Abstract
Cognitive impairment is known to be a core deficit in schizophrenia. Existing treatments for schizophrenia have limited efficacy against cognitive impairment. The ubiquitous use of nicotine in this population is thought to reflect an attempt by patients to self-medicate certain symptoms associated with the illness. Concurrently there is evidence that nicotinic receptors that have lower affinity for nicotine are more important in cognition. Therefore, a number of medications that target nicotinic acetylcholine receptors (nAChRs) have been tested or are in development. In this article we summarize the clinical evidence of nAChRs dysfunction in schizophrenia and review clinical studies testing either nicotine or nicotinic medications for the treatment of cognitive impairment in schizophrenia. Some evidence suggests beneficial effects of nAChRs based treatments for the attentional deficits associated with schizophrenia. Standardized cognitive test batteries have failed to capture consistent improvements from drugs acting at nAChRs. However, more proximal measures of brain function, such as ERPs relevant to information processing impairments in schizophrenia, have shown some benefit. Further work is necessary to conclude that nAChRs based treatments are of clinical utility in the treatment of cognitive deficits of schizophrenia.
Keywords: nAChR, nicotine, schizophrenia, cognition
INTRODUCTION
Cognitive deficits, a core feature of schizophrenia [1], include impairments in memory, attention, executive functioning vocabulary, visuospatial skills and old learning [2]. Cognitive deficits predate the onset of psychosis, become more pronounced in the prodrome and early years of diagnosis, and tend to plateau thereafter [3]. These deficits are also seen among first-degree relatives of probands with schizophrenia and tend to be stable despite improvements in positive and negative symptoms [4]. Most patients with schizophrenia have cognitive deficits [5] that range from moderate to severe [2], and are strongly correlated with functional outcome [6]. In fact, the degree of cognitive deficits is a better predictor of disability and vocational functioning than positive symptoms [7]. Existing antipsychotic drugs, all of which block dopamine 2 receptors (D2), continue to be the mainstay of treatment [8]. D2 receptor antagonists have limited efficacy for negative symptoms and cognitive deficits [9, 10]. There is a need to develop new treatments for schizophrenia including treatments for the cognitive impairments associated with the disorder. Currently strategies that are being developed to target cognitive deficits broadly consist of pharmacological agents and cognitive retraining. The putative pharmacological cognitive enhancers being tested in schizophrenia include drugs with diverse mechanisms of action such as the glutamatergic, dopaminergic and nicotinic systems [11-15]. The focus of this review is the clinical evidence on drugs acting at nicotinic acetylcholine receptors (nAChRs) as cognition enhancers in schizophrenia.
SMOKING AND SCHIZOPHRENIA
One of the most robust lines of evidence supporting the potential use of nAChRs agonists as cognition enhancers in schizophrenia is based on the observation of high rates of tobacco use in schizophrenia. Individuals with schizophrenia have the highest rates of cigarette smoking (58–88%) compared to the general population (~ 23%) [16-19]. The association between schizophrenia and tobacco smoking is stronger even after controlling for confounds such as institutionalization, socioeconomic status and medications [19-21]. Smokers with schizophrenia smoke more cigarettes and favor stronger cigarettes suggesting that they are more dependent on nicotine than other smokers reviewed in [19]. The ubiquitous use of nicotine in people with schizophrenia has led to the hypothesis that cigarette smoking is a form of self-medication to improve cognitive function, reduce primary and/or secondary negative symptoms, enhance antipsychotic response, and ameliorate treatment of side effects from antipsychotics [22]. Nicotine, one of the addictive and reinforcing ingredients in tobacco-smoke, produces its effects via agonist effects at nAChRs.
NICOTINIC RECEPTORS
The nAChRs in the central nervous system are pentameric ligand-gated ion channels that transverse the lipid bilayer to allow influx of cations. The proteins are either heteromeric subunit combinations of α2-α6 and β2-β4 or homomeric subunits. The most common configurations are the heteromeric α4β2 and the homomeric α7 [23]. The α4β2 receptor has higher affinity for nicotine compared to the α7 receptor but a much lower permeability to calcium [24, 25]. The α4β2 has high concentrations in the cortex and thalamus, while concentrations of α7 are higher in the cortex and hippocampus [26, 27]. The α4β2 receptor has about three times higher affinity for acetylcholine compared to α7 [25], but choline acts as a full agonist at the α7 receptor without effect at the α4β2 [28]. The α4β2 receptor also has different pharmacodynamic properties than most receptors. In the presence of an agonist such as nicotine the α4β2 concentrations increase or undergo upregulation [29, 30]. Nicotine also causes rapid desensitization of the receptor or tachyphylaxis [31]. Based on the current evidence the α4β2 nAChR is thought to regulate the rewarding properties of nicotine [32, 33], while the α7 nAChR is thought to regulate cognitive and sensory gating phenomenon [34, 35]. For a full review of nAChRs see Albuquerue et al (2009) and Jenson et al (2005) [28, 36].
NICOTINIC RECEPTOR ABNORMALITIES IN SCHIZOPHRENIA
Several lines of evidence have shown nAChRs abnormalities in people with schizophrenia. The postmortem receptor changes, electrophysiological evidence of altered functioning, and neuro-imaging evidence of abnormalities in schizophrenia will now be reviewed.
Postmortem
There are a number of reports of nAChRs analysis in postmortem studies of people with schizophrenia [37-50] (See Table 1). The methods employed include using a radioactive ligand with affinity to a subunit of the nAChRs to measure the availability of receptors [37-43, 45, 46, 48, 50] or measuring the nAChRs’ messenger RNA or proteins to determine the overall concentration of nAChRs both extracellularly and intracellularly [44, 46-49, 51]. All studies tried to control for demographic variables and smoking status. The results of these studies are mixed but support decreased availability of both the high affinity α4β2 and low affinity α7 nAChRs in schizophrenia. mRNA and protein studies have focused on the low affinity α7 nAChR in the dorsal lateral prefrontal cortex (DLPFC) and hippocampus. None of the studies have shown decrease in expression in the DLPFC. However, the hippocampus does not seem to express α7 nAChRs in people with schizophrenia to the same degree as typical individuals, at least in non-smokers [44, 49].
Table 1. Postmortem finding in people with schizophrenia versus healthy controls among nicotinic receptors either from ligand binding studies or messenger RNA/proteins.
| Reference | α7 Binding Ligand | α7 mRNA or Proteins | α4β2 Binding Ligand | α4β2 mRNA or Proteins |
|---|---|---|---|---|
| Freedman et al 1995 | ↓ Hipp | ↓ Hipp | ||
| Leonard et al 1998 | ↓ Hipp | ↓ Hipp* | ||
| Court et al 1999 | ↓ Thalamus | ND in many areas | ||
| Guan et al 1999 | ↓PFC, ND PC | |||
| Breese et al 2000 ** | ↓ Hipp, Thalamus, Cau | |||
| Court et al 2000 | ↑ Striatum | |||
| Durany et al 2000 | ↓ Striatum | |||
| Leonard et al 2001 | ↓ Hipp | |||
| Martutle et al 2001 | ↓ CC | ↑ CC and OFC | ||
| Martin-Ruiz et al 2003 | ND DLPFC | ↑ DLPFC | ND DLPFC | |
| De Luca et al 2006 | ND DLPFC | |||
| Mathew et al 2007 | ND DLPFC, Hipp | ND DLPFC and Hipp | ||
| Mexal et al 2010 | ↓ Hipp*** | |||
| Thomsen et al 2011 | ND Hipp |
Cau = Caudate, CC = cingulate cortex, DLPFC = dorsal lateral prefrontal cortex, Hipp = hippocampus, OFC = orbital frontal cortex, PC = parietal cortex, PFC = Prefrontal cortex, →= decrease, ↓= increase ND = no difference
did not increase with nicotine use as seen in normal controls
all comparisons smoking controls versus schizophrenia smokers
Only in non-smokers with schizophrenia
In vivo evidence
Recent nuclear imaging techniques have permitted in vivo measurement of human nAChRs. Two recent studies determined availability of β2-containing nAChRs in smokers with schizophrenia and controls [52, 53]. D’Souza et al. [52] scanned twelve smokers with schizophrenia and matched controls after a week of abstinence from smoking with a ligand that has high affinity for the β2-containing nAChR. Compared to controls, people with schizophrenia displayed a 21-26% decrease in available receptors in the frontal cortex, parietal cortex and thalamus (in descending order). To our knowledge there are no in vivo neuroreceptor imaging studies of α7 nAChRs in schizophrenia.
Besides the receptors themselves, magnetic resonance spectroscopy (MRS) has been used to measure the endogenous α7 nAChR ligand, choline. A recent meta-analysis showed no deficiency for choline in any region of the brain of people with schizophrenia [54], suggesting an alteration in the nAChR system and not the endogenous ligand.
COGNITIVE IMPAIRMENTS IN SCHIZOPHRENIA
Neuropsychiatric Testing
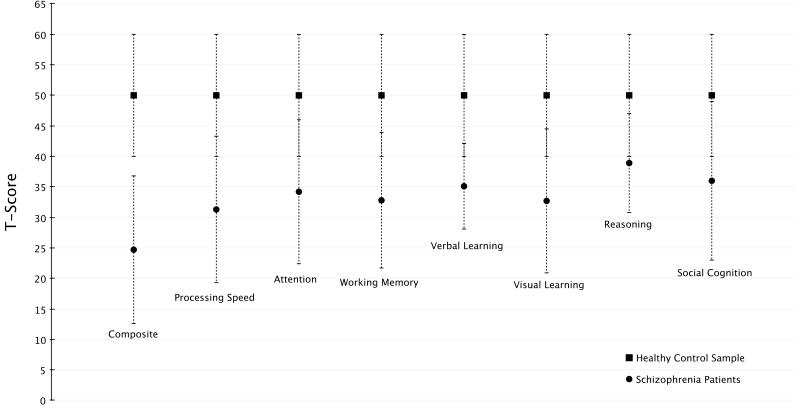
The cognitive deficits in schizophrenia are broad, present in almost every domain, and most likely represent a core symptom of the illness [55]. A recent consortium of experts defined the areas of cognition that are of most important in schizophrenia [56, 57]. The domains include processing speed, attention/vigilance, working memory, verbal learning, visual learning, reasoning and problem solving, and social cognition. Processing speed is a nonspecific area that relates to the ability to rapidly process information, which is important in many routine activities of daily living and is implicated as the cognitive deficit with the largest effect size [58, 59]. Attention/vigilance includes the ability of people to focus on a task over time. Recent evidence suggests people with schizophrenia have the most difficulty attending to stimuli when broad monitoring is required rather than focused attention [60]. Working memory is the ability to keep relevant information active for short periods of time, such as remembering a phone number to dial and forgetting it after dialing, and has been suggested to be a core deficit in schizophrenia [61]. While learning deficits are common in schizophrenia, analysis of cognitive batteries have found either verbal or visual episodic memory rather than both in almost 50% of patients [62], therefore requiring separate domains for analysis. Reasoning and problem solving is impaired in people with schizophrenia but testing suggests this area of cognition might be persevered more than other areas compared to normal controls [63]. Social cognition represents many of the skills required to successfully interact with individuals. Specific domains of social cognition correlate with functional outcomes in people with schizophrenia [64]. In Fig. 1 show the relative differences better a sample of normal controls and people with schizophrenia in these domains.
Fig. (1).
Comparison of cognitive scoring on the MATRICS consensus cognitive battery in schizophrenia patients (n=323) and a sample of healthy controls (n=300). Data shown as mean T-score ± SD from reference 57.
A variety of neuropsychiatric tests are used to measure baseline and changes in cognitive function. Subsequently several cognitive batteries have been developed to test several domains including the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) [65], the Brief Assessment of Cognition in Schizophrenia (BACS) [66, 67] and the Measurement and Treatment Research to Improve Cognition in Schizophrenia Consensus Cognitive Battery (MATRICS) [68]. The MATRICS was initiated to develop a consensus cognitive battery for developing treatments for schizophrenia [57, 68-71]. Computerized cognitive test batteries have received recent attention for both research and clinical application [72] and for use in clinical trials of schizophrenia [73]. The major benefits of computerized tests are automation, efficiency, standardization and additional test measures [74]. The MATRICS and BACS [75, 76] use a combination of paper-pencil and computerized neuropsychological tests whereas the IntegNeuro [77], CogState Schizophrenia battery [78] and the Cambridge Neuropsychological Test Automated Battery (CANTAB) [79] are completely computerized.
INFORMATION PROCESSING DEFICITS (ERPs)
In addition to the deficits in behavioral measures of cognition (attention, memory, executive functions, etc.), patients with schizophrenia also exhibit information processing deficits as indexed by eventrelated potentials (ERPs). An ERP is a stereotyped event-locked deflection (positive or negative) of the scalp potential that is characterized by the amplitude, duration, and after-stimulus latency of its peak [80]. ERPs are a manifestation of the neural activity underlying certain cognitive processes, which makes them an excellent tool for detecting and characterizing anomalies affecting these processes [80].
P50 Gating
Sensory gating refers to the ability of the brain to modulate its sensitivity to incoming sensory information. This automatic function is thought to reflect an inhibitory process that protects against overwhelming higher cortical centers with irrelevant information [81]. Sensory gating can be measured using a paired click paradigm, in which the first auditory stimulus in a pair (conditioning or S1) is followed by an identical stimulus (test or S2) presented in close proximity (500 ms) to S1 [82]. A positive voltage deflection, termed the P50, is evoked approximately fifty milliseconds after the auditory stimuli [82]. When sensory gating is intact, the amplitude of the P50 waveform following the S2 stimulus is diminished relative to the P50 following the S1 stimulus [82].
Meta-analyses support reduced P50 gating in individuals with schizophrenia, despite heterogeneity among studies [51, 83]. This gating deficit has been documented in prodromal individuals and medication-naive recent onset psychosis patients [84].
Mismatch Negativity (MMN)
The MMN is a negatively deflecting ERP component that peaks with a latency of 150-250 milliseconds. It is elicited automatically, irrespective of the direction of the subject’s attention, by an infrequent deviant (in pitch, intensity, duration, location, or pattern) auditory stimulus presented randomly in a stream of repetitive standard auditory stimuli [85-87]. It is thought to reflect auditory sensory (echoic) memory because the detection of deviance requires a representation of what has been “standard” in the auditory stream [86].
MMN abnormalities have been consistently reported in schizophrenia [88]. This finding is unlikely a treatment effect since neither typical nor atypical antipsychotics affect the amplitude or latency of this ERP [89-92]. Furthermore, it seems to be specific to schizophrenia since this is one of the few psychiatric disorders that have been consistently associated with specific MMN abnormalities [93, 94]. Most studies show that MMN abnormalities consist of an amplitude reduction to duration and frequency deviants [88, 89, 95-103]. Nevertheless, some evidence suggests that the deviants associated to an anomalous MMN change over the course of the illness. Todd et al. [104] showed that schizophrenia patients early in the course of the illness had an amplitude reduction to duration and intensity deviants but not to frequency deviants. However, patients later in the course of the illness showed a reduction to frequency and duration deviants but not to the intensity ones.
P300
The P300 is a positive ERP component arising approximately 300 milliseconds after the presentation of an infrequent (oddball) stimulus during a target detection task [reviewed in 105, 106]. Two main subcomponents of the P300 (P3a and P3b) have been distinguished, which can be evoked separately by specific stimulus and task conditions [107]. P3b is elicited by infrequent task-relevant target stimuli and is believed to reflect the process of context updating and the allocation of attentional resources, and is modulated by “top-down” cognitive processes [reviewed in 105]. The P3a is elicited by infrequent task-irrelevant stimuli. It is believed to reflect “bottom-up” processes involved in the automatic orienting of attention to novel or salient stimuli [reviewed in 105].
