Abstract
Objective:
Cyproheptadine hydrochloride (CH) is a first-generation antihistamine which is used as an appetite stimulant. This study was designed to identify the role of CH therapy on weight gain, linear growth and body mass index in children with mild to moderate undernutrition.
Methods:
Eighty-nine patients were enrolled. The present randomized, double-blinded controlled trial included 77 evaluable patients, aged 24–64 months with undernutrition. The patients were randomized to receive cyproheptadine with multivitamin, or multivitamin over a period of four weeks. The weight, height and body mass index were measured at the baseline, four weeks after intervention and four weeks after discontinuation.
Findings:
A significant higher body mass index was observed among CH-treated patients after 8 weeks intervention with cyproheptadine compared with the control group (P<0.041). Mean weight gain after eight weeks was 0.11 kg in the control group and 0.60 kg in the CH group. There were no significant differences in changes of weight and height velocity across the study between CH-treated and control group at the end of study.
Conclusion:
In our study, cyproheptadine promotes increase in body mass index in children with mild to moderate undernutrition after four weeks treatment.
Keywords: Cyproheptadine, Malnutrition, Weight Gain, Body Mass Index, Clinical Trial, Children
Introduction
Undernutrition is a common health problem in developing countries. Multidimensional factors in the etiology of childhood malnutrition are intrauterine growth retardation, lack of exclusive breast feeding, inappropriate complementary feeding, repeated attacks of infectious illnesses, inadequate food intake, and micronutrient deficiencies. Inadequate food intake may be diet scarcity and/or lack of appetite in child to take food [1–3] . The prevalence rates of undernutrition in children below 5 years were 20% and 32% in low and middle–income countries [4,5] ; however, there is high rate of malnourished children among the highest income countries, too. Finding an effective, safe and available medical treatment for increasing appetite in children with malnutrition is important because long time anorexia can impact on children’s cognitive and future growth.
Cyproheptadine hydrochloride (CH) is a histamine antagonist with appetite-stimulating effect. The probable mechanisms for appetite-stimulating effect of this drug including constant increased energy intake through more desire to eating and stimulation of growth hormone secretion by deep sleep induction [6,7] . Using CH can be a modality for improving the nutritional status in children with malnutrition.
Although different studies were performed using CH as appetite stimulant in patients with malnutrition, anorexia nervosa, cancer, cystic fibrosis, renal failure and AIDS [8–13] , clinical trials with CH in patients with undernutrition are still scarce. The present study aimed to determine whether administration of CH induces weight gain, and linear growth in children with undernutrition.
Subjects and Methods
The present randomized, double-blinded controlled trial was conducted on 89 patients aged 24–64 months with mild to moderate malnutrition who appeared normal on other parts of physical examination referred from August 2011 to April 2012. The severity of malnutrition was determined according to the Gómez classification that mild, moderate, and severe status has been equivalent to 75–90%, 60–74% and less than 60% of standard weight, respectively [14] . Exclusion criteria included subjects with a history of antihistamines intolerance, or receiving sedatives, narcotics, steroids, or appetite stimulants within one-month prior to enrollment. Patients having co-morbidities that might interfere with the changes in weight or height parameters such as urinary tract infection, metabolic disturbances, chronic renal failure and cystic fibrosis were also excluded. Celiac disease was also ruled out by measuring tissue transglutaminase antibodies (tTGA) by enzyme-linked immunosorbent assay [samples with abnormal tTGA results (titers ≥4 U/mL) were not included [15] ]. The study protocol was approved by the research and ethical committees at the Shiraz University of Medical Sciences.
After obtaining informed consent from the patients’ parents, demographic characteristics were collected. Analysis of laboratory parameters including cell blood count, blood urea nitrogen, serum creatinine, biochemical blood tests, liver function tests, fasting blood sugar were done for all included patients in the first visit. Bone age was estimated by left-hand wrist radiography. By a digital scale, weight and height were measured at the time of study, after four weeks of CH therapy and following four weeks of discontinuation of CH therapy. Body mass index (BMI) was calculated as weight (kilograms) divided by height (meters) squared in three times, too. Patients’ parents were referred to the drugstore and the study drugs were administered to the patients based on odd and even numbers. The pharmacologist selected participants by simple random sampling as CH-treated or control group.
Each patient received, either interventional protocol (CH syrup 0.25 mg/kg/day q 12h based on the recommended pediatric dose and multivitamin syrup in one opaque bottle) or placebo (multivitamin syrup in one opaque bottle) for 4 weeks [16] . Taste, smell and appearance of two types of bottle were the same. None of the patients and the physician knew the administered type of syrup. Any abnormal reactions after starting protocol were asked to report from all patients’ parents in second follow-up visit.
