Abstract
The mechanisms of furosemide (FS) hepatotoxicity were explored in mice. Specifically, C57Bl/6J mice were treated with 500 mg FS / kg bodyweight and c-Jun N-terminal kinase (JNK) activation and RIP3 expression were measured by western blotting. Co-treatment with furosemide and the JNK inhibitor SP600125 was also performed, and FS-induced liver injury was compared in wild-type and RIP3 knockout (KO) mice.
JNK phosphorylation and RIP3 expression were increased in livers from the FS-treated mice as early as 6 h after treatment, and persisted until at least 24 h. JNK phosphorylation was also observed in primary mouse hepatocytes and human HepaRG cells treated with FS.
Phosphorylated JNK translocated into mitochondria in livers, but no evidence of mitochondrial damage was observed.
SP600125 treated mice, SP600125 co-treated primary mouse hepatocytes, and RIP3 KO mice were not protected against FS hepatotoxicity. These data show that, although JNK activation and RIP3 expression are induced by FS, neither contributes to the liver injury.
INTRODUCTION
Furosemide (FS) is a loop diuretic drug that is commonly prescribed for the treatment of edema, hypertension, renal impairment and several other conditions. Although there have been no confirmed reports of FS hepatotoxicity in humans, it is known that a single large dose can cause centrilobular hepatic necrosis in mice (Mitchell et al., 1974). Use of the drug as a model hepatotoxicant in research has increased in recent years (Wong et al., 2000; Williams et al., 2007; Randle et al., 2008; Qu et al., 2014; McGill et al., 2012; 2014). Despite this, little is known about the mechanisms of FS-induced liver injury. It has been shown that the major metabolic pathway for elimination of FS in both humans and rodents is glucuronidation (Vree and van der Ven, 1999; Williams et al., 2007). Furthermore, FS appears to be metabolized by cytochrome P450s to an epoxide intermediate (Williams et al., 2007) that reacts with proteins in the liver (Mitchell et al., 1974; 1976; Williams et al., 2007). The extent of protein binding correlates with the degree of liver injury, and inhibition of P450s protects mice against FS hepatotoxicity (Mitchell et al., 1974; 1976; Williams et al., 2007). However, the downstream events are unclear. It is not yet known how the protein binding leads to the necrosis.
A number of recent studies have demonstrated a role for the c-Jun N-terminal kinases (JNK) 1/2 in various forms of liver injury (Gunawan et al., 2006; Wang et al., 2006; Latchoumycandane et al., 2007; Theruvath et al., 2008; Saito et al., 2010; Du et al., 2013; Xie et al., 2014). In particular, the JNK inhibitor SP600125 has been shown to protect against acetaminophen (APAP) toxicity in mouse hepatocytes both in vivo and in vitro (Gunawan et al., 2006; Saito et al., 2010), and in primary human hepatocytes (Xie et al., 2014). Treatment with RNA antisense for JNK isoforms also protects in vitro (Gunawan et al., 2006), and other drugs that have been shown to inhibit JNK activation also reduce APAP-induced liver injury in vivo (Latchoumycandane et al., 2007; Du et al., 2013). In addition, it has been suggested that the receptor-interacting protein kinase 3 (RIP3) can modulate JNK activation during APAP toxicity, and RIP3 knockout (KO) mice are protected (Ramachandran et al., 2013).
We hypothesized that JNK and RIP3 play a role in FS-induced liver injury. To explore this, we measured JNK phosphorylation and RIP3 expression during FS hepatotoxicity in mice, primary mouse hepatocytes, and human HepaRG cells. We also co-treated mice with FS and SP600125, and compared FS toxicity in wild-type (WT) and RIP3 KO mice. Our results show that JNK is activated and translocates into mitochondria, and that RIP3 is induced. However, neither kinase appears to be a cause of liver injury in these animals.
MATERIALS AND METHODS
Animals
Male WT C57Bl/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME). RIP3KO mice (C57Bl/6J background) were the generous gift of Dr. Vishva Dixit (Genentech, South San Francisco, CA) and were bred for 3–4 additional generations at the University of Kansas Medical Center. All animals were housed in a temperature- and humidity-controlled facility with a 12 h light/dark cycle. The mice were allowed ad libitum access to food and water throughout the studies. Experiments were performed when the mice were 8–10 weeks of age. All study protocols were approved by the Insitutional Animal Care and Use Committee of the University of Kansas Medical Center and were conducted in accordance with the criteria of the National Research Council for animal use in research.
In vivo experiments
FS was dissolved at a concentration of 15 mg/mL in 1x phosphate-buffered saline (PBS) by bringing the pH to 8.5–9.0 with a small amount of 1 N HCl and by repeated vortexing. Animals were injected i.p. with 500 mg FS/kg bodyweight or an equal volume per mouse of PBS vehicle (also at pH 8.5–9.0). Some mice were co-treated with 10 mg/kg SP600125 dissolved in 8.3% DMSO or with the DMSO vehicle. In one experiment, the animals were treated with SP600125 1 h before FS and 6 and 18 h post-FS. Mice were sacrificed under anesthesia at the indicated time points after FS treatment by cervical dislocation and exsanguination. Blood was drawn from the caudal vena cava into syringes prepared with EDTA or heparin, and plasma was obtained by centrifugation at 10,000g for 3 min. Alanine aminotransferase (ALT) was measured in plasma using a kit from Pointe Scientific (Ann Arbor, MI). Pieces of liver were harvested and flash frozen in liquid nitrogen for later western blotting and glutathione measurement, and a section from the left lateral lobe was fixed in 10% phosphate-buffered formalin and embedded in paraffin for hematoxylin and eosin (H&E) staining. In some experiments, approximately one-third of the FS-treated animals in each group did not develop any evidence of liver injury (defined as ALT > 3x ULN and necrosis in histology) and these animals were excluded from later analysis. Importantly, in the SP600125 experiments, the percentage of non-responders was the same in each treatment group, and including them in the data did not change the results.
