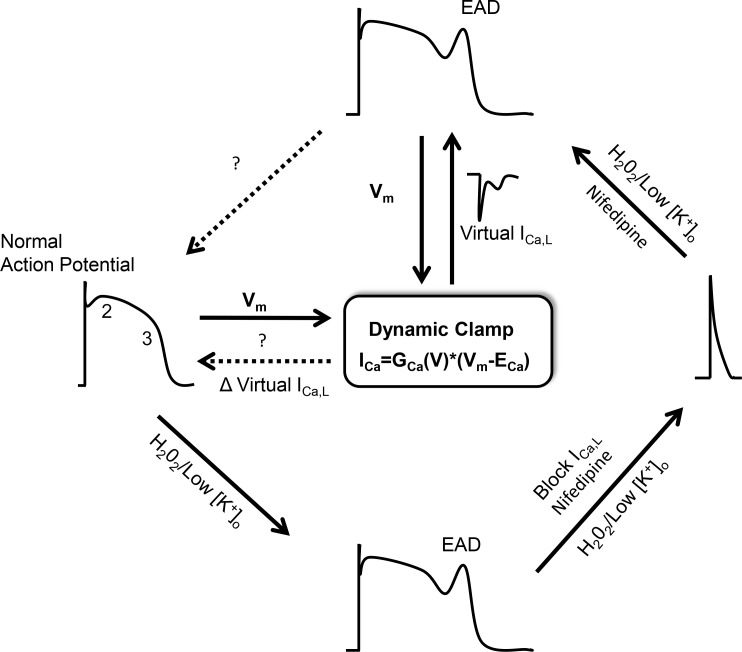
Unlike the brief action potentials (APs) in skeletal myocytes or neurons, the human cardiac AP takes 100s of milliseconds to repolarize the cell. This slow repolarization is essential for proper excitation–contraction coupling in cardiac muscle, and precise control of AP duration contributes to electrical stability. Under various pathological conditions, often when the AP duration is prolonged, repolarization can transiently fail with a sudden transient depolarization of membrane potential (Fig. 1). If such an early afterdepolarization (EAD) reaches threshold, it can trigger a premature AP and thereby initiate potentially fatal ventricular arrhythmias such as torsades de pointes (TdP) and ventricular fibrillation (Cranefield and Aronson, 1991). Thus, understanding the causes of EADs and how one might block them is of significant clinical importance.
Figure 1.
Schematic of cardiac APs studied using dynamic clamp to test properties of virtual ICa,L to eliminate EADs by Madhvani et al. (2015). A normal ventricular AP (left) with the major phases of AP repolarization indicated (2 and 3). The AP is prolonged in response to oxidative stress (H2O2) or hypokalemia (Low[K+]o), leading to an EAD (bottom). Complete block of ICa,L with a calcium channel blocker, nifedipine, dramatically shortens the AP and eliminates EAD (right). Using a computer model to generate virtual ICa,L, a dynamic clamp reconstitutes the missing ICa,L and recapitulates the long AP and EAD (top). By systematically changing the gating properties of virtual ICa,L, Madhvani et al. (2015) test for properties of the current that will preserve peak current and contraction but eliminate EADs.
Underlying ionic mechanisms responsible for EADs
The physiology underlying EADs is complex, involving multiple inward and outward ionic currents, changes in intracellular ion concentrations, and rapid regulation of ion channels. An EAD occurs when there is a reversal of the normal repolarization during phase 2 or 3 of the cardiac AP and is associated with a reduction in what has been referred to as “repolarization reserve” (Roden, 1998). Repolarization reserve is determined by the dynamic balance of outward currents and inward currents present during repolarization of the AP and implies redundancy of ionic currents in the normal heart to ensure appropriate repolarization. If there is a decrease in normal repolarization reserve, then a regenerative increase in an inward current can overcome and potentially reverse repolarization, leading to an EAD.