Results were reported as mean±standard deviation (SD) for the quantitative variables and percentages for the categorical variables. The groups were compared using the Student's t-test or Mann Whitney U test for the continuous variables and the chi-square test (or Fisher's exact test if required) for the categorical variables. The trends of the changes in study variables were examined by the repeated measure analysis of variance (RMANOVA) trend test. We calculated the sample size based on comparison of two means with α=0.05 and β=0.2, the mean and standard deviation of weight and height of two groups from the other similar study were also mentioned.
P-values of 0.05 or less were considered statistically significant. All the statistical analyses were performed using SPSS version 19.0 (SPSS Inc., Chicago, IL, USA) and SAS version 9.1 for Windows (SAS Institute Inc., Cary, NC, USA).
Findings
Parents of 82 out of 89 eligible children with criteria of mild to moderate malnutrition, agreed to participate in this study. Of these, five patients were excluded because they were unable to come to follow-up visits. Seventy-seven patients completed the study, 37 patients (10 girls, 27 boys) in the placebo group and 40 patients (15 girls, 25 boys) in the CH group (Fig. 1). Mean age of all participants was 42.10±11.76 months (range 24–64 months, median 41 months). Demographic data and laboratory results between placebo and CH groups are shown in Table 1; there were no significant differences for sex, age, weight, height, body mass index, bone age and laboratory results between the two groups.
Fig. 1:
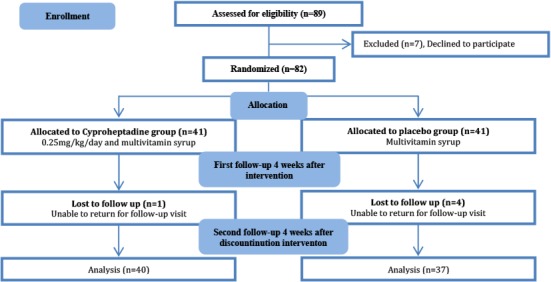
Trial profile
Table 1:
Baseline demographic data and laboratory results in study groups
| Characteristics | Cyproheptadine group (n=40) Mean (SD) | Placebo Group (n=37) Mean (SD) | P. value |
|---|---|---|---|
| Age (month) | 40.08 (11.24) | 44.30 (12.05) | 0.117 |
| Weight (kg) | 11.93 (1.58) | 12.31 (1.68) | 0.304 |
| Height (cm) | 92.35 (6.93) | 93.84 (6.89) | 0.348 |
| Body mass index (kg/m2) | 13.97 (0.81) | 13.95 (0.74) | 0.900 |
| Bone age (year) | 2.64 (0.79) | 2.93 (0.78) | 0.117 |
| White blood count (cell/mm3) | 8260 (266) | 8040 (250) | 0.743 |
| Hemoglobin (g/dL) | 12.20 (1.23) | 12.57 (0.82) | 0.121 |
| Fasting blood sugar (mg/dL) | 80.78 (8.90) | 79.32 (8.30) | 0.230 |
| Albumin (g/dL) | 4.50 (0.26) | 4.45 (0.26) | 0.439 |
| Calcium (mg/dL) | 9.30 (0.62) | 9.44 (0.62) | 0.318 |
| Phosphorus (mg/dL) | 5.09 (0.87) | 4.87 (0.85) | 0.249 |
SD: Standard Deviation
Forty patients in CH group consisted of 36 (90%) mild and 4 (10%) moderate types of undernutrition. Among 37 patients in placebo group, 30 (81%) patients had mild and 7 (19%) moderate form, with insignificant difference between CH and placebo groups.
In our study, mean weight gain after eight weeks was 0.11 kg in the control group and 0.60 kg in the CH group. Compared to baseline status, the result of average weight gain and linear growth after two-months (4 weeks CH therapy and 4 weeks CH after its discontinuation) were not significantly different between the two groups (P=0.83) (Table 2). BMI increased 0.15 kg/m2 in the placebo group and 0.83 kg/m2 in the intervention group (P<0.041) (Fig. 2).
