Abstract
Background
Anesthesia depth has been associated with mortality. The association between anesthesia depth and presurgery physical and health status, however, is currently debated. Depression is one comorbid condition that warrants investigation given its association to reduced frontal lobe activity and high prevalence in known surgery samples (e.g., gynecologic mass removal).
Purpose
This pilot study examined the hypothesis that severity of acute depressive symptoms would associate with greater sensitivity to anesthesia as measured by a frontal lobe electroencephalogram (EEG)-based monitor during the anesthesia induction phase among women undergoing gynecologic mass removal.
Method
This was a prospective and surgery anesthesia-controlled pilot investigation with 31 women undergoing surgery for removal of pelvic/gynecologic masses. Participants completed the Millon Behavioral Medicine Diagnostic (MBMD) inventory to assess depressive-related symptomatology. A Bispectral Index Score (BIS™) monitor (Aspect Medical Systems Inc., MA) was placed on the left frontal region to measure change in response from a set pre-anesthesia baseline point throughout the induction phase (6.5 min of the anesthetic). BIS™ change was calculated using a modified “area under the curve with respect to ground” formula.
Results
Greater sensitivity to anesthesia during induction was significantly associated with higher MBMD future pessimism scores and marginally associated with higher MBMD depression scores. Depressive personality, anxiety severity, tumor type, age, medication use, and comorbidity scores were not found to be predictors of BIS score change.
Conclusion
These pilot findings suggest that preoperative psychological health and anesthesia response are not independent. Acute presurgery depression and anesthesia response warrant closer empirical examination.
Keywords: Anesthesia depth, Bispectral Index Monitor, MBMD, Frontal lobe
Introduction
Preoperative health status and cumulative time in a deep hypnotic anesthetic state as measured by an electroencephalogram (EEG)-derived scale (i.e., bispectral index score (BIS™) [1] monitor) have been reported as two strong predictors of mortality, 1 [2] and 2 years [3] after noncardiac surgery and 6 months after cardiac surgery [4]. Some research suggests that deep hypnotic time under anesthesia is an independent predictor of mortality [2]. Others report that preoperative health status and duration of deep anesthesia time are not independent [3]; that is, time spent in deep anesthesia may be attributable to a patient’s underlying disease rather than increased anesthetic dose administered by the anesthesiologist [4]. These latter findings support a hypothesis that patients with less physiologic reserve (e.g., physically ill, older, cognitively impaired) are more susceptible to the depressant effects of anesthesia [5]. Such patients may not only experience greater anesthetic depth but also greater anesthesia-related postoperative difficulties and mortality.
Individuals with preoperative depressive psychological symptoms may represent one patient group potentially more “sensitive” to the effects of anesthesia. In alert and awake states, depressive symptom severity associates with neuroanatomical, functional, and structural abnormalities [6]. Reports indicate reduced electrical activity from the dorsolateral pre-frontal region as measured by EEG [7], reduced blood flow, and glucose reduction in the dorsolateral and the dorsomedial prefrontal regions [8, 9], as well as with pathological blood flow overactivation in the more emotional centers of the brain (orbitofrontal cortex, cingulate, and thalamus) [6, 10]. Some research indicates that frontal lobe asymmetry associated with depression involves reduced left frontal relative to right frontal cortical activity [11, 12]. Additional support for left frontal lobe involvement and depression comes from laterality studies of left versus right frontal lobe lesions (i.e., strokes) and depression severity [11, 13]. Depression severity could therefore indicate a vulnerability to events such as anesthesia that are known to further alter brain activity.
General anesthesia alters activation and blood flow to the brain [14]. Communication between the frontal and posterior brain regions (frontoparietal network) becomes disrupted [15]. Cerebral blood flow to the frontal cortex, parietal cortex, cingulate, and thalamus [14] is reduced. Anesthesia induction is also uniquely associated with decreased spontaneous neuronal firing within the gray matter of the cortex [16] with later firing changes occurring in the subcortical gray regions involved in sensory gating (i.e., thalamic network) [17]. Within these regions, high-frequency rhythms reduce thereby resulting in the sleep of anesthesia [18].
Under propofol sedation and anesthesia, severely depressed patients have shown greater reductions in global cerebral blood flow with particular reduction in inferior pre-frontal region relative to studies of healthy adults [14, 19]. Other mood symptoms such as anxiety have not shown the same profile [20]; patients with higher baseline preoperative anxiety have been shown to require more intraoperative anesthetic to achieve a clinically sufficient hypnotic state than patients with lower baseline preoperative anxiety [21]. These differences suggest that there are unique neuronal mechanisms associated with mood status that may explain variations in anesthesia responsiveness.
The current pilot study examined whether severity of preoperative depressive symptomatology is associated with anesthesia response during the time when there is controlled administration of anesthetic drugs on the cortex (i.e., anesthesia induction). This period of anesthesia administration is associated with electrophysiological alterations within the cortical and thalamic networks [22, 23]. Specifically, our primary objective was to investigate whether individuals with greater severity of depressive symptomatology (i.e., depression, future pessimism) showed more susceptibility to anesthesia when their frontal electrophysiological activity was measured with a common operating room device (BIS™). We also examined whether other psychiatric symptomatology (i.e., anxiety-tension) and a depressive personality style (i.e., dejected coping style) [24] were associated with anesthesia response.
For clinical relevancy and experimental rigor reasons, we focused this pilot investigation on women enrolled in a larger, ongoing, prospective investigation examining anesthetic management and mortality in women undergoing lower abdominal surgery for the removal of gynecologic masses. Primary treatment for suspected gynecological cancers (e.g., endometrial cancer, ovarian cancer) generally consists of total abdominal hysterectomy with bilateral salpingo-oophorectomy (surgical staging) and, when appropriate, cytoreduction [25]. Women undergoing surgery for suspected gynecologic cancers experience substantial rates of psychological distress, including anxiety and depression [26–29].
Overall, we expected this proof-of-principle investigation to provide preliminary evidence that psychological health status is a relevant consideration for understanding BIS-related changes within an operative room setting. Further, we expected the finding to highlight the need for a more in-depth study on how acute baseline/presurgery brain-related variables are relevant to anesthesia and surgery-related care.
Materials and Methods
Participants
We recruited participants from a larger, ongoing, prospective investigation examining anesthetic management and mortality in women scheduled for an exploratory and/or therapeutic laparotomy with general anesthesia by the same surgeon and gynecological-oncological service team. Definitive knowledge of diagnosis and gynecological mass staging was unknown at the time of the assessments or enrollment. Between September 2005 and September 2008, 51 of 106 women consented to be screened for this subinvestigation. Participants had to be over the age of 40, native English speakers, had score ≥24 on the Mini-Mental State Examination (MMSE [30]), and be willing to complete mood questionnaires prior to surgery. Exclusion criteria included the following: (1) severe cardiovascular compromise or an ejection fraction of <20 %; (2) need for regional anesthesia and/or emergency surgery; (3) malignant hyperthermia; (4) choline esterase deficiency; (5) porphyria; (6) allergy to lidocaine; (7) inability to tolerate a normal dose of hypnotic during anesthetic induction (based on the clinical judgment of the attending anesthesiologist); (8) conditions that may confound interpretation of the BIS™ data and ability to complete the psychological questionnaires such as blindness, severe hearing impairment, severe head injury, learning disability, chronic or acute substance abuse, or brain metastases; and (9) incomplete electronic anesthetic record of BIS™ during surgery. Consenting was completed according to the University of Florida Institutional Review Board guidelines and the Declaration of Helsinki.
Procedures
Participants who met the inclusion/exclusion criteria were seen in a private room during their preoperative visit routinely held 24 h prior to surgery. Participants completed the Millon Behavioral Medicine Diagnostic (MBMD [31]) and the MMSE. Comorbidity was assessed with the Charlson comorbidity index [32] and the physician-based American Society of Anesthesiologists (ASA) [33] classification. Pathology of tumor status was acquired via postsurgery medical records.
Anesthesia Management
An independent anesthesiologist not associated with the study administered the anesthetic protocol and ensured patient safety in accordance with our Patient Data Safety Monitoring Committee. All participants received 1–2 mg of midazolam IV before transport to the operating room. Providers placed the BIS™ monitor (Aspect Medical Systems Inc., MA) on the left forehead of each participant according to the manufacturer’s recommended position. Sequential compression devices were placed on the lower extremities of each participant before preoxygenation with 100 % oxygen. Participants received the same weight-based, rapid sequence induction of anesthesia using lidocaine (1 mg/kg), fentanyl (1 mcg/kg), thiopental (3 mg/kg), and succinylcholine (1 mg/kg). Dosing weight was calculated as ideal body weight+0.2 (total body weight−ideal body weight). Following intubation, anesthesia was maintained with isoflurane (end tidal concentration >0.4 %) and fentanyl infusion (1–5 mcg/kg/h). Patients received phenylephrine (40 mcg) or ephedrine (5 mg) intravenously as necessary to maintain systolic blood pressure within 20 % of preoperative values. Esmolol (10–100 mg) was used as necessary to maintain heart rate less than 100 bpm.
Psychological Measures
The MBMD [20] was used to assess depressive and anxious symptomatology. The MBMD is a 165-item, self-report, true/false questionnaire that contains 38 scales that tap into the following dimensions: response patterns, negative health habits, psychiatric indications, coping styles, and stress moderators. The MBMD has demonstrated adequate reliability and validity in health psychology research, as well as clinically to help identify factors that may impact health care delivery [31]. It has a total of 38 subscales. Based on a priori hypotheses, we selected two subscales reflecting depressive symptomatology severity (depression scale, future pessimism scale): one scale assessing depressive personality/coping style (dejected scale) and one anxiety scale (anxiety-tension scale). The final score for each scale was based on a prevalence score which reflected participants’ position on each scale as compared to the MBMD’s published normative sample (adjusted for age, sex, and education). Prevalence scores ≥75 are considered to be clinically significant.
The MBMD depression scale focuses on patients’ vegetative and mood state. It assesses the presence and extent of patients’ cognitive and somatic symptoms, as indicated by changes in appetite, feelings of hopelessness, social isolation, anhedonia, self-deprecation, and a number of other depressive symptoms. For example, “I have found few things to be pleasurable.” The MBMD future pessimism scale assesses patients’ current response to diagnosis and medical problems, rather than a lifelong trait or tendency to be pessimistic. Questions pertain to one’s outlook on health and recovery such as, “My future looks like it will be full of future problems and pain.” The MBMD dejected scale assesses chronic and personality-related styles of depression: a scale associated with chronic traits of anhedonia and pessimism. This scale identifies traits where one is chronically disheartened, glum and pessimistic, easily disposed, and has poor coping strategies. For example, “My life has always gone from bad to worse.” The MBMD anxiety-tension scale assesses patients’ general anxiety and illness-related stressors.
Cognitive Screening
The MMSE [30] was used for inclusion/exclusion cognitive screening purposes. The MMSE provides a structured approach to mental status testing and screening for general cognitive decline. It is comprised of 11 simple questions, yielding a maximum score of 30.
Physical Health Comorbidities
The Charlson comorbidity index (CCI) [32] and the ASA physical status classification [33] assessed medical comorbidity. The CCI encompasses 19 medical conditions weighted 1–6 with total scores ranging from 0 to 37. Reviews of the CCI suggest that it has good reliability and excellent correlation with mortality and progression-free survival outcomes. The final CCI score was based on medical records, participant interview, and the outcome of the tumor pathology status. The ASA classification is used to grade preoperative health of surgical patients. There are five categories (1—healthy; 2—mild systemic disease; 3—severe systemic disease; 4—severe systemic disease that is a constant threat to life; 5—a moribund person who is not expected to survive without the operation; 6—a declared brain-dead person whose organs are being removed for donor purposes).
Anesthesia Monitoring
Bispectral Index Monitor
The BIS™ (Aspect Medical Systems, Newton, MA, USA) uses a dimensionless monotonic index to record anesthesia depth on a scale from 100 (awake state) to 0 (deep coma) [1] The electrodes are integrated into a sensor that is placed on the forehead. Monitors like the BIS™ were originally designed to help detect and prevent awareness and memory formation during the surgery process. For information on reliability and validity, please see Bruhn et al. [1]. Based on research involving left frontal abnormalities with depression severity [12], the BIS sensors were placed on the left frontal region just above the eyebrow. This location also corresponds to standard BIS™ placement.
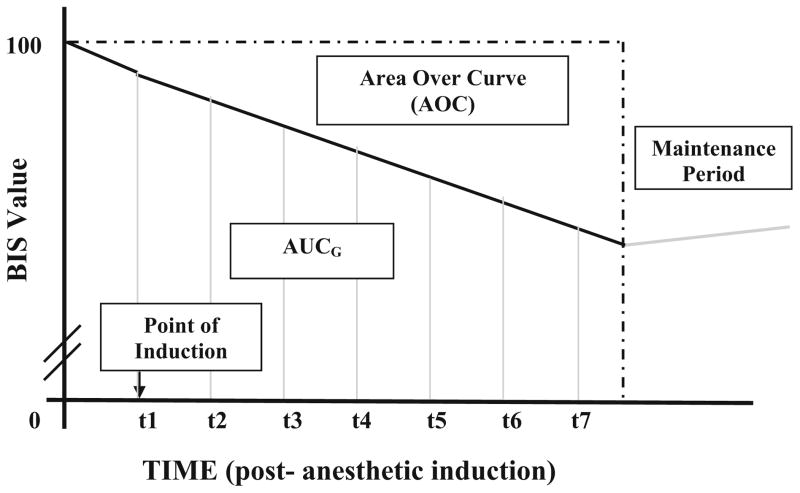
We hypothesized that individuals who were more “sensitive” to anesthesia would demonstrate a greater decline in a BIS score from baseline to an acute postanesthesia induction phase. The induction phase was operationally defined for this study as the BIS™ recorded activity from a pre-anesthesia baseline to 6.5 min postanesthetic induction. This conservative time frame ensured data collection reflecting the cortical drug effect of the induction dose based on each patient’s weight (i.e., normal anesthesia dose) rather than changes possibly due to the anesthesiologists management. BIS™ data were recorded at 30 s intervals (Figs. 1 and 2).
Fig. 1.
Illustration of “area under the curve with respect to ground” (AUCG) and “area over the curve” (AOC). t time in minutes
Fig. 2.
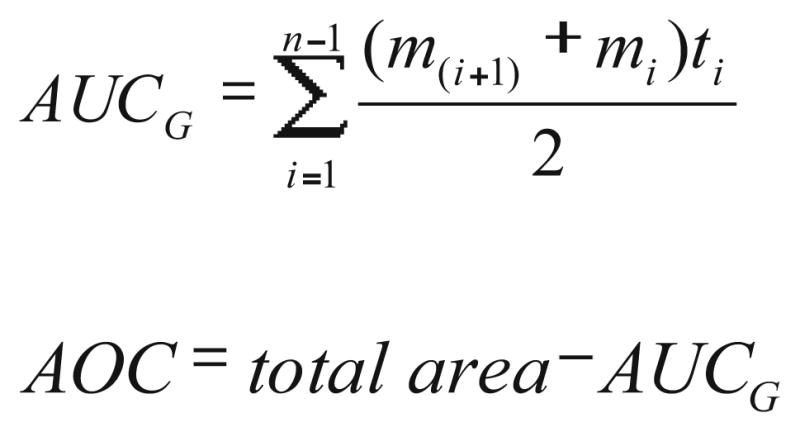
Formulas for “area under the curve with respect to ground” (AUCG) and “area over the curve” (AOC). mi represents each individual measurement of BIS, ti represents time distance between each measurement, and n the total number of measures
Area over the Curve
BIS™ values change continuously during the period of induction of anesthesia. In general, they decline from awake values of 88–98 to values of 40–60, which represent a state of general anesthesia [34]. Specifically, they fluctuate around this declining trajectory, because of drug distribution and changes in the level of sensory input, e.g., during airway management. To manage these fluctuations, we used an “area under the curve with respect to ground” formula (AUCG; [35]) to calculate BIS score changes from baseline to 6.5 min postanesthetic induction. This AUCG value was then subtracted from a hypothetical situation where the BIS score would remain at 100 the entire duration (total AUCG). This resulted in an “area over the curve” (AOC) value, which was used as our final dependent variable. The formula for AUCG was used in lieu of “area under the curve with respect to increase” (AUCI), which ignores the distance from zero for all measurements. In this case, using AUCI would discount the critical period from baseline to anesthetic induction. As such, in the present study, higher AOC reflected greater decline in BIS™ scores and, therefore, greater responsiveness to anesthesia.
Statistical Analyses
SPSS 16.0 for Windows (SPSS Inc., IL) was used to conduct the statistical analyses. Normality assumptions were examined for the outcome variables of BIS™ AOC (e.g., skewness=0.14) and for all the MBMD scale scores. Due to violations of normality assumption in some variables (i.e., MMSE; skewness=−2.24; MBMD dejected scale skewness= 2.10), nonparametric correlation analyses (i.e., Spearman’s rho, ρ) were used with these variables. Covariate considerations were examined with parametric and, where appropriate, nonparametric (Spearman’s rho, ρ) bivariate correlations to examine potential factors that might explain a significant portion of variance with the AOC variable (i.e., demographics, comorbidity, ASA status, postsurgical pathology). Two-tailed Pearson product-moment correlations were used to examine the association between preoperative depression or anxiety and anesthetic sensitivity as determined by AOC. From the r values, we reported effect sizes based on Cohen’s guidelines (small: r=0.01–0.23, medium: r=0.24–0.36, large: r=0.37 or larger; [36]) and calculate r square (r2) values to interpret percent of BIS™ change explained by mood scores.
Results
Participant Characteristics
A total of 51 women were originally consented and screened. Of the 51 consented participants, 14 were excluded because of invalid BIS records (i.e., invalid, inaccessible, or missing). Five participants did not complete the psychological measures, and one participant did not meet the minimum criteria for MMSE score ≥24. The final sample included 31 participants. This final sample did not significantly differ from the excluded participants on age, education, MMSE, CCI, ASA status, or postsurgical pathology status (all ps>0.08). The final participant sample also did not differ from the original 51 participants on whether preoperative midazolam, preoperative use of narcotics, or anti-inflammatory drugs were used prior to surgery (all ps>0.74). The final sample included 24 Caucasian participants, 4 African-American participants, 1 Hispanic participant, 1 Native American participant, and 1 participant of Pacific Island origin (Table 1).
Table 1.
Participant characteristics
| Variable | Mean | SD | Min | Max |
|---|---|---|---|---|
| Age (years) | 57.58 | 10.89 | 40.0 | 81.0 |
| Educationa | 12.86 | 2.67 | 6.0 | 18.0 |
| MMSE scoreb | 29.15 | 0.95 | 27.0 | 30.0 |
| Comorbidity scorec | 6.74 | 5.95 | 0.0 | 24.0 |
| ASA statusd | 2.74 | 0.44 | 2.0 | 3.0 |
| MBMD subscale scorese | ||||
| Depression | 44.03 | 28.79 | 10.0 | 100.0 |
| Pessimism | 49.81 | 24.70 | 10.0 | 83.0 |
| Dejected | 25.03 | 32.49 | 0.0 | 110.0 |
| Anxiety | 54.90 | 28.70 | 5.0 | 95.0 |
| BIS values | ||||
| Baseline BISf | 95.29 | 4.01 | 83.40 | 97.70 |
| BIS at inductiong | 89.75 | 10.06 | 58.00 | 97.70 |
| AOC scoreh | 547.42 | 108.63 | 358.85 | 748.75 |
Education in years (N=29)
MMSE = Mini-Mental State Exam (N=27; best score is 30 points)
Charlson comorbidity index ([32]; score range 0 to 33; maximum comorbidity=33)
American Society of Anesthesiologists (ASA) physical status classification system (range 1–6; 1 = healthy to 6 = declared brain death)
Millon Behavioral Medicine Diagnostic (MBMD) prevalence subscale scores (note: percentage of participants with a clinically significant (i.e., prevalence scores ≥75) MBMD prevalence scores: anxiety-tension (29.0 %), depression (19.4 %), dejected (12.9 %), future pessimism (9.7 %))
Bispectral index score
BIS at induction = time of administration of the induction dose of propofol
Area over the curve (see Fig. 2)
Covariate Considerations
Within our final sample, BIS™ AOC did not significantly associate with age at time of surgery (r(31)=−0.01, p=0.97), CCI (r(31)=−0.23, p=0.21), or ASA status ρ (31)=−0.01, p= 0.97). BIS™ AOC also did not differ among those postsurgically identified to have malignant (n=12; AOC value=550.63 ±113.27) versus benign (n=19) masses [t(29)=0.54, p=0.89] (Table 2).
Table 2.
Correlation matrix for AOC and physical health covariates
| Covariate | Age (years) | CCI | ASA | AOC |
|---|---|---|---|---|
| Age (years) | – | 0.34 | 0.05 | −0.01 |
| CCI total | 0.34 | – | 0.46a | −0.23 |
| ASA status | 0.05 | 0.46a | – | 0.01 |
| AOC | −0.01 | −0.23 | 0.01 | – |
ASA status based on Spearman’s rho
ASA status American Society of Anesthesiologists (ASA) physical status classification (range 1–6; 1 = healthy to 6 = declared brain death), CCI Charlson comorbidity index ([32]; score range 0 to 33; maximum comorbidity=33), AOC area over the curve
Two-tailed correlation is significant at the 0.01 level
For the MBMD subscales, there were no significant relationships between CCI and ASA status (all p values>0.11; e.g., MBMD depression and CCI: r(31)=−0.27, p=0.15; MBMD depression and ASA: r(31)=0.22, p=0.24). MBMD dejected scale was negatively associated with age (ρ(31)= −0.476, p=0.007).
Presurgery MBMD Subscale Scores and AOC
Mean (SD) prevalence scores on the MBMD depression, future pessimism, and dejected and anxiety-tension subscales are listed in Table 1. Clinically significant prevalence scores emerged among 19.4 % (n=6), 9.7 % (n=3), and 12.9 % (n= 4) of participants, respectively. Twenty-nine percent (n=9) of participants had clinically significant anxiety-tension scores.
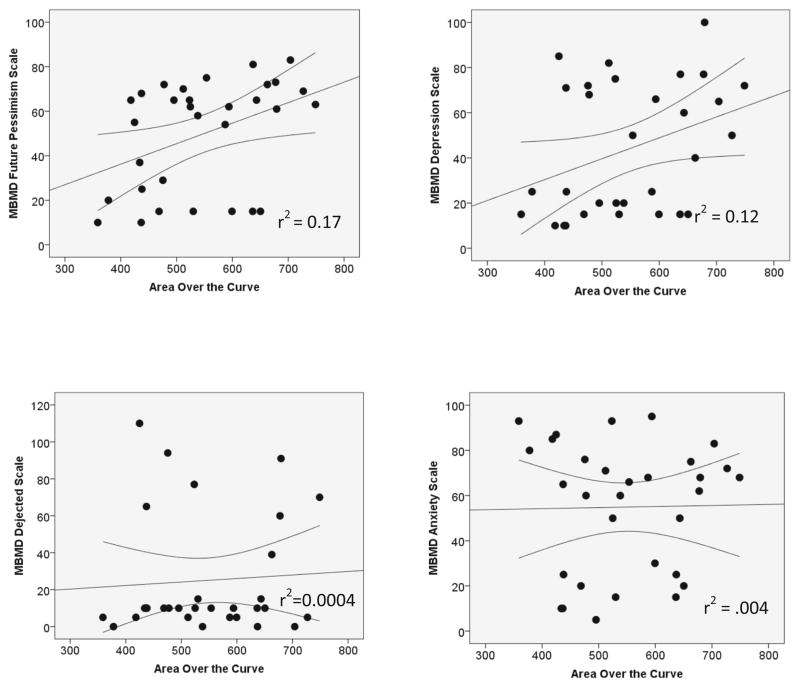
AOC was positively correlated with future pessimism scale (r(31)=0.41, p=0.024, r2=0.17, large effect size). AOC was associated positively with the MBMD depression scale (r(31)=0.35, p=0.054, r2=0.12, medium effect size). There was no significant relationship between AOC and the MBMD dejected scale (ρ(31)=0.06, p=0.730, r2=0.004, small effect size). No association was observed between the MBMD anxiety-tension scale and AOC (r(31)=0.02, p=0.925, r2= 0.0004, small effect size) (Table 3, Fig. 3).
Table 3.
Correlation matrix for AOC and MBMD depression and anxiety subscales
| Index | MBMD depression | MBMD future pessimism | MBMD dejected | MBMD anxiety-tension | AOC |
|---|---|---|---|---|---|
| MBMD depression | – | 0.64** | 0.39** | 0.52** | 0.35† |
| MBMD future pessimism | 0.64** | – | −0.01 | 0.45* | 0.41* |
| MBMD dejected | 0.39** | −0.01 | – | 0.03 | 0.07 |
| MBMD anxiety-tension | 0.52** | 0.45* | 0.03 | – | 0.02 |
| AOC | 0.35† | 0.41* | 0.07 | 0.02 | – |
MBMD dejected scale correlations based on Spearman’s rho
AOC area over the curve, MBMD Millon Behavioral Medicine Diagnostic
p<0.05;
p<0.01;
p=0.054
Fig. 3.
Scatter of Millon Behavioral Medicine Diagnostic subscale prevalence scores relative to area over the curve (AOC) values. Confidence intervals (95 %) based on the mean are shown
Discussion
This is the first prospective pilot study examining the role of preoperative psychological health on anesthesia response in a sample of women undergoing exploratory and/or therapeutic laparotomy for gynecologic mass removal. With acknowledgment regarding the pilot nature of the study, the data lend support to previous assertions that preoperative health status and anesthesia response are not independent [3]. For our sample of women undergoing tumor removal surgeries, the severity of acute preoperative depressive symptoms associated with greater declines in BIS™ scores during the anesthesia induction phase. Specifically, negative views toward diagnosis and medical problems (i.e., pessimism) were significantly associated with anesthesia response as measured by the BIS™ (large effect size), while severity of melancholic/vegetative depressive symptoms (appetite change, sleep changes, anhedonia) was marginally associated with anesthesia response (medium effect size). Scores on scales targeting chronic personality-related traits of depression or current anxiety severity did not relate to severity of BIS change. There were no significant patterns associated with age, ASA status, or tumor pathology type. These collective findings suggest that acute aspects of depression involving pessimism and vegetative/melancholic symptoms in women with gynecologic mass warrant further consideration as a contributor to anesthesia BIS™ score response and, possibly, deep hypnotic responses.
The psychological health symptoms associated with BIS™ responses in the present sample (i.e., pessimism, melancholic depression, vegetative symptoms) have neuronal significance. Optimism/pessimism and depressive symptoms associate behaviorally [37] and involve the same brain regions [38]. Pessimistic views have been associated with reduced blood oxygenation level-dependent (BOLD) signal in the (a) amygdala and the (b) rostral anterior cingulate cortex [38]—regions highly involved in emotion modulation and emotional memory (for review, see [39]). These same regions show clear irregularities in depression [6, 40], with additional evidence of abnormalities in the medial and dorsolateral regions of the prefrontal cortex [12, 38, 41–43]. These structures are also involved in the behavioral responses to anesthesia [22, 44].
Our pilot data also suggest that the clinical scales measuring chronic personality traits of anhedonia and pessimism associate less strongly with BIS™ change. Personality traits describe persistent human behavioral responses to broad classes of environmental stimuli [45]. Research in this area has not shown consistent differences in brain patterns or structures [45]. While personality traits are known to contribute to depressive episodes [46–48], the exact nature of these traits on brain function/structure remains uncertain. We encourage additional research differentiating depressive symptoms and traits with neurological profiles.
Presurgery anxiety severity also did not significantly associate with acute BIS change. This may be partially due to dissociations in electrical activity during different states of emotional distress. Unlike depression, anxiety has been most associated with right frontal hyperactivity [49–51]. Higher levels of anxiety may therefore indicate initial insensitivity to the depressant effects of anesthesia; high states of preoperative anxiety have been related to intraoperative anesthetic requirements [21]. Due to the complexity of frontal EEG with severe depressive symptoms and high levels of anxiety [52], however, more sophisticated multimodal investigations examining brain function and anesthesia [53] are needed.
We recognize several study limitations. First, the sample size was small, hence increasing the probability of type II error. In addition, the analytic sample excluded 39 % of the original individuals who consented for the study. Second, although our modest analytic sample reflects the complexity of this study from a perioperative management perioperative, it is possible that our analytic sample may not be fully generalizable to women presenting for surgical resection of a gynecologic mass. Third, our interpretation of the mechanism behind our findings is limited to the BIS™. BIS™ provides a dimensionless EEG-derived value that utilizes a unilateral sensor (integrated from two active electrodes) to obtain an electroencephalographic signal from the forehead. It differs from the traditional EEG as it is a single variable derived from several disparate descriptors of EEG [47]. A more thorough examination of baseline electrical brain activity is also required in order to validate our study findings. Fourth, we measured baseline BIS™ to 6.5 min postinduction to capture what we believed would be the most appropriate measure of pure change from baseline before variability in medication administration, length of surgery, and homeostatic differences began to vary. Other researchers are encouraged to extend beyond what we considered the induction period (designated as 6.5 min postanesthetic induction) so that duration of deep anesthesia can be examined. An extended time period may be particularly relevant to explore the relationship between physical health status/comorbidity and depressive symptoms (particularly vegetative and melancholic symptoms which showed a marginal effect in our study). Fifth, although our average baseline BIS™ range fits within the expected wake state range, two individuals showed reduced baseline values. Our protocol included a premedication with midazolam that was administered in the preoperative area before the baseline BIS™ values were collected. While the dose of midazolam was chosen to be anxiolytic and not sedative, this medication may explain the low BIS™ value in these two particularly sensitive subjects. Sixth, although the MBMD is an accepted measure of mood and personality, the measure can take some time to complete and may not be appropriate for rapid presurgical examination of mood. We encourage follow-up studies examining depressive symptomatology with more rapid screening tools (e.g., the Beck Depression Inventory [54]). Seventh, we did not conduct structured clinical interviews to diagnose current and/or past mood disorders. We do not know if our associations would remain after controlling for current/prior clinical mood diagnoses, length of time since last depressive episode, number of depressive episodes, and treatment history. Future studies should therefore incorporate a more structured examiner-driven clinical interview (SCID) examining whether diagnosis of depression at time of surgery is more sensitive to anesthesia response relative to depression symptom severity alone. Finally, the present study did not abstract detailed information about the exact type of surgery completed. Although all women were scheduled for open laparotomy for resection of a gynecologic mass, it is possible that procedures may have been altered once surgery commenced. Future research should collect detailed surgical procedure data in order to standardize the study sample.
Overall, the current pilot study suggests that psychological distress may predispose patients to altered anesthesia responses. Given others’ suggestions that deeper hypnotic levels are linked to mortality [2, 55], knowledge that depression is linked to an increase in all-cause mortality [56], and recent findings that there are neuroanatomical considerations for surgery-related outcome [57], we encourage larger and more sophisticated investigations examining presurgical patient risk factors, their associations to brain activation, and anesthesia response. These studies appear particularly necessary for women known to experience high and acute rates of psychological distress around the time of surgery, such as women undergoing gynecologic mass removal.
Acknowledgments
This project is dedicated to J. S. Gravenstein, M.D., Professor, Department of Anesthesiology, for his insightful comments and support toward this project. This work was completed in partial fulfillment of Ms. Andre’s Master of Science degree in the Department of Clinical and Health Psychology, University of Florida, Gainesville, FL, USA. This work was supported in part by the NIH/NCATS Clinical and Translational Science Award to the University of Florida UL1 TR000064, the I. Heermann Anesthesia Foundation (MH, CP), NINDS K23NS060660 (CP), and R01-NR014181 (CP).
Contributor Information
Catherine C. Price, Email: cep23@phhp.ufl.edu, Department of Clinical and Health Psychology, University of Florida, P.O. Box 100165, Gainesville, FL 32610-0165, USA. Department of Anesthesiology, University of Florida, Gainesville, FL, USA
Deidre B. Pereira, Department of Clinical and Health Psychology, University of Florida, P.O. Box 100165, Gainesville, FL 32610-0165, USA
Rachel Andre, Department of Clinical and Health Psychology, University of Florida, P.O. Box 100165, Gainesville, FL 32610-0165, USA.
Cynthia Wilson Garvan, College of Nursing, University of Florida, Gainesville, FL, USA.
Peter Nguyen, Department of Clinical and Health Psychology, University of Florida, P.O. Box 100165, Gainesville, FL 32610-0165, USA.
Mary Herman, Department of Anesthesiology, University of Florida, Gainesville, FL, USA.
Christoph Seubert, Department of Anesthesiology, University of Florida, Gainesville, FL, USA.
References
- 1.Bruhn J, Myles PS, Sneyd R, Struys MM. Depth of anaesthesia monitoring: what’s available, what’s validated and what’s next? Br J Anaesth. 2006;97(1):85–94. doi: 10.1093/bja/ael120. [DOI] [PubMed] [Google Scholar]
- 2.Monk TG, Saini V, Weldon BC, Sigl JC. Anesthetic management and one-year mortality after noncardiac surgery. Anesth Analg. 2005;100(1):4–10. doi: 10.1213/01.ANE.0000147519.82841.5E. [DOI] [PubMed] [Google Scholar]
- 3.Lindholm ML, Traff S, Granath F, Greenwald SD, Ekbom A, Lennmarken C, et al. Mortality within 2 years after surgery in relation to low intraoperative bispectral index values and preexisting malignant disease. Anesth Analg. 2009;108(2):508–12. doi: 10.1213/ane.0b013e31818f603c. [DOI] [PubMed] [Google Scholar]
- 4.Kertai MD, Pal N, Palanca BJ, Lin N, Searleman SA, Zhang L, et al. Association of perioperative risk factors and cumulative duration of low bispectral index with intermediate-term mortality after cardiac surgery in the B-Unaware Trial. Anesthesiology. 2010;112(5):1116–27. doi: 10.1097/ALN.0b013e3181d5e0a3. [DOI] [PubMed] [Google Scholar]
- 5.Muravchick S. The aging process: anesthetic implications. Acta Anaesthesiol Belg. 1998;49(2):85–90. [PubMed] [Google Scholar]
- 6.Drevets WC. Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr Opin Neurobiol. 2001;11(2):240–9. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- 7.Cornwell BR, Salvadore G, Colon-Rosario V, Latov DR, Holroyd T, Carver FW, et al. Abnormal hippocampal functioning and impaired spatial navigation in depressed individuals: evidence from whole-head magnetoencephalography. Am J Psychiatry. 2010;167(7):836–44. doi: 10.1176/appi.ajp.2009.09050614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baxter LR, Jr, Schwartz JM, Phelps ME, Mazziotta JC, Guze BH, Selin CE, et al. Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch Gen Psychiatry. 1989;46(3):243–50. doi: 10.1001/archpsyc.1989.01810030049007. [DOI] [PubMed] [Google Scholar]
- 9.Bonne O, Krausz Y, Shapira B, Bocher M, Karger H, Gorfine M, et al. Increased cerebral blood flow in depressed patients responding to electroconvulsive therapy. J Nucl Med Off Publ Soc Nucl Med. 1996;37(7):1075–80. [PubMed] [Google Scholar]
- 10.Drevets WC. Functional neuroimaging studies of depression: the anatomy of melancholia. Annu Rev Med. 1998;49:341–61. doi: 10.1146/annurev.med.49.1.341. [DOI] [PubMed] [Google Scholar]
- 11.Hama S, Yamashita H, Shigenobu M, Watanabe A, Kurisu K, Yamawaki S, et al. Post-stroke affective or apathetic depression and lesion location: left frontal lobe and bilateral basal ganglia. Eur Arch Psychiatry Clin Neurosci. 2007;257(3):149–52. doi: 10.1007/s00406-006-0698-7. [DOI] [PubMed] [Google Scholar]
- 12.Henriques JB, Davidson RJ. Left frontal hypoactivation in depression. J Abnorm Psychol. 1991;100(4):535–45. doi: 10.1037//0021-843x.100.4.535. [DOI] [PubMed] [Google Scholar]
- 13.Spalletta G, Guida G, De Angelis D, Caltagirone C. Predictors of cognitive level and depression severity are different in patients with left and right hemispheric stroke within the first year of illness. J Neurol. 2002;249(11):1541–51. doi: 10.1007/s00415-002-0885-z. [DOI] [PubMed] [Google Scholar]
- 14.Fiset P, Paus T, Daloze T, Plourde G, Meuret P, Bonhomme V, et al. Brain mechanisms of propofol-induced loss of consciousness in humans: a positron emission tomographic study. J Neurosci Off J Soc Neurosci. 1999;19(13):5506–13. doi: 10.1523/JNEUROSCI.19-13-05506.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee U, Ku S, Noh G, Baek S, Choi B, Mashour GA. Disruption of frontal-parietal communication by ketamine, propofol, and sevoflurane. Anesthesiology. 2013;118(6):1264–75. doi: 10.1097/ALN.0b013e31829103f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Velly LJ, Rey MF, Bruder NJ, Gouvitsos FA, Witjas T, Regis JM, et al. Differential dynamic of action on cortical and subcortical structures of anesthetic agents during induction of anesthesia. Anesthesiology. 2007;107(2):202–12. doi: 10.1097/01.anes.0000270734.99298.b4. [DOI] [PubMed] [Google Scholar]
- 17.Nicoll RA, Madison DV. General anesthetics hyperpolarize neurons in the vertebrate central nervous system. Science. 1982;217(4564):1055–7. doi: 10.1126/science.7112112. [DOI] [PubMed] [Google Scholar]
- 18.Steriade M. Corticothalamic resonance, states of vigilance and mentation. Neuroscience. 2000;101(2):243–76. doi: 10.1016/s0306-4522(00)00353-5. [DOI] [PubMed] [Google Scholar]
- 19.Ogawa K, Uema T, Motohashi N, Nishikawa M, Takano H, Hiroki M, et al. Neural mechanism of propofol anesthesia in severe depression: a positron emission tomographic study. Anesthesiology. 2003;98(5):1101–11. doi: 10.1097/00000542-200305000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Drevets WC. Neuroimaging studies of mood disorders. Biol Psychiatry. 2000;48(8):813–29. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- 21.Maranets I, Kain ZN. Preoperative anxiety and intraoperative anesthetic requirements. Anesth Analg. 1999;89(6):1346–51. doi: 10.1097/00000539-199912000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Verdonck O, Reed SJ, Hall J, Gotman J, Plourde G. The sensory thalamus and cerebral motor cortex are affected concurrently during induction of anesthesia with propofol: a case series with intracranial electroencephalogram recordings. Can J Anaesth. 2014;61(3):254–62. doi: 10.1007/s12630-013-0100-y. [DOI] [PubMed] [Google Scholar]
- 23.Plourde G, Garcia-Asensi A, Backman S, Deschamps A, Chartrand D, Fiset P, et al. Attenuation of the 40-hertz auditory steady state response by propofol involves the cortical and subcortical generators. Anesthesiology. 2008;108(2):233–42. doi: 10.1097/01.anes.0000299839.33721.6d. [DOI] [PubMed] [Google Scholar]
- 24.Hartlage S, Arduino K, Alloy LB. Depressive personality characteristics: state dependent concomitants of depressive disorder and traits independent of current depression. J Abnorm Psychol. 1998;107(2):349–54. doi: 10.1037//0021-843x.107.2.349. [DOI] [PubMed] [Google Scholar]
- 25.Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer Staging manual. Springer Publisher; New York, New York: [Google Scholar]
- 26.Arden-Close E, Gidron Y, Moss-Morris R. Psychological distress and its correlates in ovarian cancer: a systematic review. Psychooncology. 2008;17(11):1061–72. doi: 10.1002/pon.1363. [DOI] [PubMed] [Google Scholar]
- 27.Kain ZN, Sevarino F, Alexander GM, Pincus S, Mayes LC. Preoperative anxiety and postoperative pain in women undergoing hysterectomy. A repeated-measures design. J Psychosom Res. 2000;49(6):417–22. doi: 10.1016/s0022-3999(00)00189-6. [DOI] [PubMed] [Google Scholar]
- 28.Johnson RL, Gold MA, Wyche KF. Distress in women with gynecologic cancer. Psychooncology. 2010;19(6):665–8. doi: 10.1002/pon.1589. [DOI] [PubMed] [Google Scholar]
- 29.Posluszny DM, Edwards RP, Dew MA, Baum A. Perceived threat and PTSD symptoms in women undergoing surgery for gynecologic cancer or benign conditions. Psychooncology. 2011;20(7):783–7. doi: 10.1002/pon.1771. [DOI] [PubMed] [Google Scholar]
- 30.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 31.Millon T, Antoni MH, Millon C, Meagher S, Grossman S. Test manual for the Millon Behavioral Medicine Diagnostic (MBMD) Minneapolis: National Computer Services; 2001. [Google Scholar]
- 32.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 33.Daabiss M. American Society of Anaesthesiologists physical status classification. Indian J Anaesth. 2011;55(2):111–5. doi: 10.4103/0019-5049.79879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noirhomme Q, Boly M, Bonhomme V, Boveroux P, Phillips C, Peigneux P, et al. Bispectral index correlates with regional cerebral blood flow during sleep in distinct cortical and subcortical structures in humans. Arch Ital Biol. 2009;147(1–2):51–7. [PubMed] [Google Scholar]
- 35.Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28(7):916–31. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- 36.Cohen J. Statistical power analyses for the behavioral sciences. New York: Academic; 1969. [Google Scholar]
- 37.Strunk DR, Lopez H, DeRubeis RJ. Depressive symptoms are associated with unrealistic negative predictions of future life events. Behav Res Ther. 2006;44(6):861–82. doi: 10.1016/j.brat.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 38.Sharot T, Riccardi AM, Raio CM, Phelps EA. Neural mechanisms mediating optimism bias. Nature. 2007;450(7166):102–5. doi: 10.1038/nature06280. [DOI] [PubMed] [Google Scholar]
- 39.Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annu Rev Psychol. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- 40.Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386(6627):824–7. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 41.Davidson RJ. Anterior electrophysiological asymmetries, emotion, and depression: conceptual and methodological conundrums. Psychophysiology. 1998;35(5):607–14. doi: 10.1017/s0048577298000134. [DOI] [PubMed] [Google Scholar]
- 42.Gotlib IH, Ranganatha C, Rosenfeld P. EEG alpha asymmetry, depression, and cognitive functioning. Cogn Emot. 1998;12(3):449–78. [Google Scholar]
- 43.Zhong M, Wang X, Xiao J, Yi J, Zhu X, Liao J, et al. Amygdala hyperactivation and prefrontal hypoactivation in subjects with cognitive vulnerability to depression. Biol Psychol. 2011;88(2–3):233–42. doi: 10.1016/j.biopsycho.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 44.Leung LS, Luo T, Ma J, Herrick I. Brain areas that influence general anesthesia. Progress in Neurobiology. 2014 doi: 10.1016/j.pneurobio.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 45.Adelstein JS, Shehzad Z, Mennes M, DeYoung CG, Zuo X-N, Kelly C, et al. Personality is reflected in the brain’s intrinsic functional architecture. PloS ONE. 2011;6(11) doi: 10.1371/journal.pone.0027633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frank E, Kupfer DJ, Jacob M, Jarrett D. Personality features and response to acute treatment in recurrent depression. J Personal Disord. 1987;1:14–26. [Google Scholar]
- 47.Klein M, Wonderlich S, Shea M. Models of relationships between personality and depression: toward a framework for theory and research. In: Klein MH, Wonderlich S, Shea MT, editors. Personality and depression: a current view. New York: Guilford; 1993. pp. 1–54. [Google Scholar]
- 48.Watson D, Clark L. Depression and the melancholic temperament. Eur J Personal. 1995;9:351–66. [Google Scholar]
- 49.Mathersul D, Williams LM, Hopkinson PJ, Kemp AH. Investigating models of affect: relationships among EEG alpha asymmetry, depression, and anxiety. Emotion. 2008;8(4):560–72. doi: 10.1037/a0012811. [DOI] [PubMed] [Google Scholar]
- 50.Moscovitch DA, Santesso DL, Miskovic V, McCabe RE, Antony MM, Schmidt LA. Frontal EEG asymmetry and symptom response to cognitive behavioral therapy in patients with social anxiety disorder. Biol Psychol. 2011;87(3):379–85. doi: 10.1016/j.biopsycho.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 51.Thibodeau R, Jorgensen RS, Kim S. Depression, anxiety, and resting frontal EEG asymmetry: a meta-analytic review. J Abnorm Psychol. 2006;115(4):715–29. doi: 10.1037/0021-843X.115.4.715. [DOI] [PubMed] [Google Scholar]
- 52.Manna CB, Tenke CE, Gates NA, Kayser J, Borod JC, Stewart JW, et al. EEG hemispheric asymmetries during cognitive tasks in depressed patients with high versus low trait anxiety. Clin EEG Neurosci Off J EEG Clin Neurosci Soc. 2010;41(4):196–202. doi: 10.1177/155005941004100406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Purdon PL, Pierce ET, Bonmassar G, Walsh J, Harrell PG, Kwo J, et al. Simultaneous electroencephalography and functional magnetic resonance imaging of general anesthesia. Ann N Y Acad Sci. 2009;1157:61–70. doi: 10.1111/j.1749-6632.2008.04119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beck AT, Guth D, Steer RA, Ball R. Screening for major depression disorders in medical inpatients with the Beck Depression Inventory for primary care. Behav Res Ther. 1997;35(8):785–91. doi: 10.1016/s0005-7967(97)00025-9. [DOI] [PubMed] [Google Scholar]
- 55.Monk TG, Weldon BC. Anesthetic depth is a predictor of mortality: it’s time to take the next step. Anesthesiology. 2010;112(5):1070–2. doi: 10.1097/ALN.0b013e3181d5e0eb. [DOI] [PubMed] [Google Scholar]
- 56.Watson M, Haviland JS, Greer S, Davidson J, Bliss JM. Influence of psychological response on survival in breast cancer: a population-based cohort study. Lancet. 1999;354(9187):1331–6. doi: 10.1016/s0140-6736(98)11392-2. [DOI] [PubMed] [Google Scholar]
- 57.Price CC, Tanner JJ, Schmalfuss I, Garvan CW, Gearen P, Dickey D, et al. A pilot study evaluating presurgery neuroanatomical biomarkers for postoperative cognitive decline after total knee arthroplasty in older adults. Anesthesiology. 2014;120(3):601–13. doi: 10.1097/ALN.0000000000000080. [DOI] [PMC free article] [PubMed] [Google Scholar]