Abstract
Minimal hepatic encephalopathy (MHE) is the mildest form of the spectrum of neurocognitive impairment in cirrhosis. It is a frequent occurrence in patients of cirrhosis and is detectable only by specialized neurocognitive testing. MHE is a clinically significant disorder which impairs daily functioning, driving performance, work capability and learning ability. It also predisposes to the development of overt hepatic encephalopathy, increased falls and increased mortality. This results in impaired quality of life for the patient as well as significant social and economic burden for health providers and care givers. Early detection and treatment of MHE with ammonia lowering therapy can reverse MHE and improve quality of life.
Keywords: cirrhosis, neurocognitive testing, ammonia lowering therapy
Abbreviations: CLDQ, Chronic Liver Disease Questionnaire; CTP, Child-Turcotte-Pugh; HE, Hepatic encephalopathy; HRQOL, Health related quality of life; MHE, Minimal hepatic encephalopathy; NHP, Nottingham Health Profile; SIP, Sickness Impact Profile
Hepatic encephalopathy (HE) is a neurocognitive disorder in which brain function is impaired and is associated with both acute and chronic liver dysfunction.1 It is a major complication that develops in some form and at some stage in a majority of patients with liver cirrhosis. Minimal HE (MHE) is the mildest form of spectrum of HE which is characterized by subtle cognitive and psychomotor deficits in the absence of recognizable clinical symptoms of HE.2 It occurs in patients with liver dysfunction and/or portosystemic shunts. In MHE, neurocognitive abnormalities primarily affect attention, speed of information processing, executive control, motor ability and coordination in an individual.3
In 1970, Zeegen et al4 first described this condition when they discovered that 38% of patients who had undergone portal decompression surgery scored abnormal in Reitan trail making test (number connection test). Eight years later, the term subclinical HE5 was introduced to describe these patients. Since then, this condition was described under various names like early HE, latent HE, subclinical HE and finally minimal HE. The latest classification combines MHE and grade 1 HE into covert HE while higher grades are classified as overt HE, thus simplifying the clinical schema so that HE can be uniformly diagnosed.6
Covert HE means that the mental defect is not detectable by the clinician using conventional testing and is not noticeable to the patient. However, it is significant because these patients usually have neuropsychiatric and neurophysiological abnormalities on advanced testing which are not enough to cause disorientation or asterixis. MHE is regarded as a preclinical stage of HE and ammonia and systemic inflammation plays an important role in its pathogenesis similar to HE. Ammonia lowering therapies were used in the treatment of MHE and found to be effective.
MHE is clinically significant as it impairs daily functioning, health related quality of life (HRQOL) and driving skills, predicts the development of overt HE and is associated with poor survival.7–11 Overt HE develops in >50% of MHE patients within three years.10 These patients pose a significant burden to their care givers depending on the severity of cognitive dysfunction.2,12 Considering all these facts together, early detection and treatment of MHE is warranted and recommended.2
This review focuses on MHE and its effect on daily functioning including driving skills, sleep disturbances and HRQOL in an individual.
Epidemiology
Several studies suggest that the majority of patients with cirrhosis will develop some degree of HE at some point during the course of disease. Overt HE occurs in approximately 30%–50% of patients with cirrhosis10,13 and 10–50% of patients with transjugular intrahepatic portosystemic shunt.14 The prevalence of MHE in patients with cirrhosis who do not have the evidence of overt HE is high and has been reported to vary between 30% and 84%.2,10,15 Large variability in these studies were due to different diagnostic criteria used and population studied. Prevalence of MHE in our population varies between 48% and 67.7% as shown by our previous studies using psychometric tests.2,10,11 Patients who develop MHE are older, more often have alcohol as etiology of cirrhosis, have history of overt HE in the past, have more severe liver disease as quantified by Child Pugh score and more often have esophageal and gastric varices.16 Prevalence of MHE increases with increasing severity of liver dysfunction2,7,10,16–19 and is not affected by etiology of cirrhosis once patients with recent alcohol intake were excluded.
Pathogenesis of minimal hepatic encephalopathy
Although incompletely defined, pathogenesis of HE is considered multifactorial. It is likely that gut derived neurotoxins (ammonia, benzodiazepines, indoles, etc) acting synergistically with inflammation and oxidative stress cause HE and its associated manifestations. MHE being a preclinical stage of HE has similar pathogenesis.20–22 Ammonia is metabolized only by astrocytes in brain and increased ammonia levels causes rise in intracellular glutamine levels leading onto low-grade cerebral edema and HE.23 Ammonia also modulates glutamate neurotransmission and induces neurosteroid production in neurons, leading to a positive modulatory effect on the gamma-aminobutyric acid-A receptor.24 Ammonia lowering therapies like lactulose,11,25,26 rifaximin27 and probiotics28,29 have been shown to result in resolution of MHE and overt HE, thereby providing indirect evidence to ammonia's pathogenic role in HE. However, recent studies have shown the persistence of cognitive impairment in cirrhotics even after complete resolution of HE despite ammonia lowering therapy suggesting role of other factors.30–32 Small intestinal bacterial overgrowth correlates with MHE in patients with cirrhosis suggesting the role of gut derived bacterial toxins.32 Recent studies have implicated infection and systemic inflammation in the pathogenesis of HE.33,34 Hyperammonemia in the presence of systemic inflammation or infection results in lower cognitive scores compared to in the absence of inflammation. Inflammatory markers like TNF-α, IL-1, IL-6 and C-reactive protein were elevated in MHE patients and correlated with its severity.33,34 Inflammation increases permeability of blood brain barrier35,36 thereby leading onto increased diffusion of ammonia into brain37 and subsequently MHE. Manganese is a neurotoxin that accumulates in patients with liver cirrhosis and portosystemic shunts38 and may contribute to cerebral edema in HE.39 Its levels correlate with pallidal hyperintensity seen on MR brain scans of patients with cirrhosis, who may also demonstrate extrapyramidal signs.
