Abstract
Objective:
Unrecognized congenital hypothyroidism (CH) leads to mental retardation. Newborn screening and thyroid therapy started within 2 weeks of age can normalize cognitive development. In this systematic review, the local results of the national CH screening program in different provinces in Iran are reviewed and evaluated.
Methods:
Literature on the CH screening, the national databases including SID, Medlib, Iran Medex, Magiran as well as international databases including PubMed/Medline, ISI Web of Knowledge and web of science, EMBASE, SCOPUS and Google Scholar. Appraisal was guided by a checklist assessing clarity of aims and research questions. The 95% confidence intervals were calculated by I-square models. Meta regression was introduced to explore the heterogeneity between studies.
Findings:
We identified 25 samples including 1425124 neonates in our country. Data were Meta analyzed using random-effects models, and we found a TSH levels of 19633 babies in the first sampling were greater than the cut-off level (TSH ≥5mIU/L). The pooled recall rate was 0.014 (95 % CI: 0.013 – 0.015). According to Meta analysis the overall incidence of CH was 2/1000 (95% CI: .002 – .002). The incidence of CH did not appear to be increasing over time (P=0.08).
Conclusion:
Considering TSH ≥5mIU/L as a cut-off point for recalling neonates and low positive predictive value (14%) of this point shows that more investigation and research is needed for establishing accurate level of TSH as a criterion for recalling patients.
Keywords: Congenital Hypothyroidism, Hypothyroidism, Congenital, Meta Regression, Iran
Introduction
Congenital hypothyroidism (CH) is one of the most important causes of preventable mental retardation. The goal of neonatal CH screening programs is early diagnosis and treatment [1] . The delayed diagnosis made only on the basis of clinical findings may result in irreversible complications such as mental retardation and deafness [2,3] . The difficulty in recognizing CH and the serious consequences of delayed therapy have led to the introduction of screening programs for hypothyroidism in newborns by measuring thyroxine (T4) or thyroid-stimulating hormone (TSH or thyrotropin) in spots of blood collected via heel stick during the first few days of life [4] .
Screening programs for CH have been developed in Canada, the United States, parts of Mexico, Western Europe, Japan, Australia and New Zealand, and they are under development in parts of many countries in Eastern Europe, Asia, South America and Africa. Of the worldwide birth population of 127 million, it is estimated that 25 percent undergo screening for CH [5] . In Iran the screening program was first carried out in 1987 by Azizi et al [6] and was included to the health care service in 2005. Early diagnosis, made possible by neonatal screening, has increased the need for etiologic classification at a very young age, both for the treatment of the affected neonates and genetic counseling of the family [7] .
In Iran, preliminary studies on national CH screening program have emerged [11–35] . Within the above-mentioned background we reviewed the local results of the national CH screening program in different provinces of the country in terms of TSH cut-off level. Frequency of cases that required recall and treatment along with stages of the disease before treatment in boys and girls are evaluated.
Subjects and Methods
We identified publications estimating and reporting of results for national CH screening program among Iranian neonates in September 2013 that were published between January 1, 1995 and August 31, 2013. The review is conducted in accordance with PRISMA guidelines [8] . Literature on the CH screening program among Iranian neonates was acquired through searching the national databases including: Scientific Information Databases (SID), Global Medical Article Limberly (Medlib), Iranian Biomedical Journal (Iran Medex), Iranian Journal Database (Magiran) as well as international databases including PubMed/Medline, ISI Web of Knowledge and web of science, EMBASE, SCOPUS and Google Scholar. The search strategy was limited to the Persian and/or English language and articles published up until August 31, 2013 were considered. All publications with medical subject headings (MeSh) and keywords in title, abstract and text for words including congenital hypothyroidism and Iran were investigated. Iranian scientific databases were searched only using the keyword ‘Congenital hypothyroidism’, as these databases do not distinguish synonyms from each other and do not allow sensitive search operation using linking terms such as ‘AND’, ‘OR’ or ‘NOT’. Consequently, this single keyword search was the most practical option. The Congenital hypothyroidism, Hypothyroidism, Congenital and Iran MeSh combined with the operator “OR” vs “AND”.
