Abstract
Background/Objectives
Hepatic encephalopathy (HE) has relevant impact on the quality of life of patients and their caregivers and causes relevant costs because of hospitalizations and work days lost. Its quantification is important to perform adequate clinical trials on this relevant complication of cirrhosis and portal-systemic shunting. Clinical neurophysiology, which detects functional alterations of the nervous system, has been applied to the study of HE for over 60 years. This review aims at summarizing and clarifying the role of neurophysiologic techniques in the study of HE.
Methods
A narrative review was performed aiming at interpreting the cited papers and the techniques on the basis of their physiological and pathophysiological meaning.
Results
The potential role of EEG, quantified EEG, evoked potentials—both exogenous, endogenous and motor—have been clarified to the reader that may be unfamiliar with neurophysiology.
Conclusions
The EEG, reflecting the oscillatory changes of neural network is the preferable tool to detect and monitor HE, with the exception of its most severe stage, when EEG flattens. SSEP and MEP have indication to detect and monitor transmission alterations that are likely related to myelin changes and microedema.
Keywords: hepatic encephalopathy, cirrhosis, neurophysiology, EEG, evoked potentials
Abbreviations: BAEPs, brainstem acoustic evoked potentials; EEG, electroencephalogram; EPs, evoked potentials; ERPs, event related potentials; fVPS, flash visual evoked potentials; HE, hepatic encephalopathy; MEG, magnetoencephalogram; MEPs, motor evoked potentials; pVEPs, pattern reversal visual evoked potentials; SSEPs, somatosensory evoked potential; VEPs, visual evoked potentials
Clinical neurophysiology is the study of the central and peripheral nervous systems through the recording of bioelectrical or magnetic activity, whether spontaneous or stimulated.
The neurophysiological investigation of hepatic encephalopathy (HE) is generally performed by the electroencephalogram (EEG) and the evoked potentials (EPs). Other neurophysiologic techniques are Transcranial Magnetic Stimulation (TMS) and Magnetoencephalography (MEG). MEG is manly a research tool.
The EEG reflects the post-synaptic activity of pools of the large pyramidal cells of the fourth layer of the brain cortex and is extremely sensitive to the influence of toxic, pharmacological and metabolic factors. In fact, these impingue on electrogenesis and the oscillation of neural networks.
EPs reflect the electric summation of neuronal activity related to sensory, motor stimuli or to cognitive processes. Changes in the latency of sensory and motor EPs reflect changes in transmission time, or, in the case of cognitive EPs, in activation/synchronization of cortical areas related to cognitive processes. Changes in EPs amplitude reflect changes in the synchronization and in the amount of the activated neurons.
Clinical neurophysiological investigation can provide: 1) complementary information for the diagnosis of HE in stuporose/comatose patients with liver disease, 2) information to monitor the evolution of HE, 3) information about the existence of covert HE, 4) prognostic information on survival and on the risk of HE development over time.
The EEG
HE is characterized by alterations of the oscillatory properties of brain neural networks.1 These are easily revealed by the EEG that displays a wide spectrum of abnormalities; these alterations have been proven to be roughly related to behavioral changes in HE.2
There are two main components in the EEG: rhythmic background activity and transients. In patients with HE, the basic rhythmic activity of the EEG progressively slows down. In addition, the reactivity of the wake EEG to eye-opening reduces and finally disappears. Transients, the most important of which are called ‘triphasic waves’, can be observed in wake EEG of patients with moderate/severe HE, but are not exclusive to this metabolic encephalopathy.
Minor EEG changes that might have pathophysiological implications
A very initial stage of the wake EEG alterations seems to be characterized by an increase of the amplitude of the alpha activity in the central areas of the scalp (anteriorization).3 Of note, this is a typical neurophysiological feature of reduced vigilance in healthy individuals.4 In agreement with this finding, previous data—based on less accurate EEG mapping technique—suggested an increase the central-frontal alpha.5 Accordingly to Kullmann et al6 the very initial stage of HE may be also characterized by an increase in frontal beta activity. However, as a rule, beta activity decreases in more severe HE.7 Notably, already about fifty years ago a decrease in the EEG amplitude with replacement of alpha by beta rhythms was reported as a feature of the very initial phases of HE.2 Such observation was neglected in subsequent studies, because a low-voltage desynchronized EEG dominated by beta activity can represent a genetic feature and also the expression of anxiety or alcohol misuse.8,9 Recent data obtained by an artificial network/expert system showed a trend for an association of low-voltage, high-beta EEG with poor prognosis and bouts of HE, regardless of alcohol misuse.10 A role for enhanced gabaergic tone can be hypothesized in relation to the pathogenesis of this electric phenomenon,11 since benzodiazepines increase the amount of the beta activity of the EEG.12 The relationship of wake EEG beta activity and HE needs further formal study.
The typical EEG findings in HE
The main characteristics of the EEG features of HE are summarized in Table 1 and Figure 1. Initially EEG slowing does not characterize the whole of the EEG, and there are only runs of theta activity. This is clearly captured by quantitative EEG, which does not detect a reduction in the mean dominant frequency (MDF) of the EEG in, but only an increase of theta activity in the posterior areas of the scalp.7,13–15 The MDF is a measure of the overall frequency of an EEG interval (called epoch) and, on the computational point of view, is the mean power of each frequency weighted by the frequency rate, according to the formula:
Table 1.
EEG Changes in HE.
| 1. Normal or low frequency alpha rhythm (8.5 Hz) disturbed by random waves in the theta range (4–8 Hz) over both hemispheres. Theta waves are generally predominant in the temporal, parietal and occipital derivations, but can also be observed in the frontal derivations or be diffuse on the scalp. Eye opening reactivity can be reduced |
| 2. Background activity in the theta range (4–8) diffused over both hemispheres. Random appearance of rare high voltage waves in the delta range (<4 Hz). Eye opening reactivity is usually reduced or absent. Bursts of intermittent rhythmic delta activity (IRDA) as well as triphasic waves can appear |
| 3. Severe disorganization of EEG activity without any normal element. Asynchronous theta and delta waves diffuse over both hemispheres. Triphasic waves are usual |
| 4. High voltage arrhythmic delta activity |
| 5. Arrhythmic delta activity decreases both in frequency and amplitude |
| 6. Flat EEG |
Figure 1.