Clinical significance
MHE is clinically significant disorder as it impairs daily functioning, reduces quality of life and increases disability by affecting driving skills, learning capacity, reducing work performance and increasing the risk of falls (Figure 1).
Figure 1.
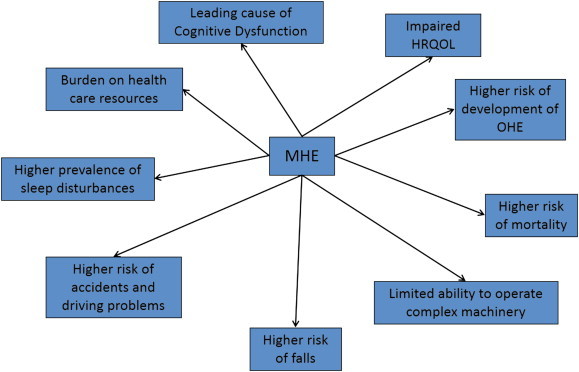
Clinical significance of minimal hepatic encephalopathy.
Effect of Minimal Hepatic Encephalopathy on Quality of Life
Quality of life (QOL) is a multidimensional concept comprehensively addressing all aspects of human well-being, encompassing physical and cognitive capabilities, functional behavior, emotional status and psychosocial adjustment.40 It is measured by using general health scales like Nottingham Health Profile (NHP), the Sickness Impact Profile (SIP), SF - 3616,41–43 and disease specific scales like Chronic Liver Disease Questionnaire (CLDQ), NIDDK-QA and the Liver Disease Quality of Life Instrument.44–47
MHE adversely affects HRQOL as shown by various studies.11,41,43,48–50 Complex activities which require attention or information processing and psychomotor skills are mainly affected, such as driving a car or planning a trip. Basic functions of day to day self-care like personal hygiene, dressing and shopping are preserved.11 Studies have shown that HRQOL scores were impaired in almost all scales of SIP in patients with MHE compared to patients without MHE. Significant impairment is seen in social interaction, alertness, emotional behavior, sleep, work, home management, and recreation and pastimes.11,43,47
In a study using SF-36 and NHP, Marchesini et al42 had shown that etiology and duration of cirrhosis had no effect on HRQOL and minor symptoms like pruritus and muscle cramps had significant negative impact on HRQOL. In decompensated cirrhotics, MHE impairs domains of activity, emotional function and global scoring on CLDQ. MHE also reduces appetite in cirrhotics and as the liver dysfunction worsens, malnutrition occurs which adversely impacts HRQOL.49 Depression, anxiety-trait and alexithymic symptoms occurs in cirrhotics and are major determinants of impaired HRQOL.50
Treatment of MHE with lactulose,11 rifaximin27,51 or probiotics29 reverses MHE in majority of patients. Significantly HRQOL also improves with treatment of MHE. Driving performance using a driving simulator also improved significantly with reversal of MHE in cirrhotic patients after 8 weeks of rifaximin therapy.51
Sleep and Health Related Quality of Life
Sleep disturbances are common in patients with MHE. Sleep disturbance is seen in 26–70% of patients with cirrhosis.52–56 Studies have confirmed higher frequency of sleep disturbances in patients of cirrhosis with MHE compared to those without MHE.11,52 Sleep is maintained by two processes namely, ‘Homeostatic process’ that determines sleep propensity in relation to waking hours and ‘Circadian process’ under the control of suprachiasmatic nucleus and its retino-hypothalamic axis which responds to light-dark cues by melatonin secretion.57 Patients with cirrhosis have unsatisfactory night sleep due to delayed sleep onset and multiple night awakenings resulting in reduced sleep time and excessive daytime sleepiness.57 Night time sleep disturbances are not related to HE and are caused by circadian rhythm abnormalities due to toxic effect on suprachiasmatic nucleus and impaired melatonin clearance.52 However, excessive daytime sleepiness correlates with ammonia levels and is associated with increased risk of HE related hospitalization and presence of portosystemic shunts.58 Sleep disturbances contribute to impairment in HRQOL in MHE patients.54,56 While some studies did not find any correlation between sleep disturbances and neurocognitive impairment, we recently demonstrated that there is significant correlation between MHE, sleep abnormalities and HRQOL.56
Memory and Learning Difficulties in Minimal Hepatic Encephalopathy
MHE is characterized by a pattern of subcortical dysfunction characterized by slowed mental processing, disturbances of attention and concentration, executive disabilities, psychomotor slowing and memory disorders.59 Memory deficits in MHE affects only short-term memory and are due to the attention deficits thereby causing an encoding defect and learning impairment.59,60 FDG PET studies in MHE patients had shown decreased glucose metabolism in parieto-occipital region involved in visual perception as against preserved metabolism in temporomesial area concerned with memory.61,62 Experimental data suggests that learning impairment was due to hyperammonemia causing decreased cGMP levels and may respond to pharmacological manipulation.63
Driving and Navigational Skills in Minimal Hepatic Encephalopathy
MHE is associated with impaired driving performance both by using real road driving tests or a driving simulator. Schomerus et al,64 in his study of 40 cirrhotic patients found that 60% of them were unfit to drive based on their psychometric performance. Similar results were reported in a study by Watanabe et al.65 However, a pilot study of 9 MHE patients using real road driving found no impairment in driving performance of these patients.66