Reductions in P300 amplitude and increased latencies have been observed in a number of neuropsychiatric disorders including schizophrenia [reviewed in 106, 108]. Deficits in P300 amplitude are one of the most replicated psychophysiological findings in schizophrenia with effect sizes above 0.8 [105].
ACUTE EFFECTS OF DRUGS ACTING AT nAChRs AND TOBACCO SMOKING IN SCHIZOPHRENIA
Since the 1970’s research has suggested a pro-cognitive effect of nicotine, although due to methodological issues the literature primarily indicated that nicotine reverses the cognitive impairing aspects of withdrawal [109]. Since the 1990’s many more studies controlling for withdrawal and other possible confounds of nicotine administration have been conducted. A meta-analysis in otherwise healthy individuals suggested procognitive effects of nicotine in several domains of attention, response time to cues, fine motor abilities, short term memory and working memory [110]. However, this study did not review the effects of nicotine in schizophrenia and since there is evidence of an altered nicotinic system in schizophrenia, studies in healthy controls cannot be extrapolated to people with schizophrenia. Given the role of the α7 nAChR in regulating cognitive and sensory gating phenomenon [34, 35], drug development has preferentially focused on this receptor [111].
CLINICAL TRIALS WITH NICOTINE FOR COGNITIVE ENHANCEMENT IN SCHIZOPHRENIA
Many clinical trials have been conducted to study the effects of nicotine and other nAChRs agonists on cognition in both healthy volunteers [110] and people with schizophrenia [112]. However, the degree of nicotine dependence, the state of nicotine satiety, the state of nicotine withdrawal, and method of administration have differed drastically across studies. Nicotine administration studies to enhance cognition in schizophrenia have used gum [113-116], transdermal patch [117-125], nasal and orally inhaled [126-131], and subcutaneous nicotine [132]. In Table 2 we review the studies in which nicotine is given to people with schizophrenia and cognitive outcomes are tested pre and post-delivery to measure effects.
Table 2. Nicotine administration studies in people with schizophrenia that measure cognition.
| Nicotine Ad- ministration |
Dose (mg) or Administration |
Duration | Design | Smokers | Antipsychotics | Enrolled | Cognitive Battery |
Results of Nicotine (N) Compared to Placebo (P) or Con- trol (C) Condition |
|
|---|---|---|---|---|---|---|---|---|---|
| Levin 1996 | Patch | 7, 14, 21 | 4 days | Placebo Crossover |
100% | Haloperidol (titrated to low, medium, and how doses) |
15 | ANAM (DMS, RT, SMT, SPRT), CPT |
SPRT: N > P, DMS: N > P, CPT: N > P (On all tasks interaction with nicotine dose and haloperidol dose) |
| Smith 2002 | Cigarettes/Nasal | 3-6 cigarettes / 50% 6 sprays (L) 50% 12 sprays (H) |
4 days | Placebo Crossover |
100% | 1st and 2nd Gen- eration |
31 | ANAM (DMS, RT, SMT, SPRT), RMT (5-items, DS, PW, SS), VF |
SPRT: Cigarettes, Nasal > P RT: H Nasal > P PW, SS: Nasal > P |
|
*Sherr 2002 |
Nasal | 2 sprays | 2 days | Crossover | 52% | 1st and 2nd Gen- eration |
29 | CPT | N = C |
|
*Depatie 2002 |
Patch | 14 | 2 days | Placebo Crossover |
100% | 1st and 2nd Generation | 15 | CPT | CPT: N > P |
|
*Myers 2004 |
Nasal | 6 sprays | 2 days | Crossover | 52% | 1st and 2nd Gen- eration |
29 | DMS, DRT | DRT: (Smokers) N > control |
|
*Jacobson 2004 |
Patch | 28 or 35 Weight based |
2 days | Placebo Crossover |
100% | 1st and 2nd Gen- eration |
13 | SAL, VWM | VWM: N > P |
| Harris 2004 |
Gum | 6 | 2 days | Placebo Crossover |
50% | 1st and 2nd Gen- eration |
20 | RBANS | Attention: (Non- Smokers) N > control |
| Smith 2005 | Nasal | 8 sprays | 4 days | Placebo Crossover |
100% | 1st and 2nd Gen- eration |
27 | ANAM (SPRT), CPT, DMT, RANDT MS |
CPT, DMT: N > P |
| *Barr 2007 | Patch | 14 | 2 days | Placebo Crossover |
0% | 1st and 2nd Gen- eration |
28 | CPT, GP, LNS, ST |
CPT, ST: N > P |
|
*Jubelt 2008 |
Patch | 14 | 2 days | Placebo Crossover |
0% | 1st and 2nd Gen- eration |
10 | EMP | EMP: N > P |
|
*Hong 2011 |
Patch | 21 or 35 based on cigarette use |
2 days | Placebo Crossover |
100% | 1st and 2nd Gen- eration |
20 | RVIP | RVIP: N > P |
| Hahn 2013 | Cigarettes/Patch | Ad libitum/14 | 3 days | Placebo Crossover |
100% | 1st and 2nd Gen- eration |
17 | SARAT, SDT |
SARAT: Patch > P SDT: Patch, Cigarettes > P |
ANAM: Neuropsychological Assessment Metrics, CPT: Continuous Performance Test, CRT: Complex Reaction Time, DMS: Delayed Matching to Sample, DMT: Dot Memory Test, DRT: Delayed Recognition, DS: Digit Span, EMP: Episodic Memory Paradigm, GP: Grooved Pegboard, LNS: Letter Number Sequence, MS: Memory Scale, N: Nicotine, P: Placebo, PW: Paired Words, RBANS: Repeatable Battery for the Assessment of Neuropsychological Status, RMT: Randt Memory test, RT: Reaction Time, RVIP: Rapid Visual Information Processing, SAL: Selective Attention Load, SARAT: Spatial Attentional Resource Allocation Task, SDT: Singleton Detection Task, SMT: Sternberg Memory Test, SPRT: Spatial Rotation Task, SS: Short Story, ST: Stroop Test, VSWM: Visuospatial Working Memory, VWM: Verbal Working Memory, WCST: Wisconsin Card Sorting Test, WSPT: Word Serial Position Test
Healthy controls enrolled but results only on schizophrenia cohort
One of the major confounds of these studies is the interaction of nicotine with antipsychotics. For example, nicotine’s cognitive effects have been reported to be modulated differentially by different doses of dopamine D2 antagonists [117]. Smoking status is another important confound as it is established that nicotine can improve/reverse cognitive impairments from nicotine withdrawal [133]. Also, nicotine increases the number of nAChRs and causes receptor desensitization. Finally, unlike long-term cognitive studies, acute administration studies only examine immediate cognitive effects and not long term.
Based on the available studies, nicotine may acutely improve attention. However, the short duration of these studies does not confirm any long-term benefits to attention. Also, the studies examined several cognitive tests with several outcomes but the vast majority failed to control for multiple comparisons. Based on the available evidence nicotine does not enhance general cognitive ability.
Event-related Potentials
P50 Gating
In a study of smokers with schizophrenia who were abstinent overnight, impaired baseline P50 gating transiently normalized upon resumption of smoking [84]. Smokers with schizophrenia, abstinent for at least 1 hour prior to P50 recording displayed gating impairments that were similar to the baseline deficits in nonsmokers with schizophrenia [134]. This gating deficit is also present in up to half of unaffected relatives [135, 136]. Nicotine (6 mg gum) administered to non-smoking unaffected relatives was found to transiently improve the gating deficit [135]. First and second generation antipsychotics did not normalize gating deficits in schizophrenia patients [51, 137, 138]. However, several studies have found that clozapine normalized gating in schizophrenia patients [137, 139, 140].
In the largest study examining P50 in non-psychiatric smokers and non-smokers, Brinkmayer et al. found that smokers who had consumed a cigarette within 1-3 hours of the P50 recording had impaired gating–relative to non-smokers [141]. Gating values for light smokers were intermediate between heavy smokers and nonsmokers [141]. Other studies have yielded conflicting results with some publications suggesting enhanced gating in smokers while others have found reduced gating [142-144]. Possible reasons for conflicting results are small sample sizes, variability in P50 technique and analysis, timing of recording in relation to last cigarette smoked and differences in smoking chronicity. In a recent study of healthy non-smokers stratified by baseline P50 gating efficiency, nicotine (6 mg gum) enhanced gating in baseline low P50 suppressors, did not affect gating in baseline medium suppressors, and reduced gating in those with high baseline efficiency [145]. Knott et al., [145] suggest that the effect of a drug on gating may differ as a function of this baseline gating efficiency in a manner similar to inverted-U dose-response relationships that have been used to characterize individual variability for other measures, such as nicotine’s effect on cognitive performance. Preclinical and clinical evidence implicate the α7 nAChR in auditory gating [146, 147] and furthermore, some α7 nAChR agonists other than nicotine have been shown to enhance sensory gating in schizophrenia (covered in following sections) [38, 148].
Mismatch Negativity (MMN)
Studies on the effect of smoking, nicotine and other nAChRs agonists and modulators in healthy subjects have shown a rather consistent amplitude increase and latency reduction of MMN in both smokers and nonsmokers [149-153]. Studies on the effects of nicotine or nAChRs modulators on MMN in schizophrenia have shown mixed results. Inami et al. [121] reported that, in contrast to non-smoking healthy controls, nicotine didn’t shorten MMN latencies in non-smoking schizophrenia patients. In turn, Dulude et al. [96] reported an increase in MMN amplitude to duration deviants in schizophrenia patients given a high dose (8 mg gum) of nicotine. However, nicotine had no effects on MMN to frequency deviants. A recent study by Fisher et al. [154] showed a significant shortening of latency for intensity deviants in schizophrenia patients receiving nicotine (6 mg gum). Interestingly, this study also found a positive correlation between the MMN amplitude to intensity deviants and the subjective clarity of auditory and visual hallucinations. This seems contradictory with reports in the literature showing an inverse relationship between MMN amplitude and symptomatology [155-157].
The variability of these results suggests that, besides the methodological differences of the studies, the course of the illness, the smoking status of individuals, the type of deviant stimuli, and the symptomatology of the sample may influence the effects of nAChRs agonists and modulators on the MMN of schizophrenia patients.
P300
In experienced tobacco users, cigarette smoking or nicotine administration has been shown to increase P300 amplitude [158-160] and/or reduce P300 latency [159, 161, 162]. However, other reports have failed to show such effects [163-166] or have found effects on visual, but not auditory, P300 [162]. In addition, some studies [160, 167, 168], but not others [169], have shown reduced P300 amplitudes in chronic smokers, suggesting a distinction between the effects of acute and chronic exposure to nicotine. A recent study [170] in typical smokers stratified by trait cognitive control found that P3b amplitude was reduced in all abstinent smokers. Greater P3b reductions were associated with higher nicotine dependence. The P3a amplitude was only reduced among the abstinent non-smokers that rated low for trait cognitive control.
The effects of smoking or nAChRs agonists and modulators on the P300 deficits known to be present in schizophrenia have not been well characterized. Using a novel EEG-informed functional magnetic resonance imaging (fMRI) technique, Mobascher et al. [171] studied the effects of nicotine (1 mg nasal) on the scalp potential and associated blood-oxygen-level dependence (BOLD) response of a visual P300 in a group of smoking healthy individuals and schizophrenia patients. Despite the fact that no effects of nicotine were found with respect to the amplitude and the latency of the P300 scalp potential, the authors found a nicotine-related increase in the P300-associated BOLD response in the anterior cingulate and the adjacent medial frontal cortex of both groups.
BOLD Response
Several fMRI studies have examined the BOLD response to nicotine in schizophrenia patients [115, 124, 125, 132, 172]. Among control smokers and smokers with schizophrenia, nicotine increased BOLD response in the anterior cingulate and thalamus in people with schizophrenia [125]. Another study showed that while nicotine increased BOLD activity in the anterior cingulate in schizophrenia, it reduced activity in controls [115]. Nicotine also reduced hippocampal activity in both groups but to a significantly greater degree in controls. However, Hong et al. [124] found that nicotine induced activation patterns did not differ between controls and people with schizophrenia. Two other studies have reported effects of nicotine on BOLD response in schizophrenia but did not have a control group for comparison [132, 172]. The differences in nicotine-induced activation between controls and people with schizophrenia are not consistent. This may be explained by the use of different tasks during the fMRI studies. Overall though, the literature suggests nicotine induced changes in the cingulate cortex and hippocampus differs among smokers with schizophrenia compared to controls.
EFFECTS OF NICOTINE IN A MODEL OF COGNITIVE DYSFUNCTION
Human pharmacologic models allow for the transient induction of symptoms including cognitive deficits in healthy controls similar to those seen in schizophrenia. Ketamine, an NMDA receptor antagonist, has become one of the most enduring pharmacologic models of schizophrenia. Ketamine can induce psychotic symptoms and cognitive deficits similar to schizophrenia in normal controls [173, 174] and exacerbates symptoms in people with schizophrenia [175]. Nicotine may reverse the cognitive deficits of ketamine by enhancing glutamatergic transmission in the prefrontal cortex through stimulation of the high affinity α4β2 nAChR [176]. Several studies have examined the interaction of ketamine and nicotine [166, 177-179]. In two of the studies intranasal nicotine and nicotine gum altered either ERP measurements or changes in neuroimaging from ketamine administration [166, 178]. The authors of these studies suggested nicotine mitigation of ketamine-induced effects is evidence that nicotine can improve symptoms of schizophrenia. However, studies using cognitive outcomes have not supported this assertion.
Knott et al. [179] tested nicotine gum in 20 smokers and 20 non-smokers with the Rapid Visual Information Processing Task (RVIP) a measure of sustained attention. In the smoking group, nicotine worsened performance on the task in the presence of ketamine. D’Souza et al. [177] used ketamine and intravenous nicotine in 37 healthy individuals in a randomized, double-blind, placebo controlled crossover study with a comprehensive cognitive battery and failed to show that nicotine reduced any of the ketamine induced cognitive deficits. In a related study, the N-methyl-D-aspartate (NMDA) antagonist memantine also failed to show an interaction between smoking cigarettes and performance on the RVIP [180].
It is difficult to extrapolate the failure of nicotine to reverse NMDA receptor antagonist induced cognitive deficits to the treatment of cognitive deficits in schizophrenia. Until a medication has shown to have cognitive enhancing effects in schizophrenia it will not be possible to completely validate the ketamine model. However, based on the general negative effects of nicotine in clinical trials, nicotine does not seem appear to improve cognitive function in schizophrenia.
CLINICAL TRIALS WITH nAChRs MEDICATIONS FOR COGNITIVE ENHANCEMENT IN SCHIZOPHRENIA
Several other nAChRs based treatments have been tested as potential therapies for the cognitive deficits associated with schizophrenia (Table 3). In the following sections we review the published clinical trials assessing the efficacy of nicotinic agents for the cognitive enhancement of schizophrenia.