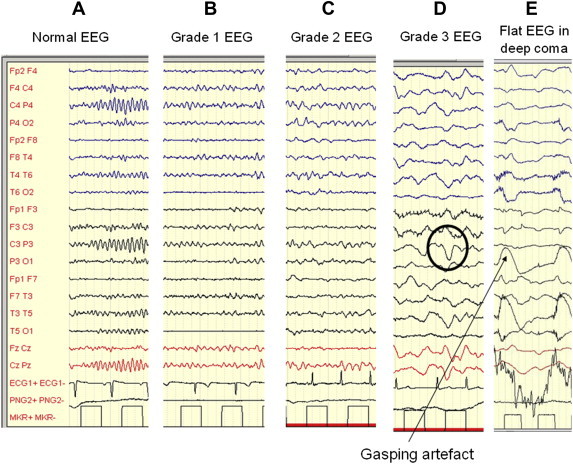
The EEG changes in HE. From the left to right (A): a normal EEG; B: low frequency, frontalized alpha activity with random theta waves; C: predominant theta activity with random delta waves; D: high voltage delta–theta activity with triphasic waves (inside circles), E: the EEG tends to flatten (the high voltage waves are gasping artefacts). Personal observations obtained by Micromed equipment.
Two kinds of transients are detectable in the EEG of HE, triphasic waves and bursts of intermittent rhythmic delta activity. The detection of these transients is more common in patients with grade 2–3 HE, but they are not found in deep coma.
Triphasic waves were first described by Bickford and Butt16 as a feature of HE, but later they were observed also in other metabolic or toxic encephalopathies.16 They are composed by a high-voltage (>70 mV), downward oriented sharp wave that are preceded and followed by upwards oriented waves of relatively lower amplitude. They tend to be diffuse and bilateral, with a degree of frontal predominance. They tend to repeat periodically every 1–2 s (Figure 2).
Figure 2.
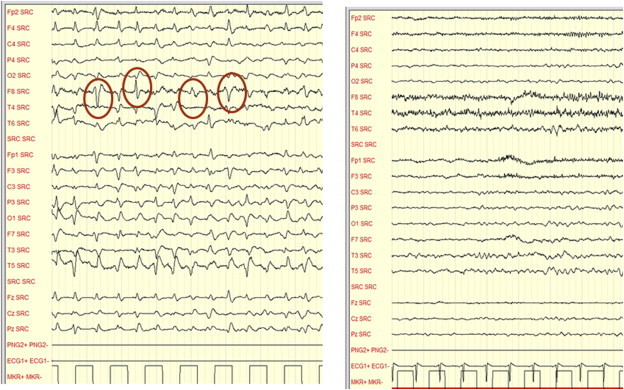
Grade 3 HE in acute on chronic liver failure before MARS (on the left) and after MARS (on the right). On the left bursts of triphasic waves are detectable. On the right triphasic waves disappear in parallel with improvement of metal state (from 3 to 1). (Personal observation obtained by a Micromed EEG equipment—Mogliano Veneto, Italy).
An association between triphasic waves and white matter alterations has been recently found,17,18 therefore it is possible that they may relate to the occurrence of white matter edema. The occurrence of triphasic waves is a marker of severe—however, not extremely severe—encephalopathy and are, therefore, a marker of increased risk of death.17,18
Other transients that can be found in patients with HE are burst of Intermittent Rhythmic Delta Activity (IRDA), which are rhythmic slow-wave discharges with a frequency of 1.5–4.0 Hz, localized mostly on the frontal derivations (FIRDA) and very rarely over the posterior, occipital, ones (OIRDA) (Figure 3). This activity is considered to reflect subcortical functional changes.19,20
Figure 3.
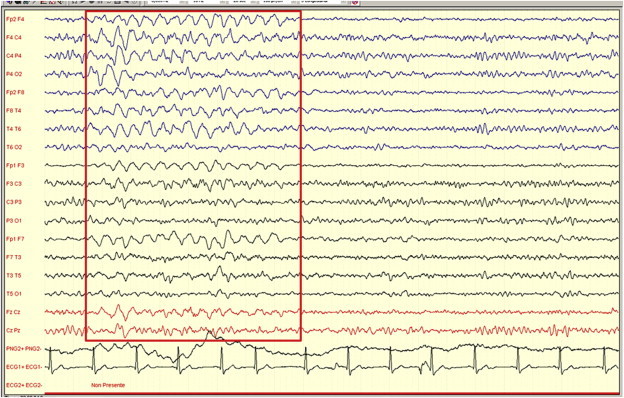
A burst of intermittent rhythmic delta activity in a cirrhotic subject with a 7 Hz background frequency and clinical signs of grade one HE (Personal observation obtained by a Micromed equipment—Mogliano Veneto, Italy).
The clinical use of the EEG in HE
The EEG can have a complementary role in the differential diagnosis of confused or comatose individuals with liver insufficiency/shunt in whom the diagnosis of HE is not sure, since it is may orient the diagnosis to other disorders.21 For example, the EEG is the only way to detect loss of consciousness due to non-convulsive seizures. In addition, it can be used to detect covert HE and monitor the severity of HE in an objective way, regardless of patient cooperation. This can be relevant in clinical trial.
Quantitative EEG
The grading of the severity of EEG alterations in HE can be based on visual pattern recognition, but this approach has limited reliability.7,10 Grading based on the simple semi-quantitative evaluation of the frequency of the background principal EEG rhythm improves repeatability.10 Spectral analysis of the digitalized EEG provides grading based on the exact quantification of the EEG components.7,10,14,15,22–25 Based on the relative power of frequency bands and MDF over approximately 60 s of EEG over posterior derivations, a classification was obtained that has prognostic value both in terms of survival and risk of development of overt HE14 (Table 2). The value of such approach was recently confirmed26 and a large, validated observational study proved that the use of the MDF of the EEG improves the predictive value of the MELD score.14,27
Table 2.
Risk of Developing Gross Overt HE or Dying, Based on EEG Spectral Analysis Classification (Modified from Amodio et al10).
| Episodes of confusion or coma |
Mortality |
|||
|---|---|---|---|---|
| Grade | Ratea | Odds ratio (CI95%) | Ratea | Odds ratio (CI95%) |
| 0 | 33 | 1 | 13 | 1 |
| 1 | 72 | 2.0 (1.06–3.65) | 41 | 4.3 (1.8–10.1) |
| 2–3 | 139 | 3.5 (1.9–6.6) | 57 | 6.3 (2.6–15.1) |
Events × 100 patients/year.