The first hint of a diminution of repolarization reserve is frequently an increase in AP duration. Conditions associated with prolongation of the AP are collectively referred to as long QT syndrome (LQTS), reflecting the longer than normal QT interval observed on the surface electrocardiogram. Both acquired and congenital forms of LQTS have been identified. Acquired LQTS occurs in the presence of certain electrolyte abnormalities, most commonly hypokalemia, as well as in response to ischemia, oxidative stress, and certain drugs. In the case of hypokalemia and QT-prolonging drugs, the reduction in repolarization reserve is primarily caused by a reduction in IKr carried by the hERG K channel. Alternatively, oxidative stress, such as that experimentally induced by H2O2 exposure, increases inward currents, including INaL (late sodium current) and ICa,L, to reduce repolarization reserve (Xie et al., 2009). Congenital LQTS is caused by mutations and dysfunction in a range of ion channels and associated regulatory proteins that either reduce outward repolarizing currents or increase inward depolarizing currents, with at least 13 such genetic defects having been identified (Ackerman et al., 2011). For example, LQTS type I is caused by loss of function mutations in KvLQT1 that reduce the IKs during AP repolarization. Thus, there are many ways to affect repolarization reserve that can contribute to the generation of EADs and triggered arrhythmias. Although the acquired forms of LQTS are generally reversible by rectifying the insult, e.g., potassium supplementation, revascularization for ischemia, or removing the offending drug, addressing the congenital forms presents more of a challenge.
The upstroke or depolarization of an EAD must be the result of a regenerative inward current, which is also necessary for the EAD to propagate at the tissue level (Zeng and Rudy, 1995). Inward currents that have been suggested to contribute to the upstroke of the EAD include ICa,L (January et al., 1988), INCX (Volders et al., 1997), and INaL (Maltsev et al., 1998); of these, ICa,L has received the greatest attention. January and Riddle (1989) first convincingly demonstrated in Purkinje fibers that there is a window current for ICa,L during which steady-state activation and inactivation curves overlap in the membrane potential range where EADs occur. In other words, as the AP repolarizes, ICa,L can reactivate and contribute to an increasing inward current. Furthermore, interventions that increase ICa,L currents, such as exposure to BayK8644, a pharmacological channel activator, lead to EADs, as can an increase in sympathetic tone, which acts, in part, by increasing ICa,L (Tanskanen et al., 2005). Likewise, activation of CaM Kinase II (CaMKII), which increased ICa,L in a mouse model by increasing mode 2 gating of the channels, also stimulated EADs (Dzhura et al., 2000). Thus, strategies to inhibit ICa,L from generating EADs comprise a logical approach to treatment and prevention of arrhythmias related to LQTS. Unfortunately, doses of classic Ca2+ channel blockers sufficient to inhibit EADs also inhibit the influx of Ca2+ necessary for excitation–contraction coupling, leading to impaired contraction. Nevertheless, Madhvani et al. (2015), in the previous issue of this journal, reasoned that if they could rationally alter gating parameters of L-type Ca2+ channels (LTCCs), then they may be able to identify a modified channel behavior that inhibits the ability of ICa,L to generate EADs while preserving their essential contribution to excitation–contraction coupling. The long-term goal of such a strategy is to identify small molecule or biological interventions that will produce this ideal channel gating to prevent EADs and thus prevent life-threatening ventricular arrhythmias.
Dynamic clamp to identify gating properties of LTCCs to eliminate EADs
The dynamic clamp technique provided the essential tool that Madhvani et al. (2015) used to systematically test the effect of changes in specific gating properties of LTCCs. In brief, these experiments used isolated rabbit ventricular myocytes that were treated with H2O2 or hypokalemic conditions to reproducibly prolong AP duration and induce EADs. After blocking all of the native ICa,L with a high concentration of nifedipine, which results in dramatic shortening of the AP duration and loss of EADs, the authors introduced a computer-generated virtual ICa,L. This virtual ICa,L was based on a mathematical model of the current, which in real-time was fed back to the cells in response to the measured voltage (Fig. 1). In a proof-of-principle study of this strategy, this group previously demonstrated that computer-simulated ICa,L successfully reconstituted the AP and the return of EADs in H2O2- or hypokalemia-treated myocytes (Madhvani et al., 2011). They also demonstrated that slight shifts in the voltage dependence of activation or inactivation of the channels could blunt EADs by reducing the window current. In the present study, however, they systematically tested a range of different channel gating properties, examining the slope of voltage-dependent activation and inactivation, the magnitude of the late current, and the time constant of activation, as well as the time constant of inactivation for ICa,L. The winning strategy was to reduce the magnitude of the late or pedestal ICa,L.
What is the late component of the L-type Ca2+ current?