In vitro experiments
Isolation of primary mouse hepatocytes was performed as previously described (Bajt et al., 2004). Cryopreserved differentiated HepaRG cells were obtained from BioPredic International (Rennes, France) and cultured according to the manufacturer’s instructions. Prior to treatment, the cells were washed and the medium was replaced with Williams’ E medium without DMSO, as previously described (McGill et al., 2011). FS was dissolved in 1x PBS at pH 8.5–9.0 and added directly to the culture medium to a final concentration of 1, 5 or 10 mM. Control cells were treated with the PBS vehicle (also at pH 8.5–9.0). In some experiments, cells were co-treated with 30 μM SP600125 dissolved in DMSO or with DMSO vehicle. Cells were harvested at the indicated time points and lactate dehydrogenase (LDH) release was measured as previously described in detail (Xie et al., 2013). For the JNK activation positive control in HepaRG cells, tert-butyl hydroperoxide was added to a final concentration of 200 μM in the culture medium and the cells were harvested 2 h later.
Subcellular fractionation
Freshly excised right and caudate liver lobes were minced and homogenized in cold isolation buffer containing 220 mM mannitol, 70mM sucrose, 2.5mM HEPES, 10mM EDTA, 1mM ethylene glycol tetraacetic acid, and 0.1% bovine serum albumin using a tight-fitting Potter-Elvehjem homogenizer. Subcellular fractions were obtained by differential centrifugation of the resulting homogenate. Briefly, the samples were centrifuged at 2,500g for 10 min to remove nuclei, red blood cells, and cell debris. The supernatant was collected and spun at 20,000g for 10 min to pellet mitochondria and other organelles. The supernatant from the 20,000g spin was collected and saved as the cytosolic fraction, while the pellet was resuspended in isolation buffer and centrifuged again at 10,000g for 10 min to obtain a crude mitochondrial fraction. After discarding the supernatant from the 10,000g spin, the mitochondrial pellet was flash frozen in liquid nitrogen and stored at −80°C until further analysis.
Western blotting
Samples were run on 4–20% Tris-glycine gels and transferred to PVDF membranes. In all cases, the membranes were blocked with 5% milk in Tris-buffered saline with 0.1% Tween 80. All primary antibodies were diluted 1:1,000 in 5% milk and were incubated overnight on the membranes. JNK antibodies were purchased from Cell Signaling (Danvers, MA). The RIP3 antibody was purchased from ProSci (Poway, CA).
GSH Measurement
Glutathione (GSH) and oxidized GSH (GSSG) were measured using a modified Tietze assay, as previously described (Jaeschke and Mitchell, 1990).
Statistics
Normality was assessed using the Shapiro-Wilk test. For normal data, comparisons were made between two groups using Student’s t-test, or between three or more groups using one-way analysis of variance (ANOVA) with Tukey’s post-hoc test. For non-normally distributed data, comparisons were made between two groups using the Mann-Whitney U-test, or between three or more groups using the Kruskal-Wallis test with Dunn’s multiple comparisons. All statistical tests were performed using SigmaPlot software (Systat, San Jose, CA).
RESULTS
FS-induced liver injury
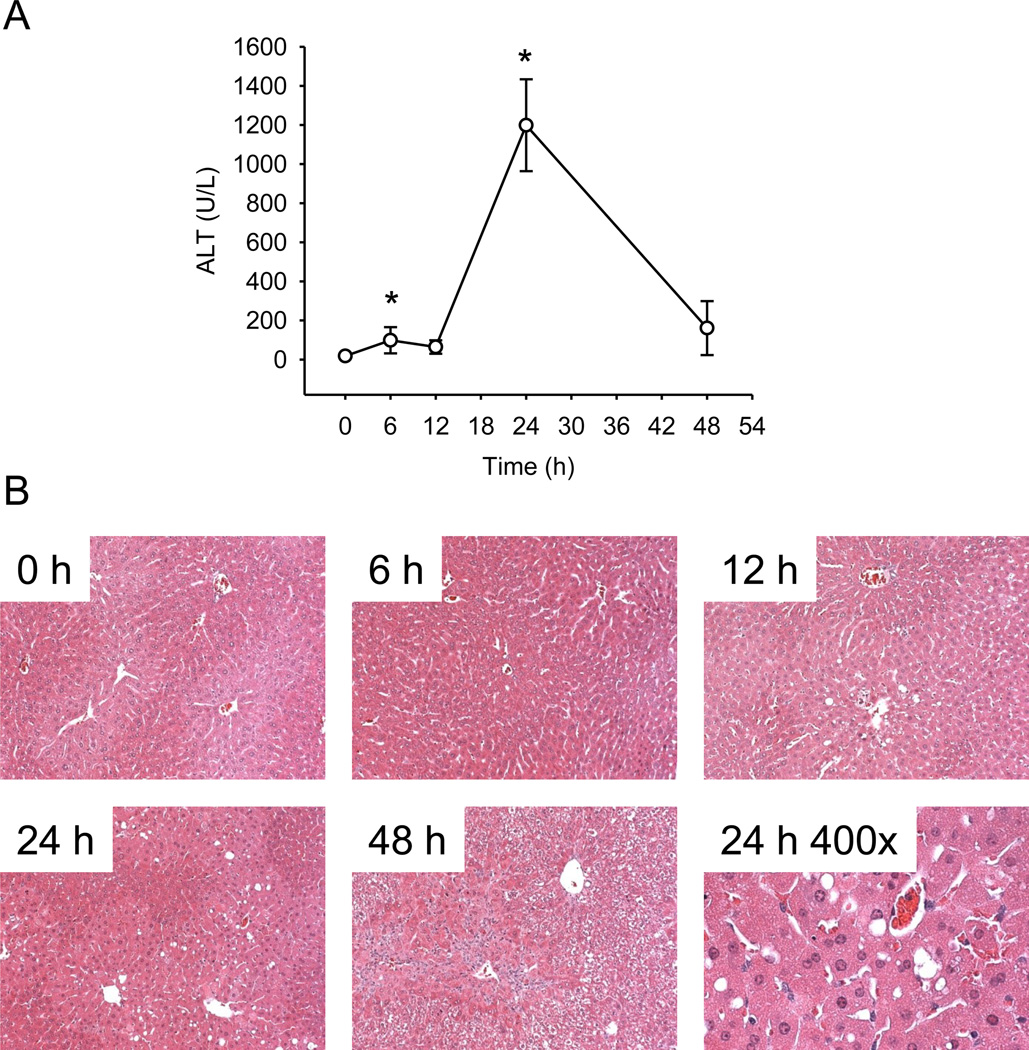
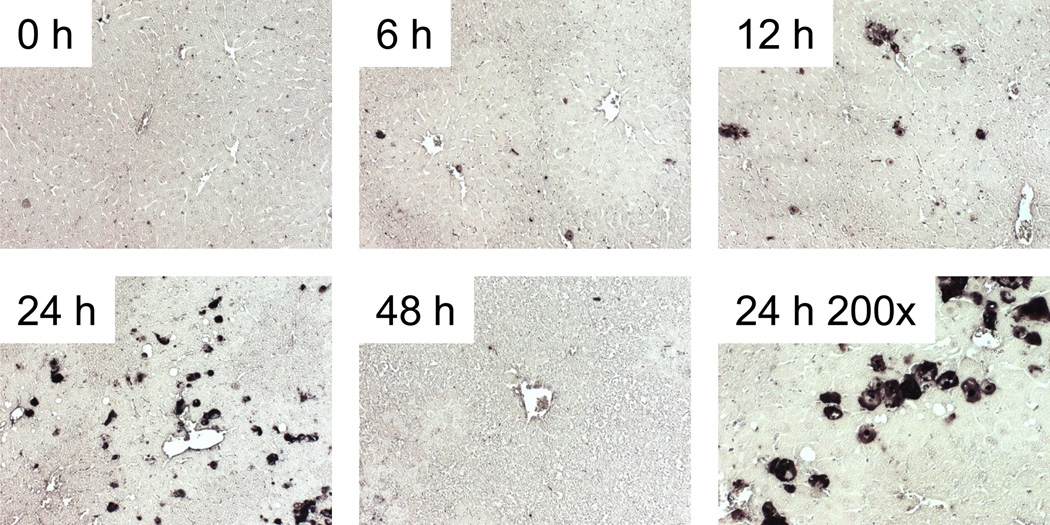
We first performed a time course study of FS hepatotoxicity. Mice were treated with 500 mg/kg FS and sacrificed at 0, 6, 12, 24 or 48 h. Plasma ALT activity was modestly increased by 6 h and remained approximately the same up to 12 h (Fig. 1A). The peak in ALT was observed at 24 h, and returned to near-control values by 48 h (Fig. 1A). Centrilobular necrosis was evident in H&E-stained liver sections, and the time course of necrosis mirrored ALT (Fig. 1B). Some TUNEL labeling of hepatocytes within the centribolular area was evident at 6 and 12 h, but increased considerably at 24 h (Fig. 2). Higher magnification images (200x) revealed that the staining was both nuclear and cytosolic, which is characteristic of cell necrosis (Cover et al., 2005).
Figure 1.
Time course of furosemide-induced liver injury in mice. Mice were treated with 500 mg/kg furosemide (FS) and sacrificed 0, 6, 12, 24 and 48 h later. (A) Plasma alanine aminotransferase (ALT). (B) H&E-stained liver sections. Data are expressed as mean ± SEM for n = 3–5. *p < 0.05 compared with 0 h.
Figure 2.
Time course of DNA damage in furosemide-induced liver injury in mice. Mice were treated with 500 mg/kg furosemide (FS) and sacrificed 0, 6, 12, 24 and 48 h later. TUNEL labeling was performed on paraffin-embedded tissue sections, as described under Materials and Methods. All images are 100x magnification, unless otherwise noted.
GSH and GSSG during FS hepatotoxicity
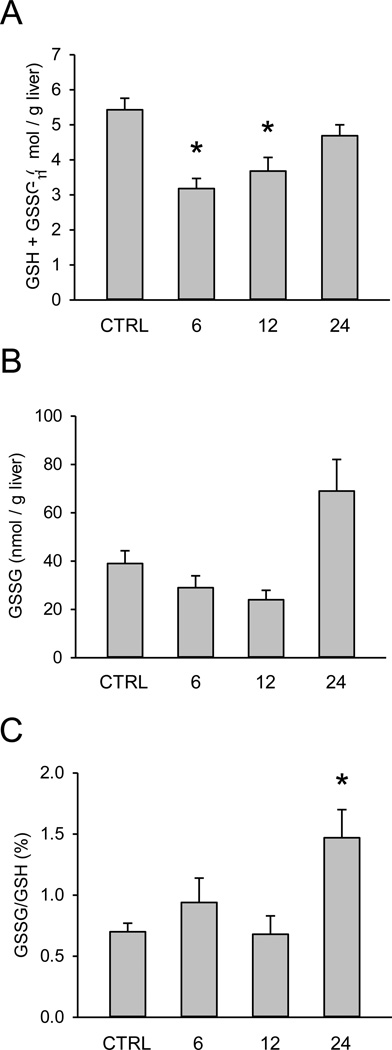
Conflicting data have been reported for liver GSH and GSSG during FS hepatotoxicity. To determine whether or not FS causes GSH depletion or oxidative stress, we measured GSH and GSSG levels in the livers of FS-treated mice. We observed depletion of total tissue GSH to about 60% of control values at 6 h (Fig. 3A). Despite this, there was no evidence of increased GSSG until 24 h (Fig. 3B,C), suggesting that oxidative stress may be a consequence of the liver injury but it is unlikely to be a cause.
Figure 3.
Time course of total and oxidized glutathione in furosemide-induced liver injury in mice. Mice were treated with 500 mg/kg furosemide (FS) and sacrificed 0, 6, 12, and 24 h later. (A) Total liver glutathione (GSH) levels. (B) Total liver oxidized GSH (GSSG) levels. (C) The percentage of total GSH in the form of GSSG. Data are expressed as mean ± SEM for n = 3–5. *p < 0.05 compared with control.
JNK activation and RIP3 induction in FS hepatotoxicity
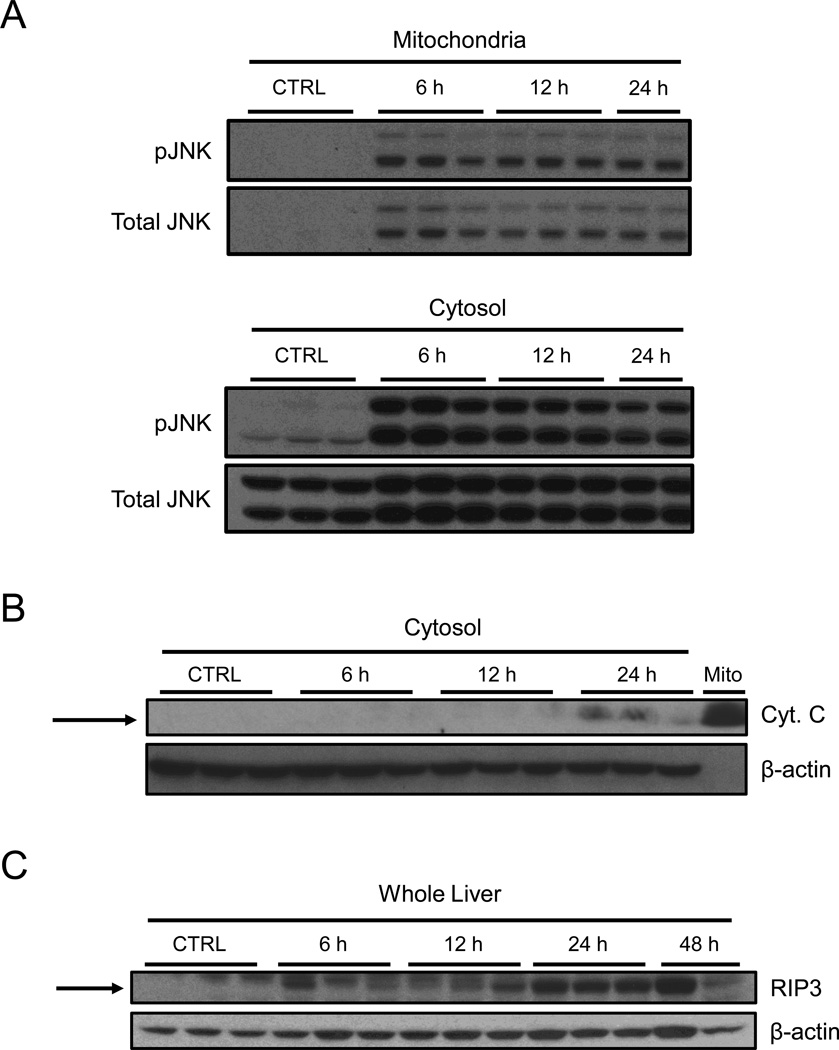
To explore the possibility that JNK plays a role in FS-induced liver injury, we performed western blotting for both phosphorylated JNK (pJNK) and total JNK in subcellular fractions from our time course study. We observed both activation of JNK and translocation into the mitochondrial fraction by 6 h, and this persisted until at least 24 h (Fig. 4A). We were surprised to find translocation of JNK into mitochondria, as FS hepatotoxicity is thought to not involve mitochondria (Wong et al., 2000; McGill et al., 2014). To explore this further, we performed immunoblotting for cytochrome c release in subcellular fractions. We could not detect any significant increase in cytochrome c levels in the cytosol until 24 h (Fig. 4B), suggesting that mitochondrial membrane integrity remains intact until the peak of liver injury. Because it is thought that RIP3 may play a role in JNK activation in the liver under pathophysiological conditions (Ramachandran et al., 2013; Gautheron et al., 2014), we also performed western blotting for RIP3 expression in total liver homogenate. Interestingly, RIP3 protein levels were increased by 6 h, and also persisted to at least 24 h (Fig. 4C).
Figure 4.
Activation and translocation of JNK and induction of RIP3 during furosemide-induced liver injury in mice. Mice were treated with 500 mg/kg furosemide (FS) and sacrificed 0, 6, 12, and 24 h later. (A) Total and phosphorylated JNK (pJNK) were measured by western blot in both mitochondrial and cytosolic fractions. (B) Cytochrome c was measured by western blot in the cytosol fractions from FS-treated mice. A control mitochondria sample is included as positive control. (C) RIP3 protein levels were measured by western blot in total liver homogenates.
