Abstract
There is large interindividual variability and ethnic differences in phenylephrine-mediated vasoconstriction. We tested the hypothesis that genetic variation in ADRA1A, the α1A adrenergic receptor gene, contributes to the variability and ethnic differences. We measured local dorsal hand vein responses to increasing doses of phenylephrine in 64 Caucasians and 42 African-Americans and genotyped for 32 ADRA1A SNPs. The ED50 ranged from 11 to 5442 ng/min, and the Emax ranged from 13.5% to 100%. The rs574647 variant was associated with a trend towards lower logED50 in each race and in the combined cohort (P=0.008). Additionally, rs1079078 was associated with a trend to higher logED50 in each race and in the combined cohort (P=0.011). Neither variant accounted for the ethnic differences in response. None of the ADRA1A haplotypes was associated with the outcomes. In conclusion, ADRA1A variants do not contribute substantially to the marked interindividual variability or ethnic differences in phenylephrine-mediated venoconstriction.
Keywords: Alpha-1A adrenergic receptor, Genetics, Vasoconstriction, Phenylephrine, Ethnicity
Introduction
The sympathetic nervous system regulates blood pressure and vascular resistance, and its effects are mediated through adrenergic receptors (AR). Vascular α1-and α2-ARs are expressed in arterial resistance and venous capacitance vessels, where they regulate blood pressure by maintaining vascular tone through vascular smooth muscle contraction.1, 2 Vascular α1-ARs appear to be the prime mediators for vascular smooth muscle contraction.3, 4
There is large interindividual variability in the vasoconstrictive response of arterial and venous vessels to the infusion of α-AR agonists,5–7 much of which is genetically determined.8,9 The two main α-AR subgroups, α1-AR and α2-AR, differ in their signal transduction pathways (coupling to the Gq or Gi subtypes of G-proteins, respectively), but share a common final effector pathway (myosin light chain kinase activation, myosin phosphorylation and cross bridging with actin filaments) to mediate vascular smooth muscle contraction.1, 10, 11 Studies finding no correlation between an individual’s sensitivity of venoconstriction in response to a selective α1-AR agonist (phenylephrine) and a selective α2-AR agonist (dexmedetomidine),12, 13 which share the distal effector pathway, suggested that an important part of the interindividual variability is likely to be explained by differences in the distinctive proximal α1-AR – and α2-AR-signaling pathways (e.g., the membrane-bound adrenergic receptors and coupling). In support of this interpretation, we found ethnic differences between whites and blacks in vascular sensitivity to α1-AR agonists5, 7 but not to the α2-AR agonist dexmedetomidine.6 Thus, genetic variation in the α1-AR could contribute to the interindividual variability in sensitivity to α1-agonists and to the observed ethnic differences.
Of the three α1-AR subtypes (α1A, α1B and α1D), the α1A-AR is the predominant subtype expressed in human vascular smooth muscle.1 There is great genetic variation in ADRA1A, the gene encoding the α1A-AR, with distinct differences between whites and blacks in single nucleotide polymorphism (SNP) prevalence and haplotype structure.14 There is little information about the functional consequences of ADRA1A genetic variation on agonist-induced vasoconstriction. In an earlier study, we found that a common ADRA1A non-synonymous SNP (rs1048101) resulting in Arg347Cys, formerly called Arg492Cys, did not affect phenylephrine-mediated venoconstriction using the hand vein model.15 However, the effect of the many other ADRA1A SNPs and haplotype blocks on agonist-induced vasoconstriction has not been defined.
We therefore set out to study venous responses to phenylephrine in a large cohort of healthy Caucasian and African American subjects using the dorsal hand vein model and define the association between vascular sensitivity and ADRA1A genotypes and haplotypes. We chose the dorsal hand vein model since it is a reproducible model for the study of local venous responses to vasoactive substances in vivo that elicits only minimal systemic cardiovascular changes, and is less invasive compared to studies in arterial vessels.6, 7, 16, 17 Our hypothesis was that genetic variation in ADRA1A, represented by 32 SNPS, affects phenylephrine-mediated venoconstriction and contributes to interindividual and ethnic differences in agonist-mediated venoconstriction.
METHODS
Subjects
Between February 2002 - April 2004 and between May 2009 – January 2011, we recruited healthy subjects to participate in phenylephrine hand vein studies. These cohorts contributed data to previous publications,7, 12, 15 which describe the study population and procedures in detail. For the current study, we included normotensive male and non-pregnant female Caucasians and African Americans aged 18–45 years who took no medications for at least 2 weeks and abstained from alcohol and caffeine for at least 5 days before the study. Each subject received a diet containing 150 mmol/day of sodium, 70 mmol/day of potassium, and 600 mmol/day of calcium for at least 4 days prior to the study day. Studies were performed in the morning after an overnight fast in a temperature-controlled room at the Vanderbilt Clinical Research Center. The Institutional Review Board of Vanderbilt University Medical Center approved the study protocol, and all subjects provided written informed consent.
Venous response to phenylephrine
Venous responses were measured in a dorsal hand vein with a linear variable differential transformer (LVDT) as previously described.7 In summary, a 24-gauge intravenous cannula was inserted into a suitable right dorsal hand vein and kept patent with saline solution infused at a flow rate of 0.4 mL/min. A LVDT (MHR 100; Shaevitz Engineering, Pennsaken, NJ) was mounted on the dorsum of the subject’s hand. A second intravenous cannula was inserted for blood sampling into the antecubital vein of the contralateral arm. After 30 minutes of saline infusion, a blood sample was taken for the determination of baseline plasma catecholamines and for genotyping. We determined the baseline vein diameter while a sphygmomanometer cuff around the upper arm was inflated to 50 mm Hg to induce venous filling. After 3 stable baseline measurements, we assessed vein constriction in response to increasing doses of phenylephrine. Phenylephrine (Elkins-Sinn, Cherry Hill, NJ) was infused through the cannula with a syringe infusion pump (Harvard Apparatus, Holliston, MA) at increasing dose rates (range, 12–12,000 ng/min). The infusion at each dose rate lasted 7 minutes, and the vein diameter was measured during the last two minutes of the infusion. The total flow rate through the vein was kept constant at 0.4 mL/min throughout the various phenylephrine dilutions. Heart rate and blood pressure were continuously monitored with a bedside cardiac monitor (Dinamap MPS; Johnson and Johnson Medical, Tampa, FL).
Analysis of hand vein response to phenylephrine
Venoconstriction was expressed as the percentage reduction in vein diameter from average baseline measurements, plotted against dose rates in individual semi-logarithm dose–response graphs, and analyzed using a sigmoid dose–response model with variable slope (GraphPad Prism 4.03, GraphPad, La Jolla, CA). The software provided the dose that produced 50% of maximal constriction (ED50, representing sensitivity to the drug) and the maximal venoconstriction (Emax, representing maximum response) for each subject.
Determination of plasma catecholamine concentrations
Blood was collected into cooled heparinized tubes that were placed on ice until centrifuged at 4°C for 10 minutes at 3000 rpm. Plasma was separated and stored at −20°C in previously cooled tubes containing 40μL of reduced glutathione (6%) until assayed. Norepinephrine and epinephrine concentrations were measured by high-performance liquid chromatography using electrochemical detection with dihydroxybenzylamine as internal standard.18
Genotyping
We genotyped 32 ADRA1A SNPs, including 23 previously described tagSNPs defining four ADRA1A haplotype blocks14 and 9 additional SNPs common in Caucasians and/or African Americans (supplementary Table 1). We genotyped these SNPs using the Sequenom platform (MassArray, San Diego, CA), except for two SNPs (rs13261054 and rs1353446) which were genotyped using allelic discrimination by TaqMan 5′-nuclease assays on an ABI 7900 HT real-time PCR system (Applied Biosystems, Foster City, CA) using validated TaqMan probes.19 For quality control, we included negative and positive controls with each genotyping run. Quality-control procedures included examination of marker and sample genotyping efficiency, allele-frequency calculations, and tests of Hardy-Weinberg equilibrium (HWE).
Statistical analysis
Our cohort was a convenience sample consisting of participants in previous studies,7, 12, 15 and the sample size was not based on a priori calculations. Our primary outcome was drug sensitivity (expressed as ED50), and drug efficacy (Emax) was the secondary outcome. ED50 values were not normally distributed and were therefore expressed as geometric means with 95% confidence intervals (CIs) following log-transformation. Other data were expressed as mean and standard deviation (SD) or median and interquartile range (IQR) as appropriate. Genotype distributions were tested for deviation from Hardy-Weinberg equilibrium using a χ2 test with one degree of freedom.
We compared the outcome, phenylephrine sensitivity (LogED50) among genotypes in a single marker analysis using one-way analysis of variance (ANOVA), first in each ethnic group separately and then in the combined cohort. We then adjusted for potential confounders that were associated with the outcome in our previous analyses7 (sex, BMI, resting norepinephrine and, for analyses of the combined cohort, ethnicity) using a multiple linear regression model. To explore the contribution of genetic markers to ethnic differences in phenylephrine sensitivity, we performed these regression analyses in the whole cohort combined, with ethnicity as additional covariate, with and without the genetic markers of interest. For all genetic analyses, we assumed an additive genetic model, coding the genotypes according to the number of variant alleles (0–2). In a sensitivity analysis, we also used a dominant model for variants with few homozygote subjects to reduce the influence of potential outliers. The secondary outcome, phenylephrine efficacy (Emax), was compared among genotypes using the non-parametric Kruskal-Wallis test. Race-specific haplotypes were derived with the R package haplo.stats which was also used to assess overall differences in the outcomes among the haplotypes.20, 21 Other statistical analyses were performed using SPSS software (v. 21, IBM® SPSS® Inc., Chicago, IL). All analyses were two-tailed, and a P-value < 0.05 was considered significant.
RESULTS
Demographic parameters and outcomes
Among 116 subjects studied, we excluded 10 (DNA not available or Asian ethnicity), and the final cohort included 106 subjects (64 Caucasians and 42 African Americans). Table 1 shows demographic data, baseline cardiovascular parameters, and outcome measures, as previously described.7 African-Americans had a higher BMI (P = 0.007) and a higher resting heart rate (P = 0.005). There was wide interindividual variability in response to phenylephrine, with the range of ED50 spanning three log units (11 to 5442 ng/min; geometric mean, 245 ng/min; 95% CI, 190 to 318 ng/min), and the Emax ranging from 13.5% to 100% (median, 87%; IQR, 76% to 96%).
Table 1.
Demographic characteristic and outcome measures
| Covariates | Caucasians n = 64 |
African Americans n = 42 |
All n = 106 |
|---|---|---|---|
| Age, years | 26.9 ± 6.8 | 28.1 ± 8.0 | 27.3± 7.3 |
| Female sex, n (%) | 31 (48) | 18 (43) | 49 (46) |
| Family history of Hypertension, n (%) | 25 (39) | 20 (48) | 45 (43) |
| Resting SBP, mmHg | 110.3 ± 11 | 111.1 ± 9.5 | 110.6 ± 10.4 |
| Resting DBP, mmHg | 60.7 ± 6.8 | 63.4 ± 8.0 | 61.8 ± 7.4 |
| Resting HR, bpm* | 59.0 ± 8.2 | 63.7 ± 8.0 | 60.9 ± 8.4 |
| Baseline Plasma norepinephrine, pg/ml | 162.3 ± 58.0 | 179.5 ± 70.8 | 168.9 ± 63.3 |
| Baseline Plasma epinephrine, pg/ml | 18.5 ± 12.5 | 21.0 ± 15.6 | 19.4 ± 13.7 |
| BMI, kg/m2* | 24.3 ± 3.8 | 26.8 ± 5.4 | 25.3 ± 4.6 |
| ED50, ng/min, Mean (95%CI)* | 310 (222 – 434) | 172 (115 – 256) | 245 (190 – 318) |
| Emax, %, Median (IQR) | 85 (75 – 95) | 89 (82 – 98) | 87 (76 – 96) |
Data presented as mean ± SD except otherwise stated. SBP = Systolic blood pressure; DBP = Diastolic blood pressure; HR = Heart rate
P value < 0.05 comparing Caucasians and African Americans.
Genotyping
We genotyped 32 ADRA1A SNPs (Supplementary Table S1). Minor allele frequencies were in the expected range, and all genotypes conformed to Hardy-Weinberg equilibrium in each ethnic group. We did not identify any carrier of the rs1496126 variant.
Genetic variants and sensitivity to phenylephrine
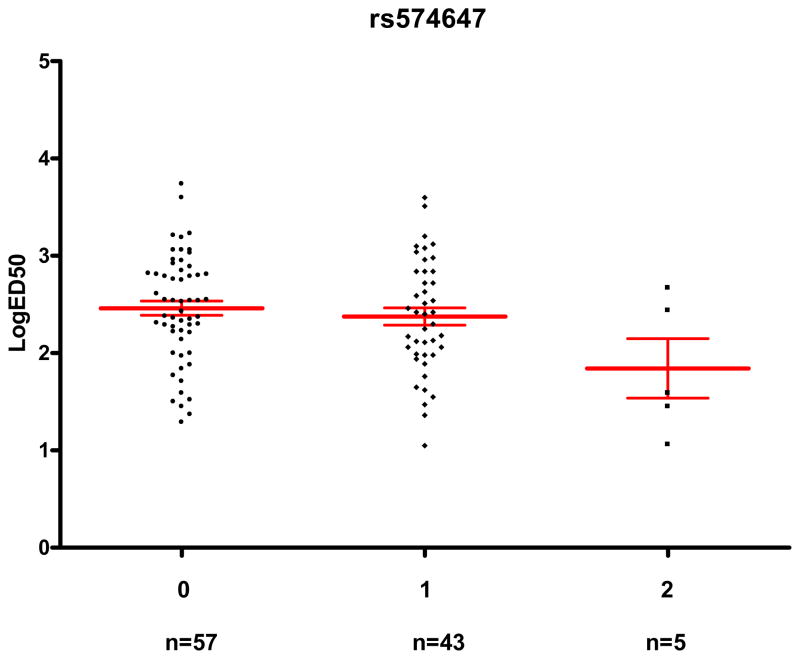
The rs574647 was associated with a trend towards lower logED50 both in Caucasians (β=−0.25; 95% CI, −0.51 to 0.02; adjusted P=0.064) and African Americans (β=−0.40; 95% CI, −0.79 to −0.02; adjusted P=0.039), and the statistical evidence for this association was stronger in the combined cohort (β=−0.29; 95% CI, −0.50 to −0.08; adjusted P=0.008). Geometric mean ED50 was 76% lower in subjects with two minor alleles (ED50=69 ng/min; 95% CI, 10 to 489 ng/min; n=5) compared to those with no minor allele (ED50=289 ng/min; 95% CI, 207 to 405 ng/min; n=57; Figure 1).
Figure 1. rs574647 genotype and its association with sensitivity to phenylephrine (ED50).
The horizontal lines represent the mean, and the whiskers represent the standard error of the mean. Subjects with variant alleles had significantly lower LogED50 (unadjusted P=0.064, adjusted P=0.008).
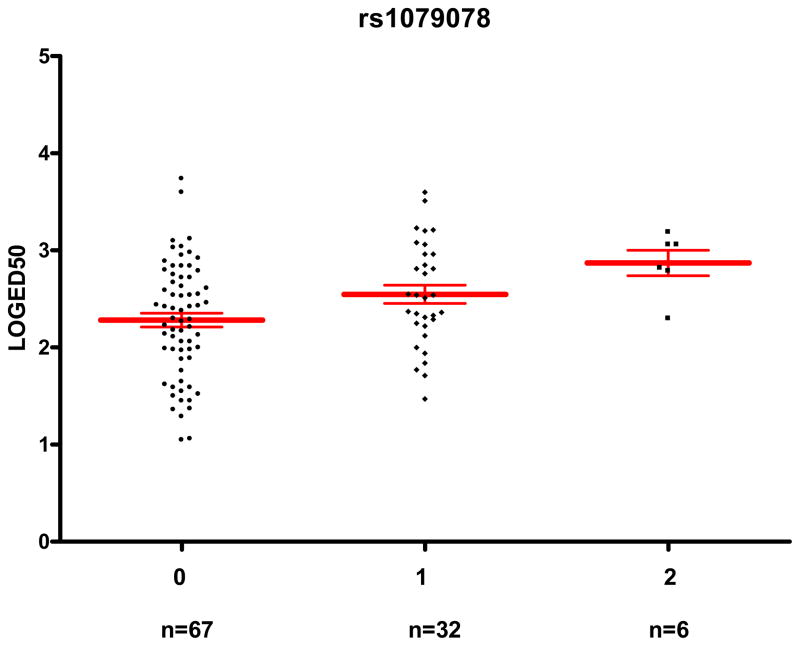
Additionally, the rs1079078 variant was associated with a trend to higher logED50 both in Caucasians (β=0.23; 95% CI, −0.01 to 0.47; adjusted P=0.055) and African Americans (β=0.31; 95% CI, −0.04 to 0.67; adjusted P=0.082), and the association was stronger in the combined group (β=0.25; 95% CI, 0.06 to 0.44; adjusted P=0.011). Thus, subjects with two variant alleles (n=6) had a geometric mean ED50 of 742 ng/min (95% CI, 345 to 1,595 ng/min), a 3.9-fold higher value compared to the 67 subjects with no minor allele (192 ng/min; 95% CI, 138 to 267 ng/min; Figure 2). In a sensitivity analysis, after combining heterozygous and homozygous variant carriers into one group assuming a dominant mode of inheritance, the associations between rs574647 and the rs1079078 with logED50 in the whole cohort remained significant(adjusted P=0.037 and 0.030, respectively).
Figure 2. rs1079078 genotype and its association with sensitivity to phenylephrine (ED50).
The horizontal lines represent the mean, and the whiskers represent the standard error of the mean. Subjects with variant alleles have significantly higher LogED50 (unadjusted P=0.003, adjusted P=0.011).
The association between rs13270252 and LogED50 was in opposite directions in the two ethnic groups and was not significant in the combined group (adjusted P=0.69) (Table 2, Supplementary Table S2). The rs580739 variant was significantly associated with LogED50 in African Americans (adjusted P=0.005) but not in Caucasians or in the combined cohort (both P>0.10). Moreover, the functional non-synonymous variant rs1048101, resulting in the Arg347Cys polymorphism, was not associated with LogED50 in any of the ethnic subgroups or the combined group (all P > 0.35).
Table 2.
Genetic variants of ADRA1A associated with sensitivity to phenylephrine (ED50)
| SNP | Caucasians | African Americans | All | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ED50, ng/min, geometric mean (95% CI) |
P-value* unadjust. (adjusted) |
ED50, ng/min, geometric mean (95% CI) |
P-value* unadjust. (adjusted) |
P-value* unadjust. (adjusted) |
|||||
| 0* | 1 | 2 | 0* | 1 | 2 | ||||
| rs574647 | 395 (248–627) | 322 (201–514) | 43 (5–360) | 0.022 (0.064) | 214 (131–350) | 98 (46–208) | 468 | 0.283 (0.039) | 0.064 (0.008) |
| N = 28 | N = 32 | N = 4 | N = 29 | N = 11 | N = 1 | ||||
|
| |||||||||
| rs1079078 | 244 (156–381) | 365 (199–671) | 963 (593– 1565) | 0.030 (0.055) | 139 (85–230) | 329 (162–667) | 201 | 0.094 (0.082) | 0.003 (0.011) |
| N = 38 | N = 21 | N = 5 | N = 29 | N = 11 | N = 1 | ||||
|
| |||||||||
| rs13270252 | 269 (186–398) | 646 (316–1318) | 324 | 0.108 (0.039) | 186 (112–302) | 204 (87–490) | 35 (4–331) | 0.207 (0.027) | 0.586 (0.686) |
| N = 53 | N = 10 | N = 1 | N = 21 | N=14 | N = 3 | ||||
|
| |||||||||
| rs580739 | 394 (245–632) | 241 (140–414) | 336 (77–1468) | 0.360 (0.623) | 267 (172–416) | 55 (32–93) | 468 | 0.007 (0.005) | 0.131 (0.103) |
| N = 29 | N = 29 | N = 6 | N = 29 | N = 11 | N = 1 | ||||
0, 1, 2 represent the number of variant alleles for each SNP.
N = number of subjects in each genotype group.
P values are before (unadjust.) and after adjustment for sex, BMI, baseline norepinephrine values and ethnicity (for analyses of the combined cohort).
Genetic variants and efficacy of phenylephrine
None of the 32 ADRA1A SNPs was associated with Emax in the ethnic subgroups, while rs17333700 was marginally associated with lower Emax in the combined group (P = 0.040, Table 3; lementary Table S3; Supplementary Figure 1).
Table 3.
Genetic variant of ADRA1A associated with efficacy to phenylephrine (Emax).
| SNP | Caucasians | African Americans | All | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Emax, %, median (IQR) | P value | Emax, %, median (IQR) | P value | P value | |||||
| 0 | 1 | 2 | 0 | 1 | 2 | ||||
| rs17333700 | 85 (75–99) | 85 (76–92) | 65 | 0.078 | 89 (83–98) | 92 (83–97) | - | 0.749 | 0.040 |
| N = 35 | N = 26 | N = 3 | N = 36 | N = 5 | |||||
0, 1, 2 is the number of variant alleles for each SNP.
N = number of subjects with each variant allele.
ADRA1A haplotypes and outcome measures
Based on a previously published ADRA1A haplotype analysis, 14 we divided the ADRA1A gene into four blocks and inferred haplotypes for each block separately in each ethnic group (Supplementary Tables S4). Haplotype frequencies were overall similar to those previously published. Phenotype-haplotype analyses did not provide any evidence for an overall association between outcomes (LogED50 or Emax) and haplotypes in any of the 4 haplotype blocks (Supplementary Tables S5 and S6).
Ethnic difference in ED50 and ADRA1A variants
As previously described,7 geometric mean ED50 was 45% lower in African Americans than in Caucasians (172 ng/min and 310 ng/min in African Americans and Caucasians respectively; adjusted P= 0.004; Table 1). Since the four ADRA1A variants associated with logED50 (rs13270252, rs580739, rs574647 and rs1079078) had significantly different prevalences in Caucasians and African Americans, we examined whether these variants contributed to ethnic differences in ED50. In the combined cohort, using a multiple linear regression model without genotypes (with logED50 as dependent variable and sex, BMI, ethnicity, and resting plasma norepinephrine concentrations as covariates), black ethnicity was associated with lower LogED50 (β=−0.36; 95% CI, −0.61 to −0.12; P=0.004). Adding the four genotypes to the model increased the coefficient of variation R2, a measure of the variation in the outcome explained by the model, from 12.9% to 18.4%, but the ethnic difference remained unaffected (β=−0.36; 95% CI, −0.64 to −0.08; P=0.013), suggesting that these genotypes did not contribute substantially to the greater phenylephrine sensitivity of African Americans compared to Caucasians.
DISCUSSION
Our study is the first to systematically examine the effect of the genetic variation in ADRA1A on vascular responses to an α1-AR agonist. Our major findings are that genetic variation in ADRA1A explains only little of the large interindividual variability in phenylephrine-mediated venoconstriction, and that it does not account for the ethnic differences in α1AR-mediated venoconstriction. In addition, our study provided some evidence for an association of two ADRA1A SNPs (rs574647 and rs1079078) with increased and decreased sensitivity to phenylephrine-mediated venoconstriction, respectively.
Family studies have found large heritability in venous response to α-AR agonists.8, 9 Veins express different α1-AR subtypes, and the α1A-AR subtype has the highest receptor density and greatest affinity for α1-AR agonists.3, 22 However, little is known about the effect of genetic variability in ADRA1A on α1-AR-mediated venoconstriction. We previously studied the effect of a single functional, non-synonymous ADRA1A SNP (rs1048101, resulting in Arg347Cys), in the hand vein model in 74 subjects (52 Caucasians, 5 African Americans and 17 other ethnicities), finding no association with phenylephrine-mediated venoconstriction.15 In our current study, with a larger sample size, we confirmed this finding. Moreover, in a systematic approach using 32 SNPS selected to better capture ADRA1A genetic variation, we found that it contributed only little at best to the great variability in phenylephrine-mediated venoconstriction and to its ethnic differences. Thus, our findings are compatible with previous studies failing to show consistent associations of ADRA1A polymorphisms with more complex phenotypes to which α1-mediated vasoregulation is thought to contribute, e.g. blood pressure response to stress23 and hypertension.24–30
We found some evidence for an association of two intronic SNPs - rs574647 and rs1079078 - with increased and decreased sensitivity to phenylephrine-mediated venoconstriction, respectively. Subjects with two minor rs574647 variant alleles had a geometric mean ED50 that was 76% lower than that of subjects without any variant allele. The rs1079078 variant was more prevalent in Caucasians, and subjects with two variant alleles had a 3.9 fold higher geometric mean ED50 (reflecting lower α1A-AR sensitivity to phenylephrine-mediated venoconstriction) compared to subjects with no variant allele. We are not aware of previous studies showing functional associations of these variants. Moreover, they may be only markers of functional haplotypes rather than SNPs primarily responsible for the observed effect. However, haplotype analysis did not provide evidence for functional effects of the haplotypes defined by these two variants.
We have previously shown increased sensitivity of venous7 and arterial5 α1-AR-mediated vasoconstriction in African Americans compared to Caucasians. In view of the ethnic differences in ADRA1A genetic variation,14 we therefore examined the hypothesis that genetic variants in α1-AR account for these ethnic differences. However, in the present study, the four variants associated with phenylephrine ED50 did not explain the ethnic difference in ED50. Thus, after excluding genetic variation in α1-AR, it remains unclear which factors contribute to the large interindividual variability in α1-AR mediated venoconstriction, and to the ethnic differences in particular. Yet, in view of the strong heritability of venous responsiveness to α-AR agonists, genetic variation in other candidate genes remains a likely cause.8, 9
In contrast to phenylephrine-mediated vasoconstriction, we previously did not find ethnic differences in α2-AR mediated venoconstriction in the hand vein model,6 suggesting that differences in the proximal signaling pathway specific to the α1-AR pathway account for ethnic differences in phenylephrine sensitivity. Thus, taken together with our current findings, genes encoding proteins involved in the early signal transduction cascade below the α1-AR level, e.g. in receptor coupling, would be reasonable candidates for explaining interindividual and ethnic differences in α1-AR mediated responsiveness.
There are several limitations to our study. Phenylephrine is not selective for the α1A-AR subtype, but acts as an agonist also at venous α1B-AR and α1D-AR. However, there is no selective α1A-AR agonist available for use in humans. Moreover, although α1B- and α1D-ARs are also expressed in human veins,31, 32 α1A-ARs are thought to be the prime mediators of vascular constriction.4 Secondly, our findings were derived using the dorsal hand vein model, and may therefore not automatically be extrapolated to other venous or arterial vascular beds, or to more complex and multifactorial phenotypes such as blood pressure. However, we found similar ethnic differences in α1-AR-mediated vasoconstriction both in venous and arterial vascular beds, suggesting that responsiveness in the dorsal hand vein model may to a certain degree also reflect that in arterial vascular beds. Finally, although our sample size was rather large for a translational study, some genotype groups were small, and we could not account for the multiple comparisons required by the large number of ADRA1A variants. Thus, we consider our findings regarding the association of the rs574647 and rs1079078 variants with responsiveness to phenylephrine preliminary rather than conclusive. Our findings should be validated in a separate cohort, and much larger cohorts would be necessary for systematic, non-candidate gene-based approaches such as genome-wide association studies.
In conclusion, we found some evidence for an association of sensitivity to phenylephrine-mediated venoconstriction with two intronic ADRA1A SNPs (rs574647 and rs1079078), but overall, genetic variation in ADRA1A does not contribute substantially to the large interindividual variation in phenylephrine-mediated venoconstriction and ethnic differences in response between African Americans and Caucasians. Future studies exploring additional candidate genes involved in the α1-AR signal transduction pathway (e.g., those involved in receptor coupling) or genes encoding other α1-AR subtypes will be of interest.
Supplementary Material
Acknowledgments
This study was supported by Vanderbilt CTSA grant 1 UL1 TR000445 from the National Center for Research Resources and P01 HL56693 from the National Institutes of Health. Dr. Stein is the recipient of the Dan May Chair in Medicine.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
References
- 1.Docherty JR. Subtypes of functional alpha1-adrenoceptor. Cell Mol Life Sci. 2010;67(3):405–417. doi: 10.1007/s00018-009-0174-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanoue A, Koshimizu TA, Tsujimoto G. Transgenic studies of alpha(1)-adrenergic receptor subtype function. Life Sci. 2002;71(19):2207–2215. doi: 10.1016/s0024-3205(02)02012-x. [DOI] [PubMed] [Google Scholar]
- 3.Rudner XL, Berkowitz DE, Booth JV, Funk BL, Cozart KL, D’Amico EB, et al. Subtype specific regulation of human vascular alpha(1)-adrenergic receptors by vessel bed and age. Circulation. 1999;100(23):2336–2343. doi: 10.1161/01.cir.100.23.2336. [DOI] [PubMed] [Google Scholar]
- 4.Leech CJ, Faber JE. Different alpha-adrenoceptor subtypes mediate constriction of arterioles and venules. Am J Physiol. 1996;270(2 Pt 2):H710–H722. doi: 10.1152/ajpheart.1996.270.2.H710. [DOI] [PubMed] [Google Scholar]
- 5.Stein CM, Lang CC, Singh I, He HB, Wood AJ. Increased vascular adrenergic vasoconstriction and decreased vasodilation in blacks. Additive mechanisms leading to enhanced vascular reactivity. Hypertension. 2000;36(6):945–951. doi: 10.1161/01.hyp.36.6.945. [DOI] [PubMed] [Google Scholar]
- 6.Muszkat M, Sofowora GG, Wood AJ, Stein CM. Alpha2-adrenergic receptor-induced vascular constriction in blacks and whites. Hypertension. 2004;43(1):31–35. doi: 10.1161/01.HYP.0000103694.30164.C7. [DOI] [PubMed] [Google Scholar]
- 7.Adefurin A, Ghimire LV, Kohli U, Muszkat M, Sofowora GG, Paranjape SY, et al. Blacks have a greater sensitivity to alpha1-adrenoceptor-mediated venoconstriction compared with Whites. Hypertension. 2013;61(4):915–920. doi: 10.1161/HYPERTENSIONAHA.111.00854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta A, Carruthers SG. Familial studies of heritability of alpha1-adrenergic receptor responsiveness in superficial veins. Clin Pharmacol Ther. 1997;62(3):322–326. doi: 10.1016/S0009-9236(97)90035-7. [DOI] [PubMed] [Google Scholar]
- 9.Luthra A, Borkowski KR, Carruthers SG. Genetic aspects of variability in superficial vein responsiveness to norepinephrine. Clin Pharmacol Ther. 1991;49(4):355–361. doi: 10.1038/clpt.1991.41. [DOI] [PubMed] [Google Scholar]
- 10.Johnson JA, Liggett SB. Cardiovascular pharmacogenomics of adrenergic receptor signaling: clinical implications and future directions. Clin Pharmacol Ther. 2011;89(3):366–378. doi: 10.1038/clpt.2010.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klabunde RE. [Last accessed on 08/08/2014];Cardiovascular physiology concepts: Vascular smooth muscle contraction and relaxation. http://www.cvphysiology.com/BloodPressure/BP026.htm.
- 12.Muszkat M, Kurnik D, Sofowora GG, Wood AJ, Stein CM. Independent regulation of alpha1 and alpha2 adrenergic receptor-mediated vasoconstriction in vivo. J Hypertens. 2011;29(2):251–256. doi: 10.1097/HJH.0b013e3283407ffd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Posti JP, Valve L, Ruohonen S, Akkila J, Scheinin M, Snapir A. Dorsal hand vein responses to the alpha-adrenoceptor agonist phenylephrine do not predict responses to the alpha-adrenoceptor agonist dexmedetomidine. Eur J Pharmacol. 2011;653(1–3):70–74. doi: 10.1016/j.ejphar.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 14.Buzas B, Belfer I, Hipp H, Lorincz I, Evans C, Phillips G, et al. Haplotype block and superblock structures of the alpha1-adrenergic receptor genes reveal echoes from the chromosomal past. Mol Genet Genomics. 2004;272(5):519–529. doi: 10.1007/s00438-004-1074-9. [DOI] [PubMed] [Google Scholar]
- 15.Sofowora GG, Dishy V, Landau R, Xie HG, Prasad HC, Byrne DW, et al. Alpha 1A-adrenergic receptor polymorphism and vascular response. Clin Pharmacol Ther. 2004;75(6):539–545. doi: 10.1016/j.clpt.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Schindler C, Grossmann M, Dobrev D, Francke K, Ravens U, Kirch W. Reproducibility of dorsal hand vein responses to phenylephrine and prostaglandin F2 alpha using the dorsal hand vein compliance method. J Clin Pharmacol. 2003;43(3):228–236. doi: 10.1177/0091270002251004. [DOI] [PubMed] [Google Scholar]
- 17.Alradi AO, Carruthers SG. Evaluation and application of the linear variable differential transformer technique for the assessment of human dorsal hand vein alpha-receptor activity. Clin Pharmacol Ther. 1985;38(5):495–502. doi: 10.1038/clpt.1985.214. [DOI] [PubMed] [Google Scholar]
- 18.He HB, Deegan RJ, Wood M, Wood AJ. Optimization of high-performance liquid chromatographic assay for catecholamines. Determination of optimal mobile phase composition and elimination of species-dependent differences in extraction recovery of 3,4-dihydroxybenzylamine. J Chromatogr. 1992;574(2):213–218. [PubMed] [Google Scholar]
- 19.Livak KJ. SNP genotyping by the 5′-nuclease reaction. Methods Mol Biol. 2003;212:129–147. doi: 10.1385/1-59259-327-5:129. [DOI] [PubMed] [Google Scholar]
- 20.Schaid DJ, Rowland CM, Tines DE, Jacobson RM, Poland GA. Score tests for association between traits and haplotypes when linkage phase is ambiguous. Am J Hum Genet. 2002;70(2):425–434. doi: 10.1086/338688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sinnwell JP, Schaid DJ. haplo.stats: Statistical Analysis of Haplotypes with Traits and Covariates when Linkage Phase is Ambiguous v.1.6.8. http://cran.rproject.org/web/packages/haplo.stats/index.html.
- 22.Theroux TL, Esbenshade TA, Peavy RD, Minneman KP. Coupling efficiencies of human alpha 1-adrenergic receptor subtypes: titration of receptor density and responsiveness with inducible and repressible expression vectors. Mol Pharmacol. 1996;50(5):1376–1387. [PubMed] [Google Scholar]
- 23.Kelsey RM, Alpert BS, Dahmer MK, Krushkal J, Quasney MW. Alpha-adrenergic receptor gene polymorphisms and cardiovascular reactivity to stress in Black adolescents and young adults. Psychophysiology. 2012;49(3):401–412. doi: 10.1111/j.1469-8986.2011.01319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie HG, Kim RB, Stein CM, Gainer JV, Brown NJ, Wood AJ. Alpha1A-adrenergic receptor polymorphism: association with ethnicity but not essential hypertension. Pharmacogenetics. 1999;9(5):651–656. [PubMed] [Google Scholar]
- 25.Freitas SR, Pereira AC, Floriano MS, Mill JG, Krieger JE. Association of alpha1a-adrenergic receptor polymorphism and blood pressure phenotypes in the Brazilian population. BMC Cardiovasc Disord. 2008;8:40. doi: 10.1186/1471-2261-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gu D, Su S, Ge D, Chen S, Huang J, Li B, et al. Association study with 33 single-nucleotide polymorphisms in 11 candidate genes for hypertension in Chinese. Hypertension. 2006;47(6):1147–1154. doi: 10.1161/01.HYP.0000219041.66702.45. [DOI] [PubMed] [Google Scholar]
- 27.Gu D, Ge D, Snieder H, He J, Chen S, Huang J, et al. Association of alpha1A adrenergic receptor gene variants on chromosome 8p21 with human stage 2 hypertension. J Hypertens. 2006;24(6):1049–1056. doi: 10.1097/01.hjh.0000226194.21311.2f. [DOI] [PubMed] [Google Scholar]
- 28.Iacoviello M, Forleo C, Sorrentino S, Romito R, De TE, Lucarelli K, et al. Alpha- and beta-adrenergic receptor polymorphisms in hypertensive and normotensive offspring. J Cardiovasc Med (Hagerstown) 2006;7(5):316–321. doi: 10.2459/01.JCM.0000223252.34611.87. [DOI] [PubMed] [Google Scholar]
- 29.Reder NP, Tayo BO, Salako B, Ogunniyi A, Adeyemo A, Rotimi C, et al. Adrenergic alpha-1 pathway is associated with hypertension among Nigerians in a pathway-focused analysis. PLoS One. 2012;7(5):e37145. doi: 10.1371/journal.pone.0037145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Franceschini N, Fox E, Zhang Z, Edwards TL, Nalls MA, Sung YJ, et al. Genome-wide association analysis of blood-pressure traits in African-ancestry individuals reveals common associated genes in African and non-African populations. Am J Hum Genet. 2013;93(3):545–554. doi: 10.1016/j.ajhg.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan M, Sun J, Bird PI, Liu DL, Grigg M, Lim YL. Alpha1A- and alpha1B-adrenoceptors are the major subtypes in human saphenous vein. Life Sci. 2001;68(10):1191–1198. doi: 10.1016/s0024-3205(00)01027-4. [DOI] [PubMed] [Google Scholar]
- 32.Giessler C, Wangemann T, Silber RE, Dhein S, Brodde OE. Noradrenaline-induced contraction of human saphenous vein and human internal mammary artery: involvement of different alpha-adrenoceptor subtypes. Naunyn Schmiedebergs Arch Pharmacol. 2002;366(2):104–109. doi: 10.1007/s00210-002-0582-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.