Madhvani et al. (2015) have found an appealing feature of ICa,L to target, but what exactly is the late ICa,L? A maintained component of ICa,L has long been recognized in ventricular myocytes, and single channel experiments suggest it is caused by multiple channel reopenings (Rose et al., 1992). LTCCs exhibit both voltage-dependent inactivation (VDI) and Ca2+-dependent inactivation (CDI; Lee et al., 1985; Peterson et al., 1999). The pedestal current reflects contributions involving both VDI and CDI mechanisms, otherwise the channel would completely inactivate. However, the relationship between VDI and CDI is incompletely defined. Do VDI and CDI share a final common pathway, or are they mediated independently (Findlay, 2004; Kim et al., 2004; Barrett and Tsien, 2008)? For example, in LQT8 or Timothy’s syndrome, mutations in Cav1.2 specifically impair VDI, leading to AP duration prolongation and EADs (Splawski et al., 2004). The study by Madhvani et al. (2015) does not distinguish the respective roles of VDI and CDI in the late ICa,L, which is modeled as a constant. Thus, it remains unclear whether interventions to reduce the pedestal current should ideally target CDI, VDI, or either of the two.
Moreover, LTCCs are not a homogeneous population of channel proteins in cardiomyocytes, making the situation even more complex. Differences in subunit composition, posttranslational modifications, and subcellular localization of channels will all contribute to the heterogeneity of channel behavior observed within a single cell. This raises the question as to whether one specific population of channels is primarily responsible for the late ICa,L and may represent the appropriate target. Although the major pore-forming LTCC subunit in ventricular cardiomyocytes is Cav1.2, different splice variants are expressed and can contribute to heterogeneity of channel gating (Liao et al., 2005). Furthermore, auxiliary subunits modulate the gating behavior of the channel (Singer et al., 1991). The auxiliary β subunit (Cavβ) is encoded by four different genes, all of which are expressed in human heart, along with multiple splice variants (Foell et al., 2004). Different Cavβ isoforms differentially regulate inactivation of ICa,L (Colecraft et al., 2002; Kobrinsky et al., 2004), so it is possible that a subpopulation of LTCCs with a distinct subunit combination may disproportionately or solely contribute to late ICa,L. Posttranslational modifications of the channel, such as phosphorylation by PKA or CaMKII, have been linked with changes in gating that can promote proarrhythmic behavior (De Ferrari et al., 1995; Dzhura et al., 2000). In fact, combining posttranslational modification with unique subunit composition may be critical to susceptibility to EAD, as suggested by a prior study demonstrating that the Cavβ2a subunit was uniquely sensitive to CaMKII modulation in response to oxidative stress, which lead to EADs (Koval et al., 2010). Finally, the distinct subcellular localization of channels in the myocytes may expose the channels to different environments and thereby influence their behavior (Balijepalli et al., 2006; Bhargava et al., 2013). For example, could a subpopulation of channels in caveolae be the source of late ICa,L
Strategies to block the late component of ICa,L
Defining the optimal way to block late ICa,L may depend on advancing our understanding of the molecular basis of this current as indicated above; nevertheless, one can speculate that the approach could use small molecules or biological therapies. A precedent for specific late current blockers has been set by the identification of compounds that block the late current conducted by voltage-gated sodium channels in the heart, INaL, without blocking the peak current. Ranolazine is the prototypic INaL blocker (Antzelevitch et al., 2004), and new more specific INaL blockers have been described that have antiarrhythmic properties (Sicouri et al., 2013). So, with this precedent, it seems possible to identify a late ICa,L blocker. Conceivably, such compounds are already available but were missed in earlier screens of compound libraries for traditional LTCC blockers that focused exclusively on the ability to block peak ICa,L. Alternatively, roscovitine, a purine-based compound that was developed as an anticancer drug (cyclin-dependent kinase inhibitor) has been demonstrated to accelerate ICa,L inactivation, although it also slows activation gating (Yarotskyy and Elmslie, 2007). Roscovitine has shown promise in the iPS cardiomyocyte model for Timothy syndrome, where it blunted a defect in VDI (Yazawa et al., 2011). Using gene therapy to express regulatory proteins or auxiliary subunits could be considered as an alternative approach. For example, overexpression of a desired Cavβ subunit in cardiomyocytes could modify the gating behavior of endogenous channels (Colecraft et al., 2002). Exactly which Cavβ isoform, or perhaps even a modified Cavβ isoform, would be optimal requires further study.
Cautiously moving forward
The study by Madhvani et al. (2015) illustrates an intriguing strategy to design new therapies to treat arrhythmia syndromes, i.e., using the dynamic clamp in a hybrid computational-experimental approach to identify modifications of ICa,L gating properties that block a trigger for arrhythmias. However, for such a strategy to succeed, the model must accurately reflect the ionic currents present and the change in ICa,L gating must achieve the goal of preventing EADs without blunting intracellular Ca2+ transients and consequently contraction. Did Madhvani et al. (2015) succeed in selectively eliminating ICa,L from the native AP to accurately test virtual ICa,L? Although nifedipine is a long-established LTCC blocker, at the high concentration necessary for complete block of ICa,L, it is not certain that off-target effects on other ion channels are not present. Testing another drug to block ICa,L could provide reassurance that the results are not biased by the particular blocker chosen. A second concern is that virtual ICa,L, unlike native ICa,L, does not lead to influx of Ca2+ nor trigger intracellular Ca2+ release and hence excitation–contraction coupling. Thus, the authors model intracellular Ca2+ transients into ICa,L gating, but it is difficult to fully recapitulate the effect of the Ca2+ transient on multiple ion channels, transporters, and regulatory pathways. In some experiments, the authors included a small fraction of virtual IKs, a current known to be modulated by intracellular [Ca2+]. However, there are certainly other currents, perhaps most importantly INCX, that could influence the results. Even more difficult to model is the regulation of the LTCCs by CaMKII, which can also be dynamically affected by the intracellular Ca2+ transients. Will the reduction in late ICa,L proposed by the investigators interfere with intracellular Ca2+ cycling? The authors argue that maintaining peak ICa,L will maintain appropriate excitation–contraction coupling, but a reduction in the late component of ICa,L will reduce overall Ca2+ influx during an AP and at steady-state likely reduce intracellular Ca2+ stores, leading to a reduction in the Ca2+ transient. Whether this will have a significant impact requires further study.
Even if the cell model functions accurately, some questions will remain. Will this intervention focused on reducing late ICa,L be effective when cardiomyocytes are coupled into a functional tissue or will new concerns/heterogeneities arise? Advancing to multiscale modeling is one approach to address this concern in future studies. How broadly applicable will a reduction in late ICa,L be to treat EADs resulting from other causes not studied here? For example, some EADs rely more heavily on INCX, and these may be more refractory to changes in late ICa,L. However, at the end of the day, existing strategies for developing antiarrhythmic drugs have largely failed, and so new, innovative approaches as described by Madhvani et al. (2015) need to be aggressively pursued and tested.
Acknowledgments
Y.S. Markandeya and T.J. Kamp are supported by funding from National Institutes of Health grant R01 HL078878.
The authors declare no competing financial interests.
Elizabeth M. Adler served as editor.
References
- Ackerman M.J., Priori S.G., Willems S., Berul C., Brugada R., Calkins H., Camm A.J., Ellinor P.T., Gollob M., Hamilton R., et al. . 2011. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA). Heart Rhythm. 8:1308–1339. [DOI] [PubMed] [Google Scholar]