Table 3. Randomized double-blinded placebo-controlled studies of nicotinic medications measuring cognition in schizophrenia.
| Experimental Medication |
Target Dose (mg/day) |
Dura- tion (Weeks) |
Design | Smokers | Antipsychotics | Randomized/ Completers |
Cognitive Battery | Results Compared to Placebo |
|
|---|---|---|---|---|---|---|---|---|---|
| Friedman 2002 |
Donepezil | 5 and 10 | 12 | Parallel | 78% | risperidone | 36/34 | CPT, DS, RAVLT, SSWMT, TMTA and B, VF, WCST |
placebo = donepezil 10 = donepezil 5 |
| Nahas 2003 | Donepezil | 10 | 12 each | Cross- over |
NA | olanzapine or risperidone |
6/6 | COWAT | placebo = donepezil |
| Tuğa 2004 | Donepezil | 5 | 6 each | Cross- over |
NA | fluphenazine or pimozide |
12/12 | TMTA and B, VF, WAIS (BD), WCST, WMS-R (DS, FG, LM, VePA, ViPA, ViR) |
placebo = donepezil |
| Freudenreich 2005 |
Donepezil | 10 | 8 | Parallel | 80% | Mostly olanzap- ine or risperi- done |
36/32 | BOWA, GP, HVLT, TMTA and B, WMS-R (DS) |
placebo = donepezil |
| Erickson 2005 |
Donepezil | 5 | 8 each | Cross- over |
NA | 1st or 2nd genera- tion |
24/15 | RAVLT, TMTA and B | RAVLT: donepezil > placebo |
| Mazeh 2006 | Donepezil | 10 | 12 | Parallel | NA | 1st or 2nd genera- tion |
20/17 | ADAS-cog | placebo = donepezil |
| Lee 2007 | Donepezil | 5 | 12 | Parallel | 58% | haloperidol | 24/23 | BNT, DS, DSST, HVLT, MMSE, RVLT, ST, TMTA and B, VF |
HVLT: donepezil > placebo MMSE, RVLT, DS: (trend) donepezil > placebo |
| Fagerlund 2007 |
Donepezil | 10 | 16 | Parallel | 64% | ziprasidone | 21/11 | BSRT, CANTAB (IED, SOC, SSP), DS, RCFT, TMTB, VF |
BSRT: placebo > donepezil SOC: (trend) placebo > donepezil |
| Kohler 2007 | Donepezil | 10 | 16 | Parallel | 46% | 2nd generation (no clozapine) |
26/22 | BDAE (AN, Reading), CPT,CVLT, DS, DSST, FT, JOLO, LNNB, MEA (Token Task, COWAT, VNT), SR, WAIS (BD), WCST, WMS-R (LM, VR) |
placebo = donepezil |
| Keefe 2008 | Donepezil | 10 | 12 | Parallel | 59% | 2nd generation (no clozapine) |
245/195 | CATIE Battery: CI, COWAT, CPT, GP, HVLT, LNTAWM, VWMT, WISC-III Mazes, WCST, WAIS (DSST) |
CI, COWAT: pla- cebo > donepezil |
| Akhindzadeh 2008 |
Donepezil | 10 | 12 | Parallel | NA | Risperidone | 30/30 | WAIS (BD), WCST, WMS-R (DS, FG, LM, VePA, ViPA, ViR) |
placebo = donepezil |
| Sharma 2006 | Rivastigmine | 12 | 24 | Parallel | 81% | Olanzapine, quetiapine, or risperidone |
40/21 | CPT, CVLT, DTS, FT, TMTA and B, VF, WCST, WAIS (DS, LNS) |
placebo = rivastig- mine |
| Kumari 2006 | Rivastigmine | 12 | 12 | Parallel | 81% | Olanzapine, quetiapine, or risperidone |
36/21 | n-back | placebo = rivastig- mine |
| Chouinard 2007 |
Rivastigmine | 9 | 24 | Parallel | 70% | 1st or 2nd genera- tion |
24/20 | CANTAB (PAL, RTI, RVIP, SOC, SWM) |
placebo = rivastigmine |
| Schubert 2006 |
Galantamine | 24 | 8 | Parallel | 94% | risperidone | 17/14 | CPT, OMMT, RBANS, TOT, UOA |
RBANS (Total score, attention and delayed memory items): galan- tamine > placebo |
| Lee 2007a | Galantamine | 16 | 12 | Parallel | NA | 1st generation | 24/22 | BNT, DS, DSST, HVLT, MMSE, RCFT, ST, TMTA, VF |
RCFT: donepezil > pla- cebo |
| Caroff 2007 | Galantamine | 8-24 | 12 each | Cross- over |
NA | 1st and 2nd generation |
38/35 | MMSE | placebo = galantamine |
| Dyer 2008 | Galantamine | 32 | 8 | Parallel | 0% | 2nd generation | 20/18 | CPT,GP, ST, WAIS (LNS) |
CPT, ST, LNS: placebo > galantamine |
| Sacco 2008 | Galantamine | 4 and 8 | 3 days | Cross- over |
57% | NA | 21/21 | CPT, DS, ST, TMTB, VSWM |
placebo = galantamine |
| Buchanan 2008 |
Galantamine | 24 | 12 | Parallel | 42% | 2nd generation (no clozapine), low dose 1st generation |
86/73 | BACS-NS, BVSMT, CPT, CVLT, GP, WAIS (DSST, LNS) |
DSST: galantamine > placebo CVLT: (trend) galan- tamine > placebo CPT: placebo > galan- tamine |
| Lindenmayer 2011 |
Galantamine | 24 | 24 | Parallel | NA | long acting injectable risperidone |
38/32 | CPT, FT, OWMT, PEAT, SST, STDT, WLMT |
placebo = galantamine |
| Hong 2011 | Varenicline | 2 | 8 | Parallel | 68% | 1st and 2nd generation |
69/59 | CPT, MCCB, WAIS (DSST) |
placebo = varenicline |
| Shim 2012 | Varenicline | 2 | 8 | Parallel | 52% | 1st and 2nd generation |
120/91 | CPT, DS, ST, VST, WAIS (DSST), WCST |
placebo = varenicline (smoking status had significant effects) |
| Velligan 2012 |
AZD3480 | 5, | 12 | Parallel | 100% | Olanzapine, quetiapine, or risperidone |
440/308 | IntegNeuro, WAIS(SS), BACS- SC |
placebo = AZD3480 |
| Olincy 2006 | DMXB-A | 150/75 or 75/37.5 |
3 days | Cross- over |
0% | 1st and 2nd generation |
13/12 | RBANS | Total Score: 75/37.5 > placebo |
| Freedman 2008 |
DMXB-A | 300 or 150 |
4 | Cross- over |
0% | 1st and 2nd generation |
31/31 | MCCB | Attention/Vigilance: 300 and 150 > placebo Working Memory: 300 > placebo, (trend) 150 > placebo Verbal Learning: placebo > 300 and 150 |
| Shiina 2010 | Tropisetror | 10 | 8 | Parallel | 28% | risperidone | 40/33 | CANTAB (DMS, IED, PRM, RVIP, SOC, SRM, SSP, SWM) |
RVIP (non-smokers): tropisetror > placebo |
| Lieberman 2013 |
TC-5619 | 25 | 12 | Parallel | 46% | quetiapine or risperidone |
185/154 | CSB, DSST, TMTA and B |
1-back, GMLT, SCI-Cog: (trend) TC-5619 > pla- cebo 1-back, GMLT (smok- ers): TC-5619 > placebo |
ADAS-Cog: Alzheimer Disease Assessment Scale – Cognitive subscale, AN: Animal naming, BACS-NS: Brief Assessment of Cognition in Schizophrenia, BD: Block Design, BDAE: Boston Diagnostic Aphasia Exam, BNT: Boston Naming Task, BOWA: Benton Oral Word Association Test, BSRT: Buschke Selective Reminding Test, BVSMT: Brief Visuospatial Memory Test, CI: Category Instances, COWAT: Controlled Oral Word Association Test, CPT: Continuous Performance Test, CSB: CogState Schizophrenia Battery, CVLT: California Verbal Learning Task, DMS: Delayed Matching to Sample, DS: Digit Span, DSST: Digit Symbol Substitution Test, DTS: Dot Test Score, FG: Figural memory, FT: Finger Tapping, GMLT: Groton Maze Learning Task, GP: Grooved Pegboard, HVLT: Hopkins Verbal Learning Task, IED: Intra-Extra Dimensional Set Shifting, JOLO: Judgment of Line Orientation, LM: Logical Memory, LNNB: Luria-Nebraska Neuropsychological Battery (stereognosis), LNS: Letter Number Sequence, LNTAWM: Letter-Number Test of Auditory Working Memory, MAE: Multilingual Aphasia Exam, MCCB: MATRICS (Measurement and Treatment Research to Improve Cognition in Schizophrenia) Consensus Cognitive Battery, MMSE: Mini-Mental State Examination, NS: Number Sequencing, OMMT: Object Matching Memory Test, OWMT: Object Working Memory Test, PAL: Paired Associates Learning, PEAT: Penn Emotional Acuity Test, PRM: Pattern Recognition Memory, RAVLT: Rey Auditory Verbal Learning Test, RBANS: Repeatable Battery for the Assessment of Neuropsychological Status, RCFT: Rey Complex Figure Test, RTI: Reaction Time, RVIP: Rapid Visual Information Processing, RVLT: Rey Visual Learning Test, SC: Symbol Coding, SGI-Cog: Subject Global Impression-Cognition, SOC: Stocking of Cambridge, SR: Seashore Rhythm, SRM: Spatial Recognition Memory, SSP: Spatial Span, SST: Set Shifting Test, SSWMT: Simple Spatial Working Memory Test, ST: Stroop Test, STDT: Strategic Target Detection Test, SWM: Spatial Working Memory, TMTA and B: Trail Making Test Part A and B, TOT: Tower of Toronto, UOA: Unirhinal Olfactory Acuity Test, VePA: Verbal Paired Associates, VF: Verbal Fluency, ViPA: Visual Paired Associates, ViR: Visual Reproduction, VNT: Visual Naming Task, VST: Visual Span Test, VSWM: Visuospatial working memory, VWMT: Visuospatial Working Memory Test, WAIS: Weschler Adult Intelligence Scale, WCST: Wisconsin Card Sorting Test, WISC-III: Wechsler Intelligence Scale for Children-Third Edition, WLMT: Word List Memory Test, WMS-R: Weschler Memory Scale-Revised
ACETYLCHOLINESTERASE INHIBITORS
Acetylcholinesterase inhibitors (AChEIs) inhibit the enzyme that breaks down acetylcholine, greatly enhancing the endogenous ligand for nAChRs. Since these medications have proven benefit in delaying the cognitive decline in dementia, they have been tested as potential treatments for the cognitive deficits of schizophrenia. While there are several open label studies and a placebo controlled case report of AChEIs in schizophrenia [181-186], only double-blind, placebo-controlled, randomized studies that evaluated the cognitive effects of AChEIs in schizophrenia/schizoaffective disorder will be reviewed.
DONEPEZIL
Donepezil is a selective AChEI that binds to acetylcholinesterase, but not peripheral butyrylcholinesterase, and is FDA approved for the treatment of mild-severe Alzheimer’s disease. Three placebo-controlled randomized studies assessed the cognitive effects of donepezil in schizophrenia [187-189]. In a crossover study over two six-week periods, 5 mg of donepezil failed to show any cognitive enhancement over placebo in a large cognitive battery [187]. Erickson et al. [188] gave participants eight-weeks of either donepezil 5 mg or placebo with a two week-washout before starting on the second study medication. Cognitive assessments included the Trails A and B and the RAVLT to assess executive functioning and verbal learning. Both placebo and donepezil improved RAVLT outcomes, but the donepezil group demonstrated statistically greater improvement in the number of words able to be recalled versus placebo. A small crossover study of six participants found no improvement in verbal fluency after 12 weeks of donepezil 10 mg versus placebo [189]. However, the study found that subjects with schizophrenia had an increased BOLD response in the frontal regions including the anterior cingulate when treated with donepezil. While one of the crossover studies reported some improvement with donepezil, all had 15 or fewer participants in the studies. Also, as shown with the one positive study, carryover effects were seen in crossover studies. The first published randomized placebo controlled parallel group study of donepezil consisted of 36 participants all on stable doses of risperidone randomized to donepezil 5 mg, 10 mg, or placebo over 12-weeks [190]. A large cognitive battery was administered at weeks 4 and 12 and showed no significant cognitive effects of either dose of donepezil versus placebo. The only positive study for the use of donepezil was also a 12-week study that used 5 mg of donepezil [191]. Lee et al. [192] studied 24 participants all stabilized on haloperidol with a large cognitive battery. After 12-weeks the donepezil arm showed significantly greater immediate recall and recognition on the HVLT (verbal memory) compared to placebo. A greater trend of improvement was also seen in the donepezil treated group on the MMSE (general cognition), the RAVLT, and DS tasks. Many other studies randomized participants with schizophrenia to donepezil 10 mg and failed to demonstrate cognitive improvement versus placebo over time periods ranging from 8-16 weeks of treatment [193-195]. Another 16-week study also reported no improvements in cognition with donepezil 10 mg but failed to report placebo response [195]. Interestingly one small study (n=11) found participants stabilized on ziprasidone showed deterioration in some aspects of cognition including planning and verbal learning after 16 weeks of treatment of donepezil compared to placebo [196]. Akhondzadeh et al. [197] studied the effects of donepezil in 30 participants stabilized on long-acting injectable risperidone. Subjects were randomized to placebo or donepezil over 12 weeks. No improvements in neurocognitive symptoms were detected.
In what is by far the largest study of the neurocognitive effects of any of the AChEIs in schizophrenia, Keefe et al. [198] randomized 245 people with schizophrenia who were stabilized on a second generation antipsychotic (excluding clozapine), to 12 weeks of donepezil titrated up to 10 mg daily. Assessments for cognition were done at weeks 6 and 12 using the CATIE neurocognitive battery [199, 200]. Almost 60% of the participants were current cigarette smokers and equally distributed between the placebo and donepezil treatment arms. A total of 195 participants completed the study with approximately the same attrition rate of around 20% in both arms. In the intent to treat sample there was no significant difference between placebo and donepezil on the CATIE Neurocognitive Battery Composite score. But the observed completers analysis showed a significant improvement in placebo greater than the donepezil arm, (cognitive improvement effect sizes for placebo, 0.45 and donepezil, 0.29; p = 0.04). At week 12 the Controlled Oral Word Association Test and the Category Instances tests were significantly more improved with placebo, no other cognitive tasks were significantly different between placebo and donepezil. A subgroup analysis found smoking status did not affect cognitive outcomes in either group.
In summary, donepezil does not seem to be an effective agent for cognitive deficits in schizophrenia. In fact, it may even interfere with learning in schizophrenia [196, 198], although it may be indicated in those with comorbid schizophrenia and dementia [181].
RIVASTIGMINE
Rivastigmine is an AChEI also approved for the treatment of mild-severe Alzheimer’s disease and for mild-moderate dementia of Parkinson’s type. Unlike donepezil, rivastigmine has affinity for both acetylcholinesterase and butyrylcholinesterase, but it is not known if this is clinically relevant.
One of the first published randomized placebo controlled studies of rivastigmine was a 12-week fMRI study [201]. After the 12-week treatment of 20 participants there was a trend for the group treated with rivastigmine 12 mg toward improved attention in one condition (p=0.075) compared to placebo. In another 24 week study of participants stabilized on risperidone, olanzapine, or quetiapine, after 12-weeks the rivastigmine treated group showed no significant improvement in an n-back task but showed some enhanced BOLD response in the visual cortex [202]. In a 24-week evaluation, the same group demonstrated no improvement in cognitive test performance [203]. The only other placebo-controlled study with rivastigmine found no cognitive enhancing effects on the CANTAB after 24-weeks with participants on either first or second generation antipsychotics [204].
One non-placebo controlled study found ERP changes elicited during a memory task that suggested rivastigmine induced information-processing benefits in people with schizophrenia [205]. In summary the results of clinical trials with rivastigmine have been disappointing. However, much larger studies would need to be conducted to conclusively determine whether rivastigmine has utility as a cognitive enhancer in schizophrenia.
GALANTAMINE
Galantamine is another AChEI that is indicated for the treatment of mild-moderate Alzheimer’s disease. However, unlike the other available AChEIs, it is also an allosteric modulator of the α4β2 and α7 nAChRs [206-209]. Interestingly, it does not cause desensitization of nicotinic receptors.
The first published randomized placebo controlled study of galantamine was an 8-week parallel group study in participants stabilized on risperidone (n = 14) [210]. After titrating up to 24 mg per day, the galantamine treated arm showed significant improvement on the RBANS total score and on subtests measuring attention and delayed memory. Lee et al. [192] found after 12 weeks of treatment with galantamine 16 mg/day participants stabilized on their first generation antipsychotic had improved visuospatial abilities on the Rey Complex Figure Task compared to placebo but not on the other cognitive tasks administered. Dyer et al. [211] used a higher dose of galantamine than any other study (32 mg/day) in 18 non-smoking participants stabilized on a second generation antipsychotic. After 8 weeks galantamine treated participants showed deterioration on a continuous performance task, Stroop task, and Letter-Number Span Task from the Weschler Adult Intelligence Scale compared to placebo treated participants. A low dose study of galantamine (8 mg/day) demonstrated no cognitive improvement compared to placebo (n = 21) [212]. A crossover study of 35 people with schizophrenia and tardive dyskinesia found no improvement on the MMSE with galantamine, but the MMSE is not meant to assess subtle cognitive changes [213]. In the longest trial (24 weeks) with galantamine in participants receiving long-acting injectable risperidone [214], there were no beneficial effects of galantamine on cognitive test performance.
Buchanan et al. [215] conducted the largest placebo-controlled randomized clinical trial with galantamine. Participants on a second-generation (excluding clozapine) or low dose first-generation antipsychotic were randomized to galantamine 24 mg/day over a 12-week period. A total of 86 participants were randomized and 73 finished the study, approximately 42% of the participants were cigarette smokers [215]. After controlling for multiple comparisons there was a significant benefit of galantamine on the Weschler Adult Intelligence Scale (WAIS) digit symbol substitution task (p = 0.02) but placebo treated individuals showed significant improvement on a continuous performance task (p =0.05).
In summary, the effects for galantamine have been more impressive than the other AChEIs, but the effects seem small at best. Larger studies need to be conducted to conclusively determine whether galantamine has utility as a cognitive enhancer in schizophrenia.
Overall, AChEIs may have beneficial effects in several domains of cognition including attention, visual memory, verbal memory and language, and executive function [216], but the effects are small and of dubious clinical significance.
α4β2 nAChR MEDICATIONS
Varenicline is a partial agonist at α4β2 and full agonist at α7 nAChRs [217] that was developed as a treatment for nicotine dependence. Clinical trials have shown it is one of the most effective treatments for nicotine addiction [218], however significant psychiatric effects have been reported with its use [219]. Most studies of varenicline in schizophrenia have focused on smoking outcomes [220-224]. In a small (n=6) pilot study varenicline did not appear to enhance P50 gating [223]. In a small (n=12) open label study of schizophrenia patients who smoked, RBANS total score and visuospatial working memory did not change significantly, but improvements in verbal learning and memory were noted [221].
Two placebo-controlled clinical trials have been conducted with varenicline in people with schizophrenia [220, 222]. Shim et al. [220] randomized approximately 120 patients to either placebo or varenicline over an 8-week period. Half of the patients in each arm were current smokers. The primary analysis showed no statistical improvement with varenicline versus placebo on several cognitive tasks including a Continuous Performance Task (CPT), the Digit Symbol Substitution Task (DSST), a Digit Span Task (DST), a Visual Span Task (VST), the Stroop Word Color Task (SWCT), and the Wisconsin Card Sorting Task (WCST), however secondary analysis looking at improvement over time found varenicline significantly improved scores on the DSST and decreased nonperseverative errors on the WCST. Smoking status also contributed to some cognitive differences, varenicline showed a significantly greater improvement in CPT reaction time in smokers but greater improvement in SWCT in non-smokers. Hong et al. [222] studied approximately 60 smoking and non-smoking people with schizophrenia over 8-weeks. After two weeks a series of biomarkers were tested and varenicline was found to reduce P50 auditory sensory gating in non-smokers and reduced startle response and antisaccadic error regardless of smoking status. However, other biomarkers including prepulse inhibition and memory saccadic error were not significantly different. After 8 weeks the varenicline treated group did not show significant improvement on the DSST or a CPT task.
None of the participants in the varenicline studies reported had adverse events related to worsening of psychosis. But some of the adverse reports of varenicline induced worsening of psychosis are in people with schizophrenia treated with an antipsychotic [219]. Therefore, the limited effects seen in cognitive enhancement along with the potential for adverse side effects will most likely limit future uses of varenicline as a cognitive enhancer. Another experimental α4β2 nAChR agonist, AZD3480, showed no cognitive enhancing effects in a study of 410 people with schizophrenia who smoke, but the medication was well tolerated [225].
ALPHA7 nAChR MEDICATIONS
Several medications that target α7 nAChR agonists have been in development as cognitive enhancers for schizophrenia, for review see Wallace, T. L. and Bertrand, D. [111]. To date few of the compounds have made it to market or published in peer-reviewed journals, although a few medication trials have been published. DMXB-A, an α7 nAChR agonist, has had many studies published [226-229]. Only two of the studies assessed changes in cognitive function in people with schizophrenia. In a three-day crossover study comparing 2 doses of DMXB-A and placebo, the lower dose of DMXB-A had statistically greater improvement on the total RBANS score than placebo [226]. A four-week crossover study of the same doses used in the proof-of-concept study showed no significant changes in cognition between placebo and DMXB-A. Practice effects were seen in the cognitive battery and a reanalysis of only the first randomization showed DMXB-A improved attention/vigilance with both doses and working memory with the low dose. Both DMXB-A studies excluded smokers from participation in the study. P50 gating was improved in non-smoking schizophrenia patients on the lower dose of DMXB-A [100]. Tropisetron, another α7 nAChR agonist, was tested in an eight-week randomized double-blind placebo controlled trial in 33 participants with schizophrenia stabilized on risperidone [230]. The primary analysis found no cognitive improvements with tropisetron but among nonsmokers sustained visual attention and P50 gating was improved compared to placebo. Lieberman et al. [231] reported on a 12-week randomized double-blind placebo controlled trial of 154 people with schizophrenia treated with TC-5619 while stabilized on risperidone or quetiapine, almost half of the participants were cigarette smokers. TC-5619 did not show any cognitive improvement in cognitive abilities on any of the cognitive tasks but a subanalysis found smokers have significant improvement in working memory and executive function. A recently published study, which did not have a cognitive battery, found that the positive α7 nAChR allosteric modulator JNJ-393934 did not affect P50, MMN or P300 [147].
CONCLUSION
Reducing the cognitive impairments associated with schizophrenia and increasing functioning remains an important goal of treatment. Several lines of evidence suggest alterations in the nAChRs system in people with schizophrenia. While nicotine use is relatively ubiquitous in people with schizophrenia, there is little evidence to support the notion that nicotine and other nAChRs-based treatments have profound cognitive enhancing qualities. Tasks measuring attention generally improve with nicotine administration and nAChRs-based treatments in schizophrenia, but the published reports often use large batteries with multiple outcomes and fail to account for multiple comparisons. The reported improvements are normally from secondary analysis and not robust signals of general cognitive improvement. Although more traditional cognitive test batteries have failed to capture consistent improvements with drugs acting at nAChRs, more proximal measures of information processing indexed by ERPs, have demonstrated some benefit.
The lack of beneficial effects of nicotine led to clinical trials with AChEIs. These drugs have also failed to show consistent, replicable, significant and clinically meaningful benefits. Larger ongoing studies for the allosteric modulator galantamine will hopefully provide more conclusive results (Clinicaltrials.gov identifier NCT01012167). Varenicline, also shows limited cognitive benefits unlike its utility as a treatment for smoking cessation in schizophrenia [232]. Initial studies with another α4β2 nAChR agonist have also been disappointing [225]. Selective α7 nAChR agonists have been tried in schizophrenia although the results are preliminary. Other α7 nAChR based drugs, such as positive allosteric modulators, are being investigated but are still early in development.
A number of factors need to be considered in developing nAChR based drugs. It is important to determine the influence of smoking status on the response to nAChRs-based medications, since the majority of people with schizophrenia smoke [16-19] and nicotine causes desensitization of nAChRs [31]. The current studies with nAChRs agonists have had varying degrees of allowance for cigarette smokers. The published studies with α7 nAChR agonist DMXB-A [226, 227] did not include any smokers in their studies while in the large study with TC-5619 [231] almost 50% of the sample were current smokers. Interestingly for TC-5619, cigarette smokers had slightly greater response to the medication than nonsmokers. This begs the question of whether nAChRs-based medications that are effective only in nonsmokers will be commercial viable given that most schizophrenia patients smoke? Furthermore, these medications are prescribed in the backdrop of antipsychotic medications that are dopamine D2 receptor antagonists and some which are potent anticholinergics [233]. Since dopamine and cholinergic signaling are important in cognition [234], it is important that we determine if nAChRs agonists work with a variety of antipsychotic medications.
A greater understanding of the nicotinic system is needed to determine if it is a therapeutic target for cognitive enhancement. But the continued perseverance in preclinical and clinical testing with new medications may lead to nAChR based treatment strategies that improve clinical outcomes in schizophrenia by enhancing cognition.
FUTURE DIRECTIONS: NICOTINIC ALLOSTERIC MODULATORS
One of the challenges in activating nAChRs is their propensity to desensitize. Ongoing work is developing compounds that bind to the α7 nAChR at alternative sites than acetylcholine, and enhance transmission without inducing desensitization, such as positive allosteric modulators [111]. While several of these compounds are in the pipeline, clinical evidence is still awaiting publication. The development and testing of these compounds may usher in clinically relevant medications for the treatment of schizophrenia.
ACKNOWLEDGEMENTS
Declared none.
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
REFERENCES
- [1].Green MF. Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. J Clin Psychiatry. 2006;67:e12. [PubMed] [Google Scholar]
- [2].Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–45. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- [3].Lewandowski KE, Cohen BM, Ongur D. Evolution of neuropsychological dysfunction during the course of schizophrenia and bipolar disorder. Psychol Med. 2011;41:225–41. doi: 10.1017/S0033291710001042. [DOI] [PubMed] [Google Scholar]
- [4].Gold JM. Cognitive deficits as treatment targets in schizophrenia. Schizophr Res. 2004;72:21–8. doi: 10.1016/j.schres.2004.09.008. [DOI] [PubMed] [Google Scholar]
- [5].Keefe RS, Eesley CE, Poe MP. Defining a cognitive function decrement in schizophrenia. Biol Psychiatry. 2005;57:688–91. doi: 10.1016/j.biopsych.2005.01.003. [DOI] [PubMed] [Google Scholar]
- [6].Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull. 2000;26:119–36. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- [7].Tsang HW, Leung AY, Chung RC, Bell M, Cheung WM. Review on vocational predictors: a systematic review of predictors of vocational outcomes among individuals with schizophrenia: an update since 1998. Aust N Z J Psychiatry. 2010;44:495–504. doi: 10.3109/00048671003785716. [DOI] [PubMed] [Google Scholar]
- [8].Kapur S, Mamo D. Half a century of antipsychotics and still a central role for dopamine D2 receptors. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1081–90. doi: 10.1016/j.pnpbp.2003.09.004. [DOI] [PubMed] [Google Scholar]
- [9].Buchanan RW, Freedman R, Javitt DC, Abi-Dargham A, Lieberman JA. Recent advances in the development of novel pharmacological agents for the treatment of cognitive impairments in schizophrenia. Schizophr Bull. 2007;33:1120–30. doi: 10.1093/schbul/sbm083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Keefe RS, Bilder RM, Davis SM, et al. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Arch Gen Psychiatry. 2007;64:633–47. doi: 10.1001/archpsyc.64.6.633. [DOI] [PubMed] [Google Scholar]
- [11].Bradley SR, Lameh J, Ohrmund L, et al. AC-260584, an orally bioavailable M(1) muscarinic receptor allosteric agonist, improves cognitive performance in an animal model. Neuropharmacology. 2010;58:365–73. doi: 10.1016/j.neuropharm.2009.10.003. [DOI] [PubMed] [Google Scholar]
- [12].O’Donnell CJ, Rogers BN, Bronk BS, et al. Discovery of 4-(5-methyloxazolo[4,5-b]pyridin-2-yl)-1,4-diazabicyclo[3.2.2]nonane (CP-810,123), a novel alpha 7 nicotinic acetylcholine receptor agonist for the treatment of cognitive disorders in schizophrenia: synthesis, SAR development, and in vivo efficacy in cognition models. J Med Chem. 2010;53:1222–37. doi: 10.1021/jm9015075. [DOI] [PubMed] [Google Scholar]
- [13].Radek RJ, Kohlhaas KL, Rueter LE, Mohler EG. Treating the cognitive deficits of schizophrenia with alpha4beta2 neuronal nicotinic receptor agonists. Curr Pharm Des. 2010;16:309–22. doi: 10.2174/138161210790170166. [DOI] [PubMed] [Google Scholar]
- [14].Homayoun H, Moghaddam B. Group 5 metabotropic glutamate receptors: role in modulating cortical activity and relevance to cognition. Eur J Pharmacol. 2010;639:33–9. doi: 10.1016/j.ejphar.2009.12.042. [DOI] [PubMed] [Google Scholar]
- [15].Arnsten AF, Cai JX, Murphy BL, Goldman-Rakic PS. Dopamine D1 receptor mechanisms in the cognitive performance of young adult and aged monkeys. Psychopharmacology (Berl) 1994;116:143–51. doi: 10.1007/BF02245056. [DOI] [PubMed] [Google Scholar]
- [16].Dalack GW, Healy DJ, Meador-Woodruff JH. Nicotine dependence in schizophrenia: clinical phenomena and laboratory findings. Am J Psychiatry. 1998;155:1490–501. doi: 10.1176/ajp.155.11.1490. [DOI] [PubMed] [Google Scholar]
- [17].Hughes J, Hatsukami D, Mitchell J, Dahlgren L. Prevalence of smoking among psychiatric outpatients. Am J Psychiatry. 1986;143:993–997. doi: 10.1176/ajp.143.8.993. [DOI] [PubMed] [Google Scholar]
- [18].Ziedonis DM, George TP. Schizophrenia and nicotine use: report of a pilot smoking cessation program and review of neurobiological and clinical issues. Schizophr Bull. 1997;23:247–54. doi: 10.1093/schbul/23.2.247. [DOI] [PubMed] [Google Scholar]
- [19].de Leon J, Diaz FJ. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr Res. 2005;76:135–57. doi: 10.1016/j.schres.2005.02.010. [DOI] [PubMed] [Google Scholar]
- [20].de Leon J, Becona E, Gurpegui M, Gonzalez-Pinto A, Diaz FJ. The association between high nicotine dependence and severe mental illness may be consistent across countries. J Clin Psychiatry. 2002;63:812–6. doi: 10.4088/jcp.v63n0911. [DOI] [PubMed] [Google Scholar]
- [21].Diwan A, Castine M, Pomerleau CS, Meador-Woodruff JH, Dalack GW. Differential prevalence of cigarette smoking in patients with schizophrenic vs mood disorders. Schizophr Res. 1998;33:113–8. doi: 10.1016/s0920-9964(98)00045-0. [DOI] [PubMed] [Google Scholar]
- [22].Kumari V, Postma P. Nicotine use in schizophrenia: the self medication hypotheses. Neuroscience & Biobehavioral Reviews. 2005;29:1021–1034. doi: 10.1016/j.neubiorev.2005.02.006. [DOI] [PubMed] [Google Scholar]
- [23].Paterson D, Nordberg A. Neuronal nicotinic receptors in the human brain. Prog Neurobiol. 2000;61:75–111. doi: 10.1016/s0301-0082(99)00045-3. [DOI] [PubMed] [Google Scholar]
- [24].Mansvelder HD, McGehee DS. Cellular and synaptic mechanisms of nicotine addiction. J Neurobiol. 2002;53:606–17. doi: 10.1002/neu.10148. [DOI] [PubMed] [Google Scholar]
- [25].Chavez-Noriega LE, Crona JH, Washburn MS, Urrutia A, Elliott KJ, Johnson EC. Pharmacological characterization of recombinant human neuronal nicotinic acetylcholine receptors h alpha 2 beta 2, h alpha 2 beta 4, h alpha 3 beta 2, h alpha 3 beta 4, h alpha 4 beta 2, h alpha 4 beta 4 and h alpha 7 expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1997;280:346–56. [PubMed] [Google Scholar]
- [26].Rueter LE, Donnelly-Roberts DL, Curzon P, Briggs CA, Anderson DJ, Bitner RS. A-85380: a pharmacological probe for the preclinical and clinical investigation of the alphabeta neuronal nicotinic acetylcholine receptor. CNS Drug Rev. 2006;12:100–12. doi: 10.1111/j.1527-3458.2006.00100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Fabian-Fine R, Skehel P, Errington ML, et al. Ultrastructural distribution of the alpha7 nicotinic acetylcholine receptor subunit in rat hippocampus. J Neurosci. 2001;21:7993–8003. doi: 10.1523/JNEUROSCI.21-20-07993.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev. 2009;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Cosgrove KP, Batis J, Bois F, et al. beta2-Nicotinic acetylcholine receptor availability during acute and prolonged abstinence from tobacco smoking. Arch Gen Psychiatry. 2009;66:666–76. doi: 10.1001/archgenpsychiatry.2009.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Breese CR, Marks MJ, Logel J, et al. Effect of smoking history on [3H] nicotine binding in human postmortem brain. J Pharmacol Exp Ther. 1997;282:7–13. [PubMed] [Google Scholar]
- [31].Yu KD, Liu Q, Wu J, Lukas RJ. Kinetics of desensitization and recovery from desensitization for human alpha4beta2-nicotinic acetylcholine receptors stably expressed in SH-EP1 cells. Acta Pharmacol Sin. 2009;30:805–17. doi: 10.1038/aps.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Mineur YS, Picciotto MR. Genetics of nicotinic acetylcholine receptors: Relevance to nicotine addiction. Biochem Pharmacol. 2008;75:323–33. doi: 10.1016/j.bcp.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Tapper AR, McKinney SL, Nashmi R, et al. Nicotine activation of alpha4* receptors: sufficient for reward, tolerance, and sensitization. Science. 2004;306:1029–32. doi: 10.1126/science.1099420. [DOI] [PubMed] [Google Scholar]
- [34].Lendvai B, Kassai F, Szajli A, Nemethy Z. alpha7 Nicotinic acetylcholine receptors and their role in cognition. Brain Res Bull. 2012 doi: 10.1016/j.brainresbull.2012.11.003. [DOI] [PubMed] [Google Scholar]
- [35].Martin LF, Freedman R. Schizophrenia and the alpha7 nicotinic acetylcholine receptor. Int Rev Neurobiol. 2007;78:225–46. doi: 10.1016/S0074-7742(06)78008-4. [DOI] [PubMed] [Google Scholar]
- [36].Jensen AA, Frolund B, Liljefors T, Krogsgaard-Larsen P. Neuronal nicotinic acetylcholine receptors: structural revelations, target identifications, and therapeutic inspirations. J Med Chem. 2005;48:4705–45. doi: 10.1021/jm040219e. [DOI] [PubMed] [Google Scholar]
- [37].Freedman R, Hall M, Adler LE, Leonard S. Evidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biol Psychiatry. 1995;38:22–33. doi: 10.1016/0006-3223(94)00252-X. [DOI] [PubMed] [Google Scholar]
- [38].Leonard S, Gault J, Adams C, et al. Nicotinic receptors, smoking and schizophrenia. Restor Neurol Neurosci. 1998;12:195–201. [PubMed] [Google Scholar]
- [39].Court J, Spurden D, Lloyd S, et al. Neuronal nicotinic receptors in dementia with Lewy bodies and schizophrenia: alpha-bungarotoxin and nicotine binding in the thalamus. J Neurochem. 1999;73:1590–7. doi: 10.1046/j.1471-4159.1999.0731590.x. [DOI] [PubMed] [Google Scholar]
- [40].Guan ZZ, Zhang X, Blennow K, Nordberg A. Decreased protein level of nicotinic receptor alpha7 subunit in the frontal cortex from schizophrenic brain. Neuroreport. 1999;10:1779–82. doi: 10.1097/00001756-199906030-00028. [DOI] [PubMed] [Google Scholar]
- [41].Breese CR, Lee MJ, Adams CE, et al. Abnormal regulation of high affinity nicotinic receptors in subjects with schizophrenia. Neuropsychopharmacology. 2000;23:351–64. doi: 10.1016/S0893-133X(00)00121-4. [DOI] [PubMed] [Google Scholar]
- [42].Court JA, Piggott MA, Lloyd S, et al. Nicotine binding in human striatum: elevation in schizophrenia and reductions in dementia with Lewy bodies, Parkinson’s disease and Alzheimer’s disease and in relation to neuroleptic medication. Neuroscience. 2000;98:79–87. doi: 10.1016/s0306-4522(00)00071-3. [DOI] [PubMed] [Google Scholar]
- [43].Durany N, Zochling R, Boissl KW, et al. Human post-mortem striatal alpha4beta2 nicotinic acetylcholine receptor density in schizophrenia and Parkinson’s syndrome. Neurosci Lett. 2000;287:109–12. doi: 10.1016/s0304-3940(00)01144-7. [DOI] [PubMed] [Google Scholar]
- [44].Leonard S, Adler LE, Benhammou K, et al. Smoking and mental illness. Pharmacol Biochem Behav. 2001;70:561–70. doi: 10.1016/s0091-3057(01)00677-3. [DOI] [PubMed] [Google Scholar]
- [45].Marutle A, Zhang X, Court J, et al. Laminar distribution of nicotinic receptor subtypes in cortical regions in schizophrenia. J Chem Neuroanat. 2001;22:115–26. doi: 10.1016/s0891-0618(01)00117-x. [DOI] [PubMed] [Google Scholar]
- [46].Martin-Ruiz CM, Haroutunian VH, Long P, et al. Dementia rating and nicotinic receptor expression in the prefrontal cortex in schizophrenia. Biol Psychiatry. 2003;54:1222–33. doi: 10.1016/s0006-3223(03)00348-2. [DOI] [PubMed] [Google Scholar]
- [47].De Luca V, Likhodi O, Van Tol HH, Kennedy JL, Wong AH. Regulation of alpha7-nicotinic receptor subunit and alpha7-like gene expression in the prefrontal cortex of patients with bipolar disorder and schizophrenia. Acta Psychiatr Scand. 2006;114:211–5. doi: 10.1111/j.1600-0447.2006.00785.x. [DOI] [PubMed] [Google Scholar]
- [48].Mathew SV, Law AJ, Lipska BK, et al. Alpha7 nicotinic acetylcholine receptor mRNA expression and binding in postmortem human brain are associated with genetic variation in neuregulin 1. Hum Mol Genet. 2007;16:2921–32. doi: 10.1093/hmg/ddm253. [DOI] [PubMed] [Google Scholar]
- [49].Mexal S, Berger R, Logel J, Ross RG, Freedman R, Leonard S. Differential regulation of alpha7 nicotinic receptor gene (CHRNA7) expression in schizophrenic smokers. J Mol Neurosci. 2010;40:185–95. doi: 10.1007/s12031-009-9233-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Thomsen MS, Weyn A, Mikkelsen JD. Hippocampal alpha7 nicotinic acetylcholine receptor levels in patients with schizophrenia, bipolar disorder, or major depressive disorder. Bipolar Disord. 2011;13:701–7. doi: 10.1111/j.1399-5618.2011.00961.x. [DOI] [PubMed] [Google Scholar]
- [51].Bramon E, Rabe-Hesketh S, Sham P, Murray RM, Frangou S. Meta-analysis of the P300 and P50 waveforms in schizophrenia. Schizophr Res. 2004;70:315–29. doi: 10.1016/j.schres.2004.01.004. [DOI] [PubMed] [Google Scholar]
- [52].D’Souza DC, Esterlis I, Carbuto M, et al. Lower Beta2*-nicotinic acetylcholine receptor availability in smokers with schizophrenia. Am J Psychiatry. 2012;169:326–34. doi: 10.1176/appi.ajp.2011.11020189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Brasic JR, Cascella N, Kumar A, et al. Positron emission tomography experience with 2-[(1)(8)F]fluoro-3-(2(S)-azetidinylmethoxy)pyridine (2-[(1)(8)F]FA) in the living human brain of smokers with paranoid schizophrenia. Synapse. 2012;66:352–68. doi: 10.1002/syn.21520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kraguljac NV, Reid M, White D, et al. Neurometabolites in schizophrenia and bipolar disorder - a systematic review and meta-analysis. Psychiatry Res. 2012;203:111–25. doi: 10.1016/j.pscychresns.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Dickinson D, Harvey PD. Systemic hypotheses for generalized cognitive deficits in schizophrenia: a new take on an old problem. Schizophr Bull. 2009;35:403–14. doi: 10.1093/schbul/sbn097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Buchanan RW, Davis M, Goff D, et al. A summary of the FDA-NIMH-MATRICS workshop on clinical trial design for neurocognitive drugs for schizophrenia. Schizophr Bull. 2005;31:5–19. doi: 10.1093/schbul/sbi020. [DOI] [PubMed] [Google Scholar]
- [57].Keefe RS, Fox KH, Harvey PD, Cucchiaro J, Siu C, Loebel A. Characteristics of the MATRICS Consensus Cognitive Battery in a 29-site antipsychotic schizophrenia clinical trial. Schizophr Res. 2011;125:161–8. doi: 10.1016/j.schres.2010.09.015. [DOI] [PubMed] [Google Scholar]
- [58].Dickinson D, Ramsey ME, Gold JM. Overlooking the obvious: a meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Arch Gen Psychiatry. 2007;64:532–42. doi: 10.1001/archpsyc.64.5.532. [DOI] [PubMed] [Google Scholar]
- [59].Evans JD, Heaton RK, Paulsen JS, Palmer BW, Patterson T, Jeste DV. The relationship of neuropsychological abilities to specific domains of functional capacity in older schizophrenia patients. Biol Psychiatry. 2003;53:422–30. doi: 10.1016/s0006-3223(02)01476-2. [DOI] [PubMed] [Google Scholar]
- [60].Hahn B, Robinson BM, Harvey AN, et al. Visuospatial attention in schizophrenia: deficits in broad monitoring. J Abnorm Psychol. 2012;121:119–28. doi: 10.1037/a0023938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Goldman-Rakic PS. Working memory dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci. 1994;6:348–57. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- [62].Nuechterlein KH, Barch DM, Gold JM, Goldberg TE, Green MF, Heaton RK. Identification of separable cognitive factors in schizophrenia. Schizophr Res. 2004;72:29–39. doi: 10.1016/j.schres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- [63].Gray BE, McMahon RP, Gold JM. General intellectual ability does not explain the general deficit in schizophrenia. Schizophr Res. 2013;147:315–9. doi: 10.1016/j.schres.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Couture SM, Penn DL, Roberts DL. The functional significance of social cognition in schizophrenia: a review. Schizophr Bull. 2006;32(Suppl 1):S44–63. doi: 10.1093/schbul/sbl029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Gold JM, Queern C, Iannone VN, Buchanan RW. Repeatable battery for the assessment of neuropsychological status as a screening test in schizophrenia I: sensitivity, reliability, and validity. Am J Psychiatry. 1999;156:1944–50. doi: 10.1176/ajp.156.12.1944. [DOI] [PubMed] [Google Scholar]
- [66].Keefe RS, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L. The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res. 2004;68:283–97. doi: 10.1016/j.schres.2003.09.011. [DOI] [PubMed] [Google Scholar]
- [67].Keefe RS, Silva SG, Perkins DO, Lieberman JA. The effects of atypical antipsychotic drugs on neurocognitive impairment in schizophrenia: a review and meta-analysis. Schizophr Bull. 1999;25:201–22. doi: 10.1093/oxfordjournals.schbul.a033374. [DOI] [PubMed] [Google Scholar]
- [68].Nuechterlein KH, Green MF, Kern RS, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165:203–13. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- [69].Kern RS, Nuechterlein KH, Green MF, et al. The MATRICS Consensus Cognitive Battery, part 2: co-norming and standardization. Am J Psychiatry. 2008;165:214–20. doi: 10.1176/appi.ajp.2007.07010043. [DOI] [PubMed] [Google Scholar]
- [70].Green MF, Nuechterlein KH. The MATRICS initiative: developing a consensus cognitive battery for clinical trials. Schizophr Res. 2004;72:1–3. doi: 10.1016/j.schres.2004.09.006. [DOI] [PubMed] [Google Scholar]
- [71].Green MF, Nuechterlein KH, Gold JM, et al. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biol Psychiatry. 2004;56:301–7. doi: 10.1016/j.biopsych.2004.06.023. [DOI] [PubMed] [Google Scholar]
- [72].Baker EL, Letz R, Fidler A. A computer-administered neuro-behavioral evaluation system for occupational and environmental epidemiology. Rationale, methodology, and pilot study results. J Occup Med. 1985;27:206–12. [PubMed] [Google Scholar]
- [73].O’Halloran JP, Kemp AS, Gooch KN, et al. Psychometric comparison of computerized and standard administration of the neurocognitive assessment instruments selected by the CATIE and MATRICS consortia among patients with schizophrenia. Schizophr Res. 2008;106:33–41. doi: 10.1016/j.schres.2007.11.015. [DOI] [PubMed] [Google Scholar]
- [74].Kline P. A handbook of test construction: Introduction to psychometric design. Methuen: 1986. [Google Scholar]
- [75].Keefe RS, Harvey PD, Goldberg TE, et al. Norms and standardization of the Brief Assessment of Cognition in Schizophrenia (BACS) Schizophr Res. 2008;102:108–15. doi: 10.1016/j.schres.2008.03.024. [DOI] [PubMed] [Google Scholar]
- [76].Hill SK, Sweeney JA, Hamer RM, et al. Efficiency of the CATIE and BACS neuropsychological batteries in assessing cognitive effects of antipsychotic treatments in schizophrenia. J Int Neuropsychol Soc. 2008;14:209–21. doi: 10.1017/S1355617708080570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Paul RH, Lawrence J, Williams LM, Richard CC, Cooper N, Gordon E. Preliminary validity of “integneuro”: a new computerized battery of neurocognitive tests. Int J Neurosci. 2005;115:1549–67. doi: 10.1080/00207450590957890. [DOI] [PubMed] [Google Scholar]
- [78].Pietrzak RH, Olver J, Norman T, Piskulic D, Maruff P, Snyder PJ. A comparison of the CogState Schizophrenia Battery and the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Battery in assessing cognitive impairment in chronic schizophrenia. J Clin Exp Neuropsychol. 2009;31:848–59. doi: 10.1080/13803390802592458. [DOI] [PubMed] [Google Scholar]
- [79].Pantelis C, Barnes TR, Nelson HE, et al. Frontal-striatal cognitive deficits in patients with chronic schizophrenia. Brain. 1997;120(Pt 10):1823–43. doi: 10.1093/brain/120.10.1823. [DOI] [PubMed] [Google Scholar]
- [80].Rugg MD, Coles MG. Electrophysiology of mind: Event-related brain potentials and cognition. Oxford University Press; 1995. [Google Scholar]
- [81].Boutros NN, Belger A, Campbell D, D’Souza C, Krystal J. Comparison of four components of sensory gating in schizophrenia and normal subjects: a preliminary report. Psychiatry Res. 1999;88:119–30. doi: 10.1016/s0165-1781(99)00074-8. [DOI] [PubMed] [Google Scholar]
- [82].Adler LE, Pachtman E, Franks RD, Pecevich M, Waldo MC, Freedman R. Neurophysiological evidence for a defect in neuronal mechanisms involved in sensory gating in schizophrenia. Biol Psychiatry. 1982;17:639–54. [PubMed] [Google Scholar]
- [83].Patterson JV, Hetrick WP, Boutros NN, et al. P50 sensory gating ratios in schizophrenics and controls: a review and data analysis. Psychiatry Res. 2008;158:226–47. doi: 10.1016/j.psychres.2007.02.009. [DOI] [PubMed] [Google Scholar]
- [84].Brockhaus-Dumke A, Schultze-Lutter F, Mueller R, et al. Sensory gating in schizophrenia: P50 and N100 gating in antipsychotic-free subjects at risk, first-episode, and chronic patients. Biol Psychiatry. 2008;64:376–84. doi: 10.1016/j.biopsych.2008.02.006. [DOI] [PubMed] [Google Scholar]
- [85].Naatanen R, Gaillard AW, Mantysalo S. Early selective-attention effect on evoked potential reinterpreted. Acta Psychol (Amst) 1978;42:313–29. doi: 10.1016/0001-6918(78)90006-9. [DOI] [PubMed] [Google Scholar]
- [86].Naatanen R, Alho K. Mismatch negativity--a unique measure of sensory processing in audition. Int J Neurosci. 1995;80:317–37. doi: 10.3109/00207459508986107. [DOI] [PubMed] [Google Scholar]
- [87].Naatanen R, Pakarinen S, Rinne T, Takegata R. The mismatch negativity (MMN): towards the optimal paradigm. Clin Neurophysiol. 2004;115:140–4. doi: 10.1016/j.clinph.2003.04.001. [DOI] [PubMed] [Google Scholar]
- [88].Näätänen R, Kähkönen S. Central auditory dysfunction in schizophrenia as revealed by the mismatch negativity (MMN) and its magnetic equivalent MMNm: A review. International Journal of Neuropsychopharmacology. 2009;12:125–135. doi: 10.1017/S1461145708009322. [DOI] [PubMed] [Google Scholar]
- [89].Umbricht D, Krljes S. Mismatch negativity in schizophrenia: a meta-analysis. Schizophr Res. 2005;76:1–23. doi: 10.1016/j.schres.2004.12.002. [DOI] [PubMed] [Google Scholar]
- [90].Umbricht D, Javitt D, Novak G, et al. Effects of clozapine on auditory event-related potentials in schizophrenia. Biol Psychiatry. 1998;44:716–25. doi: 10.1016/s0006-3223(97)00524-6. [DOI] [PubMed] [Google Scholar]
- [91].Umbricht D, Javitt D, Novak G, et al. Effects of risperidone on auditory event-related potentials in schizophrenia. Int J Neuropsychopharmacol. 1999;2:299–304. doi: 10.1017/S1461145799001595. [DOI] [PubMed] [Google Scholar]
- [92].Kasai K, Yamada H, Kamio S, et al. Do high or low doses of anxiolytics and hypnotics affect mismatch negativity in schizophrenic subjects? An EEG and MEG study. Clin Neurophysiol. 2002;113:141–50. doi: 10.1016/s1388-2457(01)00710-6. [DOI] [PubMed] [Google Scholar]
- [93].Catts SV, Shelley AM, Ward PB, et al. Brain potential evidence for an auditory sensory memory deficit in schizophrenia. American Journal of Psychiatry. 1995;152:213–219. doi: 10.1176/ajp.152.2.213. [DOI] [PubMed] [Google Scholar]
- [94].Umbricht D, Koller R, Schmid L, et al. How specific are deficits in mismatch negativity generation to schizophrenia? Biological Psychiatry. 2003;53:1120–1131. doi: 10.1016/s0006-3223(02)01642-6. [DOI] [PubMed] [Google Scholar]
- [95].Fisher DJ, Labelle A, Knott VJ. Auditory hallucinations and the mismatch negativity: Processing speech and non-speech sounds in schizophrenia. International Journal of Psychophysiology. 2008;70:3–15. doi: 10.1016/j.ijpsycho.2008.04.001. [DOI] [PubMed] [Google Scholar]
- [96].Dulude L, Labelle A, Knott VJ. Acute nicotine alteration of sensory memory impairment in smokers with schizophrenia. J Clin Psychopharmacol. 2010;30:541–8. doi: 10.1097/JCP.0b013e3181f0c9c6. [DOI] [PubMed] [Google Scholar]
- [97].Horton J, Millar A, Labelle A, Knott VJ. MMN responsivity to manipulations of frequency and duration deviants in chronic, clozapine-treated schizophrenia patients. Schizophrenia Research. 2011;126:202–211. doi: 10.1016/j.schres.2010.11.028. [DOI] [PubMed] [Google Scholar]
- [98].Rasser PE, Schall U, Todd J, et al. Gray matter deficits, mismatch negativity, and outcomes in schizophrenia. Schizophrenia Bulletin. 2011;37:131–140. doi: 10.1093/schbul/sbp060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Michie PT. What has MMN revealed about the auditory system in schizophrenia? Int J Psychophysiol. 2001;42:177–94. doi: 10.1016/s0167-8760(01)00166-0. [DOI] [PubMed] [Google Scholar]
- [100].Javitt DC, Doneshka P, Grochowski S, Ritter W. Impaired mismatch negativity generation reflects widespread dysfunction of working memory in schizophrenia. Arch Gen Psychiatry. 1995;52:550–8. doi: 10.1001/archpsyc.1995.03950190032005. [DOI] [PubMed] [Google Scholar]
- [101].Javitt DC, Grochowski S, Shelley AM, Ritter W. Impaired mismatch negativity (MMN) generation in schizophrenia as a function of stimulus deviance, probability, and interstimulus/interdeviant interval. Electroencephalogr Clin Neurophysiol. 1998;108:143–53. doi: 10.1016/s0168-5597(97)00073-7. [DOI] [PubMed] [Google Scholar]
- [102].Todd J, Michie PT, Schall U, Karayanidis F, Yabe H, Naatanen R. Deviant matters: duration, frequency, and intensity deviants reveal different patterns of mismatch negativity reduction in early and late schizophrenia. Biol Psychiatry. 2008;63:58–64. doi: 10.1016/j.biopsych.2007.02.016. [DOI] [PubMed] [Google Scholar]
- [103].Fisher DJ, Labelle A, Knott VJ. The right profile: mismatch negativity in schizophrenia with and without auditory hallucinations as measured by a multi-feature paradigm. Clin Neurophysiol. 2008;119:909–21. doi: 10.1016/j.clinph.2007.12.005. [DOI] [PubMed] [Google Scholar]
- [104].Todd J, Michie PT, Schall U, Karayanidis F, Yabe H, Näätänen R. Deviant matters: duration, frequency, and intensity deviants reveal different patterns of mismatch negativity reduction in early and late schizophrenia. Biological psychiatry. 2008;63:58–64. doi: 10.1016/j.biopsych.2007.02.016. [DOI] [PubMed] [Google Scholar]
- [105].Polich J. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118:2128–48. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Polich J, Criado JR. Neuropsychology and neuropharmacology of P3a and P3b. Int J Psychophysiol. 2006;60:172–85. doi: 10.1016/j.ijpsycho.2005.12.012. [DOI] [PubMed] [Google Scholar]
- [107].Polich J. Theoretical overview of P3a and P3b. In: Polich J, editor. Detection of Change: Event-related potential and IMRI findings. 2003. pp. 83–98. [Google Scholar]
- [108].Jeon YW, Polich J. Meta-analysis of P300 and schizophrenia: patients, paradigms, and practical implications. Psychophysiology. 2003;40:684–701. doi: 10.1111/1469-8986.00070. [DOI] [PubMed] [Google Scholar]
- [109].Heishma SJ, Taylor RC, Henningfield JE. Nicotine and smoking: A review of effects on human performance. Experimental and Clinical Psychopharmacology. 1994;2:345. [Google Scholar]
- [110].Heishman SJ, Kleykamp BA, Singleton EG. Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology. 2010;210:453–469. doi: 10.1007/s00213-010-1848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Wallace TL, Bertrand D. Alpha7 neuronal nicotinic receptors as a drug target in schizophrenia. Expert Opin Ther Targets. 2013;17:139–55. doi: 10.1517/14728222.2013.736498. [DOI] [PubMed] [Google Scholar]
- [112].D’Souza MS, Markou A. Schizophrenia and tobacco smoking comorbidity: nAChR agonists in the treatment of schizophrenia-associated cognitive deficits. Neuropharmacology. 2012;62:1564–1573. doi: 10.1016/j.neuropharm.2011.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Harris JG, Kongs S, Allensworth D, et al. Effects of nicotine on cognitive deficits in schizophrenia. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2004;29:1378. doi: 10.1038/sj.npp.1300450. [DOI] [PubMed] [Google Scholar]
- [114].Larrison-Faucher AL, Matorin AA, Sereno AB. Nicotine reduces antisaccade errors in task impaired schizophrenic subjects. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2004;28:505–516. doi: 10.1016/j.pnpbp.2004.01.002. [DOI] [PubMed] [Google Scholar]
- [115].Tanabe J, Tregellas JR, Martin LF, Freedman R. Effects of nicotine on hippocampal and cingulate activity during smooth pursuit eye movement in schizophrenia. Biological psychiatry. 2006;59:754–761. doi: 10.1016/j.biopsych.2005.09.004. [DOI] [PubMed] [Google Scholar]
- [116].Dulude L, Labelle A, Knott VJ. Acute nicotine alteration of sensory memory impairment in smokers with schizophrenia. Journal of clinical psychopharmacology. 2010;30:541. doi: 10.1097/JCP.0b013e3181f0c9c6. [DOI] [PubMed] [Google Scholar]
- [117].Levin ED, Wilson W, Rose JE, McEvoy J. Nicotine-haloperidol interactions and cognitive performance in schizophrenics. Neuropsychopharmacology. 1996;15:429–436. doi: 10.1016/S0893-133X(96)00018-8. [DOI] [PubMed] [Google Scholar]
- [118].Adler LE, Olincy A, Waldo M, et al. Schizophrenia, sensory gating, and nicotinic receptors. Schizophrenia bulletin. 1998;24:189–202. doi: 10.1093/oxfordjournals.schbul.a033320. [DOI] [PubMed] [Google Scholar]
- [119].Dalack GW, Becks L, Hill E, Pomerleau OF, Meador-Woodruff JH. Nicotine withdrawal and psychiatric symptoms in cigarette smokers with schizophrenia. Neuropsychopharmacology. 1999;21:195–202. doi: 10.1016/S0893-133X(98)00121-3. [DOI] [PubMed] [Google Scholar]
- [120].Dépatie L, O’Driscoll GA, Holahan ALV, et al. Nicotine and behavioral markers of risk for schizophrenia: a double-blind, placebo-controlled, cross-over study. Neuropsychopharmacology. 2002;27:1056–1070. doi: 10.1016/S0893-133X(02)00372-X. [DOI] [PubMed] [Google Scholar]
- [121].Inami R, Kirino E, Inoue R, Suzuki T, Arai H. Nicotine effects on mismatch negativity in nonsmoking schizophrenic patients. Neuropsychobiology. 2007;56:64–72. doi: 10.1159/000111536. [DOI] [PubMed] [Google Scholar]
- [122].Barr RS, Culhane MA, Jubelt LE, et al. The effects of transdermal nicotine on cognition in nonsmokers with schizophrenia and nonpsychiatric controls. Neuropsychopharmacology. 2007;33:480–490. doi: 10.1038/sj.npp.1301423. [DOI] [PubMed] [Google Scholar]
- [123].Jubelt LE, Barr RS, Goff DC, Logvinenko T, Weiss AP, Evins AE. Effects of transdermal nicotine on episodic memory in nonsmokers with and without schizophrenia. Psychopharmacology. 2008;199:89–98. doi: 10.1007/s00213-008-1133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Hong LE, Schroeder M, Ross TJ, et al. Nicotine enhances but does not normalize visual sustained attention and the associated brain network in schizophrenia. Schizophrenia bulletin. 2011;37:416–425. doi: 10.1093/schbul/sbp089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Jacobsen LK, D’Souza DC, Mencl WE, Pugh KR, Skudlarski P, Krystal JH. Nicotine effects on brain function and functional connectivity in schizophrenia. Biol Psychiatry. 2004;55:850–8. doi: 10.1016/j.biopsych.2003.12.023. [DOI] [PubMed] [Google Scholar]
