Abstract
Neuropathologic investigations in acute liver failure (ALF) reveal significant alterations to neuroglia consisting of swelling of astrocytes leading to cytotoxic brain edema and intracranial hypertension as well as activation of microglia indicative of a central neuroinflammatory response. Increased arterial ammonia concentrations in patients with ALF are predictors of patients at risk for the development of brain herniation. Molecular and spectroscopic techniques in ALF reveal alterations in expression of an array of genes coding for neuroglial proteins involved in cell volume regulation and mitochondrial function as well as in the transport of neurotransmitter amino acids and in the synthesis of pro-inflammatory cytokines. Liver-brain pro-inflammatory signaling mechanisms involving transduction of systemically-derived cytokines, ammonia neurotoxicity and exposure to increased brain lactate have been proposed. Mild hypothermia and N-Acetyl cysteine have both hepato-protective and neuro-protective properties in ALF. Potentially effective anti-inflammatory agents aimed at control of encephalopathy and brain edema in ALF include etanercept and the antibiotic minocycline, a potent inhibitor of microglial activation. Translation of these potentially-interesting findings to the clinic is anxiously awaited.
Keywords: hepatic encephalopathy, acute liver failure, neuroinflammation, microglial activation, intracranial hypertension
Abbreviations: ALF, acute liver failure; ATP, adenosine triphosphate; BBB, blood-brain barrier; CCL2, chemokine ligand-2; CMRO2, cerebral metabolic rate for oxygen; CNS, central nervous system; EEG, electroencephalography; GABA, gamma-aminobutyric acid; GFAP, glial fibrillary acidic protein; IgG, immunoglobulin; MRS, magnetic resonance spectroscopy; NAC, N-Acetyl cysteine; NMDA, N-methyl-d-aspartate; SIRS, systemic inflammatory response syndrome; SNATs, several neutral amino acid transport systems; TLP, translocator protein; TNFα, tumor necrosis factor alpha
Acute liver failure (ALF), also referred to as fulminant hepatic failure, invariably leads to central nervous system dysfunction that may include encephalopathy, seizures and brain edema, a major cause of intracranial hypertension and brain herniation, a leading cause of mortality in ALF. An increase in cerebral blood flow frequently accompanies the onset of brain edema.1
Neuropathological assessments of the brain in both human and experimental ALF reveals significant changes to neuroglia in general and to astrocytes and microglia, in particular. Astrocytes in brain sections from patients who died in ALF are swollen2 as are their mitochondria (Figure 1). Based upon these observations, it is generally assumed that the brain edema that accompanies ALF is primarily, if not exclusively, cytotoxic in nature. Studies in experimental animals with ALF due to toxic liver injury show a similar pattern of changes as well as alterations in expression of genes coding for key astrocytic proteins.3
Figure 1.
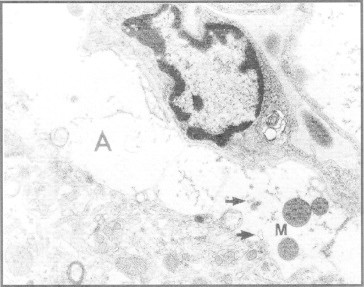
Electron micrograph of frontal cortex from a patient who died in acute liver failure. Note swelling and vacuolation of perivascular astrocyte (A) and mitochondria (M). Endoplasmic reticulum is dilated (arrows). Original magnification ×6000. From reference #2 with permission.
Although gross alterations of the blood-brain barrier (BBB) are not generally a feature of ALF, alterations of cerebrovascular endothelial cell function have occasionally been described.2,4 On the other hand, material from ALF animals in which edema and encephalopathy were precipitated by infection manifest clear alterations of both BBB function and of expression of BBB tight junction proteins.5 These latter findings suggest that, in ALF accompanied by significant infection/inflammation, brain edema may comprise both cytotoxic and vasogenic components.
Brain metabolism in acute liver failure
ALF leads to severe compromise of cerebral metabolism and includes increases of cerebral blood flow, decreases of the cerebral metabolic rate for oxygen (CMRO2) and failure of cerebrovascular autoregulation.6 These changes have been attributed to a variety of factors including ammonia, glutamine, oxidative/nitrosative stress and pro-inflammatory factors.
Ammonia
A significant positive correlation has been reported between arterial ammonia and the presence of brain herniation in patients with ALF7 and arterial ammonia concentrations may be a useful independent predictor of this complication.8 Brain ammonia removal relies almost exclusively on the synthesis of glutamine, the brain lacking an effective urea cycle. Brain glutamine synthesis from ammonia is an astrocytic responsibility since the enzyme responsible, glutamine synthetase, has a uniquely astrocytic localization.
Ammonia has multiple actions on CNS function that include direct effects of the ammonium ion (NH4+) on both excitatory and inhibitory neurotransmission,9 inhibition of glucose (pyruvate) oxidation10 and stimulation of glycolysis, altered mitochondrial function11 and impairment of key cellular transport systems.9,12
Glutamine
Brain glutamine concentrations are significantly increased in ALF whether assessed biochemically in autopsy material13 or by 1H-magnetic resonance spectroscopy (MRS).14 It was suggested, based upon these findings that the accumulation of glutamine in the brain in ALF was causally related to the encephalopathy and brain edema. Subsequent studies using 1H/13C MRS in an animal model of ALF confirmed the increase in concentrations and in synthesis of glutamine in brain.15 However, these increases were not significantly correlated with either the severity of encephalopathy or the presence of brain edema in these animals suggesting that increased brain glutamine synthesis per se is not a major cause of these neurologic disturbances as had previously been postulated. The subject of the role of glutamine in the pathogenesis of the CNS consequences of hyperammonemic disorders has been the subject of a recent review.16 In contrast, it has been proposed that the signal that triggers the increase in cerebral blood flow in ALF occurs following the generation of glutamine in the astrocytes.6 Other mechanisms proposed to explain the role of glutamine in the pathogenesis of encephalopathy and brain edema in hyperammonemia include its transamination to alpha-ketoglutaramate, a neurotoxic metabolite17 and the suggestion that glutamine, by transport into the astrocyte mitochondrion, acts as a shuttle for the production of ammonia that goes on to lead to mitochondrial energy failure, a hypothesis that has been termed “The Trojan Horse Hypothesis”.18 However direct evidence for a role for these hypotheses in the pathogenesis of the CNS consequences of ALF await further evaluation.