- Antzelevitch C., Belardinelli L., Zygmunt A.C., Burashnikov A., Di Diego J.M., Fish J.M., Cordeiro J.M., and Thomas G.. 2004. Electrophysiological effects of ranolazine, a novel antianginal agent with antiarrhythmic properties. Circulation. 110:904–910. 10.1161/01.CIR.0000139333.83620.5D [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balijepalli R.C., Foell J.D., Hall D.D., Hell J.W., and Kamp T.J.. 2006. Localization of cardiac L-type Ca2+ channels to a caveolar macromolecular signaling complex is required for β2-adrenergic regulation. Proc. Natl. Acad. Sci. USA. 103:7500–7505. 10.1073/pnas.0503465103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett C.F., and Tsien R.W.. 2008. The Timothy syndrome mutation differentially affects voltage- and calcium-dependent inactivation of CaV1.2 L-type calcium channels. Proc. Natl. Acad. Sci. USA. 105:2157–2162. 10.1073/pnas.0710501105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava A., Lin X., Novak P., Mehta K., Korchev Y., Delmar M., and Gorelik J.. 2013. Super-resolution scanning patch clamp reveals clustering of functional ion channels in adult ventricular myocyte. Circ. Res. 112:1112–1120. 10.1161/CIRCRESAHA.111.300445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colecraft H.M., Alseikhan B., Takahashi S.X., Chaudhuri D., Mittman S., Yegnasubramanian V., Alvania R.S., Johns D.C., Marban E., and Yue D.T.. 2002. Novel functional properties of Ca2+ channel beta subunits revealed by their expression in adult rat heart cells. J. Physiol. 541:435–452. 10.1113/jphysiol.2002.018515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranefield P.F., and Aronson R.S.. 1991. Torsades de pointes and early afterdepolarizations. Cardiovasc. Drugs Ther. 5:531–537. 10.1007/BF03029780 [DOI] [PubMed] [Google Scholar]
- De Ferrari G.M., Viola M.C., D’Amato E., Antolini R., and Forti S.. 1995. Distinct patterns of calcium transients during early and delayed afterdepolarizations induced by isoproterenol in ventricular myocytes. Circulation. 91:2510–2515. 10.1161/01.CIR.91.10.2510 [DOI] [PubMed] [Google Scholar]
- Dzhura I., Wu Y., Colbran R.J., Balser J.R., and Anderson M.E.. 2000. Calmodulin kinase determines calcium-dependent facilitation of L-type calcium channels. Nat. Cell Biol. 2:173–177. 10.1038/35004052 [DOI] [PubMed] [Google Scholar]
- Findlay I. 2004. Physiological modulation of inactivation in L-type Ca2+ channels: one switch. J. Physiol. 554:275–283. 10.1113/jphysiol.2003.047902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foell J.D., Balijepalli R.C., Delisle B.P., Yunker A.M., Robia S.L., Walker J.W., McEnery M.W., January C.T., and Kamp T.J.. 2004. Molecular heterogeneity of calcium channel β-subunits in canine and human heart: evidence for differential subcellular localization. Physiol. Genomics. 17:183–200. 10.1152/physiolgenomics.00207.2003 [DOI] [PubMed] [Google Scholar]
- January C.T., and Riddle J.M.. 1989. Early afterdepolarizations: mechanism of induction and block. A role for L-type Ca2+ current. Circ. Res. 64:977–990. 10.1161/01.RES.64.5.977 [DOI] [PubMed] [Google Scholar]
- January C.T., Riddle J.M., and Salata J.J.. 1988. A model for early afterdepolarizations: induction with the Ca2+ channel agonist Bay K 8644. Circ. Res. 62:563–571. 10.1161/01.RES.62.3.563 [DOI] [PubMed] [Google Scholar]
- Kim J., Ghosh S., Nunziato D.A., and Pitt G.S.. 2004. Identification of the components controlling inactivation of voltage-gated Ca2+ channels. Neuron. 41:745–754. 10.1016/S0896-6273(04)00081-9 [DOI] [PubMed] [Google Scholar]
- Kobrinsky E., Kepplinger K.J., Yu A., Harry J.B., Kahr H., Romanin C., Abernethy D.R., and Soldatov N.M.. 2004. Voltage-gated rearrangements associated with differential β-subunit modulation of the L-type Ca2+ channel inactivation. Biophys. J. 87:844–857. 10.1529/biophysj.104.041152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koval O.M., Guan X., Wu Y., Joiner M.L., Gao Z., Chen B., Grumbach I.M., Luczak E.D., Colbran R.J., Song L.S., et al. . 2010. CaV1.2 β-subunit coordinates CaMKII-triggered cardiomyocyte death and afterdepolarizations. Proc. Natl. Acad. Sci. USA. 107:4996–5000. 10.1073/pnas.0913760107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.S., Marban E., and Tsien R.W.. 1985. Inactivation of calcium channels in mammalian heart cells: joint dependence on membrane potential and intracellular calcium. J. Physiol. 364:395–411. 10.1113/jphysiol.1985.sp015752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao P., Yong T.F., Liang M.C., Yue D.T., and Soong T.W.. 2005. Splicing for alternative structures of Cav1.2 Ca2+ channels in cardiac and smooth muscles. Cardiovasc. Res. 68:197–203. 10.1016/j.cardiores.2005.06.024 [DOI] [PubMed] [Google Scholar]