Mechanistic significance of JNK and RIP3 in FS hepatotoxicity
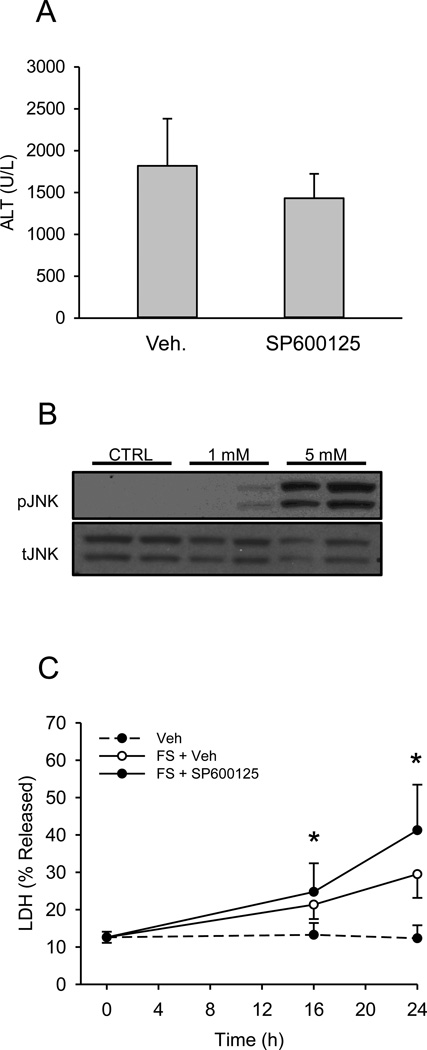
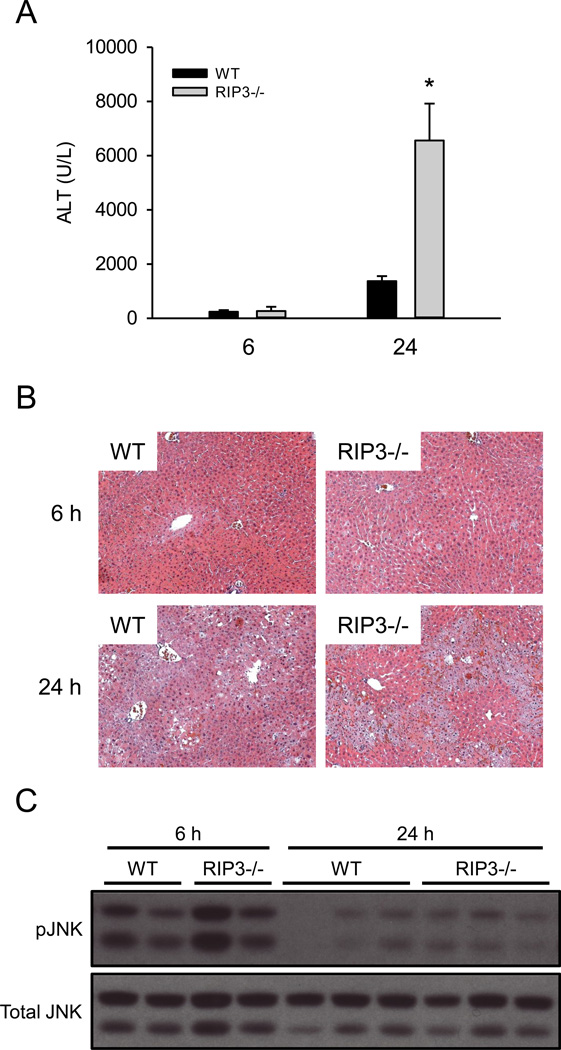
To determine whether or not JNK and RIP3 are important in FS-induced liver injury, we compared FS hepatotoxicity in WT mice and mice either co-treated with the JNK inhibitor SP600125 or that were RIP3-deficient. The dose of SP600125 chosen has repeatedly been shown to protect against APAP hepatotoxicity (Gunawan et al., 2006; Hanawa et al., 2008; Saito et al., 2010). Surprisingly, SP600125 co-treatment did not protect against FS in vivo (Fig. 5A). Importantly, this was not due to injury caused by the inhibitor itself masking protection, as treatment with SP600125 alone in preliminary experiments did not result in an increase in plasma ALT (McGill et al., unpublished data). Moreover, the JNK inhibitor is known to protect in other forms of liver injury, including APAP toxicity (Gunawan et al., 2006; Saito et al., 2012). Because SP600125 has a relatively short half-life in vivo, we also tried repeated dosing. Mice were given SP600125 or vehicle 1 h before FS and at 6 and 18 h post-FS treatment and plasma was collected at 24 h. However, although the overall ALT values were lower (possibly due to an effect of the vehicle), we still did not observe protection in the animals treated with the JNK inhibitor (ALT: 693±253 vs. 684±358 U/L for vehicle and SP600125 treatment, respectively). JNK activation was also observed in both primary mouse hepatocytes (Fig. 5B) and even at early time points in the human liver cell line HepaRG (Fig. 6) after FS treatment. However, the JNK inhibitor failed to protect primary mouse hepatocytes, despite the fact that JNK was also activated in these in vitro conditions (Fig. 5B,C). Finally, RIP3 KO mice were not protected against FS hepatotoxicity based on plasma ALT (Fig. 7A), and this was confirmed by histology (Fig. 7B). Importantly, except for the degree of necrosis, no remarkable features were observed in the tissue sections from the RIP3 KO mice compared with the WT. In fact, we observed a surprising increase in injury in these animals compared to WT mice at 24 h. To explore a possible interaction between RIP3 and JNK, we also performed western blotting for JNK in the RIP3 KO animals (Fig. 7B). However, we were unable to detect a significant difference.
Figure 5.
The JNK inhibitor SP600125 does not protect against furosemide toxicity. (A) Mice were treated with 500 mg/kg furosemide (FS) and co-treated with either vehicle or 10 m/kg SP600125 and sacrificed 24 h later. Plasma alanine aminotransferase (ALT) was measured. (B) Primary mouse hepatocytes were treated with PBS vehicle, 1 or 5 mM FS for 16 h and JNK phosphorylation was assessed by western blotting. (C) Primary mouse hepatocytes were treated with PBS vehicle or co-treated with 5 mM FS and 30 μM SP600125 or DMSO vehicle. Lactate dehydrogenase (LDH) release was measured at 0, 16 and 24 h. Data are expressed as mean ± SEM for n = 3–5. *p < 0.05 compared with control.
Figure 6.
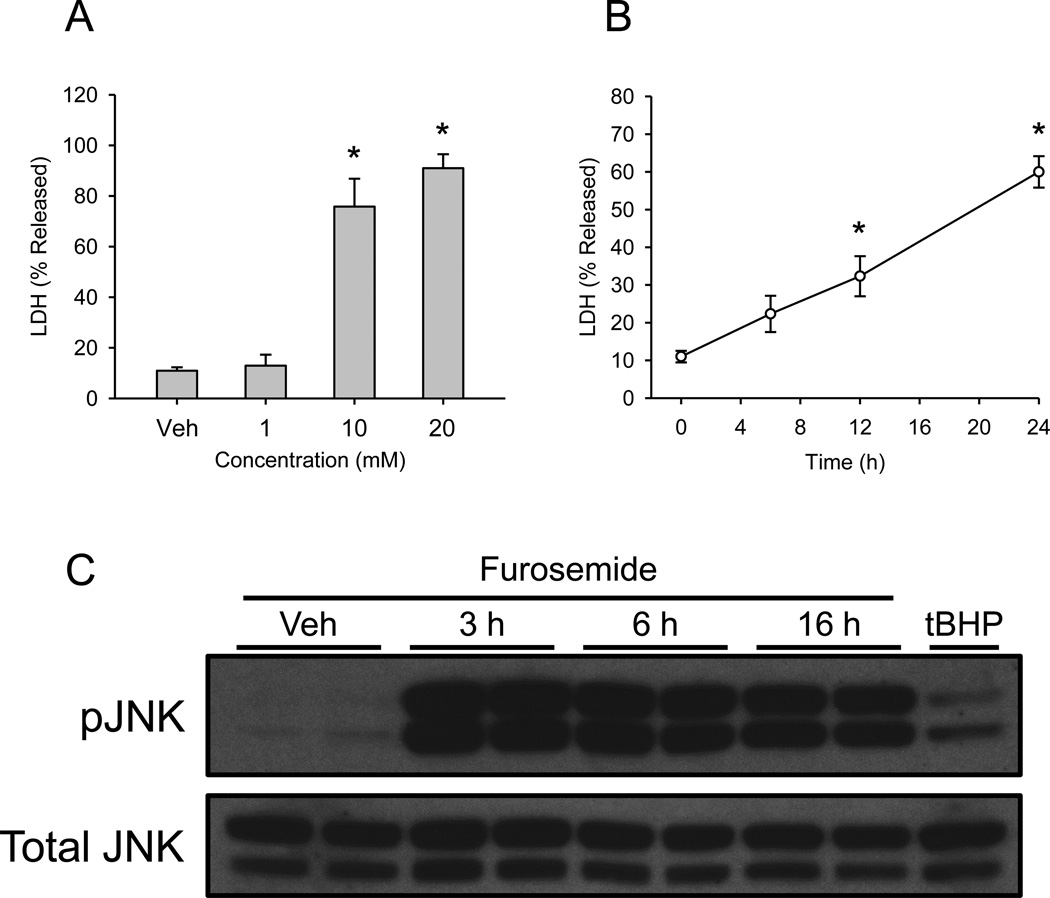
Furosemide toxicity and JNK activation in HepaRG cells. (A) Differentiated HepaRG cells were treated with increasing concentrations of furosemide (FS) or with PBS vehicle control. Lactate dehydrogenase (LDH) release was measured 24 h later. (B) HepaRG cells were treated with 10 mM FS and LDH release was measured at various time points. (C) HepaRG cells were treated with FS for various time points and JNK activation was measured by western blotting. HepaRG cells were also treated with 200 μM tert-butyl hydroperoxide (tBHP) for 2 h as a positive control. Data are expressed as mean ± SEM for n = 3 experiments. *p < 0.05 compared with control.
Figure 7.
RIP3 KO mice are not protected against furosemide toxicity. WT and RIP3 deficient mice were treated with 500 mg/kg furosemide (FS) and sacrificed 24 h later. Plasma alanine aminotransferase (ALT) was measured. (B) H&E-stained liver sections from WT and RIP3 deficient mice. (C) JNK activation was assessed by western blotting at 6 and 24 h. Data are expressed as mean ± SEM for n = 3–5. *p < 0.05 compared with WT.
DISCUSSION
FS-induced liver injury in mice was first reported 40 years ago (Mitchell et al., 1974). Since then, it has been shown that FS forms a reactive metabolite that binds to proteins, and that this is a critical first step for toxicity (Mitchell et al., 1974; 1976; Williams et al., 2007). However, despite the fact that FS hepatotoxicity is increasingly used as a model in research, little else is known about the mechanisms of FS-induced liver injury. Although it would seem plausible that the drug leads to hepatotoxicity through its diuretic effects by causing hepatic ischemia, Mitchell et al. (1974) reported that other diuretics did not cause liver injury in mice even at lethal doses. Moreover, cytochrome P450 inhibitors can prevent furosemide toxicity (Williams et al., 2007). Thus, it seems that the injury is due to a direct hepatocellular insult.
Despite the fact that FS forms a reactive intermediate, a number of studies have failed to find any depletion of GSH in the liver in vivo after FS treatment (Wong et al., 2000; Williams et al., 2007) and the drug does not appear to form a GSH conjugate in mice (Williams et al., 2007), although GSH depletion has consistently been reported in primary mouse hepatocytes treated with FS (Grewal et al., 1996; Williams et al., 2007). It is unclear why our data differ from previous in vivo studies with respect to hepatic GSH levels, but it may be due to strain differences as previous studies have relied on CD-1 mice. If so, data concerning FS metabolism from previous studies may need to be re-evaluated. It is also possible that the observed GSH depletion in our study was simply a result of liver injury and decreased GSH synthesis, rather than GSH scavenging of a reactive intermediate. In any case, because the starting concentration of GSH in hepatocytes is very high (approximately 10 mM), it seems unlikely that 40% GSH depletion would have a significant effect upon liver function or antioxidant defense. Moreover, the fact that GSSG levels did not increase until the peak of injury was achieved suggests that any oxidative stress that occurs is secondary to the injury and not a cause.
We were surprised to find that JNK translocates into mitochondria during FS-induced liver injury. In both APAP and galactosamine/endotoxin hepatotoxicity, mitochondrial JNK is thought to enhance mitochondrial damage (Hanawa et al., 2008; Win et al., 2011; Jaeschke et al., 2012). Yet,Wong et al. (2000) reported that mitochondria are unaffected by a hepatotoxic dose of furosemide before any rise in ALT occurs. Although they stopped measuring mitochondrial function at 5 h, both our previous data showing that FS has no effect on serum acylcarnitine levels as late as 24 h (McGill et al., 2014) and our data from the current study demonstrating that cytochrome c release does not occur until 24 h support the idea that mitochondrial dysfunction is not a major contributor to FS hepatotoxicity. Furthermore, the JNK inhibitor SP600125 did not protect against FS in this study, suggesting that the mitochondrial JNK does not cause the injury. Also, mice deficient in RIP3 were not protected against FS, despite the fact that RIP3 expression was induced. This is in contrast to previous reports that RIP3 may activate JNK in the liver (Ramachandran et al., 2013; Gautheron et al., 2014), although this may be due to differences in the mechanisms of injury in the different models. Surprisingly, the RIP3 KO mice had higher plasma levels of ALT. The reason for the latter is unclear. We considered the possibility that FS could induce programmed necrosis and deleting RIP3 resulted in a shift from oncotic necrosis to apoptosis with secondary necrosis. However, we were unable to detect any difference in caspase 3 cleavage or cytochrome c release in WT or RIP3 mice after FS treatment, and we were unable to find apoptotic cells in liver sections from either phenotype (McGill et al., unpublished).
Overall, our data are interesting because activation of JNK and translocation into mitochondria are generally accepted as critical events in other forms of acute liver injury, such as APAP toxicity (Gunawan et al., 2006; Saito et al., 2010; Jaeschke et al., 2012) and hepatic ischemia-reperfusion injury (Theruvath et al., 2008). Because it doesn’t seem to be important for the injury in FS hepatotoxicity, the reason for JNK translocation into mitochondria is unclear. It is possible that this is a more common feature of drug-induced liver injury than previously thought, and that something is simply preventing a negative effect of JNK on mitochondria after FS treatment. Additional work is needed to understand this result.
It is commonly thought that FS does not cause liver injury in humans (Williams et al., 2007). However, this is probably because of the low doses of FS that are usually prescribed (Mitchell et al., 1974; Williams et al., 2007) and because FS is not a recreational drug and therefore has low potential for abuse. Interestingly, we found that FS is in fact toxic in the human liver cell line HepaRG. These cells are unique among hepatoma lines because they express a full complement of drug metabolizing enzymes. Although the concentrations required to achieve toxicity were higher with HepaRG cells than with mouse hepatocytes, preliminary data from our lab demonstrate that FS is also toxic to primary human hepatocytes at the same lower concentrations as mouse cells (data not shown). Interestingly, in contrast to APAP (Xie et al., 2014), both FS and our positive control tert-butyl hydroperoxide caused JNK activation in the HepaRG cells. These data demonstrate that JNK activation is possible in this cell line, despite the fact that it doesn’t happen after APAP treatment.
Conclusions
Our data show that both JNK activation and RIP3 expression are induced in hepatocytes by a hepatotoxic dose of FS. However, inhibition or knockout of these proteins does not protect against the injury. Furthermore, we have provided some additional data that support the idea that mitochondrial damage does not play a role in FS hepatotoxicity in mice. The fact that JNK activation and translocation occur in this model, but do not seem to cause the injury may have implications for the significance of JNK in other forms of liver injury.
Acknowledgments
DECLARATION OF INTERESTS
This work was supported in part by National Institutes of Health Grants AA12916 and DK070195 (to H.J.), AA020518 (to W.D.) and by grants from the National Center for Research Resources (5P20RR021940-07) and the National Institute of General Medical Sciences (8 P20 GM103549-07) from the National Institutes of Health. Additional support was provided to M.R.M. by the “Training Program in Environmental Toxicology” (T32 ES007079-26A2) from the National Institute of Environmental Health Sciences.
References
- Bajt ML, Knight TR, Lemasters JJ, Jaeschke H. Acetaminophen-induced oxidant stress and cell injury in cultured mouse hepatocytes: protection by N-acetyl cysteine. Toxicol Sci. 2004;80:343–349. doi: 10.1093/toxsci/kfh151. [DOI] [PubMed] [Google Scholar]
- Cover C, Mansouri A, Knight TR, Bajt ML, Lemasters JJ, Pessayre D, Jaeschke H. Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J Pharmacol Exp Ther. 2005;315:879–887. doi: 10.1124/jpet.105.088898. [DOI] [PubMed] [Google Scholar]
- Du K, Williams CD, McGill MR, Xie Y, Farhood A, Vinken M, Jaeschke H. The gap junction inhibitor 2-aminoethoxy-diphenyl-borate protects against acetaminophen hepatotoxicity by inhibiting cytochrome P450 enzymes and c-Jun N-terminal kinase activation. Toxicol Appl Pharmacol. 2013;273:484–491. doi: 10.1016/j.taap.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautheron J, Vucur M, Reisinger F, Cardenas DV, Roderburg C, Koppe C, Kreggenwinkel K, Schneider AT, Bartneck M, Neumann UP, Canbay A, Reeves HL, Luedde M, Tacke F, Trautwein C, Heikenwalder M, Luedde T. A positive feedback loop between RIP3 and JNK controls non-alcoholic steatohepatitis. EMBO Mol Med. 2014;6:1062–1074. doi: 10.15252/emmm.201403856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal KK, Rafeiro E, Racz WJ. Bromobenzene and furosemide hepatotoxicity: alterations in glutathione, protein thiols, and calcium. Can J Physiol Pharmacol. 1996;74:257–264. [PubMed] [Google Scholar]