Table 2:
Variations trend of topographic variables in study groups
| Parameter | Baseline | 4 weeks CH therapy | 4 weeks after CH discontinuation Mean (SD) | P-value (group effect) | P-value (time effect) | P-value (interaction) | |
|---|---|---|---|---|---|---|---|
| Weight | CH | 11.93 (1.58) | 12.24 (2.61) | 12.53 (1.68) | 0.4 | 0.002 | 0.8 |
| Placebo | 12.31 (1.68) | 12.65 (1.83) | 12.42 (2.62) | ||||
| Height | CH | 92.35 (6.93) | 93.33 (6.71) | 93.81 (6.67) | 0.4 | <0.001 | 0.4 |
| Placebo | 93.84 (6.89) | 94.59 (7.03) | 95.05 (6.97) | ||||
| BMI | CH | 13.97 (0.81) | 14.44 (0.75) | 14.80 (0.67) | 0.3 | <0.001 | 0.04 |
| Placebo | 13.95 (0.74) | 14.12 (0.75) | 14.10 (0.75) |
CH: Cyproheptadine; BMI: Body mass index
Fig. 2:
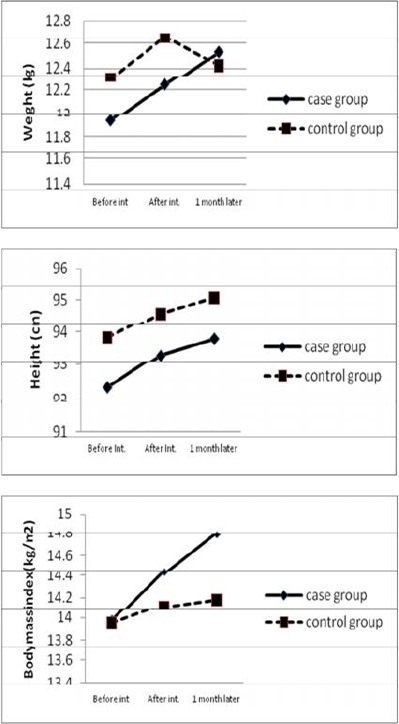
Weight, height and body mass index in cypro-treated and control groups before, 4 weeks after intervention and 1 month after discontinuation of cyproheptadine.
The most frequent adverse reaction to protocol regimen was sleepiness, 3 (8.1%) in the placebo group and 7 (17.5%) in CH-treated patients after 4 weeks therapy. Agitation was seen only in one patient in CH group. No significant difference was found between placebo and intervention group in terms of observed side effects.
Comparing willingness to eating (Table 3) showed that the unwillingness to eating after a few tablespoons of food was lower in the CH group than in the controls and attention to eating was more increased in the CH group following intervention. Also, more increase in the mean number of meals was observed after programmed intervention in the CH group compared with the placebo group.
Table 3:
Status of food intake before and after intervention in study groups
| Parameter | CH group (%) | Placebo group (%) | P. value | |
|---|---|---|---|---|
| Tend to eating | At the baseline | 7 (17.5%) | 7 (18.9%) | 0.9 |
| 4 weeks after CH therapy | 30 (75.0%) | 28 (75.5%) | 1 | |
| Unwillingness to eating after a few tablespoons of food | At the baseline | 36 (90.0%) | 33 (89.2%) | 0.9 |
| 4 weeks after CH therapy | 8 (10.0%) | 12 (32.4%) | 0.02 | |
| Attention to eating | At the baseline | 12 (30.0%) | 9 (24.3%) | 0.6 |
| 4 weeks after CH therapy | 30 (75.0%) | 18 (48.6%) | 0.02 | |
| Mean number of meals Mean (SD) | At the baseline | 3.00 (0.39) | 2.92 (0.64) | 0.5 |
| 4 weeks after CH therapy | 4.03 (0.77) | 3.70 (0.78) | 0.04 |
CH: Cyproheptadine; SD: Standard Deviation
Discussion
Normal weight and height is an important health indicator in children. Many known factors including neuropeptide Y, serotonine, glucagon like peptide 1, tumor necrotizing factor-α, some hormones like insulin and leptin are involved in regulation of anthropometric parameters in human [17,18] . Nevertheless, some antihistamines can impact height and weight regulatory processes, too. CH as an antihistamine and antiserotonin drug is administered for enhancement of height and weight in those who suffer from weight loss [19,20] . This study focused on weight, height and BMI velocity response to CH therapy in children with undernutrition.