- Madhvani R.V., Xie Y., Pantazis A., Garfinkel A., Qu Z., Weiss J.N., and Olcese R.. 2011. Shaping a new Ca2+ conductance to suppress early afterdepolarizations in cardiac myocytes. J. Physiol. 589:6081–6092. 10.1113/jphysiol.2011.219600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhvani R.V., Angelini M., Xie Y., Pantazis A., Suriany S., Borgstrom N.P., Garfinkel A., Qu Z., Weiss J.N., and Olcese R.. 2015. Targeting the late component of the cardiac L-type Ca2+ current to suppress early afterdepolarizations. J. Gen. Physiol. 145:395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltsev V.A., Sabbah H.N., Higgins R.S., Silverman N., Lesch M., and Undrovinas A.I.. 1998. Novel, ultraslow inactivating sodium current in human ventricular cardiomyocytes. Circulation. 98:2545–2552. 10.1161/01.CIR.98.23.2545 [DOI] [PubMed] [Google Scholar]
- Peterson B.Z., DeMaria C.D., Adelman J.P., and Yue D.T.. 1999. Calmodulin is the Ca2+ sensor for Ca2+-dependent inactivation of L-type calcium channels. Neuron. 22:549–558. 10.1016/S0896-6273(00)80709-6 [DOI] [PubMed] [Google Scholar]
- Roden D.M. 1998. Taking the “idio” out of “idiosyncratic”: predicting torsades de pointes. Pacing Clin. Electrophysiol. 21:1029–1034. 10.1111/j.1540-8159.1998.tb00148.x [DOI] [PubMed] [Google Scholar]
- Rose W.C., Balke C.W., Wier W.G., and Marban E.. 1992. Macroscopic and unitary properties of physiological ion flux through L-type Ca2+ channels in guinea-pig heart cells. J. Physiol. 456:267–284. 10.1113/jphysiol.1992.sp019336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicouri S., Belardinelli L., and Antzelevitch C.. 2013. Antiarrhythmic effects of the highly selective late sodium channel current blocker GS-458967. Heart Rhythm. 10:1036–1043. 10.1016/j.hrthm.2013.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer D., Biel M., Lotan I., Flockerzi V., Hofmann F., and Dascal N.. 1991. The roles of the subunits in the function of the calcium channel. Science. 253:1553–1557. 10.1126/science.1716787 [DOI] [PubMed] [Google Scholar]
- Splawski I., Timothy K.W., Sharpe L.M., Decher N., Kumar P., Bloise R., Napolitano C., Schwartz P.J., Joseph R.M., Condouris K., et al. . 2004. CaV1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 119:19–31. 10.1016/j.cell.2004.09.011 [DOI] [PubMed] [Google Scholar]
- Tanskanen A.J., Greenstein J.L., O’Rourke B., and Winslow R.L.. 2005. The role of stochastic and modal gating of cardiac L-type Ca2+ channels on early after-depolarizations. Biophys. J. 88:85–95. 10.1529/biophysj.104.051508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volders P.G., Kulcsar A., Vos M.A., Sipido K.R., Wellens H.J., Lazzara R., and Szabo B.. 1997. Similarities between early and delayed afterdepolarizations induced by isoproterenol in canine ventricular myocytes. Cardiovasc. Res. 34:348–359. 10.1016/S0008-6363(96)00270-2 [DOI] [PubMed] [Google Scholar]
- Xie L.H., Chen F., Karagueuzian H.S., and Weiss J.N.. 2009. Oxidative stress–induced afterdepolarizations and calmodulin kinase II signaling. Circ. Res. 104:79–86. 10.1161/CIRCRESAHA.108.183475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarotskyy V., and Elmslie K.S.. 2007. Roscovitine, a cyclin-dependent kinase inhibitor, affects several gating mechanisms to inhibit cardiac L-type (Ca(V)1.2) calcium channels. Br. J. Pharmacol. 152:386–395. 10.1038/sj.bjp.0707414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazawa M., Hsueh B., Jia X., Pasca A.M., Bernstein J.A., Hallmayer J., and Dolmetsch R.E.. 2011. Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature. 471:230–234. 10.1038/nature09855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng J., and Rudy Y.. 1995. Early afterdepolarizations in cardiac myocytes: mechanism and rate dependence. Biophys. J. 68:949–964. 10.1016/S0006-3495(95)80271-7 [DOI] [PMC free article] [PubMed] [Google Scholar]