effective combination, even from a pharmacodynamics standpoint, however a quantitative approach towards evaluation of outcomes is necessary. Our results, supported by the isobolographic method, are consistent with a positive outcome of combining GABAA receptor stimulatory and NMDAR inhibitory agents in TMDT seizure management. It will be of interest to apply the isobolographic method to the evaluation of these combinations in other seizure models, as advocated previously (Deckers et al., 2000, NINDS, 2013).
In conclusion, we report here that DZP and MK-801, as single treatments, dose-dependently antagonize the toxic and lethal actions of the potent neurotoxic rodenticide, TMDT. Furthermore, DZP and MK-801, when administered together after TMDT exposure, interact in a synergistic manner such that their combination is more effective than either agent administered singly. Finally, administering MK-801 several minutes after DZP provides more thorough control of the TMDT syndrome. We predict that a combination regimen with a GABAA receptor positive modulator followed by an NMDAR antagonist will constitute a superior treatment for acute TMDT poisoning.
Tetramethylenedisulfotetramine (TMDT) is implicated in thousands of poisonings.
While diazepam treatment inhibits TMDT-induced seizures, delayed death often occurs.
MK-801 better protects against delayed death, but poorly controls clonic seizures.
When co-treated, diazepam/MK-801 is synergistic, but clonic seizures persist.
A sequential diazepam/MK-801 regimen further improves control of the TMDT syndrome.
Acknowledgments
FUNDING
This work was supported by the U.S. National Institutes of Health [NS056093 to J.V., NS072966 to L.V., and 7U54AR055073 to D.E.H.]; and the New York Medical College Intramural Grant Fund [to M.P.S.]
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Mahil S. Ali, Email: mahils17@gmail.com.
Jana Velíšková, Email: jana_veliskova@nymc.edu.
Patric K. Stanton, Email: patric_stanton@nymc.edu.
Diane E. Heck, Email: diane_heck@nymc.edu.
Libor Velíšek, Email: libor_velisek@nymc.edu.
References
- Aram JA, Martin D, Tomczyk M, Zeman S, Millar J, Pohler G, Lodge D. Neocortical epileptogenesis in vitro: studies with N-methyl-D-aspartate, phencyclidine, sigma and dextromethorphan receptor ligands. The Journal of pharmacology and experimental therapeutics. 1989;248:320–328. [PubMed] [Google Scholar]
- Cao Z, Hammock BD, McCoy M, Rogawski MA, Lein PJ, Pessah IN. Tetramethylenedisulfotetramine alters Ca(2)(+) dynamics in cultured hippocampal neurons: mitigation by NMDA receptor blockade and GABA(A) receptor-positive modulation. Toxicological sciences : an official journal of the Society of Toxicology. 2012;130:362–372. doi: 10.1093/toxsci/kfs244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casida JE. Curious about pesticide action. Journal of agricultural and food chemistry. 2011;59:2762–2769. doi: 10.1021/jf102111s. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) . Poisoning by an illegally imported Chinese rodenticide containing tetramethylenedisulfotetramine--New York City, 2002. MMWR Morbidity and mortality weekly report. 2003;52:199–201. [PubMed] [Google Scholar]
- Chau CM, Leung AK, Tan IK. Tetramine poisoning. Hong Kong medical journal = Xianggang yi xue za zhi/Hong Kong Academy of Medicine. 2005;11:511–514. [PubMed] [Google Scholar]
- Chen ZK, Lu ZQ. Sodium dimercaptopropane sulfonate as antidote against non-metallic pesticides. Acta pharmacologica Sinica. 2004;25:534–544. [PubMed] [Google Scholar]
- Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer research. 2010;70:440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- Croddy E. Rat poison and food security in the People’s Republic of China: focus on tetramethylene disulfotetramine (tetramine) Archives of toxicology. 2004;78:1–6. doi: 10.1007/s00204-003-0509-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckers CL, Czuczwar SJ, Hekster YA, Keyser A, Kubova H, Meinardi H, Patsalos PN, Renier WO, Van Rijn CM. Selection of antiepileptic drug polytherapy based on mechanisms of action: the evidence reviewed. Epilepsia. 2000;41:1364–1374. doi: 10.1111/j.1528-1157.2000.tb00111.x. [DOI] [PubMed] [Google Scholar]
