Abstract
Autotaxin (ATX) is a secreted enzyme, which produces extracellular lysophosphatidate (LPA) from lysophosphatidylcholine (LPC). LPA activates six G protein-coupled receptors and this is essential for vasculogenesis during embryonic development. ATX is also involved in wound healing and inflammation, and in tumor growth, metastasis, and chemo-resistance. It is, therefore, important to understand how ATX is regulated. It was proposed that ATX activity is inhibited by its product LPA, or a related lipid called sphingosine 1-phosphate (S1P). We now show that this apparent inhibition is ineffective at the high concentrations of LPC that occur in vivo. