Abstract
Parkinson Disease (PD) is a chronic and progressive neurodegenerative disorder of unknown etiology. Autopsy findings, genetics, retrospective studies, and molecular imaging all suggest a role for inflammation in the neurodegenerative process. However, relatively little is understood about the causes and implications of neuroinflammation in PD. Understanding how inflammation arises in PD, in particular the activation state of cells of the innate immune system, may provide an exciting opportunity for novel neuroprotective therapeutics. We analyze the evidence of immune system involvement in PD susceptibility, specifically in the context of M1 and M2 activation states. Tracking and modulating these activation states may provide new insights into both PD etiology and therapeutic strategies.
Keywords: Microglia, Macrophage, Monocyte, Neurodegeneration, Genetics, Animal Models
Introduction
Parkinson Disease (PD) is a chronic, progressive neurodegenerative disorder characterized by hallmark symptoms that include bradykinesia, ataxia, rigidity, and resting tremor. Pathologically, PD is characterized by the severe loss of melanated dopaminergic neurons in the substantia nigra pars compacta (SNpc), and deposition of α-synuclein into Lewy bodies and Lewy neurites in many remaining neurons (Spillantini et al., 1997, Spillantini et al., 1998). Markers of inflammatory responses have long been noted in and around the SNpc (Nagatsu et al., 2000, Hunot and Hirsch, 2003, Khandelwal et al., 2011). Initially, post-mortem examination using immunohistochemical techniques revealed a spectrum of different types of immune cells, as well as cytokines, in PD brain tissue (McGeer et al., 1988, Boka et al., 1994, Imamura et al., 2003). Later, ligands selective for activated immunological cells also demonstrated activation and inflammatory responses, both in early and late stages of disease (Gerhard et al., 2006, Bartels et al., 2010). Retrospective studies of anti-inflammatory therapeutics also implicates inflammation in some aspect of etiology (Gagne and Power, 2010). Several possibilities exist for understanding aspects of inflammation in PD: particular immunological responses are detrimental, benign, or beneficial. PD is not an acute disorder, so inflammatory responses may show temporal association with disease progression, where an initial response is beneficial and later becomes detrimental.
Therapeutic targeting of inflammation underlying disease pathogenesis represents an exciting approach for novel neuroprotective strategies. However, an incomplete understanding of the role of inflammation in PD will likely hinder successful implementation of rationally-derived therapeutics. The canonical role of microglia as predominant resident immune cell in the brain has led to the hypothesis that these cells underlie the inflammatory processes noted in PD (Qian and Flood, 2008, Long-Smith et al., 2009). However, there is emerging evidence that peripheral immune cells may also be changed in PD (Hisanaga et al., 2001, Saunders et al., 2012, Funk et al., 2013). Understanding inflammation in the context of M1 and M2 activation paradigms may help clarify interpretation of these complex and dynamic processes.
In this review, we will discuss a context for M1 and M2 microglia and macrophage activation states. Emerging evidence for a critical role for these cells and activation states in PD will also be discussed, along with predictions about how modulating or blocking activation might be beneficial for the treatment of PD.
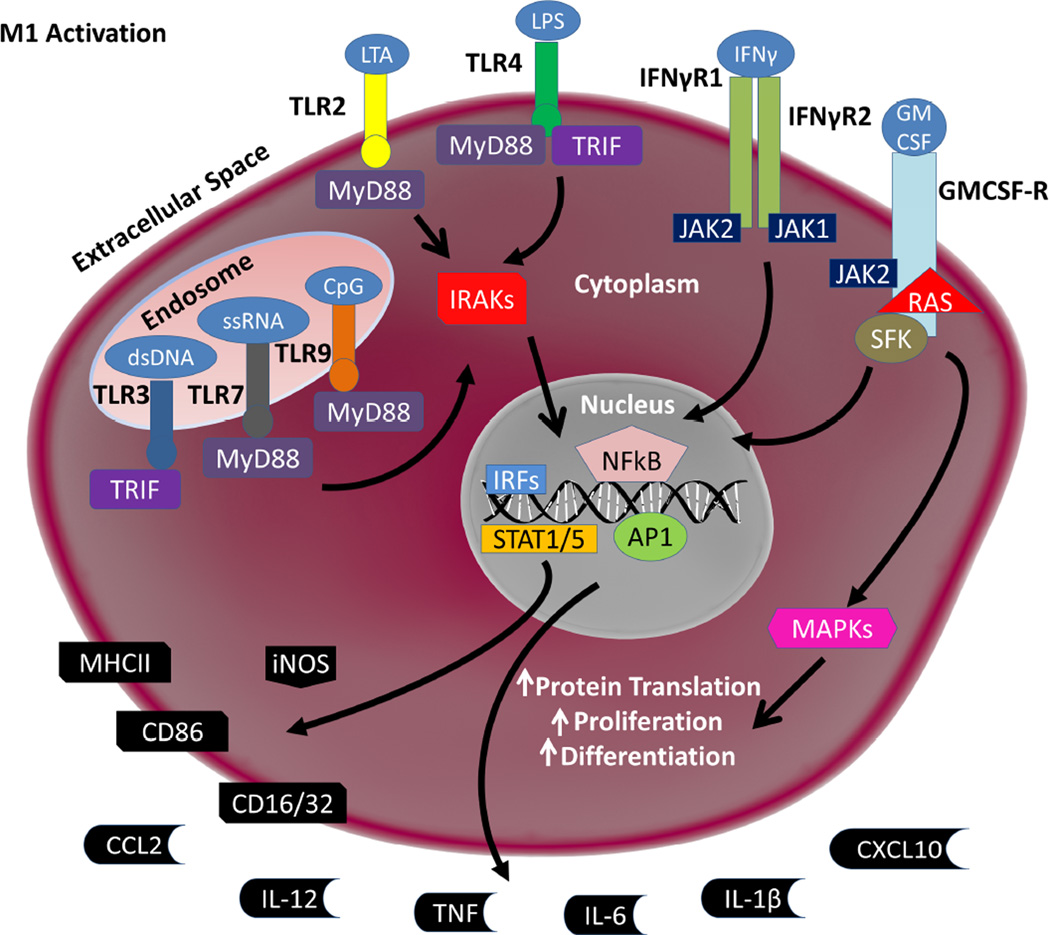
M1 Activation State
Macrophage activation states are understood within a continuum of activation paradigms that mirrors the responses of lymphocytes. The M1, or classical activation state, is associated with pro-inflammatory and pro-killing functions defined by macrophage responses to microbes. The M1 response was defined through studying the anti-microbial activity of macrophages towards Bacillus and Listeria after secondary exposure to other bacteria (Mackaness, 1962). This study highlighted an antigen dependent mechanism for macrophage activation, which has since been parsed into the prototypical M1 response.
The most common methods to track M1 responses include analysis of both secreted factors as well as cell surface and intracellular markers that increase in abundance. The M1 state causes the release of several pro-inflammatory cytokines including tumor necrosis factor (TNF), interleukin 6 (IL-6), IL-12, and IL-1β as well as several chemokines such as C-C motif ligand 2 (CCL2) and C-X-C motif ligand 10 (CXCL10). The production of these cytokines and chemokines are widely used as markers for the M1 state. Additional non-cytokine/chemokine markers of the M1 state include increased cell surface expression of major histocompatibility complex II (MHCII), increased cluster of differentiation marker 86 and 16/32 (CD86, CD16/32), and increased expression of inducible nitric oxide synthase (iNOS)(Nau et al., 2002, Martinez et al., 2006).
To induce a M1 state in macrophages in vitro and in vivo, more defined stimuli have been utilized to elucidate M1 responses in macrophages, including cytokine interferon-gamma (IFNγ) and lipopolysaccharide (LPS), an outer membrane component of gram-negative bacteria. IFNγ signals through a dimer of the IFNγ receptor 1 and 2. Activated IFNγ receptors cause the recruitment of Janus kinase 1 and 2 (JAK1/2) which in turn phosphorylates and activates STAT1 and interferon regulatory factors (IRF), mainly IRF1 (Hu and Ivashkiv, 2009). The signal transduction cascade induces transcriptional changes that up-regulate the expression of cytokines, receptors, and hundreds of other genes associated with the M1 response (Dalton et al., 1993, Huang et al., 1993, Waddell et al., 2010).
The other prototypical M1 stimulus, LPS, signals through a different class of pattern recognition receptors known as toll-like receptors (TLR). LPS binds to TLR4 along with co-receptors MD2 and CD14. Other TLR4-independent LPS activation responses have also been described (Hagar et al., 2013, Kayagaki et al., 2013). TLR4 activation stimulates the transcription factors NFKβ, STAT5, AP1, and IRFs, through MyD88 and TRIF, which go on to cause a transcriptional up-regulation of a similar set of genes as IFNγ (Hu and Ivashkiv, 2009). Other TLRs show affinity for a variety of ligands. TLR2 binds a wide variety of microbial products including LTA. TLR3 binds dsDNA, TLR7 binds ssRNA, and TLR9 binds unmethylated CpG islands in DNA. These TLR activation cascades, through MyD88 or TRIF, skew macrophages towards the M1 state (Takeda and Akira, 2004, Yamamoto and Takeda, 2010, Casanova et al., 2011).
Granulocyte-modifying colony stimulating factor (GM-CSF) is another, more recently described stimulus to the M1 activation paradigm (Lacey et al., 2012, Bayer et al., 2013). However, as opposed to LPS, GM-CSF can induce pleomorphic activation states that can show elements of both M1 and M2 activation states (Weisser et al., 2013). GM-CSF binds to a large receptor that is comprised of a dodecamer of subunits (Hansen et al., 2008). Intracellularly, GM-CSF utilizes many of the same effectors as that of the TLRs, but also utilizes ERK and AKT signal transduction pathways (Krausgruber et al., 2011). GM-CSF stimulation can produce similar cytokine responses to that of LPS, but to a much lesser extent as compared with other M1 stimuli (Lehtonen et al., 2007). GM-CSF function is understood through knockout studies in rodents as well as mutations in human populations, which highlight GM-CSF as a driver of hematopoietic (pre-cursors to myeloid lineage cells) cell differentiation and proliferation (Dranoff and Mulligan, 1994, Dirksen et al., 1997). The M1 activation state is graphically depicted in Figure 1, and listed in Table 1.
Figure 1. Schematic of M1 Signaling.
A broad array of stimuli can induce an M1 pro-inflammatory response. TLR3 binds double stranded DNA (dsDNA). TLR7 binds single stranded RNA (ssRNA). TLR9 binds unmethylated CpG islands in stretches of DNA. Broadly, TLR3, 7, and 9 defend against viral infection. TLR2 binds lipoteichoich acid (LTA) and some other microbial products. TLR4, along with co-receptors MD2 and CD14 (not shown), binds lipopolysaccharide (LPS). TLRs signal through MyD88 and/or TRIF to activate the IRAK family of kinases. IRAKs then cause the translocation of several transcription factors to the nucleus, namely NFkβ, AP1, STAT5, and IRFs. Interferon-gamma (IFNγ) binds and activates a heterodimer of the IFNγ Receptor 1 and 2 (IFNγR1/2). Activation then leads to JAK1/2 activation which leads to STAT5 translocation to the nucleus. Granulocyte modifying- colony stimulating factor (GM-CSF) binds a dodecamer of subunits that forms the GM-CSF Receptor (GMCSF-R), which in turn activates JAK2, RAS and SFK. In addition to causing STAT5 translocation to the nucleus, GM-CSF alters the RAS pathway to increase protein translation, proliferation, and differentiation in innate immune cells. NFkβ, AP1, STAT1/5, and IRFs translocation to the nucleus leads to up-regulation of iNOS as well as the cell surface markers MHCII, CD86, and CD16/32. The production and release of cytokines TNF, IL-6, IL-1β, and IL-12 and chemokines CCL2 and CXCL10 are also up-regulated.
Table 1. Summary of M1 and M2 Stimuli, Markers and Released Factors.
Summary of the different stimuli, released factors, and markers for the M1 and M2 states. These markers and factors are the canonical markers for each state, and, as such is not an exhaustive list.
| M1 | M2 | |||
|---|---|---|---|---|
| M2a | M2b | M2c | ||
| Stimulus | LPS, IFNγ, LTA, GMCSF, dsDNA, ssRNA, unmethylated CpG Islands | Il-4 | TLR and IgG | IL-10 |
| Released Factors | TNF, IL-6, IL-12, IL-1β, CCL2, CCL10 | IL-10, Polyamines | TNF, IL-6, IL- 1β, IL-10 | Matrix Proteins, TGFβ, IL-10 |
| Cell Surface Markers | CD86, CD16/32, MHCII | CD206, SRs | CD86, MHCII | SLAM, CD206 |
| Intracellular Markers | iNOS | Arg1*, YM1*, Fizz1* | NA | NA |
Denotes markers that only work in mice. YM1 and Fizz1 have no known human analogs. These markers should only be used for studies in mice.
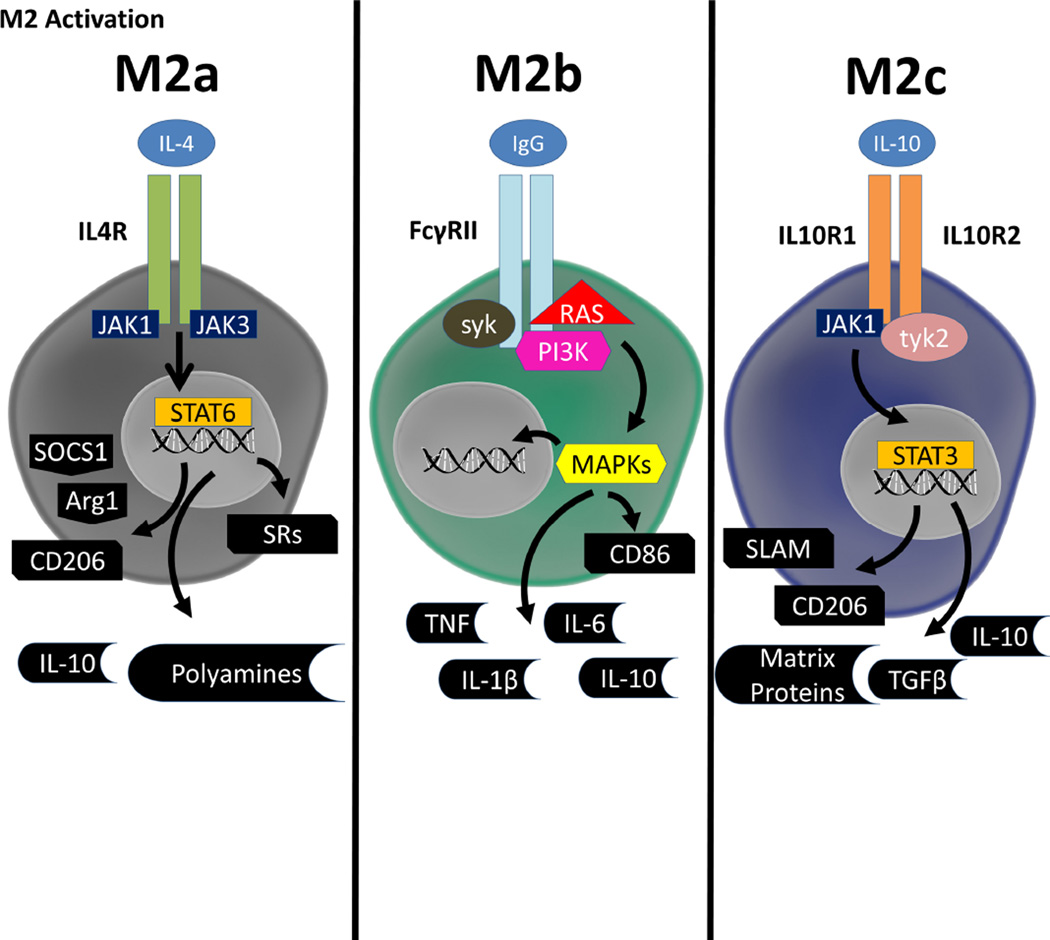
M2 Activation States
The alternative M2 activation state encompasses a broad set of responses as compared to M1 responses. Generally, the M2 activation state is associated with healing and scavenging, opposing the pro-killing state of M1 activation states. The M2 state is further subdivided into M2a, M2b, and M2c. These three states have some biochemical overlap, but have distinct activation mechanisms as well as effector outputs.
The M2a category was the first alternative activation state described and was developed as a paradigm to understand host response to parasites, and, as such, is associated with encapsulation and killing of parasites as well as allergy. IL-4 is the prototypical M2a stimulus and can bind three different receptor pairs. Each receptor pair can activate JAK1 or JAK3 which activate STAT6 leading to transcriptional changes associated with the M2a state, including; CD206 (mannose receptor), scavenger receptors (SRs), and suppressor of cytokine release 1 (SOCS1) (Edwards et al., 2006, Martinez et al., 2013). M2a macrophages will secrete polyamines and IL-10, which will block pro-inflammatory (e.g., IFNγ, IL6, and TNF) cytokine production (Lu et al., 2013). With the exception of IL-10 secretion, which is released by all the M2 states (described below) to some degree, each of these biochemical changes indicates the M2a activation state.
M2b macrophages, also referred to as type II activated macrophages, are associated with a selective up-regulation of phagocytosis as well as regulation of inflammatory responses. To stimulate this response, TLR activation is required to fuse Fcγ Receptors, especially FcγRIIB, which can then bind IgG (released from B cells)(Anderson and Mosser, 2002). The M2b state is remarkably different than the M2a state in terms of secreted cytokines and associated changes in gene expression. M2b macrophages will secret high amounts of IL10, as well as low to modest levels of typical pro-inflammatory cytokines, with CD86 highly expressed on the cell surface (Sanchez-Mejorada and Rosales, 1998, Takai, 2002, Edwards et al., 2006).
The last subcategory of M2 activation is M2c. M2c macrophages are associated with tissue repair, extracellular matrix repair, and de-activation of M1/Th1 immune responses (Fiorentino et al., 1989, Glocker et al., 2009). IL10 is the major stimulus for M2c. IL10 stimulates a dimer of the IL10 Receptor 1 and 2 subunits that causes JAK1 and subsequently STAT3 activation. This signaling results in the suppression of most M1 pro-inflammatory cytokines. IL10 also stimulates the release of CXCL13 as well as CXCL4. Several other gene products are also up-regulated including SLAM, which is a marker for this state (Park-Min et al., 2005). As compared with the M1 state, M2 stimuli are much broader and lead to a much larger array of possible responses. In summary, M2 activation states generally lead to healing and reparative responses as opposed to the pro-killing responses of the M1 state. The M2 states are graphically depicted in Figure 2, and listed in Table 1.
Figure 2. Schematic of M2 Signaling.
The M2 activation state is further broken down into three subclasses, dubbed the M2a, M2b, and M2c state, that have few overlapping characteristics. The M2a state is caused by IL-4 binding to one of three receptor pairs, which causes activation of JAK1/3. This in turn causes STAT6 translocation to the nucleus and upregulation of SOCS1, Arg1, CD206, scavenger receptors (SRs) and releases of IL-10 and polyamines. The M2b state has some characteristics of an M1 response. TLR activation is necessary to fuse the subunits of the Fcγ Receptor, which then binds IgG. Through a RAS, PI3K, and syk signaling cascade, there is increased release of typically pro-inflammatory cytokines such as TNF, IL-6 and IL-1β as well as typical M2 cytokine IL-10. Similarly to the M1 state, CD86 is up-regulated on the cell surface. The M2c state s caused by IL-10 binding to a heterodimer of IL10 Receptor subunits 1 and 2 which in turn causes activation of JAK1 and tyk. JAK1 and tyk then cause STAT3 translocation to nucleus and up-regulation of SLAM and CD206 as well as increased release of IL-10, TGFβ, and extracellular matrix proteins.
Microglia and Macrophages in PD
Microglia were originally thought to derive developmentally from origins similar to that of monocytes and macrophages (Rio-Hortega, 1939, Chan et al., 2007). However, recent studies have shown that microglia are not simply monocytes or macrophages from periphery that happen to reside in the brain. Instead, microglia arise from macrophages of the yolk sac blood islands that seed the developing brain early in development. In contrast, adult peripheral macrophages arise from the fetal liver (Ginhoux et al., 2010, Schulz et al., 2012, Kierdorf et al., 2013). Macrophages originating from the yolk sac cells remain the predominant population in the brain through life. In extreme cases such as inflammation and damage, peripheral cells will enter the brain in large numbers (de Groot et al., 1992, Ladeby et al., 2005, Mildner et al., 2007). Recently, the history and process of microglial origins has been eloquently reviewed (Ginhoux et al., 2013, Prinz and Priller, 2014)
Despite these distinct developmental origins, microglia and macrophages use remarkably similar transcription factors in development. For example, knockout of Pu.1 depletes both microglia and macrophage populations (McKercher et al., 1996, Beers et al., 2006). The cellular makeup of microglia and macrophages are very similar in terms of expressed proteins and cell surface markers, although new experimental paradigms tracking gene expression profiles are beginning to reveal differences between the two cells (Gautier et al., 2012, Butovsky et al., 2014). The M1 and M2 activation state paradigm has also been suggested to be similar in microglia and macrophages, although some differences have been noted in changes of cell surface markers such as CD206 and magnitude of responses to M2 stimuli (Durafourt et al., 2012).
This similarity between resident and peripheral cells in the brain has made the ability to distinguish resident cells from their peripheral counterparts extremely difficult. However, a few techniques have been suggested to differentiate central versus peripheral cells: CD45hi versus CD45low has been shown to mark macrophages and microglia respectively (Zhang et al., 2002, Prinz et al., 2011). Another recent study examining the gene transcription of adult microglia compared to peripheral cells has suggested that microglia lack CD169 and can be used as a staining antibody to distinguish the cell types (Butovsky et al., 2012).
In humans and animal models of PD, there is little understanding of macrophages and microglia as separate entities, even though different roles have been prescribed recently for each cell type in other disease states (Jung and Schwartz, 2012). In the context of AD, perivascular macrophages have been suggested to primarily clear protein aggregates from the brain, while microglia do not seem to significantly affect this process (Mildner et al., 2011). Recruited monocytes and macrophages increase disease severity in experimental autoimmune encephalitis (EAE), a mouse model of multiple sclerosis (MS) (Ajami et al., 2011). Additionally, recruited cells may have distinct roles from microglia, e.g., demyelination, in disease etiology (Yamasaki et al., 2014).
Despite what is not known in PD, through these correlates there is reason to believe that peripheral macrophages may be involved in PD. For example, a recent study examined the expression and number of CCR2+ cells in blood and found that number of CCR2+ cells was decreased, but the expression of CCR2 was increased in PD patients (Funk et al., 2013). This is indicative of cells migrating into a tissue, and this CCR2 mechanism has been demonstrated to control migration of peripheral cells into the CNS in EAE (Mahad and Ransohoff, 2003). These findings implicate peripheral cells being involved in PD and are reason to attempt to differentiate between macrophages and microglia in future studies.
Evidence of M1 Activation in PD Brain
In PD, enhanced microglial activation, T and B cell infiltration, and immunoglobulin deposition can be found in the substantia nigra and other brain regions associated with α-synuclein aggregation (McGeer et al., 1988, Boka et al., 1994, Imamura et al., 2003). Increases in M1 associated cytokines such as TNF and IL6, possibly from TNF activation of astrocytes (Van Wagoner et al., 1999), have been reported in serum and cerebrospinal fluid from PD patients (Boka et al., 1994, Mogi et al., 1994, Muller et al., 1998, Mogi et al., 2000). Levels of these cytokines have correlated with increasing disability and poorer prognosis (Hofmann et al., 2009, Scalzo et al., 2010). Additionally, the increasing levels of α-synuclein deposition in post mortem PD brain correlates to an increasing number of MHCII positive cells, a marker of the M1 activation state (Croisier et al., 2005). However, these observations are correlative and skewed towards late-stages of disease, so understanding the role of M1 activation in PD becomes difficult.
Positron emission tomography (PET) ligands to peripheral benzodiazepine receptors (PBR) have provided further insight into immune cell activation in PD, particularly in earlier stages of disease compared with post-mortem studies. The PBR receptor increases in expression in the outer membrane of mitochondria of activated macrophages and microglia (Chen and Guilarte, 2008, Papadopoulos and Lecanu, 2009). PET studies have shown 25–50% increases in ligand biding in several areas of the brain associated with PD, such as structures in the basal ganglia, in patients with PD compared to healthy age matched controls (Ouchi et al., 2005, Gerhard et al., 2006, Bartels et al., 2010, Edison et al., 2013, Iannaccone et al., 2013). Unfortunately, it is unclear if PBR ligands preferentially bind to M1 or M2 skewed microglia or macrophages, and interpretation must be limited to activation and not a specific state. Studies using PBR compounds indicate that PBR up-regulation may already be at maximum levels by the time of diagnosis, as patients followed for 2 years after the original PET scan did not show fluctuations in ligand binding. However, this plateau could also represent ceiling-effect technical limitation of the assay (Gerhard et al., 2006, Edison et al., 2013, Iannaccone et al., 2013). Another interpretation is that the plateau in PBR levels through disease is that PBR upregulation is important in initiation but not progression. Supporting this, PBR ligand binding did not correlate well to clinical severity of disease (Gerhard et al., 2006).
Retrospective epidemiological studies also highlight that inflammation may be important in the initiation or early progression of PD. Some studies show that non-steroidal anti-inflammatory drug (NSAID) use, especially ibuprofen, lowers PD susceptibility (Chen et al., 2003, Esposito et al., 2007, Wahner et al., 2007, Samii et al., 2009, Gagne and Power, 2010). While the preventative effect of NSAID use has failed to replicate in every study(Shaunak et al., 1995, Bornebroek et al., 2007, Becker et al., 2011), meta-analyses indicate that non-aspirin NSAID use is lowers risk for the development of PD (Samii et al., 2009, Gagne and Power, 2010, Noyce et al., 2012). Studies of NSAID use in AD highlight the complex relationship between neurodegeneration and anti-inflammatories. One study in AD prevention showed that naproxen use, a type of NSAID, was useful for prevention of AD in familial cases (Szekely et al., 2008). However, NSAID use did not modify susceptibility to AD in non-familial cases and actually was overall harmful in patients currently diagnosed with idiopathic AD (Breitner et al., 2011, ADAPT, 2013). In AD, NSAID use for currently diagnosed patients could have attenuated M2 responses, which could explain the potential worsening observed in subjects. While NSAID usage is not indicated to prevent PD or AD due to the high frequency of adverse events associated with treatment, these retrospective studies provide proof-of-principle support for the hypothesis that inflammation is not a benign process in the development of PD.
Because it is not clear when NSAID usage may provide the most benefit for patients at risk or currently suffering from PD, there is a need to understand the activation state of macrophages and microglia throughout the brain during the disease process. Instead of PBR-binding compounds, PET ligands specific for surface receptors associated with an M1 or M2 states may be more useful. Typical approaches ubiquitous in immunological studies such as flow cytometry are not possible in PD since affected brain tissue is not available during the neurodegenerative process. Studies of peripheral cells, while providing interesting clues, may not accurately reflect local microglia and/or macrophage changes in the brain. Future PET approaches with refined ligands to M1 and M2 targets would allow for longitudinal studies to help understand the presumed cycling between M1 and M2 states. Such studies would provide temporal resolution of how inflammation initiates in the disease process.
Protein Aggregates as M1 stimuli
Many neurodegenerative diseases, including PD, are defined pathologically by proteins that form insoluble aggregates in susceptible brain regions (Golde et al., 2013). How exactly macrophages or microglia become activated in PD are poorly understood. Protein aggregates comprised of α-synuclein can be generated in vitro (Giasson et al., 2001, Volpicelli-Daley et al., 2011), and these high molecular weight aggregates of α-synuclein have been shown to weakly induce an M1 response in vitro (Zhang et al., 2005b, Klegeris et al., 2008, Reynolds et al., 2008, Su et al., 2008, Freeman et al., 2013). α-Synuclein aggregates have been shown to interact with a variety of receptors including CD36, TLR2, TLR4 and CD11b, largely depending on the constituency of the protein aggregates applied to cells (Zhang et al., 2007, Su et al., 2008, Lee et al., 2010). Mounting recent evidence suggests that these protein aggregates interact mainly with TLR4 or TLR2 receptors (Beraud et al., 2011, Fellner et al., 2013, Kim et al., 2013). Monomeric forms of α-synuclein do not seem to interact strongly with immunological receptors, nor do they elicit a M1 response. Protein aggregates in other neurodegenerative disorders, such as Aβ plaques in AD, have been shown almost exclusively to interact with TLR2 to induce an M1 response (Jana et al., 2008, Tukel et al., 2009, Liu et al., 2012). Interestingly, α-synuclein has unique affinity for lipids of a variety of compositions (Burke et al., 2013, Hellstrand et al., 2013). One explanation of α-synuclein interaction with TLR4 is that these bound-lipids serve as agonists that allow for TLR4 interaction.