In well compensated cirrhotic patients the prognostic value of minor EEG changes might be lower than in patients with advanced liver disease.14,28 In fact, the attribution to HE of any change of brain functional, as well as of cognition, in these patients might be questionable (i.e., it may have low positive predictive value), because the ‘a priori’ probability of HE is low.
The magnetoencephalogram
The magnetoencephalogram (MEG) is a non-invasive, non-hazardous technique measuring the associated magnetic fields emanating from the brain as a consequence of the electrical activity that is produced by the same processes generating the EEG. It provides functional brain mapping with an excellent temporal resolution and allows recording of subcortical activity. It is an expensive, barely available research tool that has highlighted 1) the progressive reduction of oscillatory properties of neural network in parallel with the severity of HE29; 2) the relationship between the reduction of the oscillatory properties of the cortex and miniasterixes.29 In addition, the parallel decrease in frequency of brain rhythms and critical flicker frequency was considered to suggest a link between the two phenomena.30,31
The evoked potentials
The EPs can be classified as i) sensory or exogenous EPs (visual, auditory or somatosensory), ii) motor EPs, iii) cognitive or endogenous EPs (also called Event Related Potentials—ERPs).
The components of sensory EP (visual, auditory and somatosensory) are detectable even when the EEG is markedly suppressed—flat EEG—in severe coma. At odds with the EEG, short latency sensory EPs are not very much influenced by drugs.32,33 Thus, at least in principle, they are appropriate tools to monitor grade 4 HE in the Intensive Care Unit, where patients are sedated.
Sensory EPs, as well as motor EPs, produce good estimates of the transmission speed across different areas of the nervous system, providing information on focal edema and myelin fiber dysfunction.34–37 Therefore, it is reasonable that they reflect some of the mechanisms that may occur in patients with HE.
In contrast to sensory EP, cognitive EPs requires active paradigms of cognitive stimulation and the cooperation of the patients. They can reveal early brain dysfunction38 or provide insight into cognitive processing.39
Visual evoked potentials (VEPs)
VEPs are subdivided according to the nature of the eliciting stimuli into flash VEPs (fVEPs), pattern-reversal VEPs (pVEPs) and movement-related VEPs (mVEPs).40 VEPs latency depends on multisynaptic neurotransmission from the retina to the visual cortex. VEPs are influenced by optic nerve diseases, demyelinating processes, disorders of either subcortical or cortical neurons of brain hemispheres, metabolic abnormalities and the use of psychoactive medications.33,41 The latency of the P100 wave of pVEPs has been used to monitor very mild HE42; severe HE cannot be studied since pVEPs require patient cooperation. The sensitivity of P100 latency was proved to be poor, because no significant difference was observed before and after TIPS insertion, in contrast, for example to the EEG (Kuba et al, 1996).
In contrast to pVEPs, flashed-evoked VEPs (fVEPs) do not require patient cooperation; therefore, they can be elicited also in comatose subjects. The long-latency component N3 of fVEPs shows a reversible prolongation in latency which is parallel with the severity of HE; in addition, patients with grade III and IV HE show loss of N1 followed by loss of P1.43
While the prevalence (about 40%) of fVEPs alterations in cirrhotic patients with normal EEG and psychometric performance may suggest a high sensitivity of this technique in detecting HE, a clear prolongation of N3 is detected in only 50–90% of the patients with overt HE.43,44 This suggest that: i) fVEPs efficacy as a monitoring tool, is limited, if any, ii) fVEPs may reflect some phenomena which are related to HE, but not parallel to—its behavioral features.
In HE related to acute liver failure, the latencies of the late components of fVEPs (vz. N2, N3, P2) were found to have some relationship with changes in intracranial pressure, but the time-course of their changes were slower than that of intracranial pressure changes.45 The P1 component of fVEPs was used as an index of the occurrence of acute liver failure (HE) in an open, non-blind study on acute hepatitis.46 Therefore, present information does not support the clinical use of fVEPs to monitor acute HE, but justifies further studies on their applicability to the neuromonitoring of acute liver failure.
Brainstem auditory evoked potentials (BAEPs)
BAEPs are composed by five waves representing the activation of specific anatomical points along the auditory neural pathway: the cochlear nerve and nuclei (waves I and II), superior olivary nucleus (wave III), lateral lemniscus (wave IV), and inferior colliculi (wave V). One tracing from each side is obtained. The interpeak latencies between waves I and V or waves III and V are used as measures of brainstem neurotransmission. BAEPs are prolonged in diabetes, alcohol misuse and thiamine deficiency,47,48 which are common in cirrhotic patients. BAEPs are a sensitive tool to detect brainstem demyelination and neuronal loss in Wilson's disease.49,50 A considerable percentage of cirrhotic patients present delay of I–V and of III–V interpeak latencies49 and therefore BAEPs were considered as an index of minimal HE. In addition, BAEPs were found to predict the development of overt HE,51 suggesting a link between brainstem neurotransmission and HE. This link can be represented by microedema. BEARS have been also used as outcome measures in clinical trials on HE treatment.52 This use is questionable, since too many factors may influence brainstem neurotransmission and some of them are unrelated to HE.
In agreement with the skepticism in considering BAEPs a direct index of HE, there is the observation that in acute liver failure the prolongation of the III–V interpeak and to a lesser extent of the I–V interpeak was linked neither to the presence of the behavioral alterations nor to their evolution.46
Present knowledge discourages the use of BAEPs to monitor HE, even if they have the potential to detect cortical-spinal alterations. In contrast, they seem useful to detect and monitor hepatic myelopathy; however, the relationship between Hepatic myelopathy and HE is not clear. It is possible that Hepatic myelopathy is a risk factor for HE or a non-behavioral dimension of the same syndrome, but this requires further investigation.53–57
Somatosensory evoked potentials (SSEPs)
SSEPs explore both nervous transmission from the medulla oblongata to the cortex (central conduction time—i.e., the interpeak latency N13–N20-) and hemispheric transmission (middle components of the EP), therefore produce data related to the ones provided by BAEPs and fVEPs, respectively. A minor prolongation of central conduction time in cirrhosis,37,58 and therefore an alteration in brainstem nervous transmission possibly due to myelopathy or edema was confirmed by SSEPs. However, the prolongation of the middle cortical components (after P25) was found to be much more evident and related with the impairment in neuropsychiatric status37 and with the risk to develop overt HE in the follow up.59 These findings prove that HE influences hemispheric multisynaptic pathways more than subcortical brainstem neurotransmission.34,60–62
The same findings were confirmed in HE of acute liver failure: the disappearance of the middle latency cortical component (e.g., N70) precedes that of the earlier components (e.g., N20 and P25) and the increase of central conduction time. However, the delay of middle latency cortical components of SSEPs was found to be less sensitive than that of the cognitive evoked potential P300 in the model of HE caused by TIPS insertion,63 and no comparison with the EEG was produced for this indication. The disappearance N20 might be an index of brain damage so severe that it may be too late for liver transplantation.64,65 Further research on the use of SSEPs for the neuromonitoring of brain damage in acute liver failure is required before definite conclusions can be drawn.
Motor evoked potentials (MEPs)
MEPs are useful to improve the understanding of motor abnormalities that are generally caused by myelopathy associated to portal-systemic shunting.66 Motor response elicited by transcranial magnetic stimulation of the motor cortex confirmed the existence of prolongation of the central conduction time in cirrhotic patients (in agreement with BAEPs and SSEPs findings), and showed that changes in motor cortex excitability may exist in cirrhotic patients.35,36,67 Despite the opinion that MEPs may represent a tool to detect and monitor HE,68 it should be clear which brain change is studied, otherwise the assumption is simplistic.
The endogenous event related potentials (ERPs) reflect the activation of brain areas due to cognitive processes. They are relatively, but not absolutely, independent of stimuli features, but sensitive to the cognitive paradigm is presented. The most used paradigm used to elicit cognitive phenomena is the oddball task in which individuals are exposed to a series of standard visual or auditory stimuli in which small amounts (15 or 20%) of deviant stimuli (odd) are randomly nested.69 Subjects can be simply exposed to the sequence of stimuli (passive task), or asked to detect the target stimuli (active task). The passive and the active tasks evoke different ERPs components.70 The most studied component evoked by the active oddball task is the parietal P300 or P3b (Figure 4).69,70 P300 is a large positive component evoked by target (rare) stimuli.69 The functional rule attributed to this component is the context-updating of mental representation when a deviant stimulus occurs.69,71 P300 latency reflects the time needed to stimulus categorization and P300 amplitude reflects both the activation and the synchronization of cortical areas used for the execution of the task.70,72
Figure 4.
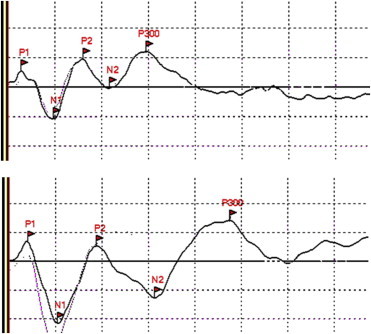
Potentials evocated by the auditory oddball paradigm in two age-matched subjects: a normal one (on the top) and a cirrhotic patient with minimal HE (on the bottom). The lines marked with flags represent the response elicited by the deviant (target and rare) stimuli, the other ones the responses elicited by the frequent stimuli. The peak of P300 in the normal subject is about 300 ms from the onset (three squares of 100 ms), in the patient with minimal HE the peak of P300 is about 480 ms. Personal observations obtained by a Micromed EEG equipment—Mogliano Veneto, Italy).
The use of P300 latency elicited by active auditory oddball paradigm was advocated as a tool to detect the onset of HE in several studies.38,73–78 Present knowledge supports the conclusion that in HE P300 latency is significantly more prolonged in HE then in normal subjects and, therefore the stimulus categorization time is prolonged. However, the value of P300 prolongation as a marker of the risk of the occurrence of overt HE in the follow up was found to be lower than that of the EEG (positive predictive value 53 vs. 75%, negative predictive value 81 vs. 86%).77
In addition, the clinical usefulness of this tool is limited by the followings: i) P300 latency is age-related,79 and therefore studies based on fixed cut off values are biased, ii) a relevant (5–10%) proportions of individuals—also healthy subjects—do not produce a reliable P300. Usually P300 is computed by the averaging of a number of trials; however, it is possible to compute P300 also on single trials.80 Recently, using this approach, we have proven that patients with minimal HE have an increased latency variability of single trial P300.72 This contribute to explain to reduction in P300 calculated by averaging techniques and highlights the variability of responses typical of minimal HE, which was also detected by psychometric testing.81
In conclusion, despite its theoretical interest, P300 latency is not recommended as a routine technique to detect and monitor minimal or covert HE by the ‘International Society for Hepatic Encephalopathy and Nitrogen Metabolism’ (ISHEN) consensus.82
Recently mismatch negativity was proposed as a good diagnostic tool for minimal HE, also because it is independence of age79 and reflects a precocious phase of the attentional processes, and these are altered in minimal HE.83 The clinical use of this potential is even more difficult and problematic than that of P300, since its elicitation is not constant and its measurement difficult.
Other cognitive potentials can be elicited by ad hoc cognitive tasks to reach insight into the time course of cognitive processes. For instance, the use of N2pc, which is a potential related to attention orientation, allowed to confirm early attention alterations in cirrhotic patients, and to prove that the impairment of top-down cognitive processes is higher than that of the bottom up processes.39 Other cognitive event related potentials explore the activation of motor areas after the presentation of a cognitive tasks requiring a motor response. The Readiness Potential was found to be reduced in amplitude in cirrhotic patients; therefore, defective activity of the cortical areas implicated in the preparation of movement was suspected.84,85 The Lateralized Readiness Potential, which is elicited contralaterally to hand movement, has been used to separate the interval between stimulus presentation and motor response. By this tool we could prove that selection defects (cognitive phenomena preceding motor activation) precede motor alterations in cirrhotic patients.85,86 Also, the study of the potentials evoked by the inhibitory control task allowed to discover the existence of a very initial phase of minimal HE in which there is an increased recruitment of cortical areas to maintain cognitive performance.87
All these techniques, based on the elicitation of potential during cognitive tasks, have mostly research interest, since they help defining cognitive processes. Their use in routine clinical practice for diagnosis or monitoring purposes, if any, is limited.
Conclusions
Neurophysiological tools reflect changes in signal transmission (mainly the exogenous potentials), cognitive phenomena (exogenous event related potentials) and cortical networks oscillatory dynamics (EEG and MEG) which are altered by HE. Therefore, they provide the opportunity to detect and monitor brain changes occurring in HE.