All identified papers were critically appraised independently by two reviewers. Disagreements between reviewers were resolved by consensus. Appraisal was guided by a checklist assessing clarity of aims and research questions. inclusion criteria were all related articles published from the local results of the national CH screening program in Iran (notates between the 3rd and 5th day of life on filter paper, by puncturing the heel) using valid databases. Studies upon neonates overlapping time intervals of sample collection from the same origin (for example articles published in both Persian and English language), inappropriate study design and inadequate reporting of results were important exclusion criteria.
The data were extracted on the year of study, geographical location, gender, author, title, setting of the study, sample size, recall rate and incidence of CH using a standardized and pre-piloted data extraction form. Data extraction was undertaken by the first reviewer and checked by a second reviewer, also the process was discussed and piloted by both reviewers. All identified papers were critically appraised independently by both reviewers. Disagreements were resolved through discussion. Appraisal was guided by a checklist assessing clarity of aims and research questions. These data-abstraction forms were reviewed and eligible papers were entered into the meta-analysis.
I-squared model was used for combining results of studies in meta-analysis [9] . Significance level was <0.1 and I-squared statistic for estimates of inconsistency within the meta-analyses [10] .
Univariate and multivariate Meta regression analyses were used to explore possible sources of heterogeneity among studies. We analyzed sources of heterogeneity by subgroup and meta regression analysis using dichotomous and continuous variables. Meta-regression was used to show trend of variation of prevalence during time. Egger’s test was conducted to examine potential publication bias. Egger’s test can reveal a symmetric or asymmetric funnel plot. The latter indicates the existence of a significant publication bias or a systematic heterogeneity between studies. Data manipulation and statistical analyses were done using STATA software, version 11.2. P values <0.05 were considered as statistically significant.
Findings
The final data-set consisted of 68 publications, 33 studies were excluded as they did not meet the inclusion criteria. There were 7 studies in English and 18 studies in Persian of the finally adopted 25 studies and were published between 1995 and 2013. The studies were conducted in 18 different provinces; these publications provided data on 1.425.124 neonates (Table 1 and Fig 1). TSH levels of 19.633 babies in the first sampling were greater than the cut-off level (TSH ≥5mIU/L). The pooled recall rate was 0.014 (95% CI: 0.013– 0.015) (TSH levels of patients whose first heel TSH level was >5 mIU/L are given in Table 1). According to Meta analysis the overall incidence of CH was 2/1000 live births (95% CI: .002 −.002) (Fig 2). According to subgroup analysis based on provinces the highest incidence occurring in the Markazi province was 0.003 (95%CI: 0.002–0.004) and lowest incidence occurring in Tehran, Gilan, Fars, Kerman, Mazandaran and Zanjan provinces was 0.001 (95%CI: 0.001–0.001) (Table 2).
Table 1:
Characteristics of included reports
| Study location (Province) | Authors | Year of study | No. of patients | Recall rate | Incidence |
|---|---|---|---|---|---|
| Khuzestan | Aminzadeh et al[11] | 2010 | 35655 | 0.0352 | 0.0020 |
| Tehran | Ordoukhani et al[12] | 2002 | 35067 | 0.0106 | 0.0010 |
| Khorasan | Namakin et al[13] | 2012 | 38987 | 0.0321 | 0.0183 |
| Gilan | Kalantari et al[14] | 2004 | 3000 | 0.0200 | 0.0010 |
| Markazi | Dorreh et al[15] | 2010 | 25658 | 0.0236 | 0.0030 |
| Fars | Karamizadeh et al[16] | 2012 | 63031 | 0.0200 | 0.0010 |
| Isfahan | Hashemipour et al[17] | 2007 | 113282 | 0.0320 | 0.0030 |
| Kerman | Eftekhari et al[18] | 2008 | 3000 | 0.0070 | 0.0010 |
| Kurdistan | Nele et al[19] | 2011 | 50539 | 0.0200 | 0.0020 |
| Mazandaran | Akhi et al[20] | 2011 | 45218 | 0.0460 | 0.0016 |
| Ghazvin | Saffari et al[21] | 2009 | 33488 | 0.0320 | 0.0020 |
| Gilan | Mohtasham et al[22] | 2007 | 9284 | 0.0315 | 0.0017 |
| Yazd | Nouri Shadkam et al[23] | 2008 | 13022 | 0.0620 | 0.0030 |
| East Azerbaijan | Zeinalzadeh et al[24] | 2011 | 62459 | 0.0250 | 0.0017 |
| Kermanshah | Khassi et al[25] | 2011 | 30265 | 0.0018 | 0.0017 |
| West Azerbaijan | Eshratkhah et al[26] | 2011 | 19141 | 0.0200 | 0.0020 |
| Tehran | Mostafavi et al[27] | 1995 | 1014 | 0.0800 | 0.0060 |
| Tehran | Ordookhani et al[28] | 2003 | 20107 | 0.0013 | 0.0010 |
| Zanjan | Valizadeh et al[29] | 2011 | 18008 | 0.0410 | 0.0010 |
| Isfahan | Honarpisheh et al[30] | 2002 | 500 | 0.0500 | 0.0100 |
| Isfahan | Hashemipour et al[31] | 2010 | 225224 | 0.0170 | 0.0020 |
| Isfahan | Hashemipour et al[32] | 2007 | 93381 | 0.0111 | 0.0030 |
| Mazandaran | Haghshenas et al[33] | 2012 | 10573 | 0.0200 | 0.0005 |
| Isfahan | Ghasemi et al[34] | 2013 | 464648 | 0.0210 | 0.0013 |
| Mazandaran | Nasehi et al[35] | 2010 | 10573 | 0.0140 | 0.0140 |
Fig. 1:
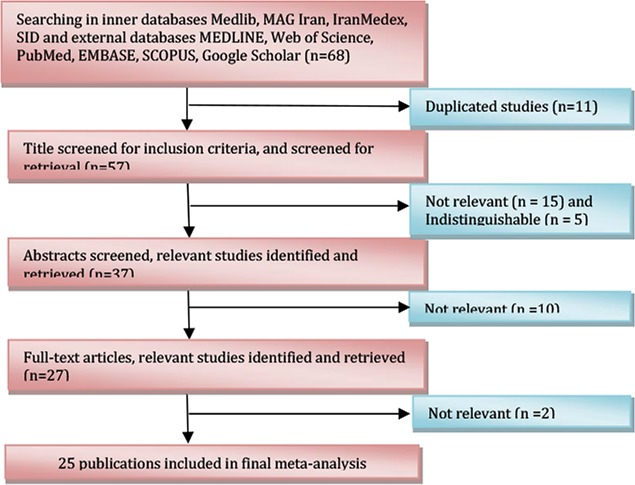
Flow diagram showing the different steps involved in searching for relevant publications (1995–2013).
Fig. 2:
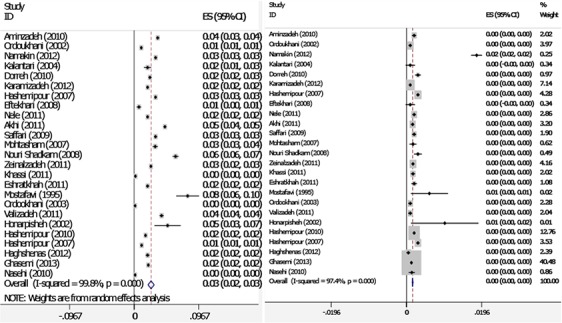
Forest plots of recall rate (A) and incidence of CH (B) for I-squared model meta-analyses (Weights are from I-squared model). The diamond represents the overall result
Table 2:
Subgroup analysis of CH Incidence based on various provinces in Iran
| Province | No. of studies | No. of Subjects | CH Incidence (95% CI) | heterogeneity | model | |
|---|---|---|---|---|---|---|
| I-squared | P value | |||||
| Khuzestan | 1 | 35655 | 0.002(0.002–0.002) | 0.00 | 0 | I-squared |
| Tehran | 3 | 56188 | 0.001(0.001–0.001) | 4.24 | 0.12 | I-squared |
| Khorasan | 1 | 38987 | 0.018(0.017–0.020) | 0.00 | 0 | I-squared |
| Gilan | 2 | 12284 | 0.001(0.001–0.002) | 0.95 | 0.33 | I-squared |
| Markazi | 1 | 25658 | 0.003(0.002–0.004) | 0.00 | 0 | I-squared |