The results of our study on 40 children with mild to moderate undernutrition who were treated with CH 0.25mg/kg/day for a 4-week period compared with 37 patients as placebo showed significant increased BMI. Patients in CH-treated group showed higher weight gain across this study, though there was no significant difference in comparison with placebo group. The effect of CH in 21 underweight children aged 2–10 years, has been reported by Mahachoklertwattana et al [21] . They reported weight and height velocities were significantly greater in the group received CH therapy for 4 months than in those of the placebo group in underweight children [21] . Rerksuppaphol and Rerksuppaphol in a double-blind, placebo controlled study determined the administration of CH in malnourished children. Seventy malnourished patients (age 6–15 years) were randomized to receive CH 0.3 mg/kg/day or placebo for eight weeks, the results show a significant weight gain in CH-treated children [8] . In the current study, the effects of CH on weight gain are nearly similar to these studies, but the low significant rate of this result in comparison with placebo might be due to short-time treatment (4 weeks) with CH in our patients.
Considering that all of the patients entering the study received multivitamin with or without CH, we expected improvement in both groups; but CH-treated patients showed a significant greater weight gain.
We could not show enhancement of linear growth after intervention between placebo and CH group. Kaplowitz et al revealed the effect of CH on increased linear velocity in six children with growth hormone deficiency but improved linear growth was achieved after a 4-month period [22] . Insignificant height velocity in the current study might be due to short time follow-up visits.
The probable mechanisms have been discussed for appetite enhancement by CH. Treatment with CH in underweight children showed increased insulin-like growth factor which is a promoting factor for growth hormone [21] . Another mechanism is related to efficacious role of CH on feeding center in hypothalamus. On the other hand, anticholinergic effect of CH causes reduction in motility of gastrointestinal tract and consequently increasing transit time of food [23] . With attention to positive effect of CH on weight gain in different studies, further investigation is required to explain exact mechanism of CH.
In the present study, the low dose of CH (0.25 mg/kg/day) was used with considerable effect on weight gain in children. Nemati et al demonstrated the effect of 5, 10 and 20 mg/kg/day CH consumption in mice with daily measuring weight and food intake. CH in lowest dose (5 mg/kg) caused weight gain and increased food intake, in 10 mg/kg had no result on weight and food intake and 20 mg/kg even caused weight loss and decreased food intake in animal models [24] . In our study, we couldn’t measure food intake in children but patients’ parents reported improved willingness to eating, attention to eating and the rate of daily meals in these children. We could not consider different doses of CH in this study because of ethical problems. In this context, administration of low dose CH is considered as an alternative treatment in poor appetite children.
Cyproheptadine has some adverse reactions especially when taken in excess amount. Some adverse reactions to CH include sedation, confusion, hallucinations, hypotension, palpitations, and tachycardia which are consistent with the anticholinergic syndrome [16] . A few number of our patients developed sleepiness, dry mouth and agitation after 4 weeks treatment with CH but none showed these symptoms in follow-up visits.
There were no significant differences for weight between interventional and control group in this study at the baseline visit, however, the control group weighed a little more. This little difference matters in children younger than 12 months due to higher rate of growth. In our study, all patients were older than 12 months with slower growth velocity.
In this study, one advantage was randomization and blindness that could exclude any potential sources of methodical and human bias.
Conclusion
In summary, administration of CH in undernourished children can show a significant increase in BMI after only four weeks. A clinical improvement in willingness to eating, attention to eating, and the rate of daily meals can also be obtained. Such findings suggest that the prescription of CH may be considered as an alternative approach for children who suffer from undernutrition.
Acknowledgment
This work was supported by a grant from Shiraz University of Medical Sciences. This trial was registered in Iran Clinical Trials Registry (IRCT) under number IRCT201201268827N1.
Authors’ Contribution
K. Najib: Concept and design, acquisition of data, analysis and interpretation of data, revised the article
M. Moghtaderi: Concept and design, acquisition of data, drafted the article, revised the article
Z. Karamizadeh: Concept and design, acquisition of data, revised the article
E. Fallahzadeh: Concept and design, acquisition of data and analysis of data
All authors approved final version of the article.