- Deckers CL, Hekster YA, Keyser A, van Lier HJ, Meinardi H, Renier WO. Monotherapy versus polytherapy for epilepsy: a multicenter double-blind randomized study. Epilepsia. 2001;42:1387–1394. doi: 10.1046/j.1528-1157.2001.30800.x. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behavior research methods. 2009;41:1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- Flomenbaum N, Goldfrank L, Hoffman R, Howland M, Lewin N, Nelson L. Goldfrank’s Toxicologic Emergencies. New York: McGraw-Hill; 2006. [Google Scholar]
- Gessner PK. Isobolographic analysis of interactions: an update on applications and utility. Toxicology. 1995;105:161–179. doi: 10.1016/0300-483x(95)03210-7. [DOI] [PubMed] [Google Scholar]
- Gessner PK, Shakarjian MP. Interactions of paraldehyde with ethanol and chloral hydrate. The Journal of pharmacology and experimental therapeutics. 1985;235:32–36. [PubMed] [Google Scholar]
- Greco WR, Bravo G, Parsons JC. The search for synergy: a critical review from a response surface perspective. Pharmacological reviews. 1995;47:331–385. [PubMed] [Google Scholar]
- Hashimoto Y, Araki H, Gomita Y. Cessation of repeated administration of MK-801 changes the anticonvulsant effect against flurothyl-induced seizure in mice. Pharmacology, biochemistry, and behavior. 2003;74:909–915. doi: 10.1016/s0091-3057(03)00013-3. [DOI] [PubMed] [Google Scholar]
- Intusoma U, Sornsrivichai V. Tetramethylenedisulfotetramine contaminated milk powder induced status epilepticus in two siblings and two dogs. Journal of the Medical Association of Thailand = Chotmaihet thangphaet. 2009;92:1393–1395. [PubMed] [Google Scholar]
- Kulkarni SK, Ticku MK. Interaction between GABAergic anticonvulsants and the NMDA receptor antagonist MK 801 against MES- and picrotoxin-induced convulsions in rats. Life sciences. 1989;44:1317–1323. doi: 10.1016/0024-3205(89)90370-6. [DOI] [PubMed] [Google Scholar]
- Lu Y, Wang X, Yan Y, Xiao Z, Stephani U. Nongenetic cause of epileptic seizures in 2 otherwise healthy Chinese families: tetramine--case presentation and literature survey. Clinical neuropharmacology. 2008;31:57–61. doi: 10.1097/WNF.0b013e3180d09983. [DOI] [PubMed] [Google Scholar]
- Martin BS, Kapur J. A combination of ketamine and diazepam synergistically controls refractory status epilepticus induced by cholinergic stimulation. Epilepsia. 2008;49:248–255. doi: 10.1111/j.1528-1167.2007.01384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazarati A, Sankar R. Status epilepticus: Danse Macabre in a ballet of subunits. Epilepsy currents/American Epilepsy Society. 2006;6:102–105. doi: 10.1111/j.1535-7511.2006.00112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara JO. Drugs Effective in the Therapy of the Epilepsies. In: Hardman JG, Limbird LE, editors. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. New York: McGraw-Hill; 2011. pp. 521–547. [Google Scholar]
- Moore M. The Telegraph. Chatham, U.K: Telegraph Media Group; 2013. Chinese kindergarten pupils die from poisoned yoghurt. [Google Scholar]
- Musteata FM, de Lannoy I, Gien B, Pawliszyn J. Blood sampling without blood draws for in vivo pharmacokinetic studies in rats. Journal of pharmaceutical and biomedical analysis. 2008;47:907–912. doi: 10.1016/j.jpba.2008.03.028. [DOI] [PubMed] [Google Scholar]
- NINDS. Anticonvulsant Screening Program - Screening Services. 2013;2013 [Google Scholar]
- Poon WT, Chan K, Lo MH, Yip KK, Lee T, Chan AY. A case of tetramine poisoning: a lethal rodenticide. Hong Kong medical journal = Xianggang yi xue za zhi/Hong Kong Academy of Medicine. 2005;11:507–509. [PubMed] [Google Scholar]
- Ratra GS, Casida JE. GABA receptor subunit composition relative to insecticide potency and selectivity. Toxicology letters. 2001;122:215–222. doi: 10.1016/s0378-4274(01)00366-6. [DOI] [PubMed] [Google Scholar]
- Rice AC, DeLorenzo RJ. N-methyl-D-aspartate receptor activation regulates refractoriness of status epilepticus to diazepam. Neuroscience. 1999;93:117–123. doi: 10.1016/s0306-4522(99)00132-3. [DOI] [PubMed] [Google Scholar]