| Fars | 1 | 63031 | 0.001(0.001–0.001) | 0.00 | 0 | I-squared |
| Isfshan | 5 | 464648 | 0.002(0.002–0.002) | 186.99 | 0.000 | I-squared |
| Kerman | 1 | 3000 | 0.001(0.001–0.002) | 0.00 | 0 | I-squared |
| Kurdisatan | 1 | 50539 | 0.002(0.002–0.002) | 0.00 | 0 | I-squared |
| Mazandaran | 3 | 66364 | 0.001(0.001–0.001) | 15.13 | 0.001 | I-squared |
| Ghazvin | 1 | 33488 | 0.002(0.002–0.002) | 0.00 | 0 | I-squared |
| Yazd | 1 | 13022 | 0.002(0.003–0.004) | 0.00 | 0 | I-squared |
| East Azerbaijan | 1 | 62459 | 0.002(0.001–0.002) | 0.00 | 0 | I-squared |
| Kermanshah | 1 | 30265 | 0.002(0.001–0.002) | 0.00 | 0 | I-squared |
| West Azerbaijan | 1 | 19141 | 0.002(0.001–0.003) | 0.00 | 0 | I-squared |
| Zanjan | 1 | 18008 | 0.001(0.001–0.001) | 0.00 | 0 | I-squared |
| Pooled of CH | 25 | 1425124 | 0.002(0.002–0.002) | 911.79 | 0.000 | I-squared |
There were 0.46 (95% CI: 0.45–0.46) female and 0.54 (95% CI: 0.54–0.54) male infants who had CH. In this study there was no statistically significant difference between males and females (P=0.5). The heterogeneity between studies was 98.7% with an I-square (I2) statistics (541.33, DF=7, P<0.001).
Fig. 3 shows that the CH incidence has had no increasing trend during 1995 till 2013. Meta-regression analysis found that sample size does not significantly affect heterogeneity for the factor ‘CH incidence’ (Reg Coef = 0.028, P=0.4). Meta-regression showed an association between the year of study and prevalence rate of CH incidence and demonstrated causes of the variability in the results of studies. Meta-regression showed that variability in CH incidence is not a significant effect for years (Reg Coef=0.031, P=0.08). There was no evidence of publication bias (Egger’s test β0: 0.26; P=0.6) (Fig. 4) so we tried to consider most of published articles in this subject.
Fig. 3:
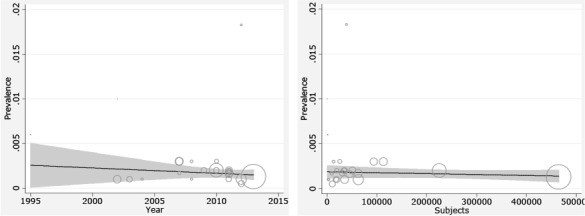
Meta-regression plots of change in CH incidence according to changes in continuous study moderator’s year and sample size
Fig. 4:
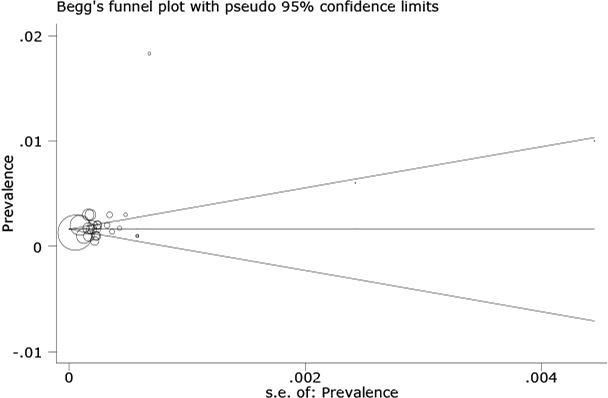
Begg’s funnel plot (pseudo 95% confidence limits) showing means difference in prevalence of hypothyroidism (CH) by standard error of mean difference
Discussion
We report a systematic review of the CH screening program in neonates based on 25 separate samples (from 68 publications) based on 1.425.124 neonates. In addition, we have, to our knowledge for the first time, reviewed reports in CH screening program in Iran and employed meta-regression analysis to explore sources of heterogeneity between studies.
The CH screening program has been implemented in Iran since 2006. It is based on TSH measurement by heel prick between the 3rd and 5th day of life. The most important goals of CH screening programs are early diagnosis and treatment of CH. Preferably, the diagnosis must be confirmed within the first 14 days, and treatment must be started. There are also some studies emphasizing the necessity of starting the treatment no later than three months (ideally 1 month) after the first sample [36] .
The pooled recall rate was (0.014 per case) according to cutoff point for recalling the patients in screening program and the overall incidence of CH was (2/1000 live births). It was reported that the prevalence of CH in the Greek Cypriot population 1990–2000 was 1/1800 [37] . The results of the screening program for CH in Italy noted the incidence of CH as 1/1446 between 1999 and 2005 [38] . Nonetheless, CH is more common in Eastern countries, for example, the incidence of permanent CH was found as 1/748 in Iran 2002–2005 [7] . CH incidence before the CH screening program in Turkey was reported as 1/2736–1/2326 [39] . A study from Konya noted that the CH incidence 1999–2007 was 1/2183 [40] . At the present time, there is a good policy and appropriate methods are available for screening of CH in Iran, but it seems that the cut-off point for TSH ≥5mIU/L in whole blood overestimates the real number of patients.
According to these findings whole blood TSH≥15mIU/L seems to be a more reliable and cost effective cutoff point for recalling patients in screening programs of CH. In Bosnia and Herzegovina, the TSH cutoff value for recall was ≥20mIU/L in whole blood [41] , ≥15mIU/L in Mexico [42] and ≥25mIU/L in Thailand [43] .
The strengths of this review include the large number of samples and neonates included, and therefore the ability to examine recall and incidence rates. In conclusion, lowering the cut-off level will probably increase the cost of the screening program; however, the number of the missed cases due to high cut-off levels is substantial. Considering TSH ≥5mIU/L as a cut-off point for recalling neonates and low positive predictive value (14%) of this point shows that more investigation and research is needed for establishing accurate level of TSH as a criterion for recalling patients.
Conclusion
Considering TSH ≥5mIU/L as a cut off point for recalling neonates and low positive predictive value (14%) of this point shows that more investigation and research is needed for establishing accurate level of TSH as a criterion for recalling patients.
Acknowledgment
This study was supported financially by Ilam University of Medical Sciences, Ilam, Iran.
Authors’ Contribution
Study design and Collection of the data: Y. Veisani and S. Rezaeian
Data analysis: K. Sayehmiri
Preparation of the manuscript: A. Delpisheh and Y. Veisani
All authors approved final version of the paper.
Conflict of Interest:
None
References
- 1. Büyükgebiz A. Congenital hypothyroidism: clinical aspects and late consequences. Pediatr Endocrinol Rev 2003; 1 (Suppl 2): 158– 90. [PubMed] [Google Scholar]
- 2. Bellman SC, Davies A, Fuggle PW, et al. Mild impairment of neuro-otological function in early treated congenital hypothyroidism. Arch Dis Child 1996; 74 (3): 215– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rouet J, Walker W, Bliss B, et al. Long term sequel of hearing impairment in congenital hypothyroidism. J Pediatr 1996; 128 (6): 776– 83. [DOI] [PubMed] [Google Scholar]
- 4. Foley TP, Klein AH, Agustin AV. Adaptation of TSH filter paper method for reorganized screening for congenital hypothyroidism. J Lab Clin Med 1977; 90 (1): 11– 7. [PubMed] [Google Scholar]
- 5. Bongers-Schockking JJ, Koot HM, Wiersma D, et al. Influence of timing and dose of thyroid hormone replacement on development in infants with congenital hypothyroidism. J Pediatr 2000;136 (3): 292– 7. [DOI] [PubMed] [Google Scholar]
- 6. Azizi F, Oladi B, Nafarabadi M. Screening for congenital hypothyroidism in Tehran. Effect of iodine deficiency on transient elevation of neonatal TSH. J Shaheed Beheshti School Med 1994; 18 (1): 24– 38. [Google Scholar]
- 7. Hashemipour M, Hovsepian S, Kelishadi R, et al. Permanent and transient congenital hypothyroidism in Isfahan-Iran. J Med Screen 2009; 16 (1): 11– 6. [DOI] [PubMed] [Google Scholar]
- 8. Grossman P, Niemann L, Schmidt S, et al. Mindfulness-based stress reduction and health benefits: A meta-analysis. J Psychosom Res 2004; 57 (1): 35– 43. [DOI] [PubMed] [Google Scholar]
- 9. Der.Simonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7 (3): 177– 88. [DOI] [PubMed] [Google Scholar]
- 10. Ades AE, Lu G, Higgins JP. The interpretation of random-effects meta-analysis in decision models. Med Decis Making 2005;25 (6): 646– 54. [DOI] [PubMed] [Google Scholar]
- 11. Aminzadeh M, Chomeili B, Aramesh MR, et al. Evaluation of possible interactive factors with thyrotropin level in screening program of congenital hypothyroidism in Ahvaz. Jundishapur Sci Med J 2010;9 (6): 553– 61. [Google Scholar]
- 12. Ordoukhani A, Hedayati SM, Mirmiran P, et al. Etiologies of transient congenital hypothyroidism in Tehran and Damavand. Iran J Endocrinol Metab 2002;6 (2): 103– 7. [Google Scholar]
- 13. Namakin N, Sedighi E, Sharifzadeh GR, Zardast M. Prevalence of congenital hypothyroidism In South Khorasan province (2006–2010). J Birjand Uni Med Sci 2012;19 (2): 191– 9. [Google Scholar]
- 14. Kalantari S. Neonatal screening for congenital hypothyroidism (CH) in Rasht. J Med Faculty Guilan Uni Med Sci 2004;13 (50): 76– 80. [Google Scholar]
- 15. Dorreh F, Mohammadi T. The relationship between recall rate and the incidence of congenital hypothyroidism in the screening program for neonatal hypothyroidism in Arak, 2006. Arak Med Uni J 2010;13 (1): 49– 55. [Google Scholar]
- 16. Karamizadeh Z, Saneifard H, Amirhakimi G, et al. Evaluation of Congenital Hypothyroidism in Fars Province, Iran. Iran J Pediatr 2012;22 (1): 107– 12. [PMC free article] [PubMed] [Google Scholar]
- 17. Hashemipour M, Amini M, Talaie R, et al. Paternal consanguinity among parents of neonates with congenital hypothyroidism in Isfahan. Eastern Mediterranean Health J 2007;13 (3): 567– 73. [PubMed] [Google Scholar]
- 18. Eftekhari N, Asadikaram Gh, Khaksari M, et al. The prevalence rate of congenital hypothyroidism in Kerman/Iran in 2005–2007. J Kerman Uni Med Sci 2008;15 (3): 243– 50. [Google Scholar]
- 19. Nele S, Ghotbi N. Congenital hypothyroidism program in Kurdistan, Iran Payesh 2011; 10 (1): 15–20. [Google Scholar]
- 20. Akhi O, Shabani M, Kowsarian M, et al. Prevalence of congenital hypothyroidism in Mazandaran Province, Iran, 2008. J Mazandaran Univ Med Sci 2011;21 (84): 63– 70. [Google Scholar]
- 21. Saffari F, Karimzadeh T, Mostafaiee M, Mahram M. Screening of congenital hypothyroidism in Qazvin Province (2006–2008). J Qazvin University Med Sci 2009;12 (4): 43– 9. [Google Scholar]
- 22. Mohtasham AZ, Mousavi M, Hosein Zadeh M. Newborn screening for congenital hypothyroidism in Rasht, North of Iran, 2007. Early Human Develop 2008; 84: 122. [Google Scholar]
- 23. Nouri Shadkam M, Jafarizade M, Mirzaei M, et al. Prevalence of congenital hypothyroidism and transient increased level of TSH in Yazd province. J Shaeed Sdoughi Uni Med Sci Yazd 2008;16 (3): 15– 20. [Google Scholar]
- 24. Zeinalzadeh AH, Kousha A, Talebi M, Akhtari M. Screening for congenital hypothyroidism in East Azerbaijan province, Iran. J Kerman Uni Med Sci 2011;18 (4): 301– 8. [Google Scholar]
- 25. Khassi K, Khademi N, Fakhri Moradi S, et al. Evaluation of neonatal hypothyroidism in 88 Kermanshah. The 4th International, 9th National Congress on Quality Improvement in Clinical Laboratories 2011 April 20–23; Tehran-Iran. [Google Scholar]
- 26. Eshratkhah A, Gholizadeh Salmasi J, Sheykhan K, et al. The survey of congenital hypothyroidism relation's amount with some factors in newborn infants in Urmia city. The 4th International, 9th National Congress on Quality Improvement in Clinical Laboratories 2011 April 20–23; Tehran-Iran. [Google Scholar]