The EEG is the technique with most clear potential for clinical applicability in the study of HE. SSEP and MEP, which reflect transmission defects caused by myelin alterations and/or edema, have clinical potentials to detect such conditions.
Conflicts of interest
All authors have none to declare.
References
- 1.Gotz T., Huonker R., Kranczioch C. Impaired evoked and resting-state brain oscillations in patients with liver cirrhosis as revealed by magnetoencephalography. NeuroImage Clin. 2013;2:873–882. doi: 10.1016/j.nicl.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parsons-Smith B.G., Summerskill W.H.J., Dawson A.M., Sherlock S. The electroencephalograph in liver disease. Lancet. 1957:867–871. doi: 10.1016/s0140-6736(57)90005-3. [DOI] [PubMed] [Google Scholar]
- 3.Montagnese S., Jackson C., Morgan M.Y. Spatio-temporal decomposition of the electroencephalogram in patients with cirrhosis. J Hepatol. 2007;46:447–458. doi: 10.1016/j.jhep.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 4.Kalauzi A., Vuckovic A., Bojic T. EEG alpha phase shifts during transition from wakefulness to drowsiness. Int J Psychophysiol. 2012;86:195–205. doi: 10.1016/j.ijpsycho.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 5.Sagales T., Gimeno V., de lC., Casellas F., Dolors M.M., Villar S.M. Brain mapping analysis in patients with hepatic encephalopathy. Brain Topogr. 1990;2:221–228. doi: 10.1007/BF01140590. [DOI] [PubMed] [Google Scholar]
- 6.Kullmann F., Hollerbach S., Lock G., Holstege A., Dierks T., Scholmerich J. Brain electrical activity mapping of EEG for the diagnosis of (sub)clinical hepatic encephalopathy in chronic liver disease. Eur J Gastroenterol Hepatol. 2001;13:513–522. doi: 10.1097/00042737-200105000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Amodio P., Marchetti P., Del Piccolo F. Spectral versus visual EEG analysis in mild hepatic encephalopathy. Clin Neurophysiol. 1999;110:1334–1344. doi: 10.1016/s1388-2457(99)00076-0. [DOI] [PubMed] [Google Scholar]
- 8.Steinlein O., Anokhin A., Yping M., Schalt E., Vogel F. Localization of a gene for the human low-voltage EEG on 20q and genetic heterogeneity. Genomics. 1992;12:69–73. doi: 10.1016/0888-7543(92)90408-k. [DOI] [PubMed] [Google Scholar]
- 9.Niedermeyer E. The normal EEG of the waking adult. In: Niedermeyer E., Lopes Da Silva F., editors. Electroencephalography. Basic Principles, Clinical Applications, and Related Fields. 3rd ed. Williams & Wilkins; Baltimore: 1999. [Google Scholar]
- 10.Amodio P., Pellegrini A., Ubiali E. The EEG assessment of low-grade hepatic encephalopathy: comparison of an artificial neural network-expert system (ANNES) based evaluation with visual EEG readings and EEG spectral analysis. Clin Neurophysiol. 2006;117:2243–2251. doi: 10.1016/j.clinph.2006.06.714. [DOI] [PubMed] [Google Scholar]
- 11.Del Piccolo F., Amodio P., Mapelli D. Rifaximin reduces EEG relative beta power in patients with minimal hepatic encephalopathy. In: Jones E.A., Meijer A.J., Chamuleau R.A.F.M., editors. Encephalopathy and Nitrogen Metabolism in Liver Failure. Kluwer Academic Publisher; Dordrecht, The Netherlands: 2003. [Google Scholar]
- 12.Hotz M.A., Ritz R., Linder L., Scollo-Lavizzari G., Haefeli W.E. Auditory and electroencephalographic effects of midazolam and alpha-hydroxy-midazolam in healthy subjects. Br J Clin Pharmacol. 2000;49:72–79. doi: 10.1046/j.1365-2125.2000.00104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marchetti P., D'Avanzo C., Orsato R. Electroencephalography in patients with cirrhosis. Gastroenterology. 2011;141:1680–1689. doi: 10.1053/j.gastro.2011.06.085. [DOI] [PubMed] [Google Scholar]
- 14.Amodio P., Del Piccolo F., Petteno E. Prevalence and prognostic value of quantified electroencephalogram (EEG) alterations in cirrhotic patients. J Hepatol. 2001;35:37–45. doi: 10.1016/s0168-8278(01)00129-5. [DOI] [PubMed] [Google Scholar]
- 15.Van der Rijt C.C., Schalm S.W., De G.G., De V.M. Objective measurement of hepatic encephalopathy by means of automated EEG analysis. Electroencephalogr Clin Neurophysiol. 1984;57:423–426. doi: 10.1016/0013-4694(84)90071-3. [DOI] [PubMed] [Google Scholar]
- 16.Bickford R.G., Butt H.R. Hepatic coma: the electroencephalographic pattern. J Clin Invest. 1955;34:790–799. doi: 10.1172/JCI103134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sutter R., Kaplan P.W. Uncovering clinical and radiological associations of triphasic waves in acute encephalopathy: a case-control study. Eur J Neurol. 2014;21:660–666. doi: 10.1111/ene.12372. [DOI] [PubMed] [Google Scholar]
- 18.Sutter R., Stevens R.D., Kaplan P.W. Significance of triphasic waves in patients with acute encephalopathy: a nine-year cohort study. Clin Neurophysiol. 2013;124:1952–1958. doi: 10.1016/j.clinph.2013.03.031. [DOI] [PubMed] [Google Scholar]
- 19.Neufeld M.Y., Chistik V., Chapman J., Korczyn A.D. Intermittent rhythmic delta activity (IRDA) morphology cannot distinguish between focal and diffuse brain disturbances. J Neurol Sci. 1999;164:56–59. doi: 10.1016/s0022-510x(99)00018-0. [DOI] [PubMed] [Google Scholar]
- 20.Watemberg N., Alehan F., Dabby R., Lerman-Sagie T., Pavot P., Towne A. Clinical and radiologic correlates of frontal intermittent rhythmic delta activity. J Clin Neurophysiol. 2002;19:535–539. doi: 10.1097/00004691-200212000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan P.W. The EEG in metabolic encephalopathy and coma. J Clin Neurophysiol. 2004;21:307–318. [PubMed] [Google Scholar]