- Gunawan BK, Liu ZX, Han D, Hanawa N, Gaarde WA, Kaplowitz N. c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology. 2006;131:165–178. doi: 10.1053/j.gastro.2006.03.045. [DOI] [PubMed] [Google Scholar]
- Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem. 2008;283:13565–13577. doi: 10.1074/jbc.M708916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, McGill MR, Ramachandran A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: lessons learned from acetaminophen hepatotoxicity. Drug Metab Rev. 2012;44:88–106. doi: 10.3109/03602532.2011.602688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, Mitchell JR. Use of isolated perfused organs in hypoxia and ischemia/reperfusion oxidant stress. Methods Enzymol. 1990;186:752–759. doi: 10.1016/0076-6879(90)86175-u. [DOI] [PubMed] [Google Scholar]
- Latchoumycandane C, Goh CW, Ong MM, Boelsterli UA. Mitochondrial protection by the JNK inhibitor leflunomide rescues mice from acetaminophen-induced liver injury. Hepatology. 2007;45:412–421. doi: 10.1002/hep.21475. [DOI] [PubMed] [Google Scholar]
- McGill MR, Li F, Sharpe MR, Williams CD, Curry SC, Ma X, Jaeschke H. Circulating acylcarnitines as biomarkers of mitochondrial dysfunction after acetaminophen overdose in mice and humans. Arch Toxicol. 2014;88:391–401. doi: 10.1007/s00204-013-1118-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Sharpe MR, Williams CD, Taha M, Curry SC, Jaeschke H. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J Clin Invest. 2012;122:1574–1583. doi: 10.1172/JCI59755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Yan HM, Ramachandran A, Murray GJ, Rollins DE, Jaeschke H. HepaRG cells: a human model to study mechanisms of acetaminophen hepatotoxicity. Hepatology. 2011;53:974–982. doi: 10.1002/hep.24132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JR, Nelson WL, Potter WZ, Sasame HA, Jollow DJ. Metabolic activation of furosemide to a chemically reactive, hepatotoxic metabolite. J Pharmacol Exp Ther. 1976;199:41–52. [PubMed] [Google Scholar]
- Mitchell JR, Potter WZ, Hinson JA, Jollow DJ. Hepatic necrosis caused by furosemide. Nature. 1974;251:508–511. doi: 10.1038/251508a0. [DOI] [PubMed] [Google Scholar]
- Qu Q, Liu J, Zhou HH, Klaassen CD. Nrf2 protects against furosemide-induced hepatotoxicity. Toxicology. 2014;324C:35–42. doi: 10.1016/j.tox.2014.02.008. [DOI] [PubMed] [Google Scholar]
- Ramachandran A, McGill MR, Xie Y, Ni HM, Ding WX, Jaeschke H. Receptor interacting protein kinase 3 is a critical early mediator of acetaminophen-induced hepatocyte necrosis in mice. Hepatology. 2013;58:2099–2108. doi: 10.1002/hep.26547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randle LE, Goldring CE, Benson CA, Metcalfe PN, Kitteringham NR, Park BK, Williams DP. Investigation of the effect of a panel of model hepatotoxins on the Nrf2-Keap1 defense response pathway in CD-1 mice. Toxicology. 2008;43:249–260. doi: 10.1016/j.tox.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Rogers LK, Valentine CJ, Szczpyka M, Smith CV. Effects of hepatotoxic doses of acetaminophen and furosemide on tissue concentrations of CoASH and CoASSG in vivo. Chem Res Toxicol. 2000;13:873–882. doi: 10.1021/tx0000926. [DOI] [PubMed] [Google Scholar]
- Saito C, Lemasters JJ, Jaeschke H. c-Jun N-terminal kinase modulates oxidant stress and peroxynitrite formation independent of inducible nitric oxide synthase in acetaminophen hepatotoxicity. Toxicol Appl Pharmacol. 2010;46:8–17. doi: 10.1016/j.taap.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theruvath TP, Snoddy MC, Zhong Z, Lemasters JJ. Mitochondrial permeability transition in liver ischemia and reperfusion: role of c-Jun N-terminal kinase 2. Transplantation. 2008;85:1500–1504. doi: 10.1097/TP.0b013e31816fefb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vree TB, van der Ven AJ. Clinical consequences of the biphasic elimination kinetics for the diuretic effect of furosemide and its acyl glucuronide in humans. J Pharm Pharmacol. 1999;51:239–248. doi: 10.1211/0022357991772402. [DOI] [PubMed] [Google Scholar]
- Williams DP, Antoine DJ, Butler PJ, Jones R, Randle L, Payne A, Howard M, Gardner I, Blagg J, Park BK. The metabolism and toxicity of furosemide in the Wistar rat and CD-1 mouse: a chemical and biochemical definition of the toxicophore. J Pharmacol Exp Ther. 2007;322:1208–1220. doi: 10.1124/jpet.107.125302. [DOI] [PubMed] [Google Scholar]
- Wang Y, Singh R, Lefkowitch JH, Rigoli RM, Czaja MJ. Tumor necrosis factor-induced toxic liver injury results from JNK2-dependent activation of caspase-8 and the mitochondrial death pathway. J Biol Chem. 2006;281:15258–15267. doi: 10.1074/jbc.M512953200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Win S, Than TA, Han D, Petrovic LM, Kaplowitz N. c-Jun N-terminal kinase (JNK)-dependent acute liver injury from acetaminophen or tumor necrosis factor (TNF) requires mitochondrial Sab protein expression in mice. J Biol Chem. 2011;286:35071–35078. doi: 10.1074/jbc.M111.276089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SG, Card JW, Racz WJ. The role of mitochondrial injury in bromobenzene and furosemide induced hepatotoxicity. Toxicol Lett. 2000;116:171–181. doi: 10.1016/s0378-4274(00)00218-6. [DOI] [PubMed] [Google Scholar]
- Xie Y, McGill MR, Dorko K, Kumer SC, Schmitt TM, Forster J, Jaeschke H. Mechanisms of acetaminophen-induced cell death in primary human hepatocytes. Toxicol Appl Pharmacol. 2014 doi: 10.1016/j.taap.2014.05.010. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Williams CD, McGill MR, Lebofsky M, Ramachandran A, Jaeschke H. Purinergic receptor antagonist A438079 protects against acetaminophen-induced liver injury by inhibiting p450 isoenzymes, not by inflammasome activation. Toxicol Sci. 2013;131:325–335. doi: 10.1093/toxsci/kfs283. [DOI] [PMC free article] [PubMed] [Google Scholar]