- 27. Mostafavi F, Rabbani A, Haghi-Ashtiani MT, Bostan T. The incidence of hypothyroidism in jaundiced newborns. Iran J Pediatr 1995;5 (17): 45– 9. [Google Scholar]
- 28. Ordookhani A, Mirmiran P, Najafi R, et al. Congenital hypothyroidism in Iran. Indian J Pediatr 2003;70 (8): 625– 8. [DOI] [PubMed] [Google Scholar]
- 29. Valizadeh M, Mazloomzadeh S, Niksirat A, et al. High incidence and recall rate of congenital hypothyroidism in Zanjan Province, a health problem or a study challenge? Int J Endocrinol Metab 2011; 9 (4): 338–42. [Google Scholar]
- 30. Honarpisheh A. Incidence of congenital hypothyroidism in newborns with prolonged jaundice. Iran J Pediatr 2002;12 (1): 50– 2. [Google Scholar]
- 31. Hashemipour M, Dehkordi EH, Hovsepian S, et al. Outcome of congenitally hypothyroid screening program in Isfahan, Iran from prevention to treatment. Int J Prev Med Spring 2010; 1 (2): 92–7. [PMC free article] [PubMed] [Google Scholar]
- 32. Hashemipour M, Amini M, Kelishadi R, et al. Seasonal variation in the incidence of congenital hypothyroidism in Isfahan, Iran. Saudi Med J 2007;28 (10): 477– 81. [PubMed] [Google Scholar]
- 33. Haghshenas M, Zahed Pasha Y, Ahmadpour-Kacho M, et al. Prevalence of permanent and transient congenital hypothyroidism in Babol City, Iran. Med Glas (Zenica) 2012;9 (2): 341– 4. [PubMed] [Google Scholar]
- 34. Ghasemi M, Hashemipour M, Hovsepian S, et al. Prevalence of transient congenital hypothyroidism in central part of Iran. J Res Med Sci 2013;18 (8): 699– 703. [PMC free article] [PubMed] [Google Scholar]
- 35. Nasehi M-M, Zakizadeh R, Mirzajani M. The prevalence of congenital hypothyroidism in north of Iran: First report of screening program. Acad J 2012; 6 (4): 1160. [Google Scholar]
- 36. Grant DB, Smith I. Survey of neonatal screening for primary hypothyroidism in England, Wales and Northern Ireland 1982–4. Br Med J (Clin Res Ed) 1988;296 (6633): 1355– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Skordis N, Toumba M, Savva SC, et al. High prevalence of congenital hypothyroidism in the Greek Cypriot population: results of the neonatal screening program 1990–2000. J Pediatr Endocrinol Metab 2005;18 (5): 453– 61. [DOI] [PubMed] [Google Scholar]
- 38. Corbetta C, Weber G, Cortinovis F, et al. A 7-year experience with low blood TSH cutoff levels for neonatal screening reveals an unsuspected frequency of congenital hypothyroidism (CH). Clin Endocrinol (Oxf) 2009;71 (5): 739– 45. [DOI] [PubMed] [Google Scholar]
- 39. Simşek E, Karabay M, Safak A, Kocabay K. Congenital hypothyroidism and iodine status in Turkey: a comparison between the data obtained from an epidemiological study in school-aged children and neonatal screening for congenital hypothyroidism in Turkey. Pediatr Endocrinol Rev 2003; 2: 155–61. [PubMed] [Google Scholar]
- 40. Ataş B, Altunhan H, Ata E, et al. Frequency of congenital hypothyroidism in neonates in the Konya region, Turkey. J Pediatr Endocrinol Metab 2011;24 (3–4): 139– 40. [DOI] [PubMed] [Google Scholar]
- 41. Tahirovic H, Toromanovic A. Neonatal screening for congenital hypothyroidism in the Federation of Bosnia and Herzegovina: eight years' experience. Eur J Pediatr 2009;168 (5): 629– 31. [DOI] [PubMed] [Google Scholar]
- 42. Rendón-Macías ME, Morales-García I, Huerta-Hernández E, et al. Birth prevalence of congenital hypothyroidism in Mexico. Paediatr Perinat Epidemiol 2008;22 (5): 478– 85. [DOI] [PubMed] [Google Scholar]
- 43. Panamonta O, Tuksapun S, Kiatchoosakun P, et al. Newborn screening for congenital hypothyroidism in Khon Kean university hospital. J Med Assoc Thai 2003;86 (10): 932– 9. [PubMed] [Google Scholar]