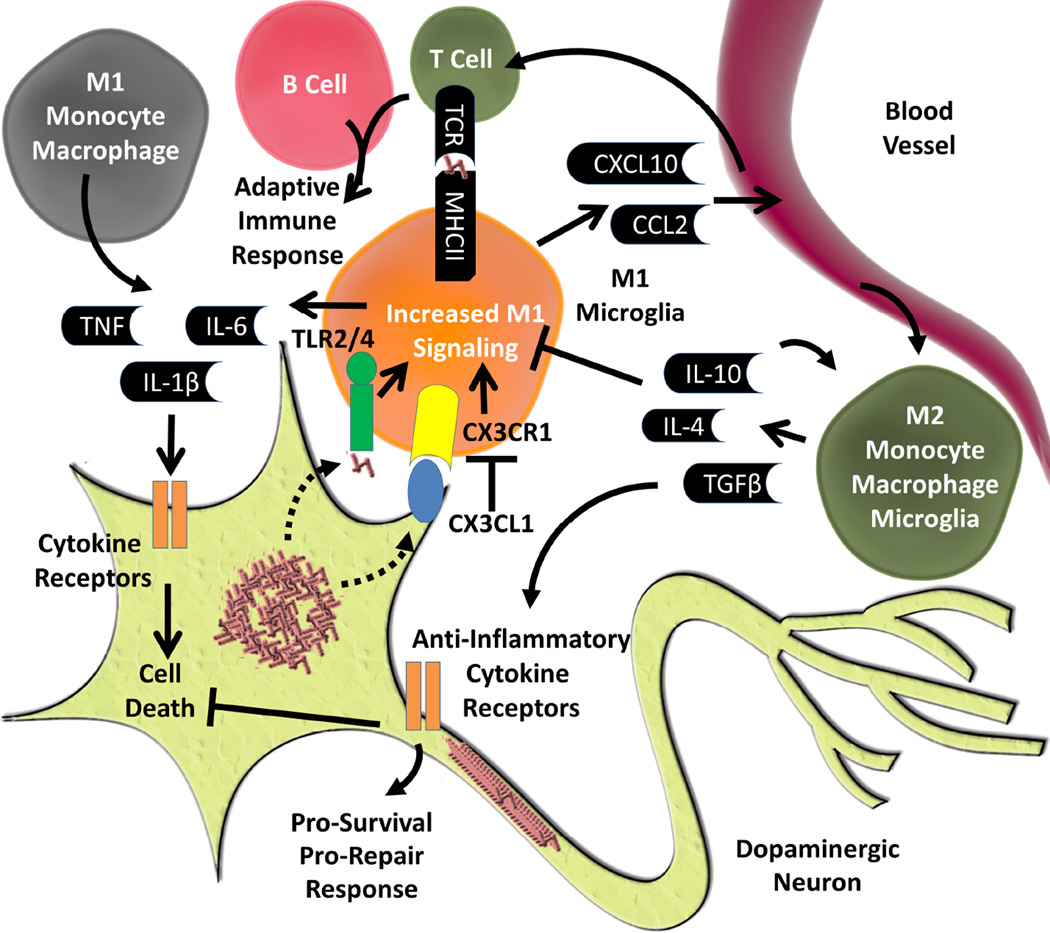
Activation states elicited by neurodegeneration-linked protein aggregates are difficult to interpret in isolated primary microglial cells. For example, if aggregates of α-synuclein are added to microglia and CD4+ T cells in co-culture, a much more robust M1/Th1 response is observed than either cell can mount on their own (Harms et al., 2013). Neurons in culture with those cells would presumably further alter immunological responses, for example through CX3CR1 signaling (Figure 3). Nevertheless, co-culture experiments point to a possible necessity for cells of the innate (e.g., macrophage or microglia) and adaptive (e.g., T-cells) immune system to work together.
Figure 3. Overview of Inflammatory Mechanisms in PD.
Inflammation is a common pathological hallmark in PD. One possible mechanism of how this arises is through direct activation of TLR2/4 by aggregated forms of α-synuclein. Another is through mechanisms by which neuronal health or dysfunction directly activates microglia. One mechanism is through CX3CR1, which is expressed on microglia, binding CX3CL1, which is expressed by neurons. Through injury, changes in health of neurons, or through α-synuclein CX3CL1 becomes down regulated which activates M1 signaling through CX3CR1 in microglia. With increased M1 signaling, microglia will release pro-inflammatory cytokines and chemokines. Chemokines will draw in innate immune cells from the peripheral immune system. These peripheral immune cells could lead to an adaptive immune response through T and B cells, or could lead to an increased M1 response through the recruitment of monocytes/macrophages and release of more pro-inflammatory cytokines and chemokines. These pro-inflammatory cytokines can act on a variety of cytokine receptors on dopaminergic neurons which could lead to cell death. Concurrently, or as a result of therapeutic intervention, M2 immune cells could release anti-inflammatory cytokines and chemokines that could decrease M1 activation and bind to anti-inflammatory cytokine receptors on neurons and promote survival and repair.
Genetics of PD Relevant to the M1 Activation State
Genome wide association studies (GWAS) have been useful to highlight genetic risk factors important for PD susceptibility (Sekiyama et al., 2014). Interestingly, the genes most strongly associated with PD (α-synuclein and tau) were already identified in neurodegeneration genetic linkage or genome wide association studies (Golbe et al., 1996, Polymeropoulos et al., 1996, Polymeropoulos et al., 1997, Martin et al., 2001, Zhang et al., 2005a). GWAS studies have identified the human leukocyte antigen-DR (HLA-DR) locus which points towards a role for inflammation in susceptibility to PD (Lampe et al., 2003, Hamza et al., 2010, Ahmed et al., 2012, Nalls et al., 2014). HLA-DR, encoding major histocompatibility complex 2 (MHCII), is expressed by a limited number of cells of the immune system, deemed antigen presenting cells (APCs). Microglia and macrophages are both APCs. The particular variant of HLA-DR associated with PD is believed to increase expression of MHCII (Wissemann et al., 2013). Of possible relevance, animal models of PD show that MHCII knockout reduces M1/Th1 inflammatory responses in response to α-synuclein overexpression, and MHCII knockout protects against dopaminergic neurodegeneration (Harms et al., 2013).
Mutations in the leucine-rich repeat kinase 2 (LRRK2) gene are the most common known genetic cause of familial PD (Paisan-Ruiz et al., 2004, Zimprich et al., 2004, Gilks et al., 2005, Healy et al., 2008, Kett and Dauer, 2012). LRRK2 shows high expression in myeloid cells (Thevenet et al., 2011) and knockdown, knockout, or pharmacological inhibition of LRRK2 decreases M1 inflammatory responses both in vivo and in vitro (Moehle et al., 2012, Daher et al., 2014). Additionally, LRRK2 knockout rodents have decreased macrophage and microglia activation and dopaminergic cell death caused by the prototypical M1-agonist LPS (Daher et al., 2014). These studies show that LRRK2 expression and activity are required for a full M1 response in model systems.
The genetic risk factors of PD implicate a role for inflammation in the etiology of disease. One recent study examined the expression of quantitative trait loci (eQTL), regions of the genome that regulate the expression of mRNA, in a wide range of inflammatory and neurodegenerative disorders in both lymphocytes and CD14+ Cd16− cells of the myeloid lineage that includes macrophages. These peripheral cells demonstrated an overrepresentation of monocyte specific eQTLs in PD. In fact, in contrast to other neurodegenerative disorders like MS, only one eQTL identified was not exclusively found in myeloid cells (Raj et al., 2014). These results suggest that myeloid cell changes predominate immunological responses in subjects with PD.
Animal Models Implicate M1 Activation in Dopaminergic Neurodegeneration
Innate immune activation, including M1 activation states, can drive dopaminergic cell loss in the SNpc in diverse models systems (Hirsch et al., 2012, Deleidi and Gasser, 2013).Through decades of work, it is thought that cells vulnerable to neurodegeneration in PD are particularly sensitive to secreted factors associated with M1 activation (Gonzalez-Hernandez et al., 2010). One hypothesis is that cells vulnerable in PD are found in brain regions enriched in cells capable of mounting M1 responses (Lawson et al., 1990). Direct injection of the canonical M1-agonist LPS into the SNpc produces robust loss of dopaminergic neurons, but not in other nearby brain regions like the ventral tegmental area that also harbor dopaminergic cells (Kim et al., 2000, Castano et al., 2002).
Another model that results in dopaminergic neurodegeneration involves rAAV mediated transduction of α-synuclein in the SNpc of rats and mice (Kirik et al., 2002, Kirik et al., 2003, St Martin et al., 2007). In this model, there is microglial activation, IgG deposition, as well as T and B cell infiltration, in addition to dopaminergic cell loss (Theodore et al., 2008, Sanchez-Guajardo et al., 2010, Barkholt et al., 2012). Interestingly, in this model, inflammation proceeds dopaminergic cell loss with pronounced inflammation 2 weeks to 3 months post injection and cell loss following at 4 to 6 months post injection (St Martin et al., 2007, Chung et al., 2009). This time course implicates that inflammation may be an initiating event in cell loss in this model. Genetically modifying the immune system in this model through MHCII, CX3CR1, or FcγRIII knockout blocks inflammation and protects from dopaminergic cell loss, but does not alter α-synuclein overexpression (Cao et al., 2010, Cao et al., 2012, Harms et al., 2013). One possibility is that inflammation is secondary or a result of α-synuclein overexpression, but is critical for over cell loss. Some transgenic models of α -synuclein overexpression, such as Thy1-α-syn (line 61), MBP1-hα-syn, and A53T or A30P mutant α-synuclein overexpressing mice (driven by chicken β-actin promoter), also have pathological findings of inflammation in areas with high expression of α-synuclein, but in general these models have not been rigorously scrutinized for markers of M1 or M2 responses (Mendritzki et al., 2010, Chesselet et al., 2012, Valera et al., 2014).
The neurotoxin 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) can also be used to model dopaminergic cell death in model systems. MPTP was identified as the neurotoxin responsible for neurodegeneration in heroin addicts that injected contaminated preparations of drug. (Langston et al., 1984b). MPTP itself is not toxic and must be metabolized by the MAO-B enzyme to MPP+ to exert toxic effects (Langston et al., 1984a). Pathogenicity of MPTP comes, in part, from decoupling constituents of the electron transport chain in the mitochondria and increases reactive oxygen and nitrogen species (Bove et al., 2005). A common finding in MPTP models of dopaminergic cell death is robust inflammation associated with neurodegeneration (Pattarini et al., 2007, Ramsey and Tansey, 2014). The precise mechanism of the inflammation in MPTP intoxicated animals is not fully understood. However, CX3CR1 interaction with its ligand CX3CL1 has been shown to modulate MPTP, with loss of the interaction through CX3R1 ablation worsening cell loss and increasing soluble CX3CL1 protecting from cell loss (Figure 3) (Cardona et al., 2006, Morganti et al., 2012).
Blocking M1 inflammation caused by MPTP offers some neuroprotection. Treatment with antiinflammatory agents as well as genetic ablation of important pro-inflammatory mediators, such as iNOS, protects from dopaminergic neurodegeneration (Wu et al., 2002, Watanabe et al., 2004, Zhao et al., 2007, Madathil et al., 2013, Thakur and Nehru, 2013). Interestingly, a recent study pre-treating MPTP intoxicated mice with GM-CSF, a typically weak M1 stimulus as described above, showed moderate protection from dopaminergic cell loss (Kosloski et al., 2013). This study indicates the possible complexity of the interplay between and pro and anti-inflammatory states in animal models of dopaminergic cell loss. Overall, neuroprotection studies in toxin models highlight the importance of inflammation to drive overt loss of neurons.
M2 Activation States in PD and Neurodegeneration
So far, the evidence presented for macrophage action in PD points to an M1 activation state contributing to susceptibility and/or progression of disease. However, it is important to note that, especially in vivo, macrophages or microglia are not necessarily only M1 or only M2, but can exist as continuums of M1 and M2 responses (Vogel et al., 2013, Martinez and Gordon, 2014). The M2 activation states in chronic disease have been the subject of intense recent interest and extensively reviewed in a series of recent publications (Shechter and Schwartz, 2013, Walker and Lue, 2013, Jiang et al., 2014, Miron and Franklin, 2014, Murray et al., 2014, Plemel et al., 2014).
Clear examples of the continuum of M1 and M2 inside neurodegenerative disease come from the prototypical neuroinflammatory disorder, multiple sclerosis, as well as acute CNS injury, which, unlike PD, have more defined periods for M1 and M2 activated cells. In contusion models of spinal cord injury in mice, there is a robust activation of resident microglia in the spinal column as well as invasion of macrophages. Despite the severity of the injury, there is a mixed M1 and M2 activation within the injury site (Shechter et al., 2009). A small and transient M2 activated cell population, defined as Arg1+ and CD206+ (M2a) localize to sites of injury (Kigerl et al., 2009). Furthermore, if this M2 population is down-regulated, lesion size and spinal cord motor neuron death are increased (Shechter et al., 2009). Conversely, supplanting the lesion site with macrophages exogenously manipulated to an M2 state, results in attenuated lesion size and spinal cord motor neuron death (Rapalino et al., 1998, Kigerl et al., 2009, Shechter et al., 2013). Similar mixed M1 and M2 populations have been identified within the cortex of brain in rodent models of traumatic brain injury (Zhang et al., 2012).
There is also very good evidence of mixed M1 and M2 populations in other chronic neuroinflammatory disorders. In MS, there is direct evidence of M2 macrophages and microglia. Arg1+CD163+ macrophages or microglia localize to both acute and chronic lesions of MS patients (M2a) (Boven et al., 2006, Zhang et al., 2011). Similar to lesion sites in acute injury, in MS models M2 cells are not the majority of innate immune cells within the lesion site. Animal models of MS, especially experimental autoimmune encephalitis (EAE), give further evidence that M1 and M2 activation states occur concurrently and can even predict some measures of disease (Mikita et al., 2011). Disease progression is dependent on M1 macrophages, since blocking M1 activation effectively blocks disease progression or initiation (King et al., 2009, Mildner et al., 2009, Moreno et al., 2014). The ratio of M1 to M2 cells has been shown to have some predictive value in determining relapses of EAE (Mikita et al., . 2011). If the M1 state is dominant, a more progressive EAE is favored (King et al., 2009, Mildner et al., 2009). Conversely, if M2 states are favored through adoptive transfer or therapeutic intervention, a less aggressive and possibly regenerative state is achieved (Weber et al., 2007, Burger et al., 2009, Liu et al., 2013). Both EAE and human MS data point to roles for M1 and M2, and not just one activation state alone, in a chronic neuroinflammatory disease.
