Abstract
Objective:
The objective of this randomized controlled trial was to compare the treatment failure of suspected early onset neonatal sepsis with either 3-day or 5-day course of empirical antibiotic therapy.
Methods:
Infants with birth weight over 1500 g and/or gestational age over 34 weeks within 7 days postnatal age with clinical symptoms of neonatal sepsis received empirical antibiotics (Ampicillin + Amikacin) in two neonatal intensive care units. After 72 hours if the result of blood culture was negative and symptoms resolved they were randomly allocated to 3-day or 5-day groups. The main outcome was treatment failure which was defined as reappearance of symptoms of sepsis within two weeks after discontinuation of antibiotics. Infants with congenital anomalies, localized infections, asphyxia, those undergoing surgery or when serum C-reactive protein levels remained abnormal despite treatment, were not included. Randomization was accomplished with simple randomization procedure.
Findings:
Sixty patients were randomized in a 1:1 ratio to either group. Baseline characteristics were similar between two groups. The follow-up period was 2 weeks with no lost to follow-up. One infant in 3-day group had treatment failure compared with no treatment failure in 5-day group (P=0.5). No serious harm was observed due to our empirical antibiotic regimen.
Conclusion:
The results of this study indicated no evidence that treatment failure differs between 3-day and 5-day course antibiotic therapy for suspected early onset uncomplicated neonatal sepsis in late preterm and term newborns.
Keywords: Neonate, Sepsis, Treatment Failure, Antibiotics, C-Reactive Protein
Introduction
The term “sepsis neonatorum” is used to describe any systemic bacterial infection documented by a positive blood culture in the first month of life. Early onset neonatal sepsis occurs in the first 7 days of life. It has a higher case-fatality rate than late-onset sepsis [1] .
In spite of decline in the case-fatality rate of neonatal sepsis over the past two decades, it is still associated with significant morbidity and mortality, justifying prompt initiation of appropriate empirical antibiotic therapy [2] . Accordingly studies have shown that between 4.4% and 10.5% of all newborns in the United States receive systemic antibiotics [3] . It is estimated that between 11 and 23 non-infected newborns have received antibiotics for each documented bacterial infection at intensive care nurseries [4] .
Such approaches could result in unnecessary use of antibiotics leading to increased cost of care, unnecessary intravenous catheterization, mother-infant separation, prolonged hospitalization, increased colonization by pathogenic organisms, emergence of drug-resistant strains, increased risk of neonatal candidiasis, medication errors, intravenous infiltrates, alteration of gut microflora, necrotizing enterocolitis and death especially with prolonged administration of antimicrobial agents (>5 days) in infants with suspected (with negative blood cultures) early onset sepsis [5–11] .
The recommendations for treatment of these infants are not based on strong evidence. This recommendation is originally based on Pichichero’s study who reported that 96% of bacteremic cultures drawn prior to antibiotic therapy are positive by 48 hours and 98% are positive by 72 hours [12] . Several studies have evaluated the role of serial CRP measurement as a guide to the duration of antibiotic therapy [13,14] . Two consecutive CRP levels <10 mg/L 24 hours apart, 8–48 hours after presentation, have a negative predictive value for sepsis of 99% [15] .
This trial was conducted because we did not find well-designed adequately powered trials evaluating the appropriate duration of empirical antimicrobial therapy in blood-culture-negative suspected early onset sepsis despite intensive searching [2,16] . We decided to determine whether a policy of stopping antibiotics with negative blood culture after 72 hours causes significantly higher treatment failure rate than 5-day course antimicrobial therapy.
Subjects and Methods
This was a parallel-group, single blind, randomized controlled trial with a 1:1 allocation ratio, conducted August 2013 to December 2013 at two level III NICUs (Neonatal Intensive Care Units) (one in a teaching hospital and the other in a private hospital) in Mazandaran/Iran province.
Participants, eligibility criteria, and settings
Eligible participants were infants with birth weight over 1500 g; and/or gestational age over 34 weeks; within 7 days postnatal age; with clinical symptoms of neonatal sepsis e.g. fever, respiratory distress, poor feeding, vomiting, lethargy, apnea, seizure (ICD-10 Code: P36.9); and/or had abnormal CBC, diff, ESR tests; and/or CXR. A minimum of 1 ml of blood using aseptic technique was taken through venipuncture and injected into biphasic blood-culture bottle. They received empirical intravenous antibiotic treatment until determination of the results of blood cultures and they were included in the study if the results of blood culture were negative and symptoms of neonatal sepsis resolved. Exclusion criteria were infants with congenital heart diseases or major congenital anomalies, clinically suspected localized infections, perinatal asphyxia, those undergoing surgery, CRP levels remained abnormal despite treatment and parental unwillingness to enter the research project.
Informed consent was obtained from the parents before recruitment of their neonates.
Interventions
Neonates who were admitted to NICU of Amirkola children’s hospital or Babol-clinic general hospital and were eligible for the study were treated with empirical antibiotics.
After 72 hours, asymptomatic neonates with negative results of blood culture were divided into two groups. For subjects in the first group who had been treated for three days with empirical intravenous antibiotics [17] (Ampicillin for infants with weight <2 Kg, 50 mg/kg/12hr and for infants with weight >2 Kg, 50mg/kg/8hr+Amikacin for infants with weight <2 Kg, 7.5 mg/kg/12hr and for infants with weight >2 Kg, 10mg/kg/12hr) antibiotics were stopped. For patients in the second group, empirical intravenous antibiotic therapy (with the same regimen) was continued for 2 other days (five days on the whole).
In both groups quantitative serum CRP levels were measured before starting and stopping antibiotics. All neonates were followed by telephone call and out-patient follow up clinic on day 2 and 15 after discontinuing antibiotics for clinical evidence of neonatal sepsis (follow up).
Outcomes
The primary outcome of this study with respect to efficacy of short course antimicrobial treatment for suspected early onset neonatal sepsis was treatment failure which was defined as reappearance of signs and symptoms of neonatal sepsis diagnosed by an expert physician (pediatrician or neonatologists) and confirmed by laboratory findings within two weeks after discontinuation of antibiotics.
Randomization, sample size and statistical analysis
Participants were randomly assigned following simple randomization procedure (Sealed-card random numbers) to 1 of 2 treatment groups. Sample size was 60 neonates (30 neonates in each group). The baseline variables were described by descriptive statistics. As all outcomes variables were categorical, χ2 test and Fisher’s Exact Test, as applicable, were used and a P. value <0.05 was taken as significant.
Findings
Out of 257 neonates assessed for eligibility, 197 could not meet inclusion criteria. Thirty-four were symptomatic after 72 hours and parents of 7 neonates declined to participate (Fig. 1). So 60 babies were randomly allocated to the 3-day and the 5-day course antibiotic groups.
Fig. 1:
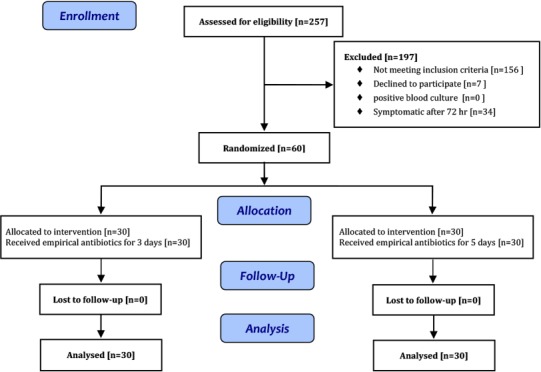
Study Flowchart
Baseline variables including age, sex, birth weight, gestational age, clinical and laboratory findings suggesting early onset sepsis were comparable between the two study groups (Table I). Over the 2 weeks follow-up period, there was no loss to follow-up in any group.
Table 1:
Comparison of baseline variables between neonatal sepsis treated for 3-day and 5-day
| Baseline variable | 3-day group (n=30) | 5-day group (n=30) | P- value |
|---|---|---|---|
| Age at the time of admission (hours) [mean (SD)] | 8.3 (10.9) | 17.5 (19.5) | 0.2 |
| Sex (male/female) | 17/13 | 19/11 | 0.6 |
| Birth weight (g)[mean (SD)] | 3098 (588) | 2984 (541) | 0.4 |
| Gestational age (wks) [mean (SD)] | 37 (1.66) | 37.2 (2.09) | 0.7 |
| Abnormal chest x-ray [n (%)] | 18 (60) | 18 (60) | 1 |
| Abnormal CBC [n (%)] | 1 (3) | 1 (3) | 1 |
| Abnormal ESR [n (%)] | 2 (6.6) | 4 (13.3) | 0.7 |
| Fever [n (%)] | 0 | 1 (3) | 0.5 |
| Respiratory distress [n (%)] | 23 (76.6) | 26 (86.6) | 0.3 |
| Poor feeding [n (%)] | 7 (23.3) | 6 (20) | 0.7 |
| Lethargy [n (%)] | 4 (13.3) | 1 (3) | 0.3 |
| Apnea [n (%)] | 1 (3) | 1 (3) | 1 |
| Hypotonia [n (%)] | 0 | 1 (3) | 0.5 |
CBC: Complete blood count; ESR: Erythrocyte sedimentation rate; SD: Standard deviation
The primary analysis was “treatment failure” and involved all patients who were randomly assigned. Infants in both groups were similar in terms of other routine cares. One infant in 3-day group had treatment failure compared with no treatment failure in 5-day group. (P=0.5). The risk difference was 0.03 (95%CI: 0.03–0.10).
This term female infant was born by cesarean section due to placental abruption. Her mother had received intravenous antibiotics before delivery. The neonate had meconium stained amniotic fluid and normal Apgar score. At 42 hours of birth, she was hospitalized due to a brief apnea and cyanosis and mild respiratory distress on physical examination. She had mild hyperaeration in CXR and normal CBC and ESR values. Empirical antibiotics were started after a blood culture. After 72 hours, she was enrolled due to negative blood culture and improvement of symptoms and was assigned to first group and intravenous antibiotics were stopped. Her serum CRP levels at time of hospitalization and antibiotic discontinuation were 1mg/L and 2mg/L respectively. She was re-evaluated due to poor feeding 48 hours after cessation of antibiotics. According to abnormal neonatal reflexes and CRP=56 mg/L, intravenous antibiotics were resumed after blood, urine and CSF cultures. After 7 days, the cultures were negative and the infant was asymptomatic, so antibiotics were stopped and she was discharged.
Discussion
In this study, “treatment failure” for 3-day and 5-day groups of treatment with intravenous antibiotics for suspected early onset neonatal sepsis was not significantly different.
It could be that the initial non-specific symptoms were not due to neonatal sepsis as evidenced by a negative blood culture [1] .
Despite the paucity of well-designed adequately powered trials evaluating the appropriate duration of empirical antimicrobial therapy in blood-culture-negative sepsis, it has been recommended that the duration of empirical antimicrobial therapy should be 48–72 hours pending culture results for suspected neonatal sepsis [2] .
The only pilot study by Saini et al. randomized 52 infants (>30weeks gestation and >1000 g at birth), with culture negative probable sepsis to either short-course (48–96 hours) or long-course (7 days) antibiotic therapy. Infants with confirmed sepsis and those who had persisting clinical symptoms were excluded. The choice of antibiotic therapy (cephalosporin, amikacin, and cloxacillin) was made by the treating physician. Three babies in the 7-day group had treatment failure (defined as reappearance of signs of sepsis within 15 days of stopping antibiotics, supported by laboratory evidence) as opposed to none in the short-course group (P=0.23) [18] .
In addition to small sample size other limitations of this study include lack of segregation between early- and late-onset sepsis, and usage of different antimicrobial regimens for patients.
Since previous studies have demonstrated that 96% of bacteremic cultures drawn prior to antibiotic therapy are positive by 48 hours and 98% are positive by 72 hours [12] , in this study, treatment duration of 72 hours was considered for first group. On the other hand, studies suggest an association between prolonged empirical treatment of preterm infants (>5 days) with higher risks of late onset sepsis, necrotizing enterocolitis and mortality [9,16] , duration of treatment in second group was limited to 5 days. In addition, serum CRP levels were measured before starting and stopping antibiotics. Although based on previous studies C-reactive protein is an excellent marker for established neonatal bacterial infections and has a negative predictive value for sepsis of 99% if two consecutive CRP levels are <10 mg/L 24 hours apart, 8–48 hours after presentation [15] , the only treatment failure occurred in spite of negative blood culture and two normal CRP values. Negative blood culture of this baby could be due to intravenous antibiotics administered to the mother before delivery [2] .
According to the difficulty of distinguishing nonspecific symptoms of neonatal sepsis in very low birth weight newborns [19] , this study was limited to infants with a gestational age >34 weeks and birth weight >1500 g. Although identifying non-inferiority or equivalence between two groups needs high power trials and thus great sample size, but considering the lack of enough studies in this context and so ethical concerns, this study was performed in 60 neonates. Due to the lack of provision of appropriate placebo for the administered antibiotics and discontinuation of intravenous lines in the majority of infants in first group, providing double-blind study was impossible.
Conclusion
According to the results of this study there is no significant difference in treatment failure between 3-day and 5-day course intravenous antibiotic therapy for suspected early onset uncomplicated neonatal sepsis in late preterm and term newborns.
Acknowledgment
Authors would like to thank all staff of NICUs and newborn services of Amirkola children’s hospital and Babol-clinic general hospital for their contribution to this study and Dr. Ali Bijani for statistical analysis of the data. The trial is registered at www.IRCT.ir , number IRCT2013051913383N1. The study was approved by Babol University of Medical Sciences Ethics Committee (project number of G/P/302458) and was supported by vice chancellery for research of that university.
Authors’ Contribution
Y. Zahed Pasha: Concept, Design, Critical revision of the manuscript.
M. Ahmadpour-kacho: Design, Critical revision of the manuscript.
R. Behmadi: Acquisition of data, Data analysis, Data Interpretation, Drafting of the manuscript.
T. Jahangir: Acquisition of data, Data analysis, Drafting of the manuscript.
All authors approved final version of the article.