- Shakarjian MP, Veliskova J, Stanton PK, Velisek L. Differential antagonism of tetramethylenedisulfotetramine-induced seizures by agents acting at NMDA and GABA(A) receptors. Toxicology and applied pharmacology. 2012;265:113–121. doi: 10.1016/j.taap.2012.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirven JI, Waterhouse E. Management of status epilepticus. American family physician. 2003;68:469–476. [PubMed] [Google Scholar]
- St Louis EK. Truly “rational” polytherapy: maximizing efficacy and minimizing drug interactions, drug load, and adverse effects. Current neuropharmacology. 2009;7:96–105. doi: 10.2174/157015909788848929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallarida RJ. Quantitative methods for assessing drug synergism. Genes & cancer. 2011;2:1003–1008. doi: 10.1177/1947601912440575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallarida RJ, Porreca F, Cowan A. Statistical analysis of drug-drug and site-site interactions with isobolograms. Life sciences. 1989;45:947–961. doi: 10.1016/0024-3205(89)90148-3. [DOI] [PubMed] [Google Scholar]
- Troupin AS, Mending JR, Cheng R, Risinger MWQ. New Anticonvulsant Drugs. London: John Libbey and Co; 1986. [Google Scholar]
- Velíšek L, Mareš P. Developmental Aspects of the Anticonvulsant Action of MK-801. In: Kamenka J-M, Domino EF, editors. Multiple Sigma and PCP Receptor Ligands: Mechanisms of Neuromodulation and Neuroprotection? Ann Arbor, MI: NPP Books; 1992. pp. 779–795. [Google Scholar]
- Velíšková J. Behavioral Characterization of Seizures in Rats. San Diego: Elsevier; 2006. [Google Scholar]
- Velíšková J, Velíšek L. Picrotoxin-induced tonic-clonic seizures and lethality are decreased by MK-801 in developing rats. Pharmacology, biochemistry, and behavior. 1992;43:291–295. doi: 10.1016/0091-3057(92)90670-b. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Serafini R, Stasi MA, Caccia S, Conti I, Tridico RV, Samanin R. Kinetics of MK-801 and its effect on quinolinic acid-induced seizures and neurotoxicity in rats. The Journal of pharmacology and experimental therapeutics. 1989;249:278–283. [PubMed] [Google Scholar]
- Walker MC, Tong X, Brown S, Shorvon SD, Patsalos PN. Comparison of single-and repeated-dose pharmacokinetics of diazepam. Epilepsia. 1998;39:283–289. doi: 10.1111/j.1528-1157.1998.tb01374.x. [DOI] [PubMed] [Google Scholar]
- Wasterlain CG, Baldwin R, Naylor DE, Thompson KW, Suchomelova L, Niquet J. Rational polytherapy in the treatment of acute seizures and status epilepticus. Epilepsia. 2011;52(Suppl 8):70–71. doi: 10.1111/j.1528-1167.2011.03243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegener N, Nagel J, Gross R, Chambon C, Greco S, Pietraszek M, Gravius A, Danysz W. Evaluation of brain pharmacokinetics of (+)MK-801 in relation to behaviour. Neuroscience letters. 2011;503:68–72. doi: 10.1016/j.neulet.2011.08.012. [DOI] [PubMed] [Google Scholar]
- Wong EH, Kemp JA, Priestley T, Knight AR, Woodruff GN, Iversen LL. The anticonvulsant MK-801 is a potent N-methyl-D-aspartate antagonist. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:7104–7108. doi: 10.1073/pnas.83.18.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff GN, Foster AC, Gill R, Kemp JA, Wong EH, Iversen LL. The interaction between MK-801 and receptors for N-methyl-D-aspartate: functional consequences. Neuropharmacology. 1987;26:903–909. doi: 10.1016/0028-3908(87)90068-2. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Su M, Tian DP. Tetramine poisoning: A case report and review of the literature. Forensic science international. 2011;204:e24–27. doi: 10.1016/j.forsciint.2010.06.025. [DOI] [PubMed] [Google Scholar]
- Zhao C, Hwang SH, Buchholz BA, Carpenter TS, Lightstone FC, Yang J, Hammock BD, Casida JE. GABAA receptor target of tetramethylenedisulfotetramine. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:8607–8612. doi: 10.1073/pnas.1407379111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolkowska D, Banks CN, Dhir A, Inceoglu B, Sanborn JR, McCoy MR, Bruun DA, Hammock BD, Lein PJ, Rogawski MA. Characterization of Seizures Induced by Acute and Repeated Exposure to Tetramethylenedisulfotetramine. The Journal of pharmacology and experimental therapeutics. 2012;341:435–446. doi: 10.1124/jpet.111.190579. [DOI] [PMC free article] [PubMed] [Google Scholar]