Similarly, a mixed M1 and M2 state could be occurring in PD, and could help to explain the heterogeneity of retrospective clinical data and observations made in model systems. Emergent data about α-synuclein’s impact on M1 and M2 balance in vitro suggest sensitization of TLR based immunity and an intermediate M1/M2 phenotype of microglia (Roodveldt et al., 2013). Unfortunately, there is a relative paucity of information on M2 markers in PD or chronic animal models of PD based on α-synuclein, so piecing together the whole puzzle of myeloid cell responses relevant to PD is not possible at this time.
Therapeutic Manipulation of M1 and M2 Responses in PD
A question arises over where and how to target inflammation in neurodegenerative disease to achieve slowing or halting progression (Hirsch and Hunot, 2009). M1 and M2 activation states are embedded within the complexity of not only other immune cells, but also the interplay between neurons, glia, and interactions at the blood-brain barrier (Rock et al., 2004). NSAID studies in PD and AD illustrate that simply blocking inflammation with relatively non-specific targets is probably not going to have overall beneficial effects. Worse, neurodegenerative phenotypes may be exacerbated if NSAIDs are used at the wrong stage of disease (Breitner et al., 2011, ADAPT, 2013). A parallel may be drawn with minocycline usage in the MS model EAE where the anti-inflammatories can block beneficial effects of an M2 response (Li et al., 2005). Macrophage activation states in neurodegeneration may need to be treated more specifically by targeting and attenuating critical and specific M1 targets, and/or promoting M2 responses.
To accomplish M1 inhibition, products of M1 activation states could be blocked or signal transduction pathways underlying M1 activation could be directly attenuated. TNF provides a good target as the molecule itself can induce cell death in neurons, particularly dopaminergic substantia nigra neurons (Frankola et al., 2011). Blocking TNF from binding its receptor through neutralizing antibody therapy (adaluminab), decoy receptors (Etanercept), or through dominant negative TNF, are approaches already used in a number of human diseases and animal models with more established M1 activation states underlying pathogenesis (Peppel et al., 1991, Kempeni, 1999, Rau, 2002, Braun et al., 2007, McCoy et al., 2008, Harms et al., 2011). These therapeutics do not directly affect the activation state of microglia or macrophages, but rather decrease the ability of TNF to act on its receptor in other cell types. These therapies all rely on large proteins (i.e., biologics), which typically do not cross the blood brain barrier. However, emerging technology to deliver biologics across the blood brain barrier using bispecific antibodies, polymers, or viruses may be on the horizon (Egleton and Davis, 2005, Xiao and Gan, 2013, Farrington et al., 2014).
Small molecules have a much better chance of crossing the blood-brain barrier to block M1 signaling in macrophages. Targeting JAK/STAT activation in signaling pathways that lead to M1 activation can effectively diminish downstream M1 responses (Mascarenhas et al., 2014). JAK/STAT inhibitors have promising efficacy in EAE models as they are able to decrease clinical score severity and associated M1 inflammatory responses (Liu et al., 2014). Whether through biological or small synthetic molecules, one important caveat with globally blocking components of the M1 response is that it could lead to decreased host responses to pathogens and greatly increase risk for infection (Kwon et al., 2014, Varley et al., 2014).
Instead, a therapy that promotes M2 responses could present a better therapeutic option in neurodegenerative disease that avoids caveats associated with blocking individual M1 responses. By polarizing microglia and macrophages into an M2 skewed phenotype, this would not only halt local M1 responses in a much more targeted and controlled way, but also promote healing and repair around the inflamed brain regions. Glimpses of benefit of an M2 targeting therapy have been seen in neurotoxin models. When IL10 was delivered virally into the midbrain or striatum of rodents undergoing MPTP or 6-OHDA intoxication, there was a robust amelioration of dopaminergic neuron loss in the substantia nigra (Schwenkgrub et al., 2013, Joniec-Maciejak et al., 2014). In models of AD, activating CD200R, a membrane glycoprotein receptor induced by M2 cytokines, has been shown to decrease inflammation as well as decrease Aβ deposits (Lyons et al., 2007). However, the best evidence for M2 therapy comes from MS therapeutics. Glatiramer acetate and beta interferons are both currently approved therapies for MS. Their mechanism of action appears to be through altering the balance of M1/Th1 and M2/Th2 cells of the immune system (Weber et al., 2007, Burger et al., 2009, Kieseier, 2011). Glatiramer and interferon treatment are believed to skew macrophages to release M2 cytokines including IL10, as well as possibly releasing neuronal growth factors, decreasing chemotaxis of cells into the CNS, and decrease release of M1 cytokines, leading to a pro-M2 effect (Yong, 2002, Ziemssen et al., 2002, Ziemssen et al., 2005, Pul et al., 2011, Kurtuncu et al., 2012, Begum-Haque et al., 2013, Peelen et al., 2013). Treatment with these compounds is associated with decreased frequency of relapses in MS and possibly decreasing the progression of disability (Johnson et al., 1998, Buttinelli et al., 2007, Ford et al., 2010, Freedman, 2011).
By drawing on parallels between MS and PD, pro-M2 therapy may prove beneficial in PD, with particular utility in slowing progression. However, any therapy, whether pro-M2 or anti-M1, will likely require the additional development of biomarkers for inflammation within the CNS that are far more sensitive or specific than currently available options, such as currently available PET ligand options. Inflammation is widely postulated to start many years before the clinical onset of symptoms. Therapeutic targeting of inflammation could begin at this time point, but could only slow the progression of disease. If a sensitive biomarker of CNS inflammation could be found, perhaps through PET, onset of clinical symptoms could be delayed by many years or possibly even prevent the clinical onset of PD.
Concluding Remarks
Better understanding M1 and M2 responses in PD presents opportunities for both enhanced clarity of pathogenic mechanisms underlying disease as well as potential therapeutic targets in neuroprotection approaches. Specifically, M1 activation may represent an insult that drives overt cell loss in the SNpc. Data from human genetic studies, pathological studies, and animal models suggest that M1 activation may have 2 possible relationships to dopaminergic cell death, ether as a secondary hit in response to α-synuclein aggregation or as primary, initiating event to inflammatory signals. Either way, in the relentless progression of neurodegenerative disease, somehow the underlying pathology subverts normal remediation of pro-inflammatory pathways or conversion to M2 pathways. Thus, therapeutic intervention to enact these responses seems well-justified. Ultimately, treating inflammation may present a unique opportunity for a disease modifying therapy in chronic neurodegeneration, but broad spectrum approaches to non-specifically attenuate immune cells seems likely to fail. Utilization of the mechanisms already in place in macrophages and microglia may represent a straightforward approach that delivers the specificity and efficacy necessary to deal with chronic neuroinflammation in PD.
-
-
Inflammation may represent an exciting novel therapeutic mechanism in PD.
-
-
M1 macrophage/microglia activation may drive overt cell loss in PD.
-
-
M2 macrophage/microglia activation may block M1 activation states and promote healing and repair
Acknowledgements
The authors thank the benevolence of John A. and Ruth R. Jurenko, as well as NIH/NINDS grants 5F31NS081963 and 5R01NS064934, for supporting this work.
Abbreviations
- PD
Parkinson Disease
- AD
Alzheimer Disease
- MS
Multiple Sclerosis
- IL
interleukin
- TNF
tumor necrosis factor
- EAE
experimental autoimmune encephalitis
- CCL
C-C motif ligand
- CXCL
C-X-C motif ligand
- iNOS
inducible nitric oxide synthase
- IFNγ
interferon-gamma
- JAK
Janus kinase
- IRFs
interferon regulatory factors
- LPS
lipopolysaccharide
- TLR
toll like receptor
- GM-CSF
Granulocyte-modifying colony stimulating factor
- SRs
scavenger receptors
- SOCS1
suppressor of cytokine release 1
- CD
cluster of differentiation
- PET
positron emission tomography
- PBR
peripheral benzodiazepine receptors
- NSAID
non-steroidal anti-inflammatory drug
- GWAS
Genome wide association studies
- HLA-DR
human leukocyte antigen-DR
- MHCII
major histocompatibility complex 2
- LRRK2
leucine-rich repeat kinase 2
- eQTL
expression of quantitative trait loci
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ADAPT. Results of a follow-up study to the randomized Alzheimer’s Disease Anti-inflammatory Prevention Trial (ADAPT) Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2013;9:714–723. doi: 10.1016/j.jalz.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed I, Tamouza R, Delord M, Krishnamoorthy R, Tzourio C, Mulot C, Nacfer M, Lambert JC, Beaune P, Laurent-Puig P, Loriot MA, Charron D, Elbaz A. Association between Parkinson’s disease and the HLA-DRB1 locus. Movement disorders : official journal of the Movement Disorder Society. 2012;27:1104–1110. doi: 10.1002/mds.25035. [DOI] [PubMed] [Google Scholar]
- Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nature neuroscience. 2011;14:1142–1149. doi: 10.1038/nn.2887. [DOI] [PubMed] [Google Scholar]
- Anderson CF, Mosser DM. A novel phenotype for an activated macrophage: the type 2 activated macrophage. Journal of leukocyte biology. 2002;72:101–106. [PubMed] [Google Scholar]
- Barkholt P, Sanchez-Guajardo V, Kirik D, Romero-Ramos M. Long-term polarization of microglia upon alpha-synuclein overexpression in nonhuman primates. Neuroscience. 2012;208:85–96. doi: 10.1016/j.neuroscience.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Bartels AL, Willemsen AT, Doorduin J, de Vries EF, Dierckx RA, Leenders KL. [11C]-PK11195 PET: quantification of neuroinflammation and a monitor of anti-inflammatory treatment in Parkinson’s disease? Parkinsonism & related disorders. 2010;16:57–59. doi: 10.1016/j.parkreldis.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Bayer C, Varani S, Wang L, Walther P, Zhou S, Straschewski S, Bachem M, Soderberg-Naucler C, Mertens T, Frascaroli G. Human cytomegalovirus infection of M1 and M2 macrophages triggers inflammation and autologous T-cell proliferation. Journal of virology. 2013;87:67–79. doi: 10.1128/JVI.01585-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker C, Jick SS, Meier CR. NSAID use and risk of Parkinson disease: a population-based case-control study. European journal of neurology : the official journal of the European Federation of Neurological Societies. 2011;18:1336–1342. doi: 10.1111/j.1468-1331.2011.03399.x. [DOI] [PubMed] [Google Scholar]
- Beers DR, Henkel JS, Xiao Q, Zhao W, Wang J, Yen AA, Siklos L, McKercher SR, Appel SH. Wild-type microglia extend survival in PU.1 knockout mice with familial amyotrophic lateral sclerosis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:16021–16026. doi: 10.1073/pnas.0607423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begum-Haque S, Christy M, Wang Y, Kasper E, Ochoa-Reparaz J, Smith JY, Haque A, Kasper LH. Glatiramer acetate biases dendritic cells towards an anti-inflammatory phenotype by modulating OPN, IL-17, and RORgammat responses and by increasing IL-10 production in experimental allergic encephalomyelitis. Journal of neuroimmunology. 2013;254:117–124. doi: 10.1016/j.jneuroim.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Beraud D, Twomey M, Bloom B, Mittereder A, Neitzke K, Ton V, Chasovskikh S, Mhyre TR, Maguire-Zeiss KA. α-Synuclein alters Toll-like receptor expression. Frontiers in Neuroscience. 2011:5. doi: 10.3389/fnins.2011.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boka G, Anglade P, Wallach D, Javoy-Agid F, Agid Y, Hirsch EC. Immunocytochemical analysis of tumor necrosis factor and its receptors in Parkinson’s disease. Neuroscience letters. 1994;172:151–154. doi: 10.1016/0304-3940(94)90684-x. [DOI] [PubMed] [Google Scholar]
- Bornebroek M, de Lau LM, Haag MD, Koudstaal PJ, Hofman A, Stricker BH, Breteler MM. Nonsteroidal anti-inflammatory drugs and the risk of Parkinson disease. Neuroepidemiology. 2007;28:193–196. doi: 10.1159/000108110. [DOI] [PubMed] [Google Scholar]
- Bove J, Prou D, Perier C, Przedborski S. Toxin-induced models of Parkinson’s disease. NeuroRx : the journal of the American Society for Experimental NeuroTherapeutics. 2005;2:484–494. doi: 10.1602/neurorx.2.3.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boven LA, Van Meurs M, Van Zwam M, Wierenga-Wolf A, Hintzen RQ, Boot RG, Aerts JM, Amor S, Nieuwenhuis EE, Laman JD. Myelin-laden macrophages are anti-inflammatory, consistent with foam cells in multiple sclerosis. Brain : a journal of neurology. 2006;129:517–526. doi: 10.1093/brain/awh707. [DOI] [PubMed] [Google Scholar]
- Braun J, McHugh N, Singh A, Wajdula JS, Sato R. Rheumatology. Vol. 46. England: Oxford; 2007. Improvement in patient-reported outcomes for patients with ankylosing spondylitis treated with etanercept 50 mg once-weekly and 25 mg twice-weekly; pp. 999–1004. [DOI] [PubMed] [Google Scholar]