Lactate
Brain energy metabolism has been the subject of intensive investigation using a variety of technical approaches over the last several decades. It is clear that brain concentrations of high energy phosphates such as phosphocreatine and adenosine triphosphate (ATP) are not significantly altered in experimental ALF until the onset of profound coma and isoelectric EEG stages.19 Similar negative observations have been reported using in vivo brain microdialysis20 or 1H-MRS.21 Glucose is the principal energy source for adult mammalian brain and there is increasing evidence to support the notion that brain glucose metabolism is modified early in the progression of the CNS consequences of ALF. Such modifications are not sufficient to result in brain energy failure but have the potential to result in abnormal CNS metabolism and function.
Brain lactate concentrations are increased in a wide range of experimental animal models of ALF resulting from ischemic19,22 or toxic23 liver injury as well as in brain microdialysates from ALF patients where increased brain lactate content was found to precede surges in intracranial hypertension.24 Worsening of neurological status in animal models of ALF is significantly correlated with increases of brain lactate concentrations15,19 and by increased de novo lactate synthesis15 (Figure 2).
Figure 2.
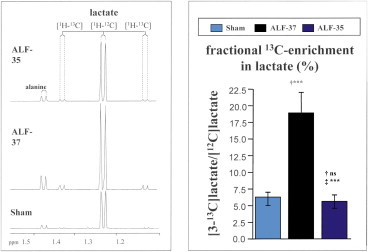
Increased de novo synthesis of lactate in brain in an animal with acute liver failure resulting from hepatic devascularisation (ALF-37) compared to a sham-operated control animal (Sham). Left hand panel shows lactate resonances [1H-12C] that appear as doublets centered at 1.25 ppm. Left hand panel shows fractional 13C-enrichment data indicative of de novo synthesis rates as mean ± SE from a group of n = 4 animals per group. Note protective effects of mild hypothermia (ALF-35) leading to attenuation of lactate resonances. Significant differences indicated by *P < 0.01 by ANOVA. Data are from reference #15 with permission.
Increases of brain lactate have been shown to be related to intracranial hypertension and a poor outcome in dogs with ALF25 suggesting a role for increased brain lactate in the pathogenesis of brain edema and, in support of such a notion, exposure of cultured astrocytes to lactate results in significant cell swelling.26
Neuroglial function in acute liver failure
Astrocytes
Astrocytes play important roles in the maintenance of CNS function by virtue of their interactions with other neural cells (neurons, endothelial cells) and their ability to modulate both excitatory and inhibitory neurotransmission being active participants in the synthesis, transport and degradation of major neurotransmitters such as glutamate and gamma-aminobutyric acid (GABA). New evidence continues to accumulate demonstrating that ALF results in alterations in expression of genes coding for key astrocytic proteins with important roles in CNS function. Three classes of astrocytic protein have so far been shown to be modified in ALF. These include structural proteins, amino acid neurotransmitter transporters and receptor proteins.
Glial fibrillary acidic protein (GFAP) constitutes the major component of astrocytic intermediate filaments implicated in the control of cell motility and morphology by providing structural stability to astrocyte processes.26,27 ALF resulting from ischemic liver failure leads to a loss of expression of the GFAP gene28 and the extent of the loss of expression is correlated with the extent of hyperammonemia and with brain edema ischemic animals. In support of a role for ammonia, exposure of primary cultures of rat astrocytes to ammonia resulted in significant cell swelling.28 Loss of GFAP expression in brain in ALF appears to be selective; expression of a second glial filamentous protein S100 beta is unaltered in the same animal model. It was proposed that the loss of GFAP in ALF has the potential to alter the visco-elastic properties of the astrocyte and consequently to facilitate cell swelling and the resulting cytotoxic brain edema.27,28 Loss of GFAP expression was recently described in autopsied brain tissue samples from patients who died as a result primarily of brain herniation due to ALF.3
The astrocyte membrane is home to a range of high affinity amino acid transporters that include transporters for glutamate (EAAT-2), glycine (GLYT-1) and glutamine (SNAT-3 and SNAT-5) Expression of genes coding for many of these proteins has been shown to be down-regulated in brain extracts from animals with ALF due to ischemic liver failure. EAAT-2 is a cloned and well characterized high affinity transporter for glutamate that is located on the astrocyte membrane primarily in forebrain of mammals. Knock-down of the EAAT-2 gene results in increased synaptic (extracellular) concentrations of glutamate and this is accompanied by brain edema and seizures, two important neurological features of ALF. Not surprisingly, experimental ALF due to liver ischemia results in a loss of expression of EAAT-2 (previously known as GLT-1) in brain12 resulting in increased extracellular brain glutamate29 at coma/edema stages of encephalopathy suggestive of a role for this loss of transporter expression in the pathogenesis of these complications. Moreover, since the astrocytic pool of glutamate is the obligate substrate for glutamine synthetase, the enzyme responsible for removal of excess brain ammonia, decreased uptake of glutamate as a result of EAAT-2 down-regulation in ALF could seriously limit the capacity of brain to remove ammonia, providing one cogent explanation for observation of brain ammonia accumulation in ALF. Loss of EAAT-2 gene and protein expression was recently reported in autopsied brain tissue from patients with ALF resulting primarily from viral hepatitis.3 Expression of a second glutamate transporter EAAT-1 was unchanged in this material.
Synaptic concentrations of the neuroactive amino acid glycine are regulated by high affinity transporters, one of which, GLYT-1 is expressed in astrocytes of the forebrain where glycine functions as agonist for a neuromodulatory site on a subclass of high affinity glutamate receptor known as the N-methyl-d-aspartate (NMDA) receptor. ALF resulting from liver ischemia leads to a loss of expression of GLYT-1 and a concomitant increase in synaptic concentrations of glycine30 favoring stimulation of NMDA receptor-mediated excitatory transmission, a phenomenon that could relate to the hyperexcitability and seizures encountered in ALF.
Several neutral amino acid transport systems (SNATs) have been characterized and have been recently renamed including SNAT-3 (sodium-coupled neutral amino acid transporter-3, previously SN-1) that favors the release of GLN, rather than its uptake, from astrocytes.31 SNAT-5 is also expressed in the brain and, like SNAT-3, shares the Na+/H− coupling mechanism. SNAT-5 is expressed exclusively by astrocyte cell bodies and their processes that surround glutamatergic, GABAergic and glycinergic nerve terminals.32 A recent study showed that ALF in the rat led to significant down-regulation of SNAT-5.33 This finding led the authors to suggest that restricted transfer of glutamine from the astrocyte (rather than its increased synthesis as had been previously suggested) offers a plausible explanation for the occurrence of brain edema in ALF. Moreover, GLN trapping within the astrocyte has the potential (by restricting its flow to neighboring nerve terminals) to limit neuronal excitability and hence encephalopathy in ALF.
GLUT-1 is a cloned and characterized glucose transporter that regulates glucose delivery to the cell and across the BBB since the transporter protein is localized on the membrane of astrocytes and cerebrovascular endothelial cells.34 It has been proposed that GLUT-1 also plays a role in the movement of water into the cell. Interestingly, GLUT-1 expression is significantly increased in parallel with brain water accumulation in experimental ALF.35 It was also proposed that the increase in GLUT-1 expression represents a compensatory mechanism relating to the reported increase of glycolytic flux and de novo lactate synthesis in brain in ALF.35
Translocator protein (TLP) is located on the outer mitochondrial membrane of the astrocyte where it functions primarily as a modulator of cholesterol uptake. Increased expression of TLP in brain has been reported in experimental ALF resulting from either ischemic36 or toxic37 liver injury where increased expression was accompanied by increased uptake of cholesterol and its metabolite pregnenolone, the precursor of a novel series of neuroactive compounds known as “neurosteroids” with potent excitatory or inhibitory properties, one of which, allopregnanolone is a potent agonist of the post-synaptic GABA receptor.
Microglia/Neuroinflammation
Microglia constitute the resident macrophages of the brain with the ability to respond (become activated) to a wide range of homeostatic challenges including tissue damage, vascular disturbances as well as changes in pH and impending energy failure. Microglial activation was first reported by Jiang et al38 in rats with ALF resulting from ischemic liver failure. Subsequent reports from several groups went on to describe similar activation of microglia in a mouse model of ALF resulting from the toxic effects of azoxymethane at various time during the progression of liver failure.39,40 Onset of coma/edema stages of encephalopathy resulted in increased expression of a range of markers of microglial activation and, in this model, deletion of the genes coding for TNF-alpha or IL-1beta delayed the onset of encephalopathy and attenuated the level of brain edema in these animals.41 Microglial activation has also been reported in autopsied brain tissue from a patient with ALF resulting from viral hepatitis.42
Studies in the ischemic liver injury model of ALF revealed that, in parallel with the activation of microglia, an accumulation in the brain of pro-inflammatory cytokines tumor necrosis factor alpha (TNF-alpha), interleukin-1beta (IL-1beta) and interleukin-6 (IL-6) occurred43 (Figure 3). These increased brain concentrations of pro-inflammatory cytokines were accompanied by increases in expression of the genes for which they encode suggesting their synthesis in situ, in the brain. Evidence for increased cytokine synthesis in the brain was also reported in patients with ALF due primarily to acetaminophen overdose using the technique of arterio-venous differences.44Systemic inflammation had already been well established prior to these reports of the presence of neuroinflammation in ALF. The presence and severity of a systemic inflammatory response syndrome (SIRS) is a major predictor of HE in. ALF6,45 and polymorphisms of the TNFa gene are known to influence the clinical outcome in ALF.46The etiology of liver disease and, in the case of toxic liver injury, the nature of the hepatotoxin appear to determine the systemic cytokine profile of the pro-inflammatory response in ALF.47 However, whether or not the systemic inflammatory response in ALF is transmitted to the brain and, if so, by what mechanism remains, to be established. Mechanisms so far proposed include the activation of traditional humoral and neural routes as well as mechanisms involving the recruitment of monocytes linked to the activation of microglia and involving TNFa signaling.48
Figure 3.
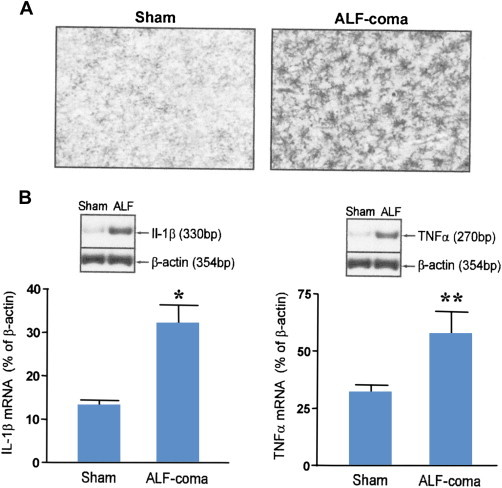
Panel A: Microglial activation indicated by increased OX-42 immunostaining in frontal cortex of a rat with acute liver failure resulting from hepatic devascularisation at coma/edema stage of encephalopathy (ALF-coma) compared to a sham-operated control (Sham). Original magnification: ×200. Panel B: Increased expression of genes coding for the pro-inflammatory cytokines interleukin-1beta (IL-1b) and tumor necrosis factor alpha (TNFa) in samples of frontal cortex from rats with acute liver failure at coma/edema stage of encephalopathy (ALF-coma) compared to sham-operated controls (Sham). Histograms represent mean ± SE values from n = 6 animals per group. Values that are significantly different from Sham indicated by *P < 0.02 **P < 0.01 by Student t test.