In a landmark study involving 48 patients with cirrhosis, Wein and his colleagues67 had shown that MHE patients had impairment of driving using a standardized 90 min on-road driving test. Compared to NMHE patients, MHE patients showed most impairment in categories like car handling, maneuvering, adaptation and cautiousness compared to NMHE patients. Intervention required by the driving instructor to avoid accidents were more in MHE patients.67 Increased risk of accidents was attributed to decline in cognitive function in MHE patients.68 They also reported to have difficulties in following a map.69 They had significantly higher self-reported rates of traffic violations and motor vehicle accidents.70 Risk of future collisions was related to the presence of MHE and history of prior collision.71 Driving performance worsens over time due to fatigue leading to more collisions during second half of driving.72
Navigation is a complex activity required for safe driving and depends on functional working memory, attention, response inhibition, visuomotor coordination, reaction time and executive control. MHE patients had impairment in navigation leading to more number of illegal turns and accidents which correlated with abnormal inhibitory control test.73 Treatment of MHE with rifaximin improves driving performance of these patients in a driving simulator, which correlates with improvement in cognition.51 Impaired driving performance in MHE patients is attributed to poor insight74 of their driving skills, prolonged reaction time, impaired navigational skills and worsening fatigue. Insight into driving skills in cirrhosis improves after driving simulation and is highest in those with navigation errors and MHE on ICT.75
Falls in Minimal Hepatic Encephalopathy
MHE is associated with increased risk of falls76,77 which result in reduced quality of life. While 12% of patients without MHE had falls, almost 40% of patients with MHE had falls resulting in an increased need for hospitalization. Risk is increased further in patients taking psychoactive drugs.76 Increased risk of falls in MHE patients is due to impaired attention and visuomotor coordination and slowed reaction time and psychomotor speed. Increased number of falls and associated osteoporosis predisposes to these patients to increased risk of fractures78–80 and associated surgery which leads on to significant morbidity, decompensation and mortality.79 This in turn has an indirect effect on patient's family and carries a high economic burden to the society.81
Employment and Socioeconomic Burden of Minimal Hepatic Encephalopathy
Working ability is an essential component of QOL, and inability to work produces a significant impact on HRQOL. Due to slowing of psychomotor function and reduced work performance in patients with MHE, almost half of them do not have regular employment, compared to only 15% of patients without MHE.16 About 60% of blue-collar workers are unfit to work compared to only 20% of white collar workers as manual labor is affected more by MHE whereas verbal intelligence remains preserved.82 Patients with MHE involved in complex occupational tasks are specially affected as they endanger themselves as well as others.83 Impact of MHE on daily life is enormous.12 Diminished work performance and lost wages also entail substantial costs. Socioeconomic implications of the profound negative effects of MHE on functioning in the workplace are significant.12
Natural History and Survival in Minimal Hepatic Encephalopathy
Patients with MHE may improve, remain unchanged or deteriorate and develop overt HE over a long-term follow-up. Frequency of MHE increases as severity of liver dysfunction increases.7,10,12,16–18 MHE predicts the development of overt HE and also adversely affects survival as shown by several studies.7,10,18,84–87 Das et al10 studied the progression of MHE to overt HE in relation to severity of liver dysfunction and found that overt HE were more frequent in patients with MHE and Child-Turcotte-Pugh (CTP) score >6. Hartmann et al7 found that survival was determined only by CTP score irrespective of presence or absence of MHE. However, we found that both abnormal psychometric hepatic encephalopathy score and CTP score were significant independent prognostic indicators associated with survival.3 In a recent study, Patidar et al187 have shown that covert HE was associated with worsened survival, increased risk of hospitalization and overt HE development after controlling for the MELD score.
Conclusion
MHE is a common condition affecting patients with cirrhosis which may be clinically silent but is associated with significant disability resulting in impaired quality of life. MHE reduces day to day functioning capability of patients by impairing driving, work capability, and learning ability resulting in increased dependency on care givers. Not only does it cause significant social and economic burden, it also predisposes to the development of overt HE and increased mortality. Early detection and treatment of MHE is recommended on case to case basis.2 Ammonia lowering therapies like lactulose, rifaximin and probiotics not only reverse MHE but also associated with improvement in HRQOL as well.
Conflicts of interest
All authors have none to declare.
References
- 1.Seyan A.S., Hughes R.D., Shawcross D.L. Changing face of hepatic encephalopathy: role of inflammation and oxidative stress. World J Gastroenterol. 2010;16:3347–3357. doi: 10.3748/wjg.v16.i27.3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dhiman R.K., Saraswat V., Duseja A., Chawla Y.K. MHE. Consensus statement of a working party of the indian national association for study of the liver. J Gastroenterol Hepatol. 2010;25:1029–1041. doi: 10.1111/j.1440-1746.2010.06318.x. [DOI] [PubMed] [Google Scholar]
- 3.Dhiman R.K., Chawla Y.K. Minimal hepatic encephalopathy. Indian J Gastroenterol. 2009;28:5–16. doi: 10.1007/s12664-009-0003-6. [DOI] [PubMed] [Google Scholar]
- 4.Zeegen R., Drinkwater J.E., Dawson A.M. Method for measuring cerebral dysfunction in patients with liver disease. Br Med J. 1970;2:633–636. doi: 10.1136/bmj.2.5710.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rikkers L., Jenko P., Rudman D., Freides D. Subclinical hepatic encephalopathy: detection, prevalence, and relationship to nitrogen metabolism. Gastroenterology. 1978;75:462–469. [PubMed] [Google Scholar]
- 6.Kappus M.R., Bajaj J.S. Covert hepatic encephalopathy: not as minimal as you might think. Clin Gastroenterol Hepatol. 2012;10:1208–1219. doi: 10.1016/j.cgh.2012.05.026. [DOI] [PubMed] [Google Scholar]
- 7.Hartmann I.J., Groeneweg M., Quero J.C. The prognostic significance of subclinical hepatic encephalopathy. Am J Gastroenterol. 2000;95:2029–2034. doi: 10.1111/j.1572-0241.2000.02265.x. [DOI] [PubMed] [Google Scholar]
- 8.Stewart C.A., Malinchoc M., Kim W.R., Kamath P.S. Hepatic encephalopathy as a predictor of survival in patients with end stage liver disease. Liver Transpl. 2007;13:1366–1371. doi: 10.1002/lt.21129. [DOI] [PubMed] [Google Scholar]
- 9.Bajaj J.S., Wade J.B., Gibson D.P. The multi-dimensional burden of cirrhosis and hepatic encephalopathy on patients and caregivers. Am J Gastroenterol. 2011;106:1646–1653. doi: 10.1038/ajg.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Das A., Dhiman R.K., Saraswat V.A., Naik S.R. Prevalence and natural history of subclinical hepatic encephalopathy in cirrhosis. J Gastroenterol Hepatol. 2001;16:531–535. doi: 10.1046/j.1440-1746.2001.02487.x. [DOI] [PubMed] [Google Scholar]
- 11.Prasad S., Dhiman R.K., Duseja A., Chawla Y., Sharma A., Agarwal R. Lactulose improves cognitive functions and health-related quality of life in cirrhotic patients with minimal hepatic encephalopathy. Hepatology. 2007;45:549–559. doi: 10.1002/hep.21533. [DOI] [PubMed] [Google Scholar]
- 12.Poordad F.F. Review article: the burden of hepatic encephalopathy. Aliment Pharmacol Ther. 2007;25(suppl 1):3–9. doi: 10.1111/j.1746-6342.2006.03215.x. [DOI] [PubMed] [Google Scholar]
- 13.Ferenci P., Lockwood A., Mullen K., Tarter R., Weissenborn k, Blei A.T. Hepatic encephalopathy—definition, nomenclature diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716–721. doi: 10.1053/jhep.2002.31250. [DOI] [PubMed] [Google Scholar]
- 14.Boyer T.D., Haskal Z.J., American Association for the Study of Liver Diseases The role of transjugular intrahepatic portosystemic shunt in the management of portal hypertension. Hepatology. 2005;41:386–400. doi: 10.1002/hep.20559. [DOI] [PubMed] [Google Scholar]
- 15.Quero J.C., Schalm S.W. Subclinical hepatic encephalopathy. Semin Liver Dis. 1996;16:321–328. doi: 10.1055/s-2007-1007244. [DOI] [PubMed] [Google Scholar]
- 16.Groeneweg M., Moerland W., Quero J.C., Hop W.C.J., Krabbe P., Schalm S.W. Screening of subclinical hepatic encephalopathy. J Hepatol. 2000;32:748–753. doi: 10.1016/s0168-8278(00)80243-3. [DOI] [PubMed] [Google Scholar]
- 17.Romero-Gomez M., Boza F., Garcia-Valdecasas M.S., García E., Aguilar-Reina J. Subclinical hepatic encephalopathy predicts the development of overt hepatic encephalopathy. Am J Gastroenterol. 2001;96:2718–2723. doi: 10.1111/j.1572-0241.2001.04130.x. [DOI] [PubMed] [Google Scholar]
- 18.Dhiman R.K., Kurmi R., Thumburu K.K. Diagnosis and prognostic significance of minimal hepatic encephalopathy in patients with cirrhosis of liver. Dig Dis Sci. 2010;55:2381–2390. doi: 10.1007/s10620-010-1249-7. [DOI] [PubMed] [Google Scholar]
- 19.Taneja S., Dhiman R.K., Khatri A. Inhibitory control test for the detection of minimal hepatic encephalopathy in patients with cirrhosis of liver. J Clin Exp Hepatol. 2012;2:306–314. doi: 10.1016/j.jceh.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cordoba J., Alonso J., Rovira A. The development of low-grade cerebral edema in cirrhosis is supported by the evolution of 1H-magnetic resonance abnormalities after liver transplantation. J Hepatol. 2001;35:598–604. doi: 10.1016/s0168-8278(01)00181-7. [DOI] [PubMed] [Google Scholar]
- 21.Lockwood A.H., Yap E.W., Wong W.H. Cerebral ammonia metabolism in patients with severe liver disease and minimal HE. J Cereb Blood Flow Metab. 1991;11:337–341. doi: 10.1038/jcbfm.1991.67. [DOI] [PubMed] [Google Scholar]
- 22.Kale R.A., Gupta R.K., Saraswat V.A. Demonstration of interstitial cerebral edema with diffusion tensor MR imaging in type C hepatic encephalopathy. Hepatology. 2006;43:698–706. doi: 10.1002/hep.21114. [DOI] [PubMed] [Google Scholar]
- 23.Butterworth R.F. Hepatic encephalopathy: a central neuroinflammatory disorder? Hepatology. 2011;53:1372–1376. doi: 10.1002/hep.24228. [DOI] [PubMed] [Google Scholar]
- 24.Ahboucha S., Butterworth R.F. The neurosteroid system: implication in the pathophysiology of hepatic encephalopathy. Neurochem Int. 2008;52:575–587. doi: 10.1016/j.neuint.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Dhiman R.K., Sawhney M.S., Chawla Y.K., Das G., Ram S., Dilawari J.B. Efficacy of lactulose in cirrhotic patients with subclinical hepatic encephalopathy. Dig Dis Sci. 2000;45:1549–1552. doi: 10.1023/a:1005556826152. [DOI] [PubMed] [Google Scholar]
- 26.Yu-qiang N., Zheng Z., Yu-yuan L., Wei-hong S., Li P., Shou-jun D. Long-term efficacy of lactulose in patients with subclinical hepatic encephalopathy. Chin J Intern Med. 2003;42:261–266. [PubMed] [Google Scholar]
- 27.Sidhu S., Goyal O., Mishra B., Sood A., Chhina R.S., Soni R.K. Rifaximin improves psychometric performance and health related quality of life in patients with minimal hepatic encephalopathy (The RIME trial. Am J Gastroenterol. 2011;106:307–316. doi: 10.1038/ajg.2010.455. [DOI] [PubMed] [Google Scholar]
- 28.Liu Q., Duon Z.P., Ha D.K., Bengmark S., Kurtovic J., Riordan S.M. Symbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatology. 2004;39:1441–1449. doi: 10.1002/hep.20194. [DOI] [PubMed] [Google Scholar]
- 29.Bajaj J.S., Saeian K., Christensen K.M. Probiotic yogurt for the treatment of minimal hepatic encephalopathy. Am J Gastroenterol. 2008;103:1707–1715. doi: 10.1111/j.1572-0241.2008.01861.x. [DOI] [PubMed] [Google Scholar]
- 30.Bajaj J.S., Schubert C.M., Heuman D.M. Persistence of cognitive impairment after resolution of overt hepatic encephalopathy. Gastroenterology. 2010;138:2332–2340. doi: 10.1053/j.gastro.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riggio O., Ridola L., Pasquale C. Evidence of persistent cognitive impairment after resolution of overt hepatic encephalopathy. Clin Gastroenterol Hepatol. 2011;9:181–183. doi: 10.1016/j.cgh.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 32.Gupta A., Dhiman R.K., Kumari S. Role of small intestinal bacterial overgrowth and delayed gastrointestinal transit time in cirrhotic patients with minimal hepatic encephalopathy. J Hepatol. 2010;53:849–855. doi: 10.1016/j.jhep.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 33.Shawcross D.L., Wright G., Olde Damink S.W., Jalan R. Role of ammonia and inflammation in minimal hepatic encephalopathy. Metab Brain Dis. 2007;22:125–138. doi: 10.1007/s11011-006-9042-1. [DOI] [PubMed] [Google Scholar]
- 34.Shawcross D.L., Davies N.A., Williams R., Jalan R. Systemic inflammatory response exacerbates the neuropsychological effects of induced hyperammonemia in cirrhosis. J Hepatol. 2004;40:247–254. doi: 10.1016/j.jhep.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 35.de Vries H.E., Blom Roosemalen M.C., van Oosten M. The influence of cytokines on the integrity of the blood-brain barrier in vitro. J Neuroimmunol. 1996;64:37–43. doi: 10.1016/0165-5728(95)00148-4. [DOI] [PubMed] [Google Scholar]
- 36.Duchini A., Govindarajan S., Santucci M., Zampi G., Hofman F.M. Effects of tumor necrosis factor-alpha and interleukin-6 on fluid-phase permeability and ammonia diffusion in CNS-derived endothelial cells. J Investig Med. 1996;44:474–482. [PubMed] [Google Scholar]
- 37.Didier N., Romero I.A., Créminon C., Wijkhuisen A., Grassi J., Mabondzo A. Secretion of interleukin-1beta by astrocytes mediates endothelin-1 and tumour necrosis factor-alpha effects on human brain microvascular endothelial cell permeability. J Neurochem. 2003;86:246–254. doi: 10.1046/j.1471-4159.2003.01829.x. [DOI] [PubMed] [Google Scholar]
- 38.Das K., Singh P., Chawla Y., Duseja A., Dhiman R.K., Suri S. Magnetic resonance imaging of brain in patients with cirrhotic and non-cirrhotic portal hypertension. Dig Dis Sci. 2008;53:2793–2798. doi: 10.1007/s10620-008-0383-y. [DOI] [PubMed] [Google Scholar]
- 39.Rama Rao K.V., Reddy P.V., Hazell A.S., Norenberg M.D. Manganese induces cell swelling in cultured astrocytes. Neurotoxicology. 2007;28:807–812. doi: 10.1016/j.neuro.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 40.Zhou Y.Q., Chen S.Y., Jiang L.D. Development and evaluation of the quality of life instrument in chronic liver disease patients with minimal hepatic encephalopathy. J Gastroenterol Hepatol. 2009;24:408–415. doi: 10.1111/j.1440-1746.2008.05678.x. [DOI] [PubMed] [Google Scholar]
- 41.Navasa M., Forns X., Sanchez V. Quality of life, major medical complications and hospital service utilization in patients with primary biliary cirrhosis after liver transplantation. J Hepatol. 1996;25:129–134. doi: 10.1016/s0168-8278(96)80064-x. [DOI] [PubMed] [Google Scholar]
- 42.Marchesini G., Bianchi G., Amodio P. Factors associated with poor health-related quality of life of patients with cirrhosis. Gastroenterology. 2001;120:170–178. doi: 10.1053/gast.2001.21193. [DOI] [PubMed] [Google Scholar]
- 43.Groeneweg M., Quero J.C., De Bruijn I. Subclinical hepatic encephalopathy impairs daily functioning. Hepatology. 1998;28:45–49. doi: 10.1002/hep.510280108. [DOI] [PubMed] [Google Scholar]
- 44.Gralnek I.M., Hays R.D., Kilbourne A. Development and evaluation of the liver disease quality of life instrument in persons with advanced, chronic liver disease-the LDQOL 1.0. Am J Gastroenterol. 2000;95:3552–3565. doi: 10.1111/j.1572-0241.2000.03375.x. [DOI] [PubMed] [Google Scholar]
- 45.Younossi Z.M., Guyatt G., Kiwi M., Boparai N., King D. Development of a disease specific questionnaire to measure health related quality of life in patients with chronic liver disease. Gut. 1999;45:295–300. doi: 10.1136/gut.45.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nie Y.Z., Zhang J.X., Li X.H. Evaluation the quality of life instrument in patients with chronic liver disease. Mod Rehabil. 2001;5:18–19. [Google Scholar]
- 47.Kim W.R., Lindor K.D., Malinchoc M., Petz J.L., Jorgensen R., Dickson E.R. Reliability and validity of the NIDDK-QA instrument in the assessment of quality of life in ambulatory patients with cholestatic liver disease. Hepatology. 2000;32:924–929. doi: 10.1053/jhep.2000.19067. [DOI] [PubMed] [Google Scholar]
- 48.Arguedas M.R., DeLawrence T.G., McGuire B.M. Influence of hepatic encephalopathy on health – related quality of life in patients with cirrhosis. Dig Dis Sci. 2003;48:1622–1626. doi: 10.1023/a:1024784327783. [DOI] [PubMed] [Google Scholar]
- 49.Mina A., Moran S., Ortiz-Olvera N., Mera R., Uribe M. Prevalence of minimal hepatic encephalopathy and quality of life in patients with decompensated cirrhosis. Hepatol Res. 2013 Aug 19 doi: 10.1111/hepr.12227. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 50.Nardelli S., Pentassuglio I., Pasquale C. Depression, anxiety and alexithymia symptoms are major determinants of health related quality of life (HRQoL) in cirrhotic patients. Metab Brain Dis. 2013 Jun;28:239–243. doi: 10.1007/s11011-012-9364-0. [DOI] [PubMed] [Google Scholar]
- 51.Bajaj J.S., Heuman D.M., Wade J.B. Rifaximin improves driving simulator performance in a randomized trial of patients with minimal hepatic encephalopathy. Gastroenterology. 2011 Feb;140:478–487. doi: 10.1053/j.gastro.2010.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cordoba J., Cabrera J., Lataif L., Penev P., Zee P., Blei A.T. High prevalence of sleep disturbance in cirrhosis. Hepatology. 1998;27:339–345. doi: 10.1002/hep.510270204. [DOI] [PubMed] [Google Scholar]
- 53.Bianchi G., Marchesini G., Nicolino F. Psychological status and depression in patients with liver cirrhosis. Dig Liver Dis. 2005;37:593–600. doi: 10.1016/j.dld.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 54.Montagnese S., Middleton B., Skene D.J., Morgan M.Y. Night-time sleep disturbance does not correlate with neuropsychiatric impairment in patients with cirrhosis. Liver Int. 2009;29:1372–1382. doi: 10.1111/j.1478-3231.2009.02089.x. [DOI] [PubMed] [Google Scholar]
- 55.Mostacci B., Ferlisi M., Baldi A.A. Sleep disturbance and daytime sleepiness in patients with cirrhosis: a case control study. Neurol Sci. 2008;29:237–240. doi: 10.1007/s10072-008-0973-7. [DOI] [PubMed] [Google Scholar]
- 56.Samanta J., Dhiman R.K., Khatri A. Correlation between degree and quality of sleep disturbance and the level of neuropsychiatric impairment in patients with liver cirrhosis. Metab Brain Dis. 2013;28:249–259. doi: 10.1007/s11011-013-9393-3. [DOI] [PubMed] [Google Scholar]
- 57.Montagnese S., De Pittà C., De Rui M. Sleep-wake abnormalities in patients with cirrhosis. Hepatology. 2013 Jun 6 doi: 10.1002/hep.26555. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 58.De Rui M., Schiff S., Aprile D. Excessive daytime sleepiness and hepatic encephalopathy: it is worth asking. Metab Brain Dis. 2013;28:245–248. doi: 10.1007/s11011-012-9360-4. [DOI] [PubMed] [Google Scholar]
- 59.Ortiz M., Córdoba J., Jacas C., Flavià M., Esteban R., Guardia J. Neuropsychological abnormalities in cirrhosis include learning impairment. J Hepatol. 2006;44:104–110. doi: 10.1016/j.jhep.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 60.Weissenborn K., Heidenreich S., Giewekemeyer K., Rückert N., Hecker H. Memory function in early hepatic encephalopathy. J Hepatol. 2003;39:320–325. doi: 10.1016/s0168-8278(03)00295-2. [DOI] [PubMed] [Google Scholar]
- 61.Weissenborn K., Burchert W., Bokemeyer M. Regional differences of cerebral glucose metabolism in patients with liver cirrhosis depending on the grade of portosystemic encephalopathy (PSE) In: Record C., Al Mardini H., editors. Advances in Hepatic Encephalopathy and Metabolism in Liver Disease. Medical Faculty of the University of Newcastle-upon-Tyne; Newcastle upon Tyne: 1997. pp. 359–363. [Google Scholar]
- 62.Lockwood A.H., Weissenborn K., Bokemeyer M., Tietge U., Burchert W. Correlations between cerebral glucose metabolism and neuropsychological test performance in non-alcoholic cirrhotics. Metab Brain Dis. 2002;17:29–40. doi: 10.1023/a:1014000313824. [DOI] [PubMed] [Google Scholar]
- 63.Erceg S., Monfort P., Hernandez-Viadel M., Rodrigo R., Montoliu C., Felipo V. Oral administration of sildenafil restores learning ability in rats with hyperammonemia and with portacaval shunts. Hepatology. 2005;41:299–306. doi: 10.1002/hep.20565. [DOI] [PubMed] [Google Scholar]
- 64.Schomerus H., Hamster W., Blunck H., Reinhard U., Mayer K., Dolle W. Latent portasystemic encephalopathy. I. Nature of cerebral functional defects and their effect on fitness to drive. Dig Dis Sci. 1981;26:622–630. doi: 10.1007/BF01367675. [DOI] [PubMed] [Google Scholar]
- 65.Watanabe A., Tuchida T., Yata Y., Kuwabara Y. Evaluation of neuropsychological function in patients with liver cirrhosis with special reference to their driving ability. Metab Brain Dis. 1995;10:239–248. doi: 10.1007/BF02081029. [DOI] [PubMed] [Google Scholar]
- 66.Srivastava A., Mehta R., Rothke S.P., Rademaker A.W., Blei A.T. Fitness to drive in patients with cirrhosis and portal-systemic shunting: a pilot study evaluating driving performance. J Hepatol. 1994;21:1023–1028. doi: 10.1016/s0168-8278(05)80612-9. [DOI] [PubMed] [Google Scholar]
- 67.Wein C., Koch H., Popp B., Oehler G., Schauder P. Minimal hepatic encephalopathy impairs fitness to drive. Hepatology. 2004;39:739–745. doi: 10.1002/hep.20095. [DOI] [PubMed] [Google Scholar]
- 68.Marotolli R.A., Cooney L.M., Wagner S., Doucette J., Tinetti M.E. Predictors of automobile crashes and moving violations among elderly drivers. Ann Intern Med. 1994;121:842–846. doi: 10.7326/0003-4819-121-11-199412010-00003. [DOI] [PubMed] [Google Scholar]
- 69.Nyberg S.L., Baskin-Bey E.S., Mitchell M.M. Early experience with HEADS: hepatic encephalopathy assessment driving simulator. Adv Transp Stud. 2006:53–62. [Google Scholar]
- 70.Bajaj J.S., Hafeezullah M., Hoffmann R.G., Saeian K. Minimal hepatic encephalopathy: a vehicle for accidents and traffic violations. Am J Gastroenterol. 2007;102:1903–1909. doi: 10.1111/j.1572-0241.2007.01424.x. [DOI] [PubMed] [Google Scholar]
- 71.Bajaj J.S., Saeian K., Schubert C.M. Minimal hepatic encephalopathy is associated with motor vehicle crashes: the reality beyond the driving test. Hepatology. 2009;50:1175–1183. doi: 10.1002/hep.23128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bajaj J.S., Hafeezullah M., Zadvornova Y. The effect of fatigue on driving skills in patients with hepatic encephalopathy. Am J Gastroenterol. 2009;104:898–905. doi: 10.1038/ajg.2009.7. [DOI] [PubMed] [Google Scholar]
- 73.Bajaj J.S., Hafeezullah M., Hoffmann R.G. Navigation skill impairment: another dimension of the driving difficulties in minimal hepatic encephalopathy. Hepatology. 2008;47:596–604. doi: 10.1002/hep.22032. [DOI] [PubMed] [Google Scholar]
- 74.Bajaj J.S., Saeian K., Hafeezullah M., Hoffmann R.G., Hammeke T.A. Patients with minimal hepatic encephalopathy have poor insight into their driving skills. Clin Gastroenterol Hepatol. 2008;6:1135–1139. doi: 10.1016/j.cgh.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 75.Bajaj J.S., Thacker L.R., Heuman D.M. Driving simulation can improve insight into impaired driving skills in cirrhosis. Dig Dis Sci. 2012;57:554–560. doi: 10.1007/s10620-011-1888-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Román E., Córdoba J., Torrens M. Minimal hepatic encephalopathy is associated with falls. Am J Gastroenterol. 2011;106:476–482. doi: 10.1038/ajg.2010.413. [DOI] [PubMed] [Google Scholar]
- 77.Soriano G., Roman E., Cordoba J. Cognitive dysfunction in cirrhosis is associated with falls: a prospective study. Hepatology. 2011;55:1922–1930. doi: 10.1002/hep.25554. [DOI] [PubMed] [Google Scholar]
- 78.Collier J. Bone disorders in chronic liver disease. Hepatology. 2007;46:1271–1278. doi: 10.1002/hep.21852. [DOI] [PubMed] [Google Scholar]
- 79.Cohen S.M., Te H.S., Levitsky J. Operative risk of total hip and knee arthroplasty in cirrhotic patients. J Arthroplasty. 2005;20:460–466. doi: 10.1016/j.arth.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 80.Diamond T., Stiel D., Lunzer M., Wilkinson M., Roche J., Posen S. Osteoporosis and skeletal fractures in chronic liver disease. Gut. 1990;31:82–87. doi: 10.1136/gut.31.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ström O., Borgstrom F., Zethraeus N. Long-term cost and effect on quality of life of osteoporosis-related fractures in Sweden. Acta Orthop. 2008;79:269–280. doi: 10.1080/17453670710015094. [DOI] [PubMed] [Google Scholar]
- 82.Schomerus H., Hamster W. Quality of life in cirrhotics with minimal hepatic encephalopathy. Metab Brain Dis. 2001;16:37–41. doi: 10.1023/a:1011610427843. [DOI] [PubMed] [Google Scholar]
- 83.Stewart C.A., Smith G.E. Minimal hepatic encephalopathy. Nat Clin Pract Gastroenterol Hepatol. 2007;4:677–685. doi: 10.1038/ncpgasthep0999. [DOI] [PubMed] [Google Scholar]
- 84.Romero-Gómez M., Córdoba J., Jover R. Value of the critical flicker frequency in patients with minimal hepatic encephalopathy. Hepatology. 2007;45:879–885. doi: 10.1002/hep.21586. [DOI] [PubMed] [Google Scholar]
- 85.Yen C.L., Liaw Y.F. Somatosensory evoked potentials and number connection test in the detection of hepatic encephalopathy. Hepatogastroenterology. 1990;37:332–334. [PubMed] [Google Scholar]
- 86.Amodio P., Del Piccolo F., Marchetti P. Clinical features and survival of cirrhotic patients with subclinical cognitive alterations detected by the number connection test and computerized psychometric tests. Hepatology. 1999;29:1662–1667. doi: 10.1002/hep.510290619. [DOI] [PubMed] [Google Scholar]
- 87.Patidar K.R., Thacker L.R., Wade J.B. Covert hepatic encephalopathy is independently associated with poor survival and increased risk of hospitalization. Am J Gastroenterol. 2014 Sep 2 doi: 10.1038/ajg.2014.264. [DOI] [PMC free article] [PubMed] [Google Scholar]