- [126].Sherr JD, Myers C, Avila MT, Elliott A, Blaxton TA, Thaker GK. The effects of nicotine on specific eye tracking measures in schizophrenia. Biological psychiatry. 2002;52:721–728. doi: 10.1016/s0006-3223(02)01342-2. [DOI] [PubMed] [Google Scholar]
- [127].Smith RC, Singh A, Infante M, Khandat A, Kloos A. Effects of cigarette smoking and nicotine nasal spray on psychiatric symptoms and cognition in schizophrenia. Neuropsychopharmacology. 2002;27:479–497. doi: 10.1016/S0893-133X(02)00324-X. [DOI] [PubMed] [Google Scholar]
- [128].Myers CS, Robles O, Kakoyannis AN, et al. Nicotine improves delayed recognition in schizophrenic patients. Psychopharmacology. 2004;174:334–340. doi: 10.1007/s00213-003-1764-8. [DOI] [PubMed] [Google Scholar]
- [129].Smith RC, Warner-Cohen J, Matute M, et al. Effects of nicotine nasal spray on cognitive function in schizophrenia. Neuropsychopharmacology. 2005;31:637–643. doi: 10.1038/sj.npp.1300881. [DOI] [PubMed] [Google Scholar]
- [130].Olincy A, Johnson LL, Ross RG. Differential effects of cigarette smoking on performance of a smooth pursuit and a saccadic eye movement task in schizophrenia. Psychiatry research. 2003;117:223–236. doi: 10.1016/s0165-1781(03)00022-2. [DOI] [PubMed] [Google Scholar]
- [131].Hahn B, Harvey AN, Concheiro-Guisan M, Huestis MA, Holcomb HH, Gold JM. A Test of the Cognitive Self-Medication Hypothesis of Tobacco Smoking in Schizophrenia. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Postma P, Gray JA, Sharma T, et al. A behavioural and functional neuroimaging investigation into the effects of nicotine on sensorimotor gating in healthy subjects and persons with schizophrenia. Psychopharmacology. 2006;184:589–599. doi: 10.1007/s00213-006-0307-5. [DOI] [PubMed] [Google Scholar]
- [133].Sacco KA, Termine A, Seyal A, et al. Effects of cigarette smoking on spatial working memory and attentional deficits in schizophrenia: involvement of nicotinic receptor mechanisms. Arch Gen Psychiatry. 2005;62:649–59. doi: 10.1001/archpsyc.62.6.649. [DOI] [PubMed] [Google Scholar]
- [134].Hong LE, Summerfelt A, Mitchell BD, et al. Sensory gating endophenotype based on its neural oscillatory pattern and heritability estimate. Arch Gen Psychiatry. 2008;65:1008–16. doi: 10.1001/archpsyc.65.9.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Adler LE, Olincy A, Waldo M, et al. Schizophrenia, sensory gating, and nicotinic receptors. Schizophr Bull. 1998;24:189–202. doi: 10.1093/oxfordjournals.schbul.a033320. [DOI] [PubMed] [Google Scholar]
- [136].Turetsky BI, Dent G, Jaeger J, Zukin SR. P50 amplitude reduction: a nicotinic receptor-mediated deficit in first-degree relatives of schizophrenia patients. Psychopharmacology (Berl) 2012;221:39–52. doi: 10.1007/s00213-011-2544-5. [DOI] [PubMed] [Google Scholar]
- [137].Adler LE, Olincy A, Cawthra EM, et al. Varied effects of atypical neuroleptics on P50 auditory gating in schizophrenia patients. Am J Psychiatry. 2004;161:1822–8. doi: 10.1176/ajp.161.10.1822. [DOI] [PubMed] [Google Scholar]
- [138].Su L, Cai Y, Wang L, Shi S. Various effects of antipsychotics on P50 sensory gating in Chinese schizophrenia patients: a metaanalysis. Psychiatr Danub. 2012;24:44–50. [PubMed] [Google Scholar]
- [139].Nagamoto HT, Adler LE, McRae KA, et al. Auditory P50 in schizophrenics on clozapine: improved gating parallels clinical improvement and changes in plasma 3-methoxy-4-hydroxyphenylglycol. Neuropsychobiology. 1999;39:10–7. doi: 10.1159/000026553. [DOI] [PubMed] [Google Scholar]
- [140].Becker J, Gomes I, Ghisolfi ES, et al. Clozapine, but not typical antipsychotics, correct P50 suppression deficit in patients with schizophrenia. Clin Neurophysiol. 2004;115:396–401. doi: 10.1016/j.clinph.2003.09.018. [DOI] [PubMed] [Google Scholar]
- [141].Brinkmeyer J, Mobascher A, Musso F, et al. P50 sensory gating and smoking in the general population. Addict Biol. 2011;16:485–98. doi: 10.1111/j.1369-1600.2010.00302.x. [DOI] [PubMed] [Google Scholar]
- [142].Crawford HJ, McClain-Furmanski D, Castagnoli N, Jr., Castagnoli K. Enhancement of auditory sensory gating and stimulus-bound gamma band (40 Hz) oscillations in heavy tobacco smokers. Neurosci Lett. 2002;317:151–5. doi: 10.1016/s0304-3940(01)02454-5. [DOI] [PubMed] [Google Scholar]
- [143].Croft RJ, Dimoska A, Gonsalvez CJ, Clarke AR. Suppression of P50 evoked potential component, schizotypal beliefs and smoking. Psychiatry Res. 2004;128:53–62. doi: 10.1016/j.psychres.2004.05.009. [DOI] [PubMed] [Google Scholar]
- [144].Wan L, Crawford HJ, Boutros N. P50 sensory gating: impact of high vs. low schizotypal personality and smoking status. Int J Psychophysiol. 2006;60:1–9. doi: 10.1016/j.ijpsycho.2005.03.024. [DOI] [PubMed] [Google Scholar]
- [145].Knott V, de la Salle S, Smith D, et al. Baseline dependency of nicotine’s sensory gating actions: similarities and differences in low, medium and high P50 suppressors. J Psychopharmacol. 2013 doi: 10.1177/0269881113490449. [DOI] [PubMed] [Google Scholar]
- [146].Simosky JK, Stevens KE, Freedman R. Nicotinic agonists and psychosis. Curr Drug Targets CNS Neurol Disord. 2002;1:149–62. doi: 10.2174/1568007024606168. [DOI] [PubMed] [Google Scholar]
- [147].Winterer G, Gallinat J, Brinkmeyer J, et al. Allosteric alpha-7 nicotinic receptor modulation and P50 sensory gating in schizophrenia: a proof-of-mechanism study. Neuropharmacology. 2013;64:197–204. doi: 10.1016/j.neuropharm.2012.06.040. [DOI] [PubMed] [Google Scholar]
- [148].Zhang XY, Liu L, Liu S, et al. Short-term tropisetron treatment and cognitive and P50 auditory gating deficits in schizophrenia. Am J Psychiatry. 2012;169:974–81. doi: 10.1176/appi.ajp.2012.11081289. [DOI] [PubMed] [Google Scholar]
- [149].Inami R, Kirino E, Inoue R, Arai H. Transdermal nicotine administration enhances automatic auditory processing reflected by mismatch negativity. Pharmacol Biochem Behav. 2005;80:453–61. doi: 10.1016/j.pbb.2005.01.001. [DOI] [PubMed] [Google Scholar]
- [150].Harkrider AW, Hedrick MS. Acute effect of nicotine on auditory gating in smokers and non-smokers. Hear Res. 2005;202:114–28. doi: 10.1016/j.heares.2004.11.009. [DOI] [PubMed] [Google Scholar]
- [151].Baldeweg T, Wong D, Stephan KE. Nicotinic modulation of human auditory sensory memory: Evidence from mismatch negativity potentials. Int J Psychophysiol. 2006;59:49–58. doi: 10.1016/j.ijpsycho.2005.07.014. [DOI] [PubMed] [Google Scholar]
- [152].Martin LF, Davalos DB, Kisley MA. Nicotine enhances automatic temporal processing as measured by the mismatch negativity waveform. Nicotine Tob Res. 2009;11:698–706. doi: 10.1093/ntr/ntp052. [DOI] [PubMed] [Google Scholar]
- [153].Dunbar G, Boeijinga PH, Demazieres A, et al. Effects of TC-1734 (AZD3480), a selective neuronal nicotinic receptor agonist, on cognitive performance and the EEG of young healthy male volunteers. Psychopharmacology (Berl) 2007;191:919–29. doi: 10.1007/s00213-006-0675-x. [DOI] [PubMed] [Google Scholar]
- [154].Fisher DJ, Grant B, Smith DM, Borracci G, Labelle A, Knott VJ. Nicotine and the hallucinating brain: Effects on mismatch negativity (MMN) in schizophrenia. Psychiatry Research. 2012;196:181–187. doi: 10.1016/j.psychres.2012.01.026. [DOI] [PubMed] [Google Scholar]
- [155].Hirayasu Y, Potts GF, Kwon JS, et al. Auditory mismatch negativity in schizophrenia: topographic evaluation with a highdensity recording montage. American Journal of Psychiatry. 1998;155:1281–1284. doi: 10.1176/ajp.155.9.1281. [DOI] [PubMed] [Google Scholar]
- [156].Youn T, Park H-J, Kim J-J, Kim MS, Kwon JS. Altered hemispheric asymmetry and positive symptoms in schizophrenia: equivalent current dipole of auditory mismatch negativity. Schizophrenia research. 2003;59:253–260. doi: 10.1016/s0920-9964(02)00154-8. [DOI] [PubMed] [Google Scholar]
- [157].Fisher DJ, Grant B, Smith DM, Borracci G, Labelle A, Knott VJ. Effects of auditory hallucinations on the mismatch negativity (MMN) in schizophrenia as measured by a modified ‘optimal’ multi-feature paradigm. International Journal of Psychophysiology. 2011;81:245–251. doi: 10.1016/j.ijpsycho.2011.06.018. [DOI] [PubMed] [Google Scholar]
- [158].Houlihan ME, Pritchard WS, Robinson JH. Effects of smoking/nicotine on performance and event-related potentials during a short-term memory scanning task. Psychopharmacology (Berl) 2001;156:388–96. doi: 10.1007/s002130100751. [DOI] [PubMed] [Google Scholar]
- [159].Knott V, Bosman M, Mahoney C, Ilivitsky V, Quirt K. Transdermal nicotine: single dose effects on mood, EEG, performance, and event-related potentials. Pharmacol Biochem Behav. 1999;63:253–61. doi: 10.1016/s0091-3057(99)00006-4. [DOI] [PubMed] [Google Scholar]
- [160].Kodama E, Morita K, Kinoshita S, Miyahira A, Nakamura J, Maeda H. Inhibitory effect of smoking on event-related potentials in schizophrenic patients: Comparison with healthy subjects. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 1998;22:1275–1288. [Google Scholar]
- [161].Edwards JA, Wesnes K, Warburton DM, Gale A. Evidence of more rapid stimulus evaluation following cigarette smoking. Addict Behav. 1985;10:113–26. doi: 10.1016/0306-4603(85)90017-6. [DOI] [PubMed] [Google Scholar]
- [162].Houlihan ME, Pritchard WS, Robinson JH. Faster P300 latency after smoking in visual but not auditory oddball tasks. Psychopharmacology (Berl) 1996;123:231–8. doi: 10.1007/BF02246577. [DOI] [PubMed] [Google Scholar]
- [163].Michel C, Hasenfratz M, Nil R, Battig K. Cardiovascular, electrocortical, and behavioral effects of nicotine chewing gum. Klin Wochenschr. 1988;66(Suppl 11):72–9. [PubMed] [Google Scholar]
- [164].Lindgren M, Stenberg G, Rosen I. Effects of nicotine in a bimodal attention task. Neuropsychobiology. 1998;38:42–9. doi: 10.1159/000026515. [DOI] [PubMed] [Google Scholar]
- [165].Lindgren M, Molander L, Verbaan C, Lunell E, Rosen I. Electroencephalographic effects of intravenous nicotine--a dose-response study. Psychopharmacology (Berl) 1999;145:342–50. doi: 10.1007/s002130051067. [DOI] [PubMed] [Google Scholar]
- [166].Knott V, McIntosh J, Millar A, et al. Nicotine and smoker status moderate brain electric and mood activation induced by ketamine, an N-methyl-D-aspartate (NMDA) receptor antagonist. Pharmacol Biochem Behav. 2006;85:228–42. doi: 10.1016/j.pbb.2006.08.005. [DOI] [PubMed] [Google Scholar]
- [167].Anokhin AP, Vedeniapin AB, Sirevaag EJ, et al. The P300 brain potential is reduced in smokers. Psychopharmacology (Berl) 2000;149:409–13. doi: 10.1007/s002130000387. [DOI] [PubMed] [Google Scholar]
- [168].Neuhaus A, Bajbouj M, Kienast T, et al. Persistent dysfunctional frontal lobe activation in former smokers. Psychopharmacology (Berl) 2006;186:191–200. doi: 10.1007/s00213-006-0366-7. [DOI] [PubMed] [Google Scholar]
- [169].Ascioglu M, Dolu N, Golgeli A, Suer C, Ozesmi C. Effects of cigarette smoking on cognitive processing. Int J Neurosci. 2004;114:381–90. doi: 10.1080/00207450490270668. [DOI] [PubMed] [Google Scholar]
- [170].Evans DE, Maxfield ND, Van Rensburg KJ, Oliver JA, Jentink KG, Drobes DJ. Nicotine Deprivation Influences P300 Markers of Cognitive Control. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Mobascher A, Warbrick T, Brinkmeyer J, et al. Nicotine effects on anterior cingulate cortex in schizophrenia and healthy smokers as revealed by EEG-informed fMRI. Psychiatry Research: Neuroimaging. 2012;204:168–177. doi: 10.1016/j.pscychresns.2012.09.005. [DOI] [PubMed] [Google Scholar]
- [172].Tregellas JR, Tanabe JL, Martin LF, Freedman R. fMRI of response to nicotine during a smooth pursuit eye movement task in schizophrenia. American Journal of Psychiatry. 2005;162:391–393. doi: 10.1176/appi.ajp.162.2.391. [DOI] [PubMed] [Google Scholar]
- [173].Breier A, Malhotra AK, Pinals DA, Weisenfeld NI, Pickar D. Association of ketamine-induced psychosis with focal activation of the prefrontal cortex in healthy volunteers. Am J Psychiatry. 1997;154:805–11. doi: 10.1176/ajp.154.6.805. [DOI] [PubMed] [Google Scholar]
- [174].Krystal JH, D’Souza DC, Karper LP, et al. Interactive effects of subanesthetic ketamine and haloperidol in healthy humans. Psychopharmacology (Berl) 1999;145:193–204. doi: 10.1007/s002130051049. [DOI] [PubMed] [Google Scholar]
- [175].Lahti AC, Holcomb HH, Medoff DR, Tamminga CA. Ketamine activates psychosis and alters limbic blood flow in schizophrenia. Neuroreport. 1995;6:869–72. doi: 10.1097/00001756-199504190-00011. [DOI] [PubMed] [Google Scholar]
- [176].Lambe EK, Picciotto MR, Aghajanian GK. Nicotine induces glutamate release from thalamocortical terminals in prefrontal cortex. Neuropsychopharmacology. 2003;28:216–25. doi: 10.1038/sj.npp.1300032. [DOI] [PubMed] [Google Scholar]
- [177].D’Souza DC, Ahn K, Bhakta S, et al. Nicotine fails to attenuate ketamine-induced cognitive deficits and negative and positive symptoms in humans: implications for schizophrenia. Biol Psychiatry. 2012;72:785–94. doi: 10.1016/j.biopsych.2012.05.009. [DOI] [PubMed] [Google Scholar]
- [178].Rowland LM, Beason-Held L, Tamminga CA, Holcomb HH. The interactive effects of ketamine and nicotine on human cerebral blood flow. Psychopharmacology (Berl) 2010;208:575–84. doi: 10.1007/s00213-009-1758-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Knott VJ, Millar AM, McIntosh JF, et al. Separate and combined effects of low dose ketamine and nicotine on behavioural and neural correlates of sustained attention. Biol Psychol. 2011;88:83–93. doi: 10.1016/j.biopsycho.2011.06.012. [DOI] [PubMed] [Google Scholar]
- [180].Jackson A, Nesic J, Groombridge C, Clowry O, Rusted J, Duka T. Differential involvement of glutamatergic mechanisms in the cognitive and subjective effects of smoking. Neuropsychopharmacology. 2009;34:257–65. doi: 10.1038/npp.2008.50. [DOI] [PubMed] [Google Scholar]
- [181].Stryjer R, Strous RD, Bar F, et al. Beneficial effect of donepezil augmentation for the management of comorbid schizophrenia and dementia. Clin Neuropharmacol. 2003;26:12–7. doi: 10.1097/00002826-200301000-00004. [DOI] [PubMed] [Google Scholar]
- [182].Buchanan RW, Summerfelt A, Tek C, Gold J. An open-labeled trial of adjunctive donepezil for cognitive impairments in patients with schizophrenia. Schizophr Res. 2003;59:29–33. doi: 10.1016/s0920-9964(01)00387-5. [DOI] [PubMed] [Google Scholar]
- [183].Mendelsohn E, Rosenthal M, Bohiri Y, Werber E, Kotler M, Strous RD. Rivastigmine augmentation in the management of chronic schizophrenia with comorbid dementia: an open-label study investigating effects on cognition, behaviour and activities of daily living. Int Clin Psychopharmacol. 2004;19:319–24. doi: 10.1097/00004850-200411000-00001. [DOI] [PubMed] [Google Scholar]
- [184].Lenzi A, Maltinti E, Poggi E, Fabrizio L, Coli E. Effects of rivastigmine on cognitive function and quality of life in patients with schizophrenia. Clin Neuropharmacol. 2003;26:317–21. doi: 10.1097/00002826-200311000-00011. [DOI] [PubMed] [Google Scholar]
- [185].Bora E, Veznedaroglu B, Kayahan B. The effect of galantamine added to clozapine on cognition of five patients with schizophrenia. Clin Neuropharmacol. 2005;28:139–41. doi: 10.1097/01.wnf.0000162555.68729.04. [DOI] [PubMed] [Google Scholar]
- [186].Risch SC, McGurk S, Horner MD, et al. A double-blind placebo-controlled case study of the use of donepezil to improve cognition in a schizoaffective disorder patient: functional MRI correlates. Neurocase. 2001;7:105–10. doi: 10.1093/neucas/7.2.105. [DOI] [PubMed] [Google Scholar]
- [187].Tugal O, Yazici KM, Anil Yagcioglu AE, Gogus A. A double-blind, placebo controlled, cross-over trial of adjunctive donepezil for cognitive impairment in schizophrenia. Int J Neuropsychopharmacol. 2004;7:117–23. doi: 10.1017/S1461145703004024. [DOI] [PubMed] [Google Scholar]
- [188].Erickson SK, Schwarzkopf SB, Palumbo D, Badgley-Fleeman J, Smirnow AM, Light GA. Efficacy and tolerability of low-dose donepezil in schizophrenia. Clin Neuropharmacol. 2005;28:179–84. doi: 10.1097/01.wnf.0000173714.61744.e6. [DOI] [PubMed] [Google Scholar]
- [189].Nahas Z, George MS, Horner MD, et al. Augmenting atypical antipsychotics with a cognitive enhancer (donepezil) improves regional brain activity in schizophrenia patients: a pilot double-blind placebo controlled BOLD fMRI study. Neurocase. 2003;9:274–82. doi: 10.1076/neur.9.3.274.15563. [DOI] [PubMed] [Google Scholar]
- [190].Friedman JI, Adler DN, Howanitz E, et al. A double blind placebo controlled trial of donepezil adjunctive treatment to risperidone for the cognitive impairment of schizophrenia. Biol Psychiatry. 2002;51:349–57. doi: 10.1016/s0006-3223(01)01342-7. [DOI] [PubMed] [Google Scholar]
- [191].Lee BJ, Lee JG, Kim YH. A 12-week, double-blind, placebo-controlled trial of donepezil as an adjunct to haloperidol for treating cognitive impairments in patients with chronic schizophrenia. J Psychopharmacol. 2007;21:421–7. doi: 10.1177/0269881106070996. [DOI] [PubMed] [Google Scholar]
- [192].Lee SW, Lee JG, Lee BJ, Kim YH. A 12-week, double-blind, placebo-controlled trial of galantamine adjunctive treatment to conventional antipsychotics for the cognitive impairments in chronic schizophrenia. Int Clin Psychopharmacol. 2007;22:63–8. doi: 10.1097/YIC.0b013e3280117feb. [DOI] [PubMed] [Google Scholar]
- [193].Freudenreich O, Herz L, Deckersbach T, et al. Added donepezil for stable schizophrenia: a double-blind, placebo-controlled trial. Psychopharmacology (Berl) 2005;181:358–63. doi: 10.1007/s00213-005-2235-1. [DOI] [PubMed] [Google Scholar]
- [194].Mazeh D, Zemishlani H, Barak Y, Mirecki I, Paleacu D. Donepezil for negative signs in elderly patients with schizophrenia: an add-on, double-blind, crossover, placebo-controlled study. Int Psychogeriatr. 2006;18:429–36. doi: 10.1017/S1041610205003017. [DOI] [PubMed] [Google Scholar]
- [195].Kohler CG, Martin EA, Kujawski E, Bilker W, Gur RE, Gur RC. No effect of donepezil on neurocognition and social cognition in young persons with stable schizophrenia. Cogn Neuropsychiatry. 2007;12:412–21. doi: 10.1080/13546800701307263. [DOI] [PubMed] [Google Scholar]
- [196].Fagerlund B, Soholm B, Fink-Jensen A, Lublin H, Glenthoj BY. Effects of donepezil adjunctive treatment to ziprasidone on cognitive deficits in schizophrenia: a double-blind, placebo-controlled study. Clin Neuropharmacol. 2007;30:3–12. doi: 10.1097/01.WNF.0000240940.67241.F6. [DOI] [PubMed] [Google Scholar]
- [197].Akhondzadeh S, Gerami M, Noroozian M, et al. A 12-week, double-blind, placebo-controlled trial of donepezil adjunctive treatment to risperidone in chronic and stable schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1810–5. doi: 10.1016/j.pnpbp.2008.08.001. [DOI] [PubMed] [Google Scholar]
- [198].Keefe RS, Malhotra AK, Meltzer HY, et al. Efficacy and safety of donepezil in patients with schizophrenia or schizoaffective disorder: significant placebo/practice effects in a 12-week, randomized, double-blind, placebo-controlled trial. Neuropsychopharmacology. 2008;33:1217–28. doi: 10.1038/sj.npp.1301499. [DOI] [PubMed] [Google Scholar]
- [199].Keefe RS, Mohs RC, Bilder RM, et al. Neurocognitive assessment in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) project schizophrenia trial: development, methodology, and rationale. Schizophr Bull. 2003;29:45–55. doi: 10.1093/oxfordjournals.schbul.a006990. [DOI] [PubMed] [Google Scholar]
- [200].Keefe RS, Bilder RM, Harvey PD, et al. Baseline neurocognitive deficits in the CATIE schizophrenia trial. Neuropsychopharmacology. 2006;31:2033–46. doi: 10.1038/sj.npp.1301072. [DOI] [PubMed] [Google Scholar]
- [201].Aasen I, Kumari V, Sharma T. Effects of rivastigmine on sustained attention in schizophrenia: an FMRI study. J Clin Psychopharmacol. 2005;25:311–7. doi: 10.1097/01.jcp.0000169267.36797.76. [DOI] [PubMed] [Google Scholar]
- [202].Kumari V, Aasen I, ffytche D, Williams SC, Sharma T. Neural correlates of adjunctive rivastigmine treatment to antipsychotics in schizophrenia: a randomized, placebo-controlled, double-blind fMRI study. Neuroimage. 2006;29:545–56. doi: 10.1016/j.neuroimage.2005.08.013. [DOI] [PubMed] [Google Scholar]
- [203].Sharma T, Reed C, Aasen I, Kumari V. Cognitive effects of adjunctive 24-weeks Rivastigmine treatment to antipsychotics in schizophrenia: a randomized, placebo-controlled, double-blind investigation. Schizophr Res. 2006;85:73–83. doi: 10.1016/j.schres.2006.03.037. [DOI] [PubMed] [Google Scholar]
- [204].Chouinard S, Stip E, Poulin J, et al. Rivastigmine treatment as an add-on to antipsychotics in patients with schizophrenia and cognitive deficits. Curr Med Res Opin. 2007;23:575–83. doi: 10.1185/030079906X167372. [DOI] [PubMed] [Google Scholar]
- [205].Guillem F, Chouinard S, Poulin J, et al. Are cholinergic enhancers beneficial for memory in schizophrenia? An event-related potentials (ERPs) study of rivastigmine add-on therapy in a crossover trial. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:934–45. doi: 10.1016/j.pnpbp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- [206].Lopes C, Pereira EF, Wu HQ, et al. Competitive antagonism between the nicotinic allosteric potentiating ligand galantamine and kynurenic acid at alpha7* nicotinic receptors. J Pharmacol Exp Ther. 2007;322:48–58. doi: 10.1124/jpet.107.123109. [DOI] [PubMed] [Google Scholar]
- [207].Schilstrom B, Ivanov VB, Wiker C, Svensson TH. Galantamine enhances dopaminergic neurotransmission in vivo via allosteric potentiation of nicotinic acetylcholine receptors. Neuropsychopharmacology. 2007;32:43–53. doi: 10.1038/sj.npp.1301087. [DOI] [PubMed] [Google Scholar]
- [208].Samochocki M, Hoffle A, Fehrenbacher A, et al. Galantamine is an allosterically potentiating ligand of neuronal nicotinic but not of muscarinic acetylcholine receptors. J Pharmacol Exp Ther. 2003;305:1024–36. doi: 10.1124/jpet.102.045773. [DOI] [PubMed] [Google Scholar]
- [209].Dajas-Bailador FA, Heimala K, Wonnacott S. The allosteric potentiation of nicotinic acetylcholine receptors by galantamine is transduced into cellular responses in neurons: Ca2+ signals and neurotransmitter release. Mol Pharmacol. 2003;64:1217–26. doi: 10.1124/mol.64.5.1217. [DOI] [PubMed] [Google Scholar]
- [210].Schubert MH, Young KA, Hicks PB. Galantamine improves cognition in schizophrenic patients stabilized on risperidone. Biol Psychiatry. 2006;60:530–3. doi: 10.1016/j.biopsych.2006.04.006. [DOI] [PubMed] [Google Scholar]
- [211].Dyer MA, Freudenreich O, Culhane MA, et al. High-dose galantamine augmentation inferior to placebo on attention, inhibitory control and working memory performance in nonsmokers with schizophrenia. Schizophr Res. 2008;102:88–95. doi: 10.1016/j.schres.2007.12.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Sacco KA, Creeden C, Reutenauer EL, George TP. Effects of galantamine on cognitive deficits in smokers and non-smokers with schizophrenia. Schizophr Res. 2008;103:326–7. doi: 10.1016/j.schres.2008.05.004. [DOI] [PubMed] [Google Scholar]
- [213].Caroff SN, Walker P, Campbell C, et al. Treatment of tardive dyskinesia with galantamine: a randomized controlled crossover trial. J Clin Psychiatry. 2007;68:410–5. doi: 10.4088/jcp.v68n0309. [DOI] [PubMed] [Google Scholar]
- [214].Lindenmayer JP, Khan A. Galantamine augmentation of long-acting injectable risperidone for cognitive impairments in chronic schizophrenia. Schizophr Res. 2011;125:267–77. doi: 10.1016/j.schres.2010.08.021. [DOI] [PubMed] [Google Scholar]
- [215].Buchanan RW, Conley RR, Dickinson D, et al. Galantamine for the treatment of cognitive impairments in people with schizophrenia. Am J Psychiatry. 2008;165:82–9. doi: 10.1176/appi.ajp.2007.07050724. [DOI] [PubMed] [Google Scholar]
- [216].Singh J, Kour K, Jayaram MB. Acetylcholinesterase inhibitors for schizophrenia. Cochrane Database Syst Rev. 2012;1:CD007967. doi: 10.1002/14651858.CD007967.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Mol Pharmacol. 2006;70:801–5. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- [218].Fiore M. Treating tobacco use and dependence: 2008 update: Clinical practice guideline. DIANE Publishing; 2008. [Google Scholar]
- [219].Ahmed AI, Ali AN, Kramers C, Harmark LV, Burger DM, Verhoeven WM. Neuropsychiatric adverse events of varenicline: a systematic review of published reports. J Clin Psychopharmacol. 2013;33:55–62. doi: 10.1097/JCP.0b013e31827c0117. [DOI] [PubMed] [Google Scholar]
- [220].Shim JC, Jung DU, Jung SS, et al. Adjunctive varenicline treatment with antipsychotic medications for cognitive impairments in people with schizophrenia: a randomized double-blind placebo-controlled trial. Neuropsychopharmacology. 2012;37:660–8. doi: 10.1038/npp.2011.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Smith RC, Lindenmayer JP, Davis JM, et al. Cognitive and antismoking effects of varenicline in patients with schizophrenia or schizoaffective disorder. Schizophr Res. 2009;110:149–55. doi: 10.1016/j.schres.2009.02.001. [DOI] [PubMed] [Google Scholar]
- [222].Hong LE, Thaker GK, McMahon RP, et al. Effects of moderate-dose treatment with varenicline on neurobiological and cognitive biomarkers in smokers and nonsmokers with schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 2011;68:1195–206. doi: 10.1001/archgenpsychiatry.2011.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Waldo MC, Woodward L, Adler LE. Varenicline and P50 auditory gating in medicated schizophrenic patients: a pilot study. Psychiatry Res. 2010;175:179–80. doi: 10.1016/j.psychres.2009.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Dutra SJ, Stoeckel LE, Carlini SV, Pizzagalli DA, Evins AE. Varenicline as a smoking cessation aid in schizophrenia: effects on smoking behavior and reward sensitivity. Psychopharmacology (Berl) 2012;219:25–34. doi: 10.1007/s00213-011-2373-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Velligan D, Brenner R, Sicuro F, et al. Assessment of the effects of AZD3480 on cognitive function in patients with schizophrenia. Schizophr Res. 2012;134:59–64. doi: 10.1016/j.schres.2011.10.004. [DOI] [PubMed] [Google Scholar]
- [226].Olincy A, Harris JG, Johnson LL, et al. Proof-of-concept trial of an alpha7 nicotinic agonist in schizophrenia. Arch Gen Psychiatry. 2006;63:630–8. doi: 10.1001/archpsyc.63.6.630. [DOI] [PubMed] [Google Scholar]
- [227].Freedman R, Olincy A, Buchanan RW, et al. Initial phase 2 trial of a nicotinic agonist in schizophrenia. Am J Psychiatry. 2008;165:1040–7. doi: 10.1176/appi.ajp.2008.07071135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Tregellas JR, Olincy A, Johnson L, et al. Functional magnetic resonance imaging of effects of a nicotinic agonist in schizophrenia. Neuropsychopharmacology. 2010;35:938–42. doi: 10.1038/npp.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Tregellas JR, Tanabe J, Rojas DC, et al. Effects of an alpha 7-nicotinic agonist on default network activity in schizophrenia. Biol Psychiatry. 2011;69:7–11. doi: 10.1016/j.biopsych.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Shiina A, Shirayama Y, Niitsu T, et al. A randomised, double-blind, placebo-controlled trial of tropisetron in patients with schizophrenia. Ann Gen Psychiatry. 2010;9:27. doi: 10.1186/1744-859X-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Lieberman JA, Dunbar G, Segreti AC, et al. A randomized exploratory trial of an alpha-7 nicotinic receptor agonist (TC-5619) for cognitive enhancement in schizophrenia. Neuropsychopharmacology. 2013;38:968–75. doi: 10.1038/npp.2012.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Williams JM, Anthenelli RM, Morris CD, et al. A randomized, double-blind, placebo-controlled study evaluating the safety and efficacy of varenicline for smoking cessation in patients with schizophrenia or schizoaffective disorder. J Clin Psychiatry. 2012;73:654–60. doi: 10.4088/JCP.11m07522. [DOI] [PubMed] [Google Scholar]
- [233].Ozbilen M, Adams CE, Marley J. Anticholinergic effects of oral antipsychotic drugs of typicals versus atypicals over medium- and long-term: systematic review and meta-analysis. Curr Med Chem. 2012;19:5214–8. doi: 10.2174/092986712803530476. [DOI] [PubMed] [Google Scholar]
- [234].Stormer VS, Passow S, Biesenack J, Li SC. Dopaminergic and cholinergic modulations of visual-spatial attention and working memory: insights from molecular genetic research and implications for adult cognitive development. Dev Psychol. 2012;48:875–89. doi: 10.1037/a0026198. [DOI] [PubMed] [Google Scholar]