Abstract
Objective:
Acute bacterial meningitis in pediatrics remains a serious and potentially lethal disease. Its prognosis is critically dependent on rapid diagnosis and treatment. The use of biological markers, like procalcitonin, has been proposed to facilitate the accuracy of the initial diagnosis of bacterial meningitis. The aim of this study was assessment the diagnostic values of serum procalcitonin (PCT) assay in the diagnosis and differentiation of acute bacterial from non bacterial meningitis.
Methods:
45 patients with suspicion of meningitis were enrolled in the study and were clinically evaluated and investigated by lumbar punctures for cerebrospinal fluid analysis, C-reactive protein and differential leukocyte count. Patients with clinical and laboratory suggestion of bacterial causes were regarded as bacterial meningitis group (29 patients), and those who were suggestive of nonbacterial causes were regarded as nonbacterial group (16 patients).
Findings:
Serum procalcitonin levels were significantly higher in bacterial meningitis group (637±325 pg/ml) compared with non-bacterial meningitis (380±170 pg/ml); P<0.001. Procalcitonin levels were more sensitive and specific (79%, 81%) than C-reactive protein (76%, 75%) and white blood cell count (72%, 75%) in the diagnosis of bacterial meningitis.
Conclusion:
Elevated serum procalcitonin level could be a predictor of bacterial causes of meningitis and is more sensitive and specific than other diagnostic predictors.
Keywords: Bacterial Meningitis, Nonbacterial, Procalcitonon, C-Reactive Protein, White Blood Cell
Introduction
Many disorders may show signs of meningeal irritation and increased ICP including the infectious causes of meningitis either bacterial, viral, rheumatic diseases, hemorrhage and malignancies [1] . Among these causes infectious causes of meningitis are still an important cause of morbidity and mortality in children worldwide, so high index of suspicion, prompt diagnosis, and aggressive management are essential to reduce mortality and morbidity [2] . Acute bacterial meningitis in pediatrics remains a serious and potentially lethal disease. Its prognosis is critically dependent on rapid diagnosis and treatment [3] . Routine analysis of cerebrospinal fluid (CSF) is not always efficient for distinguishing bacterial from a bacterial meningitis [4] . The use of biological markers, especially cytokines and acute phase proteins, has been proposed to facilitate the accuracy of the initial diagnosis of meningitis but the results were conflicting and none of them are used in clinical routine [5,6] . Several serum biomarkers have been identified in recent years that have the potential to help diagnose local and systemic infections, differentiate bacterial and fungal infections from viral syndromes or noninfectious conditions, prognosticate, and ultimately guide management, particularly of antibiotic therapy. Among these, procalcitonin is the most extensively studied biomarker [7,8] . Numerous studies have investigated the potential roles of procalcitonin in diagnosing and managing local and systemic infections [9] . There is some evidence that procalcitonin is more specific for bacterial infections, with serum levels rising at the onset of infection and falling rapidly as the infection resolves, as compared with other markers [10] . In contrast to most classical pro inflammatory cytokines which increase only briefly and intermittently in severe bacterial infections [11] such as ; C-reactive protein (CRP) that reaches its maximum serum levels only 24–48 hours after the onset of infections [12] , serum procalcitonin (PCT) levels increase early (within 4 hours) after injection of bacterial endotoxins and it is stable with long half-life [13,14] . Experimental studies revealed that PCT acts as a modulator of the inflammatory, immunologic host reaction and the treatment with PCT-reactive antiserum increased the survival of septic animals [15, 16] . PCT is more than just a marker in bacterial infection; it might offer a new hope for more effective treatment options in sepsis [14] .
Subjects and Methods
A prospective study of forty five patients with clinical suspicion of acute meningitis (26 males and 19 females; age range 1 to 60 months). All patients were admitted to Al-Zahra teaching hospital in Al-Najaf city in the period from April to October 2013 for suspicion of acute meningitis. Patients with evidence of other systemic or local infection or evidence of thyroid or parathyroid disorders were excluded.