Conflict of Interest:
None
References
- 1. Edwards MS. Postnatal Bacterial Infections. In: Martin RJ, Fanaroff AA, Walsh MC. (eds). Fanaroff and Martin's Neonatal-Perinatal Medicine: Diseases of the Fetus and Infant. 9th ed. Missouri: Mosby; 2010; Pp: 793–6. [Google Scholar]
- 2. Sivanandan S, Soraisham AS, Swarnam K. Choice and duration of antimicrobial therapy for neonatal sepsis and meningitis. Int J Pediatr 2011; 2011: 712150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Escobar GJ, Zukin T, Usatin MS, et al. Early discontinuation of antibiotic treatment in newborn admitted to rule out sepsis: a decision rule. Pediatr Infect Dis J 1994; 13 (10): 860– 6 [DOI] [PubMed] [Google Scholar]
- 4. Eichenwald EC. Perinatally transmitted neonatal bacterial infections. Infect Dis Clin North Am 1997; 11 (1): 223– 39. [DOI] [PubMed] [Google Scholar]
- 5. Sormunen P, Kallio MJ, Kilpi T, et al. C-reactive protein is useful in distinguishing Gram stain–negative bacterial meningitis from viral meningitis in children. J Pediatr 1999; 134 (6): 725– 9. [DOI] [PubMed] [Google Scholar]
- 6. Philip AGS, Hewitt JR. Early diagnosis of neonatal sepsis. Pediatrics 1980; 65 (5): 1036– 41. [PubMed] [Google Scholar]
- 7. Lacey RW. Evolution of microorganisms and antibiotic resistance. Lancet 1984; 2 (8410): 1022– 5. [DOI] [PubMed] [Google Scholar]
- 8. Goldmann DA, Leclair J, Macone A. Bacterial colonization of neonates admitted to an intensive care environment. J Pediatr 1978; 93 (2): 288– 93. [DOI] [PubMed] [Google Scholar]
- 9. Cotten CM, Taylor S, Stoll B, et al. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics 2009; 123 (1): 58– 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cotten CM, McDonald S, Stoll B, et al. The association of third-generation cephalosporin use and invasive candidiasis in extremely low birth-weight infants. Pediatrics 2006; 118 (2): 717– 22. [DOI] [PubMed] [Google Scholar]
- 11. Gewolb IH, Schwalbe RS, Taciak VL, et al. Stool microflora in extremely low birthweight infants. Arch Disease Child Fetal Neonatal Ed 1999; 80 (3): F167– 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pichichero ME, Todd JK. Detection of neonatal bacteremia. J Pediatr 1979; 94 (6): 958– 60. [DOI] [PubMed] [Google Scholar]
- 13. Jaswal RS, Kaushal RK, Goel A, et al. Role of C-reactive protein in deciding duration of antibiotic therapy in neonatal septicemia. Indian Pediatr 2003; 40 (9): 880– 3. [PubMed] [Google Scholar]
- 14. Philip AGS, Mills PC. Use of C-reactive protein in minimizing antibiotic exposure: experience with infants initially admitted to a well-baby nursery. Pediatrics 2000; 106 (1): E4. [DOI] [PubMed] [Google Scholar]
- 15. Bomela HN, Ballot DE, Cory BJ, et al. Use of C-reactive protein to guide duration of empiric antibiotic therapy in suspected early neonatal sepsis. Pediatr Infect Dis J 2000; 19 (6): 531– 5. [DOI] [PubMed] [Google Scholar]
- 16. Polin RA, Papile LA, Baley JE, et al. Management of neonates with suspected or proven early-onset bacterial sepsis. Pediatrics 2012; 129 (5): 1006– 15. [DOI] [PubMed] [Google Scholar]
- 17. Young TE. Therapeutic agents. In: Martin RJ, Fanaroff AA, Walsh MC. (eds). Fanaroff and Martin's Neonatal-Perinatal Medicine: Diseases of the Fetus and Infant. 9th ed. Missouri: Mosby; 2010; Pp: 1803–12. [Google Scholar]
- 18. Saini SS, Dutta S, Ray P, et al. Short course versus 7-day course of intravenous antibiotics for probable neonatal septicemia: a pilot, open-label, randomized controlled trial. Indian Pediatr 2011; 48 (1): 19– 24. [DOI] [PubMed] [Google Scholar]
- 19. Hornik CP, Fort P, Clark RH, et al. Early and late onset sepsis in very-low-birth-weight infants from a large group of neonatal intensive care units. Early Hum Dev 2012; 88 (Suppl 2): S69– S74. [DOI] [PMC free article] [PubMed] [Google Scholar]