- 22.Bahn E., Nolte W., Kurth C., Ramadori G., Ruther E., Wiltfang J. Quantification of the electroencephalographic theta/alpha ratio for the assessment of portal-systemic encephalopathy following implantation of transjugular intrahepatic portosystemic stent shunt (TIPSS) Metab Brain Dis. 2002;17:19–28. doi: 10.1023/a:1014048229754. [DOI] [PubMed] [Google Scholar]
- 23.Ciancio A., Marchet A., Saracco G. Spectral electroencephalogram analysis in hepatic encephalopathy and liver transplantation. Liver Transpl. 2002;8:630–635. doi: 10.1053/jlts.2002.33971. [DOI] [PubMed] [Google Scholar]
- 24.De la Torre G.S., Garcia de Leon A.M., Bello M.G. Evaluation of hepatic encephalopathy using computerized electroencephalography. Rev Esp Enferm Apar Dig. 1989;76:138–143. [PubMed] [Google Scholar]
- 25.Epstein C.M., Riether A.M., Henderson R.M., Cotsonis G.A. EEG in liver transplantation: visual and computerized analysis. Electroencephalogr Clin Neurophysiol. 1992;83:367–371. doi: 10.1016/0013-4694(92)90072-p. [DOI] [PubMed] [Google Scholar]
- 26.Koziarska D., Wunsch E., Milkiewicz M., Wojcicki M., Nowacki P., Milkiewicz P. Mini-mental state examination in patients with hepatic encephalopathy and liver cirrhosis: a prospective, quantified electroencephalography study. BMC Gastroenterol. 2013;13:107. doi: 10.1186/1471-230X-13-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montagnese S., De R.M., Schiff S. Prognostic benefit of the addition of a quantitative index of hepatic encephalopathy to the MELD score: the MELD-EEG. Liver Int. 2014 doi: 10.1111/liv.12490. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28.Hartmann I.J., Groeneweg M., Quero J.C. The prognostic significance of subclinical hepatic encephalopathy. Am J Gastroenterol. 2000;95:2029–2034. doi: 10.1111/j.1572-0241.2000.02265.x. [DOI] [PubMed] [Google Scholar]
- 29.Butz M., Timmermann L., Gross J. Cortical activation associated with asterixis in manifest hepatic encephalopathy. Acta Neurol Scand. 2013 doi: 10.1111/ane.12217. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 30.Timmermann L., Butz M., Gross J. Impaired cerebral oscillatory processing in hepatic encephalopathy. Clin Neurophysiol. 2008;119:265–272. doi: 10.1016/j.clinph.2007.09.138. [DOI] [PubMed] [Google Scholar]
- 31.Kahlbrock N., Butz M., May E.S. Lowered frequency and impaired modulation of gamma band oscillations in a bimodal attention task are associated with reduced critical flicker frequency. NeuroImage. 2012;61:216–227. doi: 10.1016/j.neuroimage.2012.02.063. [DOI] [PubMed] [Google Scholar]
- 32.Bonhomme V., Hans P. Monitoring depth of anaesthesia: is it worth the effort? Eur J Anaesthesiol. 2004;21:423–428. doi: 10.1017/s0265021504006027. [DOI] [PubMed] [Google Scholar]
- 33.Guerit J.M. Neuromonitoring in the operating room: why, when, and how to monitor? Electroencephalogr Clin Neurophysiol. 1998;106:1–21. doi: 10.1016/s0013-4694(97)00077-1. [DOI] [PubMed] [Google Scholar]
- 34.Chu N.S., Yang S.S. Portal-systemic encephalopathy: alterations in somatosensory and brainstem auditory evoked potentials. J Neurol Sci. 1988;84:41–50. doi: 10.1016/0022-510x(88)90172-4. [DOI] [PubMed] [Google Scholar]
- 35.Cordoba J., Raguer N., Flavia M. T2 hyperintensity along the cortico-spinal tract in cirrhosis relates to functional abnormalities. Hepatology. 2003;38:1026–1033. doi: 10.1053/jhep.2003.50406. [DOI] [PubMed] [Google Scholar]
- 36.Oria M., Raguer N., Chatauret N. Functional abnormalities of the motor tract in the rat after portocaval anastomosis and after carbon tetrachloride induction of cirrhosis. Metab Brain Dis. 2006;21:297–308. doi: 10.1007/s11011-006-9036-z. [DOI] [PubMed] [Google Scholar]
- 37.Yang S.S., Wu C.H., Chiang T.R., Chen D.S. Somatosensory evoked potentials in subclinical portosystemic encephalopathy: a comparison with psychometric tests. Hepatology. 1998;27:357–361. doi: 10.1002/hep.510270207. [DOI] [PubMed] [Google Scholar]
- 38.Weissenborn K., Scholz M., Hinrichs H., Wiltfang J., Schmidt F.W., Kunkel H. Neurophysiological assessment of early hepatic encephalopathy. Electroencephalogr Clin Neurophysiol. 1990;75:289–295. doi: 10.1016/0013-4694(90)90107-u. [DOI] [PubMed] [Google Scholar]
- 39.Mapelli D., Iannizzi P., Biancardi A. Neuropsychological dysfunction in minimal hepatic encephalopathy: a review compared with own experience. In: Haussinger D., Kircheis G., Schliess F., editors. Hepatic Encephalopathy and Nitrogen Metabolism. Springer; Dordrecht, The Netherlands: 2006. pp. 467–473. [Google Scholar]
- 40.Odom J.V., Bach M., Barber C. Visual evoked potentials standard. Doc Ophthalmol. 2004;2004(108):115–123. doi: 10.1023/b:doop.0000036790.67234.22. [DOI] [PubMed] [Google Scholar]
- 41.Guérit J.M. Masson; Paris: 1993. Les Potentiales Évoqués. [Google Scholar]
- 42.Sandford N.L., Saul R.E. Assessment of hepatic encephalopathy with visual evoked potentials compared with conventional methods. Hepatology. 1988;8:1094–1098. doi: 10.1002/hep.1840080519. [DOI] [PubMed] [Google Scholar]
- 43.Zeneroli M.L., Pinelli G., Gollini G. Visual evoked potential: a diagnostic tool for the assessment of hepatic encephalopathy. Gut. 1984;25:291–299. doi: 10.1136/gut.25.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davies M.G., Rowan M.J., MacMathuna P., Keeling P.W., Weir D.G., Feely J. The auditory P300 event-related potential: an objective marker of the encephalopathy of chronic liver disease. Hepatology. 1990;12:688–694. doi: 10.1002/hep.1840120412. [DOI] [PubMed] [Google Scholar]
- 45.Davenport A., Bramley P.N. Cerebral function analyzing monitor and visual evoked potentials as a noninvasive method of detecting cerebral dysfunction in patients with acute hepatic and renal failure treated with intermittent machine hemofiltration. Ren Fail. 1993;15:515–522. doi: 10.3109/08860229309054967. [DOI] [PubMed] [Google Scholar]
- 46.Sawhney I.M., Verma P.K., Dhiman R.K. Visual and auditory evoked responses in acute severe hepatitis. J Gastroenterol Hepatol. 1997;12:554–559. doi: 10.1111/j.1440-1746.1997.tb00484.x. [DOI] [PubMed] [Google Scholar]
- 47.Begleiter H., Porjesz B., Chou C.L. Auditory brainstem potentials in chronic alcoholics. Science. 1981;211:1064–1066. doi: 10.1126/science.7466379. [DOI] [PubMed] [Google Scholar]
- 48.Fedele D., Martini A., Cardone C. Impaired auditory brainstem-evoked responses in insulin-dependent diabetic subjects. Diabetes. 1984;33:1085–1089. doi: 10.2337/diab.33.11.1085. [DOI] [PubMed] [Google Scholar]
- 49.Grimm G., Oder W., Prayer L., Ferenci P., Madl C. Evoked potentials in assessment and follow-up of patients with Wilson's disease. Lancet. 1990;336:963–964. doi: 10.1016/0140-6736(90)92419-i. [DOI] [PubMed] [Google Scholar]
- 50.Grimm G., Madl C., Katzenschlager R., Oder W., Ferenci P., Gangl A. Detailed evaluation of evoked potentials in Wilson's disease. Electroencephalogr Clin Neurophysiol. 1992;82:119–124. doi: 10.1016/0013-4694(92)90154-a. [DOI] [PubMed] [Google Scholar]
- 51.Romero-Gomez M., Boza F., Garcia-Valdecasas M.S., Garcia E., Aguilar-Reina J. Subclinical hepatic encephalopathy predicts the development of overt hepatic encephalopathy. Am J Gastroenterol. 2001;96:2718–2723. doi: 10.1111/j.1572-0241.2001.04130.x. [DOI] [PubMed] [Google Scholar]
- 52.Liu Q., Duan Z.P., Ha dK., Bengmark S., Kurtovic J., Riordan S.M. Synbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatology. 2004;39:1441–1449. doi: 10.1002/hep.20194. [DOI] [PubMed] [Google Scholar]
- 53.Baccarani U., Zola E., Adani G.L. Reversal of hepatic myelopathy after liver transplantation: fifteen plus one. Liver Transpl. 2010;16:1336–1337. doi: 10.1002/lt.22149. [DOI] [PubMed] [Google Scholar]
- 54.Nardone R., Orioli A., Holler Y. Central motor and sensory conduction in patients with hepatic myelopathy. Spinal Cord. 2014;52(6):439–443. doi: 10.1038/sc.2014.61. [DOI] [PubMed] [Google Scholar]
- 55.Weissenborn K. Portosystemic encephalopathy. Handb Clin Neurol. 2014;120:661–674. doi: 10.1016/B978-0-7020-4087-0.00045-0. [DOI] [PubMed] [Google Scholar]
- 56.Sobukawa E., Sakimura K., Hoshino S., Hoshino M., Miyoshi K. Hepatic myelopathy: an unusual neurological complication of advanced hepatic disease. Intern Med. 1994;33:718–722. doi: 10.2169/internalmedicine.33.718. [DOI] [PubMed] [Google Scholar]
- 57.Mendoza G., Marti-Fabregas J., Kulisevsky J., Escartin A. Hepatic myelopathy: a rare complication of portacaval shunt. Eur Neurol. 1994;34:209–212. doi: 10.1159/000117040. [DOI] [PubMed] [Google Scholar]
- 58.Kono I., Ueda Y., Nakajima K., Araki K., Kagawa K., Kashima K. Subcortical impairment in subclinical hepatic encephalopathy. J Neurol Sci. 1994;126:162–167. doi: 10.1016/0022-510x(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 59.Yen C.L., Liaw Y.F. Somatosensory evoked potentials and number connection test in the detection of subclinical hepatic encephalopathy. Hepatogastroenterology. 1990;37:332–334. [PubMed] [Google Scholar]
- 60.Yang S.S., Chu N.S., Liaw Y.F. Somatosensory evoked potentials in hepatic encephalopathy. Gastroenterology. 1985;89:625–630. doi: 10.1016/0016-5085(85)90460-3. [DOI] [PubMed] [Google Scholar]
- 61.Yang S.S., Chu N.S., Liaw Y.F. Brainstem auditory evoked potentials in hepatic encephalopathy. Hepatology. 1986;6:1352–1355. doi: 10.1002/hep.1840060622. [DOI] [PubMed] [Google Scholar]
- 62.Chu N.S., Yang S.S., Liaw Y.F. Evoked potentials in liver diseases. J Gastroenterol Hepatol. 1997;12:S288–S293. doi: 10.1111/j.1440-1746.1997.tb00512.x. [DOI] [PubMed] [Google Scholar]
- 63.Kramer L., Bauer E., Gendo A. Neurophysiological evidence of cognitive impairment in patients without hepatic encephalopathy after transjugular intrahepatic portosystemic shunts. Am J Gastroenterol. 2002;97:162–166. doi: 10.1111/j.1572-0241.2002.05441.x. [DOI] [PubMed] [Google Scholar]
- 64.Madl C., Grimm G., Ferenci P. Serial recording of sensory evoked potentials: a noninvasive prognostic indicator in fulminant liver failure. Hepatology. 1994;20:1487–1494. doi: 10.1002/hep.1840200618. [DOI] [PubMed] [Google Scholar]
- 65.Yang S.S., Chu N.S., Wu C.H. Disappearance of N20 and P25 components of somatosensory evoked potential: an ominous sign in severe acute hepatitis. J Formos Med Assoc. 1993;92:46–49. [PubMed] [Google Scholar]
- 66.Nardone R., Buratti T., Oliviero A., Lochmann A., Tezzon F. Corticospinal involvement in patients with a portosystemic shunt due to liver cirrhosis: a MEP study. J Neurol. 2006;253:81–85. doi: 10.1007/s00415-005-0930-9. [DOI] [PubMed] [Google Scholar]
- 67.Nolano M., Guardascione M.A., Amitrano L. Cortico-spinal pathways and inhibitory mechanisms in hepatic encephalopathy. Electroencephalogr Clin Neurophysiol. 1997;105:72–78. doi: 10.1016/s0924-980x(96)96571-6. [DOI] [PubMed] [Google Scholar]
- 68.Oria M., Chatauret N., Chavarria L. Motor-evoked potentials in awake rats are a valid method of assessing hepatic encephalopathy and of studying its pathogenesis. Hepatology. 2010;52:2077–2085. doi: 10.1002/hep.23938. [DOI] [PubMed] [Google Scholar]
- 69.Polich J. Neuropsychology of P3a and P3b: a theoretical overview. In: Moore N.C., Arikan K., editors. Brainwaves and Mind: Recent Developments. Kjellberg Inc.; Wheaton, IL: 2004. pp. 15–29. [Google Scholar]
- 70.Kok A. Age-related changes in involuntary and voluntary attention as reflected in components of the event-related potential (ERP) Biol Psychol. 2000;54:107–143. doi: 10.1016/s0301-0511(00)00054-5. [DOI] [PubMed] [Google Scholar]
- 71.Donchin E. The P300 as a metric for mental workload. Electroencephalogr Clin Neurophysiol Suppl. 1987;39:338–343. [PubMed] [Google Scholar]
- 72.Bisiacchi P., Cona G., Tarantino V. Assessing inter- and intra-individual cognitive variability in patients at risk for cognitive impairment: the case of minimal hepatic encephalopathy. Metab Brain Dis. 2014 doi: 10.1007/s11011-014-9529-0. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 73.Amodio P., Valenti P., Del P.F. P300 latency for the diagnosis of minimal hepatic encephalopathy: evidence that spectral EEG analysis and psychometric tests are enough. Dig Liver Dis. 2005;37:861–868. doi: 10.1016/j.dld.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 74.Gallai V., Alberti A., Balo S. Cognitive event-related potential in hepatic encephalopathy. Acta Neurol Scand. 1995;91:358–361. doi: 10.1111/j.1600-0404.1995.tb07021.x. [DOI] [PubMed] [Google Scholar]
- 75.Kullmann F., Hollerbach S., Holstege A., Scholmerich J. Subclinical hepatic encephalopathy: the diagnostic value of evoked potentials. J Hepatol. 1995;22:101–110. doi: 10.1016/0168-8278(95)80267-3. [DOI] [PubMed] [Google Scholar]
- 76.Kügler C.F., Petter J., Taghavy A. Dynamics of cognitive brain dysfunction in patients with cirrhotic liver disease: an event-related P300 potential perspective. Electroencephalogr Clin Neurophysiol. 1994;91:33–41. doi: 10.1016/0013-4694(94)90016-7. [DOI] [PubMed] [Google Scholar]
- 77.Saxena N., Bhatia M., Joshi Y.K., Garg P.K., Tandon R.K. Auditory P300 event-related potentials and number connection test for evaluation of subclinical hepatic encephalopathy in patients with cirrhosis of the liver: a follow-up study. J Gastroenterol Hepatol. 2001;16:322–327. doi: 10.1046/j.1440-1746.2001.02388.x. [DOI] [PubMed] [Google Scholar]
- 78.Saxena N., Bhatia M., Joshi Y.K., Garg P.K., Dwivedi S.N., Tandon R.K. Electrophysiological and neuropsychological tests for the diagnosis of subclinical hepatic encephalopathy and prediction of overt encephalopathy. Liver. 2002;22:190–197. doi: 10.1034/j.1600-0676.2002.01431.x. [DOI] [PubMed] [Google Scholar]
- 79.Schiff S., Valenti P., Andrea P. The effect of aging on auditory components of event-related brain potentials. Clin Neurophysiol. 2008;119:1795–1802. doi: 10.1016/j.clinph.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 80.D'Avanzo C., Schiff S., Amodio P., Sparacino G.A. Bayesian method to estimate single-trial event-related potentials with application to the study of the P300 variability. J Neurosci Methods. 2011;198:114–124. doi: 10.1016/j.jneumeth.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 81.Lauridsen M.M., Gronbaek H., Naeser E.B., Leth S.T., Vilstrup H. Gender and age effects on the continuous reaction times method in volunteers and patients with cirrhosis. Metab Brain Dis. 2012;27:559–565. doi: 10.1007/s11011-012-9318-6. [DOI] [PubMed] [Google Scholar]
- 82.Guerit J.M., Amantini A., Fischer C. Neurophysiological investigations of hepatic encephalopathy: ISHEN practice guidelines. Liver Int. 2009;29:789–796. doi: 10.1111/j.1478-3231.2009.02030.x. [DOI] [PubMed] [Google Scholar]
- 83.Felipo V., Ordono J.F., Urios A. Patients with minimal hepatic encephalopathy show impaired mismatch negativity correlating with reduced performance in attention tests. Hepatology. 2012;55:530–539. doi: 10.1002/hep.24704. [DOI] [PubMed] [Google Scholar]
- 84.Kulisevsky J., Conill J., Avila A., Pujol J., Balanzo J., Capdevila A. Abnormalities of the Bereitschaftspotential and MRI pallidal signal in non-encephalopathic cirrhotic patients. Electroencephalogr Clin Neurophysiol. 1995;94:425–431. doi: 10.1016/0013-4694(94)00331-e. [DOI] [PubMed] [Google Scholar]
- 85.Polich J. Meta-analysis of P300 normative aging studies. Psychophysiology. 1996;33:334–353. doi: 10.1111/j.1469-8986.1996.tb01058.x. [DOI] [PubMed] [Google Scholar]
- 86.Schiff S., Vallesi A., Mapelli D. Impairment of response inhibition precedes motor alteration in the early stage of liver cirrhosis: a behavioral and electrophysiological study. Metab Brain Dis. 2005;20:381–392. doi: 10.1007/s11011-005-7922-4. [DOI] [PubMed] [Google Scholar]
- 87.Cona G., Montagnese S., Bisiacchi P.S. Early markers of neural dysfunction and compensation: a model from minimal hepatic encephalopathy. Clin Neurophysiol. 2014;125(6):1138–1144. doi: 10.1016/j.clinph.2013.10.048. [DOI] [PubMed] [Google Scholar]