Taking clinical history and thorough physical examination was done in all patients by senior house officer resident doctor. Lumbar puncture was done using sterile needles and the CSF sample analyzed for glucose, protein and cytology. 2 ml of blood was collected from each patient in an EDTA tube and sent for complete blood count analyzer (Sysmex automated hematology analyzer, KX-21N, Japan), another 5 ml of blood was collected in a plan serum tube and sent for CRP level using (enzymatic heterogeneous sandwich immunoassay) and 2 ml of blood for procalcitonin level using an immunoluminometric assay ELIZA M6, USA in which the serum was separated and frozen to −20 centigrade until the time of measurement (not more than 3 months) when dissolved and analyzed.
Depending on the clinical history and physical examination (fever, abnormal movement, irritability, bulging fontanel, meningeal irritation signs) and findings of CSF (increased protein >0.2g/l, decreased glucose ratio <0.4, leukocyte count >1500×106/L and polymorph nuclear leukocyte domination), 29 patients were included in the suspicious of bacterial meningitis group and 16 patients with none of the above findings were regarded as nonbacterial meningitis group. Due to relatively high rate of false negative CSF cultures in our hospital’s laboratory and delay in maintaining results, the CSF culture was not regarded as parameter in differentiating the cases in both groups.
All patients with suspicion of meningitis admitted to pediatric ward were treated with the standard management and followed until discharge.
Parents of eligible newborns were approached for participation in the study and informed consent was obtained from them. Study was approved by ethical and scientific committee of the hospital and college of medicine, University of Kufa.
Primary outcome was to evaluate the procalcitonin as a marker for diagnosis of bacterial meningitis, secondary outcome to compare and assess procalcitonin sensitivity and specificity.
Statistical analysis was done using SPSS version 20.0 (Statistical Package for the Social Sciences, Chicago, IL). Data were expressed as number and percentage for qualitative variables, mean±standard deviation for quantitative ones, chi square test and Student t test were used to compare and ROC curve used for the sensitivity and specificity. P. value less than 0.05 was considered significant.
Findings
From the 45 patients enrolled in this study, 26 patients were clinically diagnosed as bacterial meningitis and 19 patients with no evidence of bacterial causes of meningitis. The mean age of the studied population was (17.7±17.6) months and 58% were females. The most common clinical presentations were fever (97.7%), vomiting (93.3%) and convulsions (73.3%), as shown in Table 1.
Table 1:
Characteristics and clinical features of all studied groups
| Clinical features | Total patients (n=45) | Bacterial meningitis (n=26) | Non-bacterial meningitis (n=19) | P. value | |
|---|---|---|---|---|---|
| Age(month) | 17.7 (17.6) | 17.6 (20.2) | 13.7 (10.9) | 0.4 | |
| Sex | Male | 26 (58%) | 17 (65%) | 9 (47%) | 0.9 |
| Female | 19 (42%) | 12 (35%) | 7 (53%) | ||
| Disturbed Consciousness | 8 (17.7%) | 3 (10%) | 5 (31%) | 0.07 | |
| Irritability | 23 (51.1%) | 13 (44%) | 10 (62%) | 0.2 | |
| Lethargy | 16 (35.5%) | 12 (41%) | 4 (13%) | 0.3 | |
| Convulsion | 33 (73.3%) | 19 (65%) | 14 (87%) | 0.1 | |
| Fever | 44 (97.7%) | 28 (96%) | 14 (87%) | 0.4 | |
| Vomiting | 42 (93.3%) | 27 (93%) | 15 (93%) | 0.9 | |
| Bulging fontanel | 16 (35.5%) | 11 (37%) | 5 (31%) | 0.6 | |
| Headache | 12 (26.6%) | 11 (37%) | 1 (6%) | 0.02 | |
| Photophobia | 4 (0.8%) | 4 (13%) | 0 (0%) | 0.1 | |
| Skin rash | 3 (0.6%) | 3 (10%) | 0 (0%) | 0.2 | |
| Neck stiffness | 14 (31.1%) | 8 (27%) | 4 (13%) | 0.06 | |
| Kernig sign | 2 (0.2%) | 2 (6%) | 0 (0%) | 0.2 | |
| Brudzuniski sign | 2 (0.2%) | 2 (6%) | 0 (0%) | 0.2 | |
The CSF neutrophil cell count and protein were higher in bacterial meningitis group than in nonbacterial (P<0.05) and glucose level was lower (P<0.05). The total white blood cell (WBC) count and neutrophil count also were significantly higher in bacterial group (P<0.05).