Brain lactate increases in ALF are significantly correlated with neurological symptoms and electroencephalographic changes as well as the extent of microglial activation.15,38 Brain concentrations of lactate as high as 12–15 mM have been recorded at coma stages of encephalopathy in both experimental and human ALF (see above) and cultured microglial cells exposed to lactate concentrations of this magnitude have been shown to cause release of TNFa, IL-1b and IL-6 from these cells.49 These findings suggest that cellular energy status may afford a possible trigger for the activation of microglia and the ensuing pro-inflammatory response observed in ALF.
Therapeutic implications
A comprehensive knowledge of key mechanisms implicated in the pathogenesis of the CNS complications of ALF continues to stimulate the discovery of novel therapeutic strategies. Such strategies fall into one of three categories namely ammonia-lowering strategies, those aimed at modulation of neurotransmitter action and those aimed at the modulation of inflammation.
Ammonia-Lowering Strategies
In spite of the consistent findings relating increases in circulating and brain concentrations of ammonia in ALF, strategies aimed at the lowering of gut-produced ammonia using standard agents such as lactulose, antibiotics and L-ornithine L-aspartate have, to date, been shown to be of questionable benefit. For example, a review of published studies in 2003 suggested that the use of lactulose may result in short-term (but not a long-term) survival advantage.50 Moreover, it was concluded that, although prophylactic use of antibiotics was widely in use, improvements in outcome had not been convincingly demonstrated. L-ornithine L-aspartate is an effective ammonia-lowering agent that has been successfully used in the treatment of hepatic encephalopathy in chronic liver failure where it acts my stimulation of residual hepatic urea formation as well as stimulating muscle ammonia removal in the form of glutamine. The agent is also effective in the reduction of circulating ammonia in rats with ischemic ALF where it reduces encephalopathy grade and prevents brain edema.51 On the other hand, a recent study of its effects in ALF patients failed to demonstrate any significant effects on encephalopathy grade or survival.52 However, this latter study was potentially flawed since most patients were treated simultaneously with antibiotics and the dose of L-ornithine L-aspartate chosen for the study was insufficient to cause lowering of blood ammonia. In the meantime, a novel ammonia-lowering agent made up of a combination of L-ornithine with phenylacetate (the latter agent has been shown to be effective in the lowering of ammonia in children with urea cycle enzymopathies) was shown to be effective in lowering circulating ammonia and to reduce brain edema in pigs with ischemic liver failure.53 Clinical trials with this agent are currently ongoing.
Mild Hypothermia
Two or three degrees of hypothermia have been shown to be effective in extending survival time and preventing brain edema and its complications in animal models of ALF54 as well as in patients with ALF.55 Multiple mechanisms have been proposed to explain the beneficial effects of hypothermia in the prevention of the CNS complications of ALF and these mechanisms include (i) the reduction of blood-brain ammonia transfer55 and (ii) decreased brain lactate synthesis56 consistent with improvement in brain energy status. Mild hypothermia also possesses anti-inflammatory properties; it reduces microglial activation in the brain in experimental ALF38,43 with concomitant reduction of brain pro-inflammatory cytokines. Hypothermia also reduces the extent of acetaminophen-induced liver injury in mice57 with the potential to do so in ALF patients. Although mild hypothermia is used in an ad hoc manner as a bridge to transplantation in some tertiary centers, tragically, a lack of clear guidelines precludes its more widespread use in the management of ALF and its complications at this time.58
N-Acetyl Cysteine (NAC)
NAC is widely used as an early antidote following acetaminophen overdose and improvement of both hepatic and neurological damage and dysfunction have been reported following its use.59,60 Both anti-oxidant61 and central anti-inflammatory60 properties of NAC have been proposed to explain its beneficial action. Moreover, NAC has been shown to be beneficial in both acetaminophen and non-acetaminophen-induced ALF in experimental animals involving similar mechanisms of action.60
Anti-inflammatory Strategies
As discussed in an earlier section of this review, there is now a hard body of evidence suggesting that neuroinflammation, (the concept of “the inflamed brain”) plays a key role in the pathogenesis of the CNS complications of ALF. Consequently, an array of agents shown to modulate the neuroinflammatory response has been shown to be beneficial in the prevention of encephalopathy and brain edema in ALF models. Etanercept is a fusion protein consisting of two ligand binding domains of the soluble human TNF receptor linked to human immunoglobulin (IgG) that, when bound to TNFa, renders it inactive. Treatment of ALF mice with etanercept delays the onset of severe encephalopathy while reducing the neuroinflammatory response.62 A previous study in the same animal model showed that TNFa gene deletion likewise led to attenuation of the neurological complications.41 As stated above, mild hypothermia and NAC both have the potential to reduce ALF-related increases of the brain production of pro-inflammatory cytokines including TNFa.43,60
Minocycline is a semi-synthetic tetracycline antibiotic with potent anti-inflammatory properties that are independent of the agents' antimicrobial action. Minocycline is a potent inhibitor of microglial activation that is currently under investigation in a wide range of disorders of the CNS in which significant pro-inflammatory mechanisms have been identified. In this regard, minocycline has been shown to limit microglial activation in the brains of animals with ischemic liver failure leading to reduction in brain edema and a slowing of the progression of encephalopathy38 (Figure 4).
Figure 4.
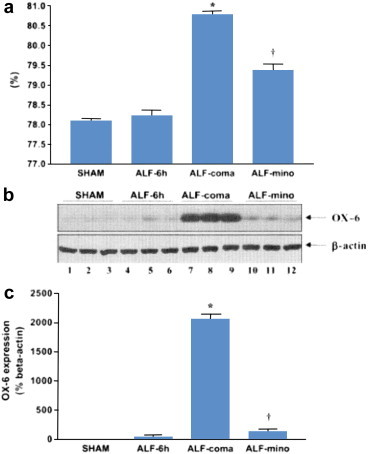
Minocycline (mino) inhibits microglial activation as assessed by OX-6 expression (shown in panels b and c) and, in so doing leads to a reduction in per cent brain water content (%), panel (a) in frontal cortex of rats with acute liver failure (ALF) resulting from hepatic devascularisation compared to sham-operated control animals (Sham). ALF-6 h: animals at 6 h after devascularisation, no coma, no edema. ALF-coma: animals at coma/edema stages of encephalopathy, ALF-mino : animals treated with minocycline (22.5 mg/kg). Significant differences indicated by *P < 0.01 compared to Sham, +P < 0.02 compared to AKLF-coma by ANOVA. From reference #39 with permission.
Other Neuropharmacological Approaches
Based largely on the results of studies in animal models, a range of pharmacological approaches have been suggested to be of potential value in the management of the CNS complications of ALF. One such example is memantine, an inhibitor of the N-methyl-d-aspartate (NMDA) subclass of glutamate receptor, a receptor located on both neuronal and astrocytic membranes in the brain. Studies in an animal model of ALF resulting from liver ischemia revealed that memantine was effective in reducing encephalopathy grade.63 There is still evidence to suggest that central GABA-related benzodiazepine receptor antagonist flumazenil may have beneficial actions in ALF in animal models of ALF due to toxic liver injury.64 However, negative reports have subsequently appeared.65,66 It has been suggested that the beneficial effects of flumazenil that appear to occur in a sub-group of patients may relate to allosteric effects on the neurosteroid modulatory site that is adjacent to the benzodiazepine site on the GABA-A receptor complex.67 This hypothesis has not been tested in ALF. A recent study in mice with ALF resulting from toxic liver injury showed clear evidence of neuronal accumulation of chemokine ligand-2 (CCL2) in brain resulting from microglial activation. Moreover, inhibition of its receptor led to slowing of the progression of encephalopathy in this animal model.40
In summary, acute liver failure is characterized neuropathologically by alterations of neuroglial morphology consisting of astrocyte swelling resulting in cytotoxic brain edema and microglial activation indicative of a central neuroinflammatory response. Increased arterial ammonia concentrations in patients with ALF are predictors of patients at risk for the development of brain herniation. Molecular techniques in ALF reveal alterations in expression of genes coding for neuroglial proteins involved in cell volume regulation and brain metabolism as well as in the transport of amino acids and in the synthesis of pro-inflammatory cytokines. Liver-brain pro-inflammatory signaling mechanisms involve the transduction of systemically-derived cytokines as well as the gliotoxic effects of ammonia and lactate. Mild hypothermia and N-Acetyl cysteine have both hepato-protective and neuro-protective properties in ALF. Effective anti-inflammatory agents in experimental ALF include etanercept and the antibiotic minocycline, a potent inhibitor of microglial activation. Continued search for new therapeutic agents that target the brain together with more robust attempts at translational research will undoubtedly lead to novel approaches to the management and treatment of the cerebral complications of ALF.
Conflicts of interest
The author has none to declare.
References
- 1.Aggarwal S., Kramer D., Yonas H. Cerebral hemodynamic and metabolic changes in fulminant hepatic failure: a retrospective study. Hepatology. 1994;19:80–87. [PubMed] [Google Scholar]
- 2.Kato M., Hughes R.D., Keays R.T., Williams R. Electron microscopic study of brain capillaries in cerebral edema from fulminant hepatic failure. Hepatology. 1992;15:1060–1066. doi: 10.1002/hep.1840150615. [DOI] [PubMed] [Google Scholar]
- 3.Thumburu K.K., Dhiman R.K., Vasishta R.K. Expression of astrocytic genes coding for proteins implicated in neural excitation and brain edema is altered after acute liver failure. J Neurochem. 2014;128:617–627. doi: 10.1111/jnc.12511. [DOI] [PubMed] [Google Scholar]
- 4.Potvin M., Morrison H.F., Hinchey E.J. Cerebral abnormalities in hepatectomised rats with acute hepatic coma. Lab Invest. 1984;50:560–564. [PubMed] [Google Scholar]
- 5.Chastre A., Belanger M., Nguyen B.N., Butterworth R.F. Lipo-polysaccharide precipitates hepatic encephalopathy and increases blood-brain barrier permeability in mice with acute liver failure. Liver Int. 2014;34:353–361. doi: 10.1111/liv.12252. [DOI] [PubMed] [Google Scholar]
- 6.Vaquero J., Chung C., Cahill M.E., Blei A.T. Pathogenesis of hepatic encephalopathy in acute liver failure. Semin Liver Dis. 2003;23:259–269. doi: 10.1055/s-2003-42644. [DOI] [PubMed] [Google Scholar]
- 7.Clemmesen J.O., Larsen F.S., Kondrup J. Cerebral herniation in patients with acute liver failure is correlated with arterial ammonia concentrations. Hepatology. 1999;29:648–653. doi: 10.1002/hep.510290309. [DOI] [PubMed] [Google Scholar]
- 8.Bernal W., Hall C., Karvellas C.J. Arterial ammonia and clinical risk factors for encephalopathy and intracranial hypertension in acute liver failure. Hepatology. 2007;46:1844–1852. doi: 10.1002/hep.21838. [DOI] [PubMed] [Google Scholar]
- 9.Butterworth R.F. Pathophysiology of hepatic encephalopathy: a new look at ammonia. Metab Brain Dis. 2002;17:221–227. doi: 10.1023/a:1021989230535. [DOI] [PubMed] [Google Scholar]
- 10.Lai J.C.K., Cooper A.J.L. Alpha-ketoglutarate dehydrogenase complex: kinetic properties, regional distribution and effects of inhibitors. J Neurochem. 1986;47:1376–1386. doi: 10.1111/j.1471-4159.1986.tb00768.x. [DOI] [PubMed] [Google Scholar]
- 11.Bai G., Rama Rao K.V., Murthy C.R. Ammonia induces the mitochondrial permeability transition in primary cultures of rat astrocytes. J Neurosci Res. 2001;66:981–991. doi: 10.1002/jnr.10056. [DOI] [PubMed] [Google Scholar]
- 12.Knecht K., Michalak A., Rose C., Butterworth R.F. Decreased glutamate transporter (GLT-1) expression in frontal cortex of rats with acute liver failure. Neurosci Lett. 1997;229:201–203. doi: 10.1016/s0304-3940(97)00444-8. [DOI] [PubMed] [Google Scholar]
- 13.Record C.O., Buxton B., Chase R.A. Plasma and brain amino acids in fulminant hepatic failure and their relationship to hepatic encephalopathy. Eur J Clin Invest. 1976;6:387–394. doi: 10.1111/j.1365-2362.1976.tb00533.x. [DOI] [PubMed] [Google Scholar]
- 14.McConnell J.R., Antonson D.L., Ong C.S. Proton spectroscopy of brain glutamine in acute liver failure. Hepatology. 1995;22:69–74. [PubMed] [Google Scholar]
- 15.Zwingmann C., Chatauret N., Liebfritz D., Butterworth R.F. Selective increase in brain lactate synthesis in experimental acute liver failure: results of a 1H-13C NMR study. Hepatology. 2003;37:420–428. doi: 10.1053/jhep.2003.50052. [DOI] [PubMed] [Google Scholar]
- 16.Butterworth R.F. Pathophysiology of brain dysfunction in hyperammonemic syndromes: the many faces of glutamine. Metab Genet Med. 2014 doi: 10.1016/j.ymgme.2014.06.003. in press. [DOI] [PubMed] [Google Scholar]
- 17.Cooper A.J.L., Gross M. The glutamate transaminase-w-amidase system in rat and human brain. J Neurochem. 1977;28:771–778. doi: 10.1111/j.1471-4159.1977.tb10626.x. [DOI] [PubMed] [Google Scholar]
- 18.Albrecht J., Norenberg M.D. Glutamine: a Trojan horse in ammonia neurotoxicity. Hepatology. 2006;44:788–794. doi: 10.1002/hep.21357. [DOI] [PubMed] [Google Scholar]
- 19.Mans A.M., DeJoseph M.R., Hawkins R.A. Metabolic abnormalities and grade of encephalopathy in acute liver failure. J Neurochem. 1994;63:1829–1838. doi: 10.1046/j.1471-4159.1994.63051829.x. [DOI] [PubMed] [Google Scholar]
- 20.Deutz N.E.P., DeGraaf A.A., De Haan J.G. In vivo brain 1H-NMR spectroscopy (1H-MRS) during acute hepatic encephalopathy (HE) In: Soeters P.B., editor. Advances in Ammonia Metabolism and Hepatic Encephalopathy. Excerpta Medica; Amsterdam: 1988. pp. 439–446. [Google Scholar]
- 21.Chatauret N., Rose C., Therrien G., Butterworth R.F. Mild hypothermia prevents cerebral edema and CSF lactate accumulation in acute liver failure. Metab Brain Dis. 2001;16:95–102. doi: 10.1023/a:1011622830569. [DOI] [PubMed] [Google Scholar]
- 22.Peeling J., Shoemaker L., Gauthier T. Cerebral metabolic and histological effects of thioacetamide-induced liver failure. Am J Phys. 1993;265:G572–G578. doi: 10.1152/ajpgi.1993.265.3.G572. [DOI] [PubMed] [Google Scholar]
- 23.Tofteng F., Jorgensen L., Hansen B.A. Cerebral microdialysis in patients with fulminant hepatic failure. Hepatology. 2002;36:1333–1340. doi: 10.1053/jhep.2002.36944. [DOI] [PubMed] [Google Scholar]
- 24.Nyberg S.L., Cerra F.B., Gruetter R. Brain lactate by magnetic resonance spectroscopy during fulminant hepatic failure in the dog. Liver Transpl Surg. 1998;4:158–165. doi: 10.1002/lt.500040203. [DOI] [PubMed] [Google Scholar]
- 25.Staub F., Baethmann, Peters J. Effects of lactic acidosis on glial cell volume and viability. J Cereb Blood Flow Metab. 1990;10:866–876. doi: 10.1038/jcbfm.1990.143. [DOI] [PubMed] [Google Scholar]
- 26.Eng L.F., GhirnikarR S., Lee Y.L. Glial fibrillary acidic protein: GFAP-31 years (1969–2000) Neurochem Res. 2000;25:1439–1451. doi: 10.1023/a:1007677003387. [DOI] [PubMed] [Google Scholar]
- 27.Belanger M., Desjardins P., Chatauret N., Butterworth R.F. Loss of expression of glial fibrillary acidic protein in acute hyperammonemia. Neurochem Int. 2002;41:155–160. doi: 10.1016/s0197-0186(02)00037-2. [DOI] [PubMed] [Google Scholar]
- 28.Norenberg N.D., Neary J.T., Norenberg L.O. Ammonia-induced decrease in glial fibrillary acidic protein in cultured astrocytes. J Neuropathol Exp Neurol. 1990;49:399–405. doi: 10.1097/00005072-199007000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Michalak A., Rose C., Butterworth J., Butterworth R.F. Neuroactive amino acids and glutamate(NMDA) receptors in frontal cortex of rats with experimental acute liver failure. Hepatology. 1996;24:908–913. doi: 10.1002/hep.510240425. [DOI] [PubMed] [Google Scholar]
- 30.Zwingmann C., Desjardins P., Hazell A., Butterworth R.F. Reduced expression of astrocytic glycine transporter GLYT-1 in acute liver failure. Metab Brain Dis. 2002;17:263–273. doi: 10.1023/a:1021997532352. [DOI] [PubMed] [Google Scholar]
- 31.Chaudhry F.A., Krisaj D., Larsson P. Coupled and uncoupled proton movement by amino acid transport system N. EMBO J. 2001;20:7041–7051. doi: 10.1093/emboj/20.24.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cubelos B., Gonzales-Gonzales I.M., Gimenez C. Amino acid transporter SNAT-5 localises to glial cells in the rat brain. Glia. 2005;49:230–244. doi: 10.1002/glia.20106. [DOI] [PubMed] [Google Scholar]
- 33.Desjardins P., Du T., Jiang W. Pathogenesis of hepatic encephalopathy and brain edema in acute liver failure: role of glutamine redefined. Neurochem Int. 2012;60:690–696. doi: 10.1016/j.neuint.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Fischbarq J., Kuang K.Y., Vera J.C. Glucose transporters serve as water channels. Proc Natl Acad Sci USA. 1990;87:3244–3247. doi: 10.1073/pnas.87.8.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Belanger M., Desjardins P., Chatauret N. Selectively increased expression of the astrocytiv/endothelial glucose transporter protein GLUT1 in acute liver failure. Glia. 2006;53:557–562. doi: 10.1002/glia.20310. [DOI] [PubMed] [Google Scholar]
- 36.Belanger M., Desjardins P., Chatauret N. Mild hypothermia prevents brain edema and attenuates up-regulation of the astrocytic benzodiazepine receptor in experimental acute liver failure. J Hepatol. 2005;42:694–699. doi: 10.1016/j.jhep.2004.12.029. [DOI] [PubMed] [Google Scholar]
- 37.Itzhak Y., Roig-Cantisano A., Dombro R.S. Acute liver failure and hyperammonemia increase peripheral-type benzodiazepine receptor binding and pregnenolone synthesis in mouse brain. Brain Res. 1995;705:345–348. doi: 10.1016/0006-8993(95)01244-3. [DOI] [PubMed] [Google Scholar]
- 38.Jiang W., Desjardins P., Butterworth R.F. Cerebral inflammation contributes to encephalopathy and brain edema in acute liver failure: protective effect of minocycline. J Neurochem. 2009;109:485–493. doi: 10.1111/j.1471-4159.2009.05981.x. [DOI] [PubMed] [Google Scholar]
- 39.McMillin M., Galindo C., Frampton G.A. Increased neuronal chemokine (CCL2/MCP1) expression is associated with hepatic encephalopathy and contributes to neurological decline. Hepatology. 2012;56(suppl 1):958A. [Google Scholar]
- 40.Thrane V.R., Thrane A.S., Chanag J. Real-time analysis of microglial activation and motility in hepatic and hyperammonemic encephalopathy. Neuroscience. 2012;220:247–255. doi: 10.1016/j.neuroscience.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bemeur C., Qu H., Desjardins P., Butterworth R.F. IL-1 or TNF receptor gene deletion delays onset of encephalopathy and attenuates brain edema in experimental acute liver failure. Neurochem Int. 2010;56:213–216. doi: 10.1016/j.neuint.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 42.Butterworth R.F. Hepatic encephalopathy: a central neuroinflammatory disorder? Hepatology. 2011;53:1372–1376. doi: 10.1002/hep.24228. [DOI] [PubMed] [Google Scholar]
- 43.Jiang W., Desjardins P., Butterworth R.F. Direct evidence for central proinflammatory mechanisms in rats with experimental acute liver failure: protective effect of hypothermia. J Cereb Blood Flow Metab. 2009;29:944–952. doi: 10.1038/jcbfm.2009.18. [DOI] [PubMed] [Google Scholar]
- 44.Wright G., Shawcross D., Olde Daminck S.W., Jalan R. Brain cytokine flux in acute liver failure and its relationship with intracranial hypertension. Metab Brain Dis. 2007;22:375–388. doi: 10.1007/s11011-007-9071-4. [DOI] [PubMed] [Google Scholar]
- 45.Rolando N., Wade J., Davalos M. The systemic inflammatory response syndrome in acute liver failure. Hepatology. 2000;32:734–739. doi: 10.1053/jhep.2000.17687. [DOI] [PubMed] [Google Scholar]
- 46.Bernal W., Donaldson P., Underhill J. Tumor necrosis factor genomic polymorphism and outcome of acetaminophen (paracetamol)-induced acute liver failure. J Hepatol. 1998;29:53–59. doi: 10.1016/s0168-8278(98)80178-5. [DOI] [PubMed] [Google Scholar]
- 47.Bemeur C., Butterworth R.F. Liver-brain proinflammatory signalling in acute liver failure: role in the pathogenesis of hepatic encephalopathy and brain edema. Metab Brain Dis. 2013;28:145–150. doi: 10.1007/s11011-012-9361-3. [DOI] [PubMed] [Google Scholar]
- 48.D'Mello C., Le T., Swain M.G. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factor a signalling during peripheral organ inflammation. J Neurosci. 2009;29:2089–2102. doi: 10.1523/JNEUROSCI.3567-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Andersson A.K., Ronnback L., Hansson E. Lactate induces tumor necrosis factor-alpha, interleukin-6 and interleukin-1beta release in microglial and astroglial-enriched primary cultures. J Neurochem. 2005;93:1327–1333. doi: 10.1111/j.1471-4159.2005.03132.x. [DOI] [PubMed] [Google Scholar]
- 50.Lee W.M. Acute liver failure in the United States. Semin Liver Dis. 2003;23:217–226. doi: 10.1055/s-2003-42641. [DOI] [PubMed] [Google Scholar]
- 51.Rose C., Michalak A., Pannunzio Mild hypothermia delays the onset of coma and prevents brain edema and extracellular brain glutamate accumulation in rats with acute liver failure. Hepatology. 2000;31:872–877. doi: 10.1053/he.2000.5923. [DOI] [PubMed] [Google Scholar]
- 52.Acharya S.K., Bhatia V., Sreenivas V. Efficacy of L-ornithine L-aspartate in acute liver failure: a double-blind randomized, placebo-controlled trial. Gastroenterology. 2009;136:2159–2168. doi: 10.1053/j.gastro.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 53.Ytrebo L.M., Kristiansen R.G., Maehre H. L-ornithine phenylacetate attenuates increased arterial and extracellular brain ammonia and prevents intracranial hypertension in pigs with acute liver failure. Hepatology. 2009;50:165–174. doi: 10.1002/hep.22917. [DOI] [PubMed] [Google Scholar]
- 54.Traber P., DalCanto M., Ganger D. Effect of body temperature on brain edema and encephalopathy in the rat after hepatic devascularization. Gastroenterology. 1989;96:885–891. [PubMed] [Google Scholar]
- 55.Jalan R., Olde Daminck S.W., Deutz N.E. Moderate hypothermia for uncontrolled intracranial hypertension in acute liver failure. Lancet. 1999;1:1164–1168. doi: 10.1016/s0140-6736(98)12440-6. [DOI] [PubMed] [Google Scholar]
- 56.Chatauret N., Zwingmann C., Rose C. Effects of hypothermia on brain glucose metabolism in acute liver failure : a H/C nuclear magnetic resonance study. Gastroenterology. 2003;125:815–824. doi: 10.1016/s0016-5085(03)01054-0. [DOI] [PubMed] [Google Scholar]
- 57.Vaquero J., Belanger M., James L. Mild hypothermia attenuates liver injury and improves survival in mice with acetaminophen toxicity. Gastroenterology. 2007;132:3722–3733. doi: 10.1053/j.gastro.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 58.Vaquero J., Butterworth R.F. Mild hypothermia for the treatment of acute liver failure: what are we waiting for? Nat Clin Pract Gastroenterol Hepatol. 2007;10:528–529. doi: 10.1038/ncpgasthep0927. [DOI] [PubMed] [Google Scholar]
- 59.Harrison P.M., Keays R., Bray G.P. Improved outcome of paracetamol-induced fulminant hepatic failure by late administration of acetylcysteine. Lancet. 1990;335:1572–1573. doi: 10.1016/0140-6736(90)91388-q. [DOI] [PubMed] [Google Scholar]
- 60.Bemeur C., Vaquero J., Desjardins P., Butterworth R.F. N-acetyl cysteine attenuates cerebral complications of non-acetaminophen-induced acute liver failure in mice: antioxidant and anti-inflammatory mechanisms. Metab Brain Dis. 2010;25:241–249. doi: 10.1007/s11011-010-9201-2. [DOI] [PubMed] [Google Scholar]
- 61.James L.P., McCullough S.S., Lamps L.W. Effect of N-acetyl cysteine on acetaminophen toxicity in mice: relationship to reactive nitrogen and cytokine formation. Toxicol Sci. 2003;75:458–467. doi: 10.1093/toxsci/kfg181. [DOI] [PubMed] [Google Scholar]
- 62.Chastre A., Belanger M., Beauchesne E. Inflammatory cascades driven by tumor necrosis factor alpha play a major role in the progression of acute liver failure and its neurological complications. PLos One. 2012;7:e49670. doi: 10.1371/journal.pone.0049670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vogels B.A., Maas M.A., Daalhuisen J. Memantine, a non-competitive NMDA receptor antagonist improves hyperammonemia-induced encephalopathy and acute liver failure encephalopathy in rats. Hepatology. 1997;25:820–827. doi: 10.1002/hep.510250406. [DOI] [PubMed] [Google Scholar]
- 64.Basile A.S., Hughes R.D., Harrison P.M. Elevated brain concentrations of 1,4 benzodiazepines in fulminant hepatic failure. N Engl J Med. 1991;325:473–478. doi: 10.1056/NEJM199108153250705. [DOI] [PubMed] [Google Scholar]
- 65.Van der Rigt C.C., de Knegt R.J., Schalm S.W. Flumazenil does not improve hepatic encephalopathy associated with acute ischemic liver failure in the rabbit. Metab Brain Dis. 1990;5:131–141. doi: 10.1007/BF00999840. [DOI] [PubMed] [Google Scholar]
- 66.Widler P., Fisch H.U., Schoch P. Increased benzodiazepine-like activity is neither necessary nor sufficient to explain acute hepatic encephalopathy in the thioacetamide-treated rat. Hepatology. 1993;18:1459–1464. [PubMed] [Google Scholar]
- 67.Ahboucha S., Coyne L., Hirakawa R. An interaction between benzodiazepines and neuroactive steroids at GABA-A receptors in cultured hippocampal neurons. Neurochem Int. 2006;48:703–707. doi: 10.1016/j.neuint.2005.12.006. [DOI] [PubMed] [Google Scholar]