Conflict of Interest:
None
References
- 1. Jeong SJM. Nutritional approach to failure to thrive. Korean J Pediatr 2011; 54 (7): 277– 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sharghi A, Kamran A, Faridan M. Evaluating risk factors for protein-energy malnutrition in children under the age of six years: a case-control study from Iran. Int J Gen Med 2011; 4: 607– 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sharifzadeh G, Mehrjoofard H, Raghebi S. Prevalence of malnutrition in under 6-year olds in South Khorasan, Iran. Iran J Pediatr 2010; 20 (4): 435– 41. [PMC free article] [PubMed] [Google Scholar]
- 4. Payandeh A, Saki A, Safarian M, et al. Prevalence of malnutrition among preschool children in northeast of Iran, a result of a population based study. Glob J Health Sci 2013; 5 (2): 208– 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bergman P, Graham J. An approach to failure to thrive. Aust Fam Physician 2005; 34 (9): 725– 9. [PubMed] [Google Scholar]
- 6. Bergen SS., Jr Appetite Stimulating Properties of Cyproheptadine. Am J Dis Child 1964; 108: 270–3. [DOI] [PubMed] [Google Scholar]
- 7. Halmi KA, Goldberg SC. Cyproheptadine in anorexia nervosa. Psychopharmacol Bull 1978; 14 (2): 31– 3. [PubMed] [Google Scholar]
- 8. Rerksuppaphol S, Rerksuppaphol L. Effect of cyproheptadine on weight gain in malnourished children: a randomized, controlled trial. Asian Biomed 2010; 4: 977– 82. [Google Scholar]
- 9. Powers PS, Santana C. Available pharmacological treatments for anorexia nervosa. Expert Opin Pharmacother 2004; 5 (11): 2287– 92. [DOI] [PubMed] [Google Scholar]
- 10. Couluris M, Mayer JL, Freyer DR, et al. The effect of cyproheptadine hydrochloride (periactin) and megestrol acetate (megace) on weight in children with cancer/treatment-related cachexia. J Pediatr Hematol Oncol 2008; 3 0: 791– 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Epifanio M, Marostica PC, Mattiello R, et al. A randomized, double-blind, placebo-controlled trial of cyproheptadine for appetite stimulation in cystic fibrosis. J Pediatr (Rio J) 2012; 88 (2): 155– 60. [DOI] [PubMed] [Google Scholar]
- 12. Aguilera A, Selgas R, Diéz JJ, et al. Anorexia in end-stage renal disease: pathophysiology and treatment. Expert Opin Pharmacother 2001; 2 (11): 1825– 38. [DOI] [PubMed] [Google Scholar]
- 13. Dabaghzadeh F, Khalili H, Ghaeli P, et al. Potential benefits of cyproheptadine in HIV-positive patients under treatment with antiretroviral drugs including efavirenz. Expert Opin Pharmacother 2012; 13 (18): 2613– 24. [DOI] [PubMed] [Google Scholar]
- 14. Gómez F, Ramos-Galvan R, Frenk S, et al. Mortality in second and third degree malnutrition. J Trop Pediatr (Lond) 1956; 2 (2): 77– 83. [DOI] [PubMed] [Google Scholar]
- 15. Rubio-Tapia A, Kyle RA, Kaplan EL, et al. Increased prevalence and mortality in undiagnosed celiac disease. Gastroenterology 2009; 137 (1): 88– 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Periactin® package insert. Whitehouse Station, NJ: Merck & Co; 2002. [Google Scholar]
- 17. Crowley VE, Spiegelman BM. Obesity therapy: Altering the energy intake and expenditure balance sheet. Nat Rev Drug Disc 2002; 1 (4): 276– 81. [DOI] [PubMed] [Google Scholar]
- 18. Flier JS, Maratos-Flier E. Obesity. In: Kasper DL, et al. Harrison's principles of internal medicine. 16th ed. New York: McGraw-Hill; 2005; pp 422–9. [Google Scholar]
- 19. Herfindal ET, Gourley DR. Textbook of therapeutics drug and disease management. 7th ed. New York: Lippincott Williams and Wilkins; 2000; pp 1277–80. [Google Scholar]
- 20. Ferriols LF, Tordera BM. Wasting syndrome in cancer patients: Pathophysiology, manifestations and drug therapy. Farm Hosp 2003; 27 (5): 308– 16. [PubMed] [Google Scholar]
- 21. Mahachoklertwattana P, Wanasuwankul S, Poomthavorn P, et al. Short-term cyproheptadine therapy in underweight children: effects on growth and serum insulin-like growth factor-I. J Pediatr Endocrinol Metab 2009; 22 (5): 425– 32. [DOI] [PubMed] [Google Scholar]
- 22. Kaplowitz PB, Jennings S. Enhancement of linear growth and weight gain by cyproheptadine in children with hypopituitarism receiving growth hormone therapy. J Pediatr 1987; 110 (1): 140– 3. [DOI] [PubMed] [Google Scholar]
- 23. Daviss WB, Scott J. A chart review of cyproheptadine for stimulant-induced weight loss. J Child Adolesc Psychopharmacol 2004; 14 (1): 65– 73. [DOI] [PubMed] [Google Scholar]
- 24. Nemati M, Habibi AsL B, Sharifi K. Effect of ketotifen and cyproheptadine on appetite and weight changes in mice. Iran J Pharmaceut Sci 2006; 2: 123– 8. [Google Scholar]