The procalcitonon serum level was significantly higher in bacterial group as well as CRP positive cases (P=0.001) as shown in Table 2. Procalcitonin test has higher sensitivity, specificity (79%, 81%) and positive and negative predictive values than CRP and WBC count for diagnosis of bacterial meningitis as shown in Table 3. Serum procalcitonin has more area in ROC curve than WBC count and CRP (0.769, 95%CI: 0.62 - 0.91), as shown in Fig. 1.
Table 2:
Laboratory findings of CSF and blood tests in all studied groups
| Parameter | All patients (n=45) | Bacterial meningitis (n=29) | Non-bacterial meningitis (n=16) | P-value | |
|---|---|---|---|---|---|
| Cerebrospinal fluid | Neutrophils | 34.5 (81.5) | 53.1 (97) | 0.63 (0.9) | 0.007 |
| Lymphocytes | 15.1 (24.8) | 16 (30.9) | 13.4 (4.9) | 0.7 | |
| Proteins | 439 (432) | 567 (490) | 206 (100) | 0.001 | |
| Sugar | 54.8 (19.98) | 45 (14.6) | 72.56 (15.83) | <0.001 | |
| Blood | WBC count | 13.38 (6.365) | 15.06 (6.99) | 10.35 (3.49) | 0.003 |
| Neutrophils (%) | 59.05 (17.73) | 63.90 (14.57) | 50.27 (19.96) | 0.03 | |
| Lymphocytes (%) | 30.68 (15.52) | 26.89 (12.62) | 37.55 (18.19) | 0.5 | |
| Hemoglobin (g/dl) | 11.59 (1.33) | 11.57 (1.525) | 11.63 (0.93) | 0.9 | |
| Hematocrit (%) | 36.07 (5.69) | 34.86 (5.55) | 38.26 (5.45) | 0.06 | |
| Procalcitonin (pg/dl) | 564 (305) | 637 (326) | 380 (170) | 0.001 | |
| CRP positive (%) | 26 (58) | 22 (75) | 4 (25) | 0.001 |
WBC: White Blood Cell; CRP: C-Reactive Protein
Table 3:
Validity of procalcitonin, CRP and WBC in diagnosis of bacterial meningitis
| Validity test | WBC count | CRP | Procalcitonin |
|---|---|---|---|
| Sensitivity | 72.4% | 75.8% | 79.3% |
| Specificity | 75% | 75% | 81.2% |
| Positive predictive value | 84% | 84.6% | 88.4% |
| Negative predictive value | 60% | 63.1% | 72.2% |
| Area under the curve | 0.737 | 0.754 | 0.769 |
| 95% Confidence Interval | 0.591 - 0.883 | 0.601 - 0.908 | 0.624 - 0.915 |
| P-value | 0.009 | 0.005 | 0.003 |
WBC: White Blood cell; CRP: C-Reactive Protein
Fig. 1:
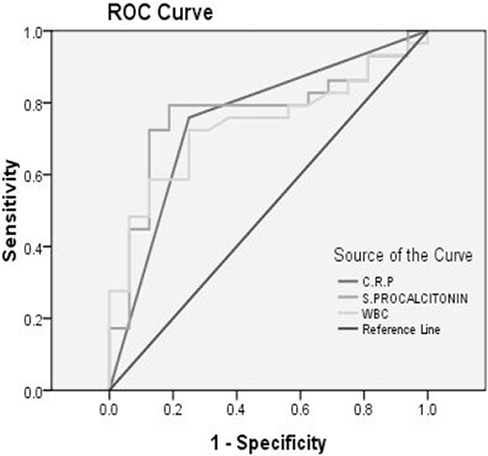
ROC curve for WBC: white blood cell, CRP: C-reactive protein and serum procalcitonin
Discussion
In this prospective analytic study of a group of pediatric patients with clinical diagnosis of meningitis, it is confirmed that a high serum PCT level is the best biological predictor for distinguishing between bacterial and aseptic meningitis in children presenting to ED.
Clinical criteria are unable to distinguish between bacterial and aseptic meningitis with high sensitivity [17,18] . Gram-stain and bacterial antigen testing of cerebrospinal fluid (CSF) is highly specific, but not 100% sensitive [19–22] Biologic markers in blood (C-reactive protein [CRP], white blood cell [WBC] count, neutrophil count) or CSF (protein, glucose, WBCs, and neutrophils) may improve sensitivity for the etiologic diagnosis [20,21] . Serum procalcitonin (PCT) appears to have sensitive and specific early predictive value for bacterial infection [23–26] . We assessed the value of biologic tests in predicting the etiology of meningitis in children with clinical acute meningitis and CSF pleocytosis. The results of ROC curve comparisons and univariate and multivariate analyses of potential biologic tests identify serum PCT cut off (500 pg/dl), and it would be necessary to lower the threshold of PCT to (200 pg/dl) to get 100% sensitivity for the diagnosis of bacterial meningitis.
All studies on procalcitonin in children with sepsis, septic shock, or meningitis report that procalcitonin is an excellent marker of severe bacterial infection and that it has a diagnostic performance significantly greater than that of CRP concentration and leucocyte count [25] . Sensitivity and specificity of procalcitonin varied from 83% to 100% and from 70% to 100%, respectively. For CRP, sensitivity and specificity were in a lower range (73–88% and 50–89%, respectively) [27–30] which is in agreement with results in this study (sensitivity 79.3%, specificity 81.2%, ROC 0.769, 95% CI 0.624 - 0.915, p=0.003), all more than CRP, and WBC count. The diagnostic value of procalcitonin was excellent, both for discriminating between viral and bacterial infections and between invasive and localized bacterial infections.
Although PCT level is probably the best biological predictor currently available to distinguish between bacterial and aseptic meningitis, it cannot be used alone with 100% sensitivity and good specificity regardless of the threshold chosen. Some rare pediatric [19,31] and adult [32] patients with bacterial meningitis may have an initially low PCT level and some investigators have not found the PCT level to be this sensitive, especially in adult populations [27,33] . The use of a recently developed and more sensitive PCT assay (Kryptor; Brahms Diagnostica) and a follow-up measurement (within hours after admission) may increase the sensitivity of the PCT level. Another way to increase the sensitivity of a diagnosis tool while retaining high specificity might be the combination of PCT level at admission with other predictors in a clinical decision rule [34].
The introduction of the PCT in a modified-BMS might enhance sensitivity while maintaining high specificity [35].
This study has a limitation, we couldn’t use a strong evidence to confirm bacterial meningitis like CSF culture or PCR. The CSF culture had high rate of false negative results in our situation and also it was a late method to confirm bacterial meningitis which did not go with our goal to find out an early diagnostic method for bacterial infection and also it was a known fact that negative culture does not rule out bacterial infection.
We recommend to use PCR as a confirmatory parameter for bacterial meningitis in a future study for more evaluation of procalcitonin as an early marker for bacterial meningitis.
Conclusion
Serum PCT assay might be considered as a simple, easy, sensitive and specific marker for an early diagnosis of acute bacterial meningitis. It can differentiate between bacterial and a bacterial etiologies.
Acknowledgment
We would like to thank all lab technicians for helping in samples analysis.
Authors’ Contribution
R.M.R. Umran: Concept, design, interpretation of data and preparation of the manuscript.
N.H. Radhi: Data collection and writing and data analysis
All author approved final version of the manuscript.
Conflict of Interest:
None
References
- 1. Prober CG, Dyner L. Central Nervous System Infections. In: Kliegman RM, Behman RE, jenson HB. Nelson Textbook of Pediatrics. 19th ed. Philadelphia: Saunders; 2011; Pp: 2086–98. [Google Scholar]
- 2. Black S, Shinefield H, Fireman B, et al. Efficacy, safety and immunogenicity of pneumococcal conjugate vaccine in children. Northern California Kaiser Premanente Vaccine Study Center Group. Pediatr Infect Dis J 2000; 19 (3): 187– 95. [DOI] [PubMed] [Google Scholar]
- 3. Durand ML, Caldewood SB, Weber DJ, et al. Acute bacterial meningitis in adults. A review of 493 episodes. N Engl J Med 1993; 328 (1): 21– 8. [DOI] [PubMed] [Google Scholar]
- 4. Marc E, Menager C, Moulin F, et al. Procalcitonin and viral meningitis reduction of unnecessary antibiotics by measurement during an outbreak. Arch Pediatr 2002; 9 (4): 358– 64. [DOI] [PubMed] [Google Scholar]
- 5. Lopez-Cortes LF, Cruz-Ruiz M, Gomez-Mateos J. Interleukin 6 in cerebrospinal fluid of patients with meningitis is not a useful diagnostic marker in the differential diagnosis of meningitis. Ann Clin Biochem 1997; 34 (Pt 2): 165– 9. [DOI] [PubMed] [Google Scholar]
- 6. Mary R, Veinberg F, Coudrec R. Acute meningitides, acute phase proteins and procalcitonin. Ann Bio Clin (Paris) 2003; 61 (2): 127– 37. [PubMed] [Google Scholar]
- 7. Pierrakos C, Vincent JL. Sepsis biomarkers: a review. Crit Care 2010; 14 (1): R 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marshall JC, Reinhart K. Biomarkers of sepsis. Crit Care Med 2009; 37 (7): 2290– 8. [DOI] [PubMed] [Google Scholar]
- 9. Becker KL, Snider R, Nylen ES. Procalcitonin in sepsis and systemic inflammation: a harmful biomarker and a therapeutic target. Br J Pharmacol 2010; 159 (2): 253– 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brunkhorst FM, Heinz U, Forycki ZF. Kinetics of procalcitonin in iatrogenic sepsis. Intensive Care Med 1998; 24 (8): 888– 9. [DOI] [PubMed] [Google Scholar]
- 11. Cbrist-Crain M, Muller B. Procalcitonin in bacterial infections-hype, hope, more or less. Swiss Med Wkly 2005; 135 (31–32): 451– 60. [DOI] [PubMed] [Google Scholar]
- 12. Muller B, White JC, Nylen ES, et al. Ubiquitous expression of the calcitonin-i gene in multiple tissues in response to sepsis. J Clin Endocrinol Metab 2001; 86 (1): 396– 404. [DOI] [PubMed] [Google Scholar]
- 13. Casado Flores J, Blanco Quirós A. Procalcitonin. A new marker for bacterial infections. An ESP Pediatr 2001; 54 (1): 69– 73. [PubMed] [Google Scholar]
- 14. Dandana P, Nix D, Wilson MF, et al. Procalcitonin increase after endotoxin injection in normal subjects. J Clin Endocrinol Metab 1994; 79 (6): 1605– 8. [DOI] [PubMed] [Google Scholar]
- 15. Nylen ES, Whang KT, Snider RH, Jr, et al. Mortality is increased by PCT and decreased by an antiserum reactive to PCT in experimental sepsis. Crit Care Med 1998; 26 (6): 1001– 6. [DOI] [PubMed] [Google Scholar]
- 16. Hoffmann G, Czechowski M, Schlosser M, et al. Procalcitonin ampilifies inducible NO synthase gene expression and nitric oxide production in vascular smooth muscle cell. Crit Care Med 2002; 30 (9): 2091– 5. [DOI] [PubMed] [Google Scholar]
- 17. Nigrovic LE, Kuppermann N, Malley R. Development and validation of a multivariable predictive model to distinguish bacterial from aseptic meningitis in children in the post-Haemophilus influenzae era. Pediatrics 2002; 110 (4): 712– 9. [DOI] [PubMed] [Google Scholar]
- 18. Saez-Llorens X, McCracken GH., Jr Bacterial meningitis in children. Lancet 2003; 361 (9375): 2139– 48. [DOI] [PubMed] [Google Scholar]
- 19. Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis 2004; 39 (9): 1267– 84. [DOI] [PubMed] [Google Scholar]
- 20. Michelow IC, Nicol M, Tiemessen C, et al. Value of cerebrospinal fluid leukocyte aggregation in distinguishing the causes of meningitis in children. Pediatr Infect Dis J 2000; 19 (1): 66– 72. [DOI] [PubMed] [Google Scholar]
- 21. Tatara R, Imai H. Serum C-reactive protein in the differential diagnosis of childhood meningitis. Pediatr Int 2000; 42 (5): 541– 6. [DOI] [PubMed] [Google Scholar]
- 22. Maxson S, Lewno MJ, Schutze GE. Clinical usefulness of cerebrospinal fluid bacterial antigen studies. J Pediatr 1994; 125 (2): 235– 8. [DOI] [PubMed] [Google Scholar]
- 23. Assicot M, Gendrel D, Carsin H, et al. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet 1993; 341 (8844): 515– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gendrel D, Raymond J, Assicot M, et al. Measurement of procalcitonin levels in children with bacterial or viral meningitis. Clin Infect Dis 1997; 24 (6): 1240– 2. [DOI] [PubMed] [Google Scholar]
- 25. van Rossum AM, Wulkan RW, Oudesluys-Murphy AM. Procalcitonin as an early marker of infection in neonates and children. Lancet Infect Dis 2004; 4 (10): 620– 30. [DOI] [PubMed] [Google Scholar]
- 26. Simon L, Gauvin F, Amre DK, et al. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin Infect Dis 2004; 39 (2): 206– 17. [DOI] [PubMed] [Google Scholar]
- 27. Enguix A, Rey C, Concha A, et al. Comparison of procalcitonin with C-reactive protein and serum amyloid for the early diagnosis of bacterial sepsis in critically ill neonates and children. Intensive Care Med 2001; 27 (1): 211– 5. [DOI] [PubMed] [Google Scholar]
- 28. Fernández Lopez A1, Luaces Cubells C, García García JJ, et al. Procalcitonin in pediatric emergency departments for the early diagnosis of invasive bacterial infections in febrile infants: results of a multicenter study and utility of a rapid qualitative test for this marker. Pediatr Infect Dis J 2003; 22 (10): 895– 903. [DOI] [PubMed] [Google Scholar]
- 29. Prat C, Dominguez J, Rodrigo C, et al. Use of quantitative and semiquantitative procalcitonin measurements to identify children with sepsis and meningitis. Eur J Clin Microbiol Infect Dis 2004; 23 (2): 136– 8. [DOI] [PubMed] [Google Scholar]
- 30. Carrol ED, Newland P, Riordan FA, et al. Procalcitonin as a diagnostic marker of meningococcal disease in children presenting with fever and a rash. Arch Dis Child 2002; 86 (4): 282– 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Korczowski B, Bijos A, Rybak A. Procalcitonin in diagnosis of purulent and aseptic meningitis in children. Pol Merkur Lekarski 2000; 9 (53): 755– 7. [In Polish] [PubMed] [Google Scholar]
- 32. Hoffmann O, Reuter U, Masuhr F, et al. Low sensitivity of serum procalcitonin in bacterial meningitis in adults. Scand J Infect Dis 2001; 33 (3): 215– 8. [DOI] [PubMed] [Google Scholar]
- 33. Knudsen TB, Larsen K, Kristiansen TB, et al. Diagnostic value of soluble CD163 serum levels in patients suspected of meningitis: comparison with CRP and procalcitonin. Scand J Infect Dis 2007; 39 (6–7): 542– 53. [DOI] [PubMed] [Google Scholar]
- 34. Dubos F, Korczowski B, Aygun DA, et al. Serum procalcitonin level and other biological markers to distinguish between bacterial and aseptic meningitis in children. Arch Pediatr Adolesc Med 2008; 162 (12): 1157– 63. [DOI] [PubMed] [Google Scholar]
- 35. Dubos F, Rocque F, Levy C. Sensitivity of the bacterial meningitis score in 889 children with bacterial meningitis. J Pediatr 2008; 152 (3): 378– 82. [DOI] [PubMed] [Google Scholar]