- Breitner JC, Baker LD, Montine TJ, Meinert CL, Lyketsos CG, Ashe KH, Brandt J, Craft S, Evans DE, Green RC, Ismail MS, Martin BK, Mullan MJ, Sabbagh M, Tariot PN. Extended results of the Alzheimer’s disease anti-inflammatory prevention trial. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2011;7:402–411. doi: 10.1016/j.jalz.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger D, Molnarfi N, Weber MS, Brandt KJ, Benkhoucha M, Gruaz L, Chofflon M, Zamvil SS, Lalive PH. Glatiramer acetate increases IL-1 receptor antagonist but decreases T cell-induced IL-1β in human monocytes and multiple sclerosis. Proceedings of the National Academy of Sciences. 2009;106:4355–4359. doi: 10.1073/pnas.0812183106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke KA, Yates EA, Legleiter J. Biophysical insights into how surfaces, including lipid membranes, modulate protein aggregation related to neurodegeneration. Frontiers in neurology. 2013;4:17. doi: 10.3389/fneur.2013.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G, Koeglsperger T, Dake B, Wu PM, Doykan CE, Fanek Z, Liu L, Chen Z, Rothstein JD, Ransohoff RM, Gygi SP, Antel JP, Weiner HL. Identification of a unique TGF-beta-dependent molecular and functional signature in microglia. Nature neuroscience. 2014;17:131–143. doi: 10.1038/nn.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O, Siddiqui S, Gabriely G, Lanser AJ, Dake B, Murugaiyan G, Doykan CE, Wu PM, Gali RR, Iyer LK, Lawson R, Berry J, Krichevsky AM, Cudkowicz ME, Weiner HL. Modulating inflammatory monocytes with a unique microRNA gene signature ameliorates murine ALS. The Journal of clinical investigation. 2012;122:3063–3087. doi: 10.1172/JCI62636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttinelli C, Clemenzi A, Borriello G, Denaro F, Pozzilli C, Fieschi C. Mitoxantrone treatment in multiple sclerosis: a 5-year clinical and MRI follow-up. European journal of neurology : the official journal of the European Federation of Neurological Societies. 2007;14:1281–1287. doi: 10.1111/j.1468-1331.2007.01969.x. [DOI] [PubMed] [Google Scholar]
- Cao S, Standaert DG, Harms AS. The gamma chain subunit of Fc receptors is required for alpha-synuclein-induced pro-inflammatory signaling in microglia. Journal of neuroinflammation. 2012;9:259. doi: 10.1186/1742-2094-9-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao S, Theodore S, Standaert DG. Fcgamma receptors are required for NF-kappaB signaling, microglial activation and dopaminergic neurodegeneration in an AAV-synuclein mouse model of Parkinson’s disease. Molecular neurodegeneration. 2010;5:42. doi: 10.1186/1750-1326-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, Lee J-C, Cook DN, Jung S, Lira SA, Littman DR, Ransohoff RM. Control of microglial neurotoxicity by the fractalkine receptor. Nature neuroscience. 2006;9:917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- Casanova JL, Abel L, Quintana-Murci L. Human TLRs and IL-1Rs in host defense: natural insights from evolutionary, epidemiological, and clinical genetics. Annual review of immunology. 2011;29:447–491. doi: 10.1146/annurev-immunol-030409-101335. [DOI] [PubMed] [Google Scholar]
- Castano A, Herrera AJ, Cano J, Machado A. The degenerative effect of a single intranigral injection of LPS on the dopaminergic system is prevented by dexamethasone, and not mimicked by rh-TNF-alpha, IL-1beta and IFN-gamma. Journal of neurochemistry. 2002;81:150–157. doi: 10.1046/j.1471-4159.2002.00799.x. [DOI] [PubMed] [Google Scholar]
- Chan WY, Kohsaka S, Rezaie P. The origin and cell lineage of microglia: new concepts. Brain research reviews. 2007;53:344–354. doi: 10.1016/j.brainresrev.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Chen H, Zhang SM, Hernan MA, Schwarzschild MA, Willett WC, Colditz GA, Speizer FE, Ascherio A. Nonsteroidal anti-inflammatory drugs and the risk of Parkinson disease. Archives of neurology. 2003;60:1059–1064. doi: 10.1001/archneur.60.8.1059. [DOI] [PubMed] [Google Scholar]
- Chen MK, Guilarte TR. Translocator protein 18 kDa (TSPO): molecular sensor of brain injury and repair. Pharmacology & therapeutics. 2008;118:1–17. doi: 10.1016/j.pharmthera.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesselet MF, Richter F, Zhu C, Magen I, Watson MB, Subramaniam SR. A progressive mouse model of Parkinson’s disease: the Thy1-aSyn (“Line 61”) mice. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2012;9:297–314. doi: 10.1007/s13311-012-0104-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung CY, Koprich JB, Siddiqi H, Isacson O. Dynamic changes in presynaptic and axonal transport proteins combined with striatal neuroinflammation precede dopaminergic neuronal loss in a rat model of AAV alpha-synucleinopathy. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:3365–3373. doi: 10.1523/JNEUROSCI.5427-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croisier E, Moran LB, Dexter DT, Pearce RK, Graeber MB. Microglial inflammation in the parkinsonian substantia nigra: relationship to alpha-synuclein deposition. Journal of neuroinflammation. 2005;2:14. doi: 10.1186/1742-2094-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daher JP, Volpicelli-Daley LA, Blackburn JP, Moehle MS, West AB. Abrogation of alpha-synuclein-mediated dopaminergic neurodegeneration in LRRK2-deficient rats. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:9289–9294. doi: 10.1073/pnas.1403215111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton DK, Pitts-Meek S, Keshav S, Figari IS, Bradley A, Stewart TA. Science. Vol. 259. New York, NY: 1993. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes; pp. 1739–1742. [DOI] [PubMed] [Google Scholar]
- de Groot CJ, Huppes W, Sminia T, Kraal G, Dijkstra CD. Determination of the origin and nature of brain macrophages and microglial cells in mouse central nervous system, using non-radioactive in situ hybridization and immunoperoxidase techniques. Glia. 1992;6:301–309. doi: 10.1002/glia.440060408. [DOI] [PubMed] [Google Scholar]
- Deleidi M, Gasser T. The role of inflammation in sporadic and familial Parkinson’s disease. Cellular and molecular life sciences : CMLS. 2013;70:4259–4273. doi: 10.1007/s00018-013-1352-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirksen U, Nishinakamura R, Groneck P, Hattenhorst U, Nogee L, Murray R, Burdach S. Human pulmonary alveolar proteinosis associated with a defect in GM-CSF/IL-3/IL-5 receptor common beta chain expression. The Journal of clinical investigation. 1997;100:2211–2217. doi: 10.1172/JCI119758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dranoff G, Mulligan RC. Activities of granulocyte-macrophage colony-stimulating factor revealed by gene transfer and gene knockout studies. Stem cells (Dayton, Ohio) 1994;1(12 Suppl):173–182. discussion 182–174. [PubMed] [Google Scholar]
- Durafourt BA, Moore CS, Zammit DA, Johnson TA, Zaguia F, Guiot MC, Bar-Or A, Antel JP. Comparison of polarization properties of human adult microglia and blood-derived macrophages. Glia. 2012;60:717–727. doi: 10.1002/glia.22298. [DOI] [PubMed] [Google Scholar]
- Edison P, Ahmed I, Fan Z, Hinz R, Gelosa G, Ray Chaudhuri K, Walker Z, Turkheimer FE, Brooks DJ. Microglia, amyloid, and glucose metabolism in Parkinson’s disease with and without dementia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38:938–949. doi: 10.1038/npp.2012.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JP, Zhang X, Frauwirth KA, Mosser DM. Biochemical and functional characterization of three activated macrophage populations. Journal of leukocyte biology. 2006;80:1298–1307. doi: 10.1189/jlb.0406249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egleton RD, Davis TP. Development of neuropeptide drugs that cross the blood-brain barrier. NeuroRx : the journal of the American Society for Experimental NeuroTherapeutics. 2005;2:44–53. doi: 10.1602/neurorx.2.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito E, Di Matteo V, Benigno A, Pierucci M, Crescimanno G, Di Giovanni G. Non-steroidal antiinflammatory drugs in Parkinson’s disease. Experimental neurology. 2007;205:295–312. doi: 10.1016/j.expneurol.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Farrington GK, Caram-Salas N, Haqqani AS, Brunette E, Eldredge J, Pepinsky B, Antognetti G, Baumann E, Ding W, Garber E, Jiang S, Delaney C, Boileau E, Sisk WP, Stanimirovic DB. A novel platform for engineering blood-brain barrier-crossing bispecific biologics. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2014 doi: 10.1096/fj.14-253369. [DOI] [PubMed] [Google Scholar]
- Fellner L, Irschick R, Schanda K, Reindl M, Klimaschewski L, Poewe W, Wenning GK, Stefanova N. Toll-like receptor 4 is required for alpha-synuclein dependent activation of microglia and astroglia. Glia. 2013;61:349–360. doi: 10.1002/glia.22437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. The Journal of experimental medicine. 1989;170:2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford C, Goodman AD, Johnson K, Kachuck N, Lindsey JW, Lisak R, Luzzio C, Myers L, Panitch H, Preiningerova J, Pruitt A, Rose J, Rus H, Wolinsky J. Multiple sclerosis. Vol. 16. England: Houndmills, Basingstoke; 2010. Continuous long-term immunomodulatory therapy in relapsing multiple sclerosis: results from the 15-year analysis of the US prospective open-label study of glatiramer acetate; pp. 342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankola KA, Greig NH, Luo W, Tweedie D. Targeting TNF-alpha to elucidate and ameliorate neuroinflammation in neurodegenerative diseases. CNS & neurological disorders drug targets. 2011;10:391–403. doi: 10.2174/187152711794653751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman MS. Long-term follow-up of clinical trials of multiple sclerosis therapies. Neurology. 2011;76:S26–S34. doi: 10.1212/WNL.0b013e318205051d. [DOI] [PubMed] [Google Scholar]
- Freeman D, Cedillos R, Choyke S, Lukic Z, McGuire K, Marvin S, Burrage AM, Sudholt S, Rana A, O’Connor C, Wiethoff CM, Campbell EM. Alpha-synuclein induces lysosomal rupture and cathepsin dependent reactive oxygen species following endocytosis. PloS one. 2013;8:e62143. doi: 10.1371/journal.pone.0062143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk N, Wieghofer P, Grimm S, Schaefer R, Buhring HJ, Gasser T, Biskup S. Characterization of peripheral hematopoietic stem cells and monocytes in Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society. 2013;28:392–395. doi: 10.1002/mds.25300. [DOI] [PubMed] [Google Scholar]
- Gagne JJ, Power MC. Anti-inflammatory drugs and risk of Parkinson disease: a meta-analysis. Neurology. 2010;74:995–1002. doi: 10.1212/WNL.0b013e3181d5a4a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, Helft J, Chow A, Elpek KG, Gordonov S, Mazloom AR, Ma’ayan A, Chua WJ, Hansen TH, Turley SJ, Merad M, Randolph GJ. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nature immunology. 2012;13:1118–1128. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhard A, Pavese N, Hotton G, Turkheimer F, Es M, Hammers A, Eggert K, Oertel W, Banati RB, Brooks DJ. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson’s disease. Neurobiology of disease. 2006;21:404–412. doi: 10.1016/j.nbd.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Giasson BI, Murray IV, Trojanowski JQ, Lee VM. A hydrophobic stretch of 12 amino acid residues in the middle of alpha-synuclein is essential for filament assembly. The Journal of biological chemistry. 2001;276:2380–2386. doi: 10.1074/jbc.M008919200. [DOI] [PubMed] [Google Scholar]
- Gilks WP, Abou-Sleiman PM, Gandhi S, Jain S, Singleton A, Lees AJ, Shaw K, Bhatia KP, Bonifati V, Quinn NP, Lynch J, Healy DG, Holton JL, Revesz T, Wood NW. A common LRRK2 mutation in idiopathic Parkinson’s disease. The Lancet. 2005;365:415–416. doi: 10.1016/S0140-6736(05)17830-1. [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Science. Vol. 330. New York, NY: 2010. Fate mapping analysis reveals that adult microglia derive from primitive macrophages; pp. 841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Lim S, Hoeffel G, Low D, Huber T. Origin and differentiation of microglia. Frontiers in Cellular Neuroscience. 2013:7. doi: 10.3389/fncel.2013.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glocker EO, Kotlarz D, Boztug K, Gertz EM, Schaffer AA, Noyan F, Perro M, Diestelhorst J, Allroth A, Murugan D, Hatscher N, Pfeifer D, Sykora KW, Sauer M, Kreipe H, Lacher M, Nustede R, Woellner C, Baumann U, Salzer U, Koletzko S, Shah N, Segal AW, Sauerbrey A, Buderus S, Snapper SB, Grimbacher B, Klein C. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. The New England journal of medicine. 2009;361:2033–2045. doi: 10.1056/NEJMoa0907206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golbe LI, Di Iorio G, Sanges G, Lazzarini AM, La Sala S, Bonavita V, Duvoisin RC. Clinical genetic analysis of Parkinson’s disease in the Contursi kindred. Annals of neurology. 1996;40:767–775. doi: 10.1002/ana.410400513. [DOI] [PubMed] [Google Scholar]
- Golde TE, Borchelt DR, Giasson BI, Lewis J. Thinking laterally about neurodegenerative proteinopathies. The Journal of clinical investigation. 2013;123:1847–1855. doi: 10.1172/JCI66029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Hernandez T, Cruz-Muros I, Afonso-Oramas D, Salas-Hernandez J, Castro-Hernandez J. Vulnerability of mesostriatal dopaminergic neurons in Parkinson’s disease. Frontiers in neuroanatomy. 2010;4:140. doi: 10.3389/fnana.2010.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagar JA, Powell DA, Aachoui Y, Ernst RK, Miao EA. Science. Vol. 341. New York, NY: 2013. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock; pp. 1250–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamza TH, Zabetian CP, Tenesa A, Laederach A, Montimurro J, Yearout D, Kay DM, Doheny KF, Paschall J, Pugh E, Kusel VI, Collura R, Roberts J, Griffith A, Samii A, Scott WK, Nutt J, Factor SA, Payami H. Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson’s disease. Nature genetics. 2010;42:781–785. doi: 10.1038/ng.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen G, Hercus TR, McClure BJ, Stomski FC, Dottore M, Powell J, Ramshaw H, Woodcock JM, Xu Y, Guthridge M, McKinstry WJ, Lopez AF, Parker MW. The structure of the GM-CSF receptor complex reveals a distinct mode of cytokine receptor activation. Cell. 2008;134:496–507. doi: 10.1016/j.cell.2008.05.053. [DOI] [PubMed] [Google Scholar]
- Harms AS, Barnum CJ, Ruhn KA, Varghese S, Trevino I, Blesch A, Tansey MG. Delayed Dominant-Negative TNF Gene Therapy Halts Progressive Loss of Nigral Dopaminergic Neurons in a Rat Model of Parkinson’s Disease. Mol Ther. 2011;19:46–52. doi: 10.1038/mt.2010.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms AS, Cao S, Rowse AL, Thome AD, Li X, Mangieri LR, Cron RQ, Shacka JJ, Raman C, Standaert DG. MHCII is required for alpha-synuclein-induced activation of microglia, CD4 T cell proliferation, and dopaminergic neurodegeneration. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:9592–9600. doi: 10.1523/JNEUROSCI.5610-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy DG, Falchi M, O’Sullivan SS, Bonifati V, Durr A, Bressman S, Brice A, Aasly J, Zabetian CP, Goldwurm S, Ferreira JJ, Tolosa E, Kay DM, Klein C, Williams DR, Marras C, Lang AE, Wszolek ZK, Berciano J, Schapira AH, Lynch T, Bhatia KP, Gasser T, Lees AJ, Wood NW. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson’s disease: a case-control study. Lancet neurology. 2008;7:583–590. doi: 10.1016/S1474-4422(08)70117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrand E, Grey M, Ainalem M-L, Ankner J, Forsyth VT, Fragneto G, Haertlein M, Dauvergne M-T, Nilsson H, Brundin P, Linse S, Nylander T, Sparr E. Adsorption of α-Synuclein to Supported Lipid Bilayers: Positioning and Role of Electrostatics. ACS Chemical Neuroscience. 2013;4:1339–1351. doi: 10.1021/cn400066t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch EC, Hunot S. Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet neurology. 2009;8:382–397. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- Hirsch EC, Vyas S, Hunot S. Neuroinflammation in Parkinson’s disease. Parkinsonism & related disorders. 2012;1(18 Suppl):S210–S212. doi: 10.1016/S1353-8020(11)70065-7. [DOI] [PubMed] [Google Scholar]
- Hisanaga K, Asagi M, Itoyama Y, Iwasaki Y. Increase in peripheral CD4 bright+ CD8 dull+ T cells in Parkinson disease. Archives of neurology. 2001;58:1580–1583. doi: 10.1001/archneur.58.10.1580. [DOI] [PubMed] [Google Scholar]
- Hofmann KW, Schuh AF, Saute J, Townsend R, Fricke D, Leke R, Souza DO, Portela LV, Chaves ML, Rieder CR. Interleukin-6 serum levels in patients with Parkinson’s disease. Neurochemical research. 2009;34:1401–1404. doi: 10.1007/s11064-009-9921-z. [DOI] [PubMed] [Google Scholar]
- Hu X, Ivashkiv LB. Cross-regulation of signaling pathways by interferon-gamma: implications for immune responses and autoimmune diseases. Immunity. 2009;31:539–550. doi: 10.1016/j.immuni.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel RM, Aguet M. Science. Vol. 259. New York, NY: 1993. Immune response in mice that lack the interferon-gamma receptor; pp. 1742–1745. [DOI] [PubMed] [Google Scholar]
- Hunot S, Hirsch EC. Neuroinflammatory processes in Parkinson’s disease. Annals of neurology. 2003;3(53 Suppl.):S49–S58. doi: 10.1002/ana.10481. discussion S58–60. [DOI] [PubMed] [Google Scholar]
- Iannaccone S, Cerami C, Alessio M, Garibotto V, Panzacchi A, Olivieri S, Gelsomino G, Moresco RM, Perani D. In vivo microglia activation in very early dementia with Lewy bodies, comparison with Parkinson’s disease. Parkinsonism & related disorders. 2013;19:47–52. doi: 10.1016/j.parkreldis.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Imamura K, Hishikawa N, Sawada M, Nagatsu T, Yoshida M, Hashizume Y. Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson’s disease brains. Acta neuropathologica. 2003;106:518–526. doi: 10.1007/s00401-003-0766-2. [DOI] [PubMed] [Google Scholar]
- Jana M, Palencia CA, Pahan K. Fibrillar amyloid-beta peptides activate microglia via TLR2: implications for Alzheimer’s disease. Journal of immunology. 2008;181:7254–7262. doi: 10.4049/jimmunol.181.10.7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Jiang JX, Zhang GX. Macrophages: a double-edged sword in experimental autoimmune encephalomyelitis. Immunology letters. 2014;160:17–22. doi: 10.1016/j.imlet.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KP, Brooks BR, Cohen JA, Ford CC, Goldstein J, Lisak RP, Myers LW, Panitch HS, Rose JW, Schiffer RB, Vollmer T, Weiner LP, Wolinsky JS. Extended use of glatiramer acetate (Copaxone) is well tolerated and maintains its clinical effect on multiple sclerosis relapse rate and degree of disability. Copolymer 1 Multiple Sclerosis Study Group. Neurology. 1998;50:701–708. doi: 10.1212/wnl.50.3.701. [DOI] [PubMed] [Google Scholar]
- Joniec-Maciejak I, Ciesielska A, Wawer A, Sznejder-Pacholek A, Schwenkgrub J, Cudna A, Hadaczek P, Bankiewicz KS, Czlonkowska A, Czlonkowski A. The influence of AAV2-mediated gene transfer of human IL-10 on neurodegeneration and immune response in a murine model of Parkinson’s disease. Pharmacological reports : PR. 2014;66:660–669. doi: 10.1016/j.pharep.2014.03.008. [DOI] [PubMed] [Google Scholar]
- Jung S, Schwartz M. Non-identical twins - microglia and monocyte-derived macrophages in acute injury and autoimmune inflammation. Frontiers in immunology. 2012;3:89. doi: 10.3389/fimmu.2012.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayagaki N, Wong MT, Stowe IB, Ramani SR, Gonzalez LC, Akashi-Takamura S, Miyake K, Zhang J, Lee WP, Muszynski A, Forsberg LS, Carlson RW, Dixit VM. Science. Vol. 341. New York, NY: 2013. Noncanonical inflammasome activation by intracellular LPS independent of TLR4; pp. 1246–1249. [DOI] [PubMed] [Google Scholar]
- Kempeni J. Preliminary results of early clinical trials with the fully human anti-TNFalpha monoclonal antibody D2E7. Annals of the rheumatic diseases. 1999;1(58 Suppl):I70–I72. doi: 10.1136/ard.58.2008.i70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kett LR, Dauer WT. Leucine-rich repeat kinase 2 for beginners: six key questions. Cold Spring Harbor perspectives in medicine. 2012;2:a009407. doi: 10.1101/cshperspect.a009407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandelwal PJ, Herman AM, Moussa CE. Inflammation in the early stages of neurodegenerative pathology. Journal of neuroimmunology. 2011;238:1–11. doi: 10.1016/j.jneuroim.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierdorf K, Erny D, Goldmann T, Sander V, Schulz C, Perdiguero EG, Wieghofer P, Heinrich A, Riemke P, Holscher C, Muller DN, Luckow B, Brocker T, Debowski K, Fritz G, Opdenakker G, Diefenbach A, Biber K, Heikenwalder M, Geissmann F, Rosenbauer F, Prinz M. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nature neuroscience. 2013;16:273–280. doi: 10.1038/nn.3318. [DOI] [PubMed] [Google Scholar]
- Kieseier BC. The mechanism of action of interferon-beta in relapsing multiple sclerosis. CNS drugs. 2011;25:491–502. doi: 10.2165/11591110-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Ho DH, Suk JE, You S, Michael S, Kang J, Joong Lee S, Masliah E, Hwang D, Lee HJ, Lee SJ. Neuron-released oligomeric alpha-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nature communications. 2013;4:1562. doi: 10.1038/ncomms2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WG, Mohney RP, Wilson B, Jeohn GH, Liu B, Hong JS. Regional difference in susceptibility to lipopolysaccharide-induced neurotoxicity in the rat brain: role of microglia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:6309–6316. doi: 10.1523/JNEUROSCI.20-16-06309.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King IL, Dickendesher TL, Segal BM. Circulating Ly-6C+ myeloid precursors migrate to the CNS and play a pathogenic role during autoimmune demyelinating disease. Blood. 2009;113:3190–3197. doi: 10.1182/blood-2008-07-168575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirik D, Annett LE, Burger C, Muzyczka N, Mandel RJ, Bjorklund A. Nigrostriatal alpha-synucleinopathy induced by viral vector-mediated overexpression of human alpha-synuclein: a new primate model of Parkinson’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:2884–2889. doi: 10.1073/pnas.0536383100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirik D, Rosenblad C, Burger C, Lundberg C, Johansen TE, Muzyczka N, Mandel RJ, Bjorklund A. arkinson-like neurodegeneration induced by targeted overexpression of alpha-synuclein in the nigrostriatal system. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:2780–2791. doi: 10.1523/JNEUROSCI.22-07-02780.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klegeris A, Pelech S, Giasson BI, Maguire J, Zhang H, McGeer EG, McGeer PL. Alpha-synuclein activates stress signaling protein kinases in THP-1 cells and microglia. Neurobiology of aging. 2008;29:739–752. doi: 10.1016/j.neurobiolaging.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Kosloski LM, Kosmacek EA, Olson KE, Mosley RL, Gendelman HE. GM-CSF induces neuroprotective and anti-inflammatory responses in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine intoxicated mice. Journal of neuroimmunology. 2013;265:1–10. doi: 10.1016/j.jneuroim.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krausgruber T, Blazek K, Smallie T, Alzabin S, Lockstone H, Sahgal N, Hussell T, Feldmann M, Udalova IA. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nature immunology. 2011;12:231–238. doi: 10.1038/ni.1990. [DOI] [PubMed] [Google Scholar]
- Kurtuncu M, Tuzun E, Turkoglu R, Petek-Balci B, Icoz S, Pehlivan M, Birisik O, Ulusoy C, Shugaiv E, Akman-Demir G, Eraksoy M. Effect of short-term interferon-beta treatment on cytokines in multiple sclerosis: significant modulation of IL-17 and IL-23. Cytokine. 2012;59:400–402. doi: 10.1016/j.cyto.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Kwon M, Sung M, Kwon YJ, Song YG, Lee SW, Park MC, Park YB, Lee SK, Song JJ. Active tuberculosis risk with tumor necrosis factor inhibitors after treating latent tuberculosis. Journal of clinical rheumatology : practical reports on rheumatic & musculoskeletal diseases. 2014;20:68–73. doi: 10.1097/RHU.0000000000000074. [DOI] [PubMed] [Google Scholar]
- Lacey DC, Achuthan A, Fleetwood AJ, Dinh H, Roiniotis J, Scholz GM, Chang MW, Beckman SK, Cook AD, Hamilton JA. Defining GM-CSF- and macrophage-CSF-dependent macrophage responses by in vitro models. Journal of immunology. 2012;188:5752–5765. doi: 10.4049/jimmunol.1103426. [DOI] [PubMed] [Google Scholar]
- Ladeby R, Wirenfeldt M, Garcia-Ovejero D, Fenger C, Dissing-Olesen L, Dalmau I, Finsen B. Microglial cell population dynamics in the injured adult central nervous system. Brain research Brain research reviews. 2005;48:196–206. doi: 10.1016/j.brainresrev.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Lampe JB, Gossrau G, Herting B, Kempe A, Sommer U, Fussel M, Weber M, Koch R, Reichmann H. HLA typing and Parkinson’s disease. European neurology. 2003;50:64–68. doi: 10.1159/000072500. [DOI] [PubMed] [Google Scholar]
- Langston JW, Irwin I, Langston EB, Forno LS. 1-Methyl-4-phenylpyridinium ion (MPP+): identification of a metabolite of MPTP, a toxin selective to the substantia nigra. Neuroscience letters. 1984a;48:87–92. doi: 10.1016/0304-3940(84)90293-3. [DOI] [PubMed] [Google Scholar]
- Langston JW, Langston EB, Irwin I. MPTP-induced parkinsonism in human and non-human primates--clinical and experimental aspects. Acta neurologica Scandinavica Supplementum. 1984b;100:49–54. [PubMed] [Google Scholar]
- Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39:151–170. doi: 10.1016/0306-4522(90)90229-w. [DOI] [PubMed] [Google Scholar]
- Lee E-J, Woo M-S, Moon P-G, Baek M-C, Choi I-Y, Kim W-K, Junn E, Kim H-S. α-Synuclein Activates Microglia by Inducing the Expressions of Matrix Metalloproteinases and the Subsequent Activation of Protease-Activated Receptor-1. The Journal of Immunology. 2010;185:615–623. doi: 10.4049/jimmunol.0903480. [DOI] [PubMed] [Google Scholar]
- Lehtonen A, Ahlfors H, Veckman V, Miettinen M, Lahesmaa R, Julkunen I. Gene expression profiling during differentiation of human monocytes to macrophages or dendritic cells. Journal of leukocyte biology. 2007;82:710–720. doi: 10.1189/jlb.0307194. [DOI] [PubMed] [Google Scholar]
- Li WW, Setzu A, Zhao C, Franklin RJ. Minocycline-mediated inhibition of microglia activation impairs oligodendrocyte progenitor cell responses and remyelination in a non-immune model of demyelination. Journal of neuroimmunology. 2005;158:58–66. doi: 10.1016/j.jneuroim.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Liu C, Li Y, Yu J, Feng L, Hou S, Liu Y, Guo M, Xie Y, Meng J, Zhang H, Xiao B, Ma C. Targeting the shift from M1 to M2 macrophages in experimental autoimmune encephalomyelitis mice treated with fasudil. PloS one. 2013;8:e54841. doi: 10.1371/journal.pone.0054841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Liu Y, Hao W, Wolf L, Kiliaan AJ, Penke B, Rube CE, Walter J, Heneka MT, Hartmann T, Menger MD, Fassbender K. TLR2 is a primary receptor for Alzheimer’s amyloid beta peptide to trigger neuroinflammatory activation. Journal of immunology. 2012;188:1098–1107. doi: 10.4049/jimmunol.1101121. [DOI] [PubMed] [Google Scholar]
- Liu Y, Holdbrooks AT, De Sarno P, Rowse AL, Yanagisawa LL, McFarland BC, Harrington LE, Raman C, Sabbaj S, Benveniste EN, Qin H. Therapeutic efficacy of suppressing the Jak/STAT pathway in multiple models of experimental autoimmune encephalomyelitis. Journal of immunology. 2014;192:59–72. doi: 10.4049/jimmunol.1301513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long-Smith CM, Sullivan AM, Nolan YM. The influence of microglia on the pathogenesis of Parkinson’s disease. Progress in neurobiology. 2009;89:277–287. doi: 10.1016/j.pneurobio.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Lu J, Cao Q, Zheng D, Sun Y, Wang C, Yu X, Wang Y, Lee VW, Zheng G, Tan TK, Wang X, Alexander SI, Harris DC, Wang Y. Discrete functions of M2a and M2c macrophage subsets determine their relative efficacy in treating chronic kidney disease. Kidney international. 2013;84:745–755. doi: 10.1038/ki.2013.135. [DOI] [PubMed] [Google Scholar]
- Lyons A, Downer EJ, Crotty S, Nolan YM, Mills KH, Lynch MA. CD200 ligand receptor interaction modulates microglial activation in vivo and in vitro: a role for IL-4. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:8309–8313. doi: 10.1523/JNEUROSCI.1781-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness GB. Cellular resistance to infection. The Journal of experimental medicine. 1962;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madathil KS, Karuppagounder SS, Haobam R, Varghese M, Rajamma U, Mohanakumar KP. Nitric oxide synthase inhibitors protect against rotenone-induced, oxidative stress mediated parkinsonism in rats. Neurochemistry international. 2013;62:674–683. doi: 10.1016/j.neuint.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Mahad DJ, Ransohoff RM. The role of MCP-1 (CCL2) and CCR2 in multiple sclerosis and experimental autoimmune encephalomyelitis (EAE) Seminars in immunology. 2003;15:23–32. doi: 10.1016/s1044-5323(02)00125-2. [DOI] [PubMed] [Google Scholar]
- Martin ER, Scott WK, Nance MA, Watts RL, Hubble JP, Koller WC, Lyons K, Pahwa R, Stern MB, Colcher A, Hiner BC, Jankovic J, Ondo WG, Allen FH, Jr, Goetz CG, Small GW, Masterman D, Mastaglia F, Laing NG, Stajich JM, Ribble RC, Booze MW, Rogala A, Hauser MA, Zhang F, Gibson RA, Middleton LT, Roses AD, Haines JL, Scott BL, Pericak-Vance MA, Vance JM. Association of single-nucleotide polymorphisms of the tau gene with late-onset Parkinson disease. JAMA : the journal of the American Medical Association. 2001;286:2245–2250. doi: 10.1001/jama.286.18.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000prime reports. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. Journal of immunology. 2006;177:7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- Martinez FO, Helming L, Milde R, Varin A, Melgert BN, Draijer C, Thomas B, Fabbri M, Crawshaw A, Ho LP, Ten Hacken NH, Cobos Jimenez V, Kootstra NA, Hamann J, Greaves DR, Locati M, Mantovani A, Gordon S. Genetic programs expressed in resting and IL-4 alternatively activated mouse and human macrophages: similarities and differences. Blood. 2013;121:e57–e69. doi: 10.1182/blood-2012-06-436212. [DOI] [PubMed] [Google Scholar]
- Mascarenhas JO, Cross NC, Mesa RA. The future of JAK inhibition in myelofibrosis and beyond. Blood reviews. 2014 doi: 10.1016/j.blre.2014.06.002. [DOI] [PubMed] [Google Scholar]
- McCoy MK, Ruhn KA, Martinez TN, McAlpine FE, Blesch A, Tansey MG. Intranigral Lentiviral Delivery of Dominant-negative TNF Attenuates Neurodegeneration and Behavioral Deficits in Hemiparkinsonian rats. Mol Ther. 2008;16:1572–1579. doi: 10.1038/mt.2008.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology. 1988;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- McKercher SR, Torbett BE, Anderson KL, Henkel GW, Vestal DJ, Baribault H, Klemsz M, Feeney AJ, Wu GE, Paige CJ, Maki RA. Targeted disruption of the PU.1 gene results in multiple hematopoietic abnormalities. The EMBO journal. 1996;15:5647–5658. [PMC free article] [PubMed] [Google Scholar]
- Mendritzki S, Schmidt S, Sczepan T, Zhu XR, Segelcke D, Lubbert H. Spinal cord pathology in alpha-synuclein transgenic mice. Parkinson’s disease. 2010;2010:375462. doi: 10.4061/2010/375462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikita J, Dubourdieu-Cassagno N, Deloire MS, Vekris A, Biran M, Raffard G, Brochet B, Canron MH, Franconi JM, Boiziau C, Petry KG. Multiple sclerosis. Vol. 17. England: Houndmills, Basingstoke; 2011. Altered M1/M2 activation patterns of monocytes in severe relapsing experimental rat model of multiple sclerosis. Amelioration of clinical status by M2 activated monocyte administration; pp. 2–15. [DOI] [PubMed] [Google Scholar]
- Mildner A, Mack M, Schmidt H, Brück W, Djukic M, Zabel MD, Hille A, Priller J, Prinz M. CCR2+Ly− 6Chi monocytes are crucial for the effector phase of autoimmunity in the central nervous system. Brain : a journal of neurology. 2009;132:2487–2500. doi: 10.1093/brain/awp144. [DOI] [PubMed] [Google Scholar]
- Mildner A, Schlevogt B, Kierdorf K, Bottcher C, Erny D, Kummer MP, Quinn M, Bruck W, Bechmann I, Heneka MT, Priller J, Prinz M. Distinct and non-redundant roles of microglia and myeloid subsets in mouse models of Alzheimer’s disease. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:11159–11171. doi: 10.1523/JNEUROSCI.6209-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mildner A, Schmidt H, Nitsche M, Merkler D, Hanisch UK, Mack M, Heikenwalder M, Bruck W, Priller J, Prinz M. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nature neuroscience. 2007;10:1544–1553. doi: 10.1038/nn2015. [DOI] [PubMed] [Google Scholar]
- Miron VE, Franklin RJ. Macrophages and CNS remyelination. Journal of neurochemistry. 2014;130:165–171. doi: 10.1111/jnc.12705. [DOI] [PubMed] [Google Scholar]
- Moehle MS, Webber PJ, Tse T, Sukar N, Standaert DG, DeSilva TM, Cowell RM, West AB. LRRK2 inhibition attenuates microglial inflammatory responses. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:1602–1611. doi: 10.1523/JNEUROSCI.5601-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogi M, Harada M, Riederer P, Narabayashi H, Fujita K, Nagatsu T. Tumor necrosis factor-alpha (TNF-alpha) increases both in the brain and in the cerebrospinal fluid from parkinsonian patients. Neuroscience letters. 1994;165:208–210. doi: 10.1016/0304-3940(94)90746-3. [DOI] [PubMed] [Google Scholar]
- Mogi M, Togari A, Kondo T, Mizuno Y, Komure O, Kuno S, Ichinose H, Nagatsu T. Caspase activities and tumor necrosis factor receptor R1 (p55) level are elevated in the substantia nigra from parkinsonian brain. Journal of neural transmission. 2000;107:335–341. doi: 10.1007/s007020050028. [DOI] [PubMed] [Google Scholar]
- Moreno M, Bannerman P, Ma J, Guo F, Miers L. Conditional ablation of astroglial CCL2 suppresses CNS accumulation of M1 macrophages and preserves axons in mice with MOG peptide EAE. 2014;34:8175–8185. doi: 10.1523/JNEUROSCI.1137-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morganti JM, Nash KR, Grimmig BA, Ranjit S, Small B, Bickford PC, Gemma C. The soluble isoform of CX3CL1 is necessary for neuroprotection in a mouse model of Parkinson’s disease. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:14592–14601. doi: 10.1523/JNEUROSCI.0539-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller T, Blum-Degen D, Przuntek H, Kuhn W. Interleukin-6 levels in cerebrospinal fluid inversely correlate to severity of Parkinson’s disease. Acta neurologica Scandinavica. 1998;98:142–144. doi: 10.1111/j.1600-0404.1998.tb01736.x. [DOI] [PubMed] [Google Scholar]
- Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA. Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatsu T, Mogi M, Ichinose H, Togari A. Changes in cytokines and neurotrophins in Parkinson’s disease. Journal of neural transmission Supplementum. 2000:277–290. doi: 10.1007/978-3-7091-6301-6_19. [DOI] [PubMed] [Google Scholar]
- Nalls MA, Pankratz N, Lill CM, Do CB, Hernandez DG, Saad M, DeStefano AL, Kara E, Bras J, Sharma M, Schulte C, Keller MF, Arepalli S, Letson C, Edsall C, Stefansson H, Liu X, Pliner H, Lee JH, Cheng R, Ikram MA, Ioannidis JP, Hadjigeorgiou GM, Bis JC, Martinez M, Perlmutter JS, Goate A, Marder K, Fiske B, Sutherland M, Xiromerisiou G, Myers RH, Clark LN, Stefansson K, Hardy JA, Heutink P, Chen H, Wood NW, Houlden H, Payami H, Brice A, Scott WK, Gasser T, Bertram L, Eriksson N, Foroud T, Singleton AB. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nature genetics. 2014;46:989–993. doi: 10.1038/ng.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nau GJ, Richmond JF, Schlesinger A, Jennings EG, Lander ES, Young RA. Human macrophage activation programs induced by bacterial pathogens. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:1503–1508. doi: 10.1073/pnas.022649799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyce AJ, Bestwick JP, Silveira-Moriyama L, Hawkes CH, Giovannoni G, Lees AJ, Schrag A. Metaanalysis of early nonmotor features and risk factors for Parkinson disease. Annals of neurology. 2012;72:893–901. doi: 10.1002/ana.23687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchi Y, Yoshikawa E, Sekine Y, Futatsubashi M, Kanno T, Ogusu T, Torizuka T. Microglial activation and dopamine terminal loss in early Parkinson’s disease. Annals of neurology. 2005;57:168–175. doi: 10.1002/ana.20338. [DOI] [PubMed] [Google Scholar]
- Paisan-Ruiz C, Jain S, Evans EW, Gilks WP, Simon J, van der Brug M, Lopez de Munain A, Aparicio S, Gil AM, Khan N, Johnson J, Martinez JR, Nicholl D, Carrera IM, Pena AS, de Silva R, Lees A, Marti-Masso JF, Perez-Tur J, Wood NW, Singleton AB. Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron. 2004;44:595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V, Lecanu L. Translocator protein (18 kDa) TSPO: an emerging therapeutic target in neurotrauma. Experimental neurology. 2009;219:53–57. doi: 10.1016/j.expneurol.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park-Min KH, Antoniv TT, Ivashkiv LB. Regulation of macrophage phenotype by long-term exposure to IL-10. Immunobiology. 2005;210:77–86. doi: 10.1016/j.imbio.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Pattarini R, Smeyne RJ, Morgan JI. Temporal mRNA profiles of inflammatory mediators in the murine 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrimidine model of Parkinson’s disease. Neuroscience. 2007;145:654–668. doi: 10.1016/j.neuroscience.2006.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelen E, Thewissen M, Knippenberg S, Smolders J, Muris AH, Menheere P, Tervaert JW, Hupperts R, Damoiseaux J. Fraction of IL-10+ and IL-17+ CD8 T cells is increased in MS patients in remission and during a relapse, but is not influenced by immune modulators. Journal of neuroimmunology. 2013;258:77–84. doi: 10.1016/j.jneuroim.2013.02.014. [DOI] [PubMed] [Google Scholar]
- Peppel K, Crawford D, Beutler B. A tumor necrosis factor (TNF) receptor-IgG heavy chain chimeric protein as a bivalent antagonist of TNF activity. The Journal of experimental medicine. 1991;174:1483–1489. doi: 10.1084/jem.174.6.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plemel JR, Wee Yong V, Stirling DP. Immune modulatory therapies for spinal cord injury - Past, present and future. Experimental neurology. 2014;258c:91–104. doi: 10.1016/j.expneurol.2014.01.025. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Higgins JJ, Golbe LI, Johnson WG, Ide SE, Di Iorio G, Sanges G, Stenroos ES, Pho LT, Schaffer AA, Lazzarini AM, Nussbaum RL, Duvoisin RC. Science. Vol. 274. New York, NY: 1996. Mapping of a gene for Parkinson’s disease to chromosome 4q21-q23; pp. 1197–1199. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Science. Vol. 276. New York, NY: 1997. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease; pp. 2045–2047. [DOI] [PubMed] [Google Scholar]
- Prinz M, Priller J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci. 2014;15:300–312. doi: 10.1038/nrn3722. [DOI] [PubMed] [Google Scholar]
- Prinz M, Priller J, Sisodia SS, Ransohoff RM. Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nature neuroscience. 2011;14:1227–1235. doi: 10.1038/nn.2923. [DOI] [PubMed] [Google Scholar]
- Pul R, Moharregh-Khiabani D, Skuljec J, Skripuletz T, Garde N, Voss EV, Stangel M. Glatiramer acetate modulates TNF-alpha and IL-10 secretion in microglia and promotes their phagocytic activity. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2011;6:381–388. doi: 10.1007/s11481-010-9248-1. [DOI] [PubMed] [Google Scholar]
- Qian L, Flood PM. Microglial cells and Parkinson’s disease. Immunologic research. 2008;41:155–164. doi: 10.1007/s12026-008-8018-0. [DOI] [PubMed] [Google Scholar]
- Raj T, Rothamel K, Mostafavi S, Ye C, Lee MN, Replogle JM, Feng T, Lee M, Asinovski N, Frohlich I, Imboywa S, Von Korff A, Okada Y, Patsopoulos NA, Davis S, McCabe C, Paik HI, Srivastava GP, Raychaudhuri S, Hafler DA, Koller D, Regev A, Hacohen N, Mathis D, Benoist C, Stranger BE, De Jager PL. Science. Vol. 344. New York, NY: 2014. Polarization of the effects of autoimmune and neurodegenerative risk alleles in leukocytes; pp. 519–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey CP, Tansey MG. A survey from 2012 of evidence for the role of neuroinflammation in neurotoxin animal models of Parkinson’s disease and potential molecular targets. Experimental neurology. 2014;256:126–132. doi: 10.1016/j.expneurol.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapalino O, Lazarov-Spiegler O, Agranov E, Velan GJ, Yoles E, Fraidakis M, Solomon A, Gepstein R, Katz A, Belkin M, Hadani M, Schwartz M. Implantation of stimulated homologous macrophages results in partial recovery of paraplegic rats. Nature medicine. 1998;4:814–821. doi: 10.1038/nm0798-814. [DOI] [PubMed] [Google Scholar]
- Rau R. Adalimumab (a fully human anti-tumour necrosis factor alpha monoclonal antibody) in the treatment of active rheumatoid arthritis: the initial results of five trials. Annals of the rheumatic diseases. 2002;2(61 Suppl):i70–i73. doi: 10.1136/ard.61.suppl_2.ii70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AD, Glanzer JG, Kadiu I, Ricardo-Dukelow M, Chaudhuri A, Ciborowski P, Cerny R, Gelman B, Thomas MP, Mosley RL, Gendelman HE. Nitrated alpha-synuclein-activated microglial profiling for Parkinson’s disease. Journal of neurochemistry. 2008;104:1504–1525. doi: 10.1111/j.1471-4159.2007.05087.x. [DOI] [PubMed] [Google Scholar]
- Rio-Hortega P. THE MICROGLIA. The Lancet. 1939;233:1023–1026. [Google Scholar]
- Rock RB, Gekker G, Hu S, Sheng WS, Cheeran M, Lokensgard JR, Peterson PK. Role of microglia in central nervous system infections. Clinical microbiology reviews. 2004;17:942–964. doi: 10.1128/CMR.17.4.942-964.2004. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roodveldt C, Labrador-Garrido A, Gonzalez-Rey E, Lachaud CC, Guilliams T, Fernandez-Montesinos R, Benitez-Rondan A, Robledo G, Hmadcha A, Delgado M, Dobson CM, Pozo D. Preconditioning of microglia by alpha-synuclein strongly affects the response induced by toll-like receptor (TLR) stimulation. PloS one. 2013;8:e79160. doi: 10.1371/journal.pone.0079160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samii A, Etminan M, Wiens MO, Jafari S. NSAID use and the risk of Parkinson’s disease: systematic review and meta-analysis of observational studies. Drugs & aging. 2009;26:769–779. doi: 10.2165/11316780-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Sanchez-Guajardo V, Febbraro F, Kirik D, Romero-Ramos M. Microglia acquire distinct activation profiles depending on the degree of alpha-synuclein neuropathology in a rAAV based model of Parkinson’s disease. PloS one. 2010;5:e8784. doi: 10.1371/journal.pone.0008784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Mejorada G, Rosales C. Signal transduction by immunoglobulin Fc receptors. Journal of leukocyte biology. 1998;63:521–533. doi: 10.1002/jlb.63.5.521. [DOI] [PubMed] [Google Scholar]
- Saunders JA, Estes KA, Kosloski LM, Allen HE, Dempsey KM, Torres-Russotto DR, Meza JL, Santamaria PM, Bertoni JM, Murman DL, Ali HH, Standaert DG, Mosley RL, Gendelman HE. CD4+ regulatory and effector/memory T cell subsets profile motor dysfunction in Parkinson’s disease. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2012;7:927–938. doi: 10.1007/s11481-012-9402-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalzo P, Kummer A, Cardoso F, Teixeira AL. Serum levels of interleukin-6 are elevated in patients with Parkinson’s disease and correlate with physical performance. Neuroscience letters. 2010;468:56–58. doi: 10.1016/j.neulet.2009.10.062. [DOI] [PubMed] [Google Scholar]
- Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, Frampton J, Liu KJ, Geissmann F. Science. Vol. 336. New York, NY: 2012. A lineage of myeloid cells independent of Myb and hematopoietic stem cells; pp. 86–90. [DOI] [PubMed] [Google Scholar]
- Schwenkgrub J, Joniec-Maciejak I, Sznejder-Pacholek A, Wawer A, Ciesielska A, Bankiewicz K, Czlonkowska A, Czlonkowski A. Effect of human interleukin-10 on the expression of nitric oxide synthases in the MPTP-based model of Parkinson’s disease. Pharmacological reports : PR. 2013;65:44–49. doi: 10.1016/s1734-1140(13)70962-9. [DOI] [PubMed] [Google Scholar]
- Sekiyama K, Takamatsu Y, Waragai M, Hashimoto M. Role of genomics in translational research for Parkinson’s disease. Biochemical and biophysical research communications. 2014 doi: 10.1016/j.bbrc.2014.06.028. [DOI] [PubMed] [Google Scholar]
- Shaunak S, Brown P, Morgan-Hughes JA. Exacerbation of idiopathic Parkinson’s disease by naproxen. BMJ (Clinical research ed) 1995;311:422. doi: 10.1136/bmj.311.7002.422b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter R, London A, Varol C, Raposo C, Cusimano M, Yovel G, Rolls A, Mack M, Pluchino S, Martino G, Jung S, Schwartz M. Infiltrating Blood-Derived Macrophages Are Vital Cells Playing an Anti-inflammatory Role in Recovery from Spinal Cord Injury in Mice. PLoS Med. 2009;6:e1000113. doi: 10.1371/journal.pmed.1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter R, Miller O, Yovel G, Rosenzweig N, London A, Ruckh J, Kim KW, Klein E, Kalchenko V, Bendel P, Lira SA, Jung S, Schwartz M. Recruitment of beneficial M2 macrophages to injured spinal cord is orchestrated by remote brain choroid plexus. Immunity. 2013;38:555–569. doi: 10.1016/j.immuni.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter R, Schwartz M. Harnessing monocyte-derived macrophages to control central nervous system pathologies: no longer ‘if’ but ‘how’. The Journal of Pathology. 2013;229:332–346. doi: 10.1002/path.4106. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- St Martin JL, Klucken J, Outeiro TF, Nguyen P, Keller-McGandy C, Cantuti-Castelvetri I, Grammatopoulos TN, Standaert DG, Hyman BT, McLean PJ. Dopaminergic neuron loss and up-regulation of chaperone protein mRNA induced by targeted over-expression of alpha-synuclein in mouse substantia nigra. Journal of neurochemistry. 2007;100:1449–1457. doi: 10.1111/j.1471-4159.2006.04310.x. [DOI] [PubMed] [Google Scholar]
- Su X, Maguire-Zeiss KA, Giuliano R, Prifti L, Venkatesh K, Federoff HJ. Synuclein activates microglia in a model of Parkinson’s disease. Neurobiology of aging. 2008;29:1690–1701. doi: 10.1016/j.neurobiolaging.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekely CA, Breitner JC, Fitzpatrick AL, Rea TD, Psaty BM, Kuller LH, Zandi PP. NSAID use and dementia risk in the Cardiovascular Health Study: role of APOE and NSAID type. Neurology. 2008;70:17–24. doi: 10.1212/01.wnl.0000284596.95156.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai T. Roles of Fc receptors in autoimmunity. Nature reviews Immunology. 2002;2:580–592. doi: 10.1038/nri856. [DOI] [PubMed] [Google Scholar]
- Takeda K, Akira S. TLR signaling pathways. Seminars in immunology. 2004;16:3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Thakur P, Nehru B. Anti-inflammatory properties rather than anti-oxidant capability is the major mechanism of neuroprotection by sodium salicylate in a chronic rotenone model of Parkinson’s disease. Neuroscience. 2013;231:420–431. doi: 10.1016/j.neuroscience.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Theodore S, Cao S, McLean PJ, Standaert DG. Targeted overexpression of human alpha-synuclein triggers microglial activation and an adaptive immune response in a mouse model of Parkinson disease. Journal of neuropathology and experimental neurology. 2008;67:1149–1158. doi: 10.1097/NEN.0b013e31818e5e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevenet J, Pescini Gobert R, Hooft van Huijsduijnen R, Wiessner C, Sagot YJ. Regulation of LRRK2 expression points to a functional role in human monocyte maturation. PloS one. 2011;6:e21519. doi: 10.1371/journal.pone.0021519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukel C, Wilson RP, Nishimori JH, Pezeshki M, Chromy BA, Baumler AJ. Responses to amyloids of microbial and host origin are mediated through toll-like receptor 2. Cell host & microbe. 2009;6:45–53. doi: 10.1016/j.chom.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera E, Ubhi K, Mante M, Rockenstein E, Masliah E. Antidepressants reduce neuroinflammatory responses and astroglial alpha-synuclein accumulation in a transgenic mouse model of multiple system atrophy. Glia. 2014;62:317–337. doi: 10.1002/glia.22610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wagoner NJ, Oh JW, Repovic P, Benveniste EN. Interleukin-6 (IL-6) production by astrocytes: autocrine regulation by IL-6 and the soluble IL-6 receptor. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:5236–5244. doi: 10.1523/JNEUROSCI.19-13-05236.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varley CD, Deodhar AA, Ehst BD, Bakke A, Blauvelt A, Vega R, Yamashita S, Winthrop KL. Rheumatology. Vol. 53. England: Oxford; 2014. Persistence of Staphylococcus aureus colonization among individuals with immune-mediated inflammatory diseases treated with TNF-alpha inhibitor therapy; pp. 332–337. [DOI] [PubMed] [Google Scholar]
- Vogel DY, Vereyken EJ, Glim JE, Heijnen PD, Moeton M, van der Valk P, Amor S, Teunissen CE, van Horssen J, Dijkstra CD. Macrophages in inflammatory multiple sclerosis lesions have an intermediate activation status. Journal of neuroinflammation. 2013;10:35. doi: 10.1186/1742-2094-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli-Daley Laura A, Luk Kelvin C, Patel Tapan P, Tanik Selcuk A, Riddle Dawn M, Stieber A, Meaney David F, Trojanowski John Q, Lee Virginia MY. Exogenous α-Synuclein Fibrils Induce Lewey Body Pathology Leading to Synaptic Dysfunction and Neuron Death. Neuron. 2011;72:57–71. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell SJ, Popper SJ, Rubins KH, Griffiths MJ, Brown PO, Levin M, Relman DA. Dissecting interferon-induced transcriptional programs in human peripheral blood cells. PloS one. 2010;5:e9753. doi: 10.1371/journal.pone.0009753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahner AD, Bronstein JM, Bordelon YM, Ritz B. Nonsteroidal anti-inflammatory drugs may protect against Parkinson disease. Neurology. 2007;69:1836–1842. doi: 10.1212/01.wnl.0000279519.99344.ad. [DOI] [PubMed] [Google Scholar]
- Walker DG, Lue LF. Understanding the neurobiology of CD200 and the CD200 receptor: a therapeutic target for controlling inflammation in human brains? Future neurology. 2013:8. doi: 10.2217/fnl.13.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Muramatsu Y, Kurosaki R, Michimata M, Matsubara M, Imai Y, Araki T. Protective effects of neuronal nitric oxide synthase inhibitor in mouse brain against MPTP neurotoxicity: an immunohistological study. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2004;14:93–104. doi: 10.1016/S0924-977X(03)00065-8. [DOI] [PubMed] [Google Scholar]
- Weber MS, Prod’homme T, Youssef S, Dunn SE, Rundle CD, Lee L, Patarroyo JC, Stuve O, Sobel RA, Steinman L, Zamvil SS. Type II monocytes modulate T cell-mediated central nervous system autoimmune disease. Nature medicine. 2007;13:935–943. doi: 10.1038/nm1620. [DOI] [PubMed] [Google Scholar]
- Weisser SB, McLarren KW, Kuroda E, Sly LM. Methods in molecular biology. Vol. 946. Clifton, NJ: 2013. Generation and characterization of murine alternatively activated macrophages; pp. 225–239. [DOI] [PubMed] [Google Scholar]
- Wissemann WT, Hill-Burns EM, Zabetian CP, Factor SA, Patsopoulos N, Hoglund B, Holcomb C, Donahue RJ, Thomson G, Erlich H, Payami H. Association of Parkinson disease with structural and regulatory variants in the HLA region. American journal of human genetics. 2013;93:984–993. doi: 10.1016/j.ajhg.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu DC, Jackson-Lewis V, Vila M, Tieu K, Teismann P, Vadseth C, Choi DK, Ischiropoulos H, Przedborski S. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:1763–1771. doi: 10.1523/JNEUROSCI.22-05-01763.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao G, Gan L-S. Receptor-Mediated Endocytosis and Brain Delivery of Therapeutic Biologics. International Journal of Cell Biology. 2013;2013:14. doi: 10.1155/2013/703545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Takeda K. Current views of toll-like receptor signaling pathways. Gastroenterology research and practice. 2010;2010:240365. doi: 10.1155/2010/240365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki R, Lu H, Butovsky O, Ohno N, Rietsch AM, Cialic R, Wu PM, Doykan CE, Lin J, Cotleur AC, Kidd G, Zorlu MM, Sun N, Hu W, Liu L, Lee JC, Taylor SE, Uehlein L, Dixon D, Gu J, Floruta CM, Zhu M, Charo IF, Weiner HL, Ransohoff RM. Differential roles of microglia and monocytes in the inflamed central nervous system. The Journal of experimental medicine. 2014;211:1533–1549. doi: 10.1084/jem.20132477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong VW. Differential mechanisms of action of interferon-beta and glatiramer aetate in MS. Neurology. 2002;59:802–808. doi: 10.1212/wnl.59.6.802. [DOI] [PubMed] [Google Scholar]
- Zhang GX, Li J, Ventura E, Rostami A. Parenchymal microglia of naive adult C57BL/6J mice express high levels of B7.1, B7.2, and MHC class II. Experimental and molecular pathology. 2002;73:35–45. doi: 10.1006/exmp.2002.2441. [DOI] [PubMed] [Google Scholar]
- Zhang J, Song Y, Chen H, Fan D. The tau gene haplotype h1 confers a susceptibility to Parkinson’s disease. European neurology. 2005a;53:15–21. doi: 10.1159/000082956. [DOI] [PubMed] [Google Scholar]
- Zhang W, Dallas S, Zhang D, Guo JP, Pang H, Wilson B, Miller DS, Chen B, Zhang W, McGeer PL, Hong JS, Zhang J. Microglial PHOX and Mac-1 are essential to the enhanced dopaminergic neurodegeneration elicited by A30P and A53T mutant alpha-synuclein. Glia. 2007;55:1178–1188. doi: 10.1002/glia.20532. [DOI] [PubMed] [Google Scholar]
- Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, Wilson B, Zhang W, Zhou Y, Hong JS, Zhang J. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2005b;19:533–542. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zhang ZY, Schittenhelm J, Wu Y, Meyermann R, Schluesener HJ. Parenchymal accumulation of CD163+ macrophages/microglia in multiple sclerosis brains. Journal of neuroimmunology. 2011;237:73–79. doi: 10.1016/j.jneuroim.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zhang ZY, Wu Y, Schluesener HJ. Lesional accumulation of CD163+ macrophages/microglia in rat traumatic brain injury. Brain research. 2012;1461:102–110. doi: 10.1016/j.brainres.2012.04.038. [DOI] [PubMed] [Google Scholar]
- Zhao C, Ling Z, Newman MB, Bhatia A, Carvey PM. TNF-alpha knockout and minocycline treatment attenuates blood-brain barrier leakage in MPTP-treated mice. Neurobiology of disease. 2007;26:36–46. doi: 10.1016/j.nbd.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemssen T, Kumpfel T, Klinkert WE, Neuhaus O, Hohlfeld R. Glatiramer acetate-specific T-helper 1- and 2-type cell lines produce BDNF: implications for multiple sclerosis therapy. Brain-derived neurotrophic factor. Brain : a journal of neurology. 2002;125:2381–2391. doi: 10.1093/brain/awf252. [DOI] [PubMed] [Google Scholar]
- Ziemssen T, Kumpfel T, Schneider H, Klinkert WE, Neuhaus O, Hohlfeld R. Secretion of brain-derived neurotrophic factor by glatiramer acetate-reactive T-helper cell lines: Implications for multiple sclerosis therapy. Journal of the neurological sciences. 2005;233:109–112. doi: 10.1016/j.jns.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, Kachergus J, Hulihan M, Uitti RJ, Calne DB, Stoessl AJ, Pfeiffer RF, Patenge N, Carbajal IC, Vieregge P, Asmus F, Muller-Myhsok B, Dickson DW, Meitinger T, Strom TM, Wszolek ZK, Gasser T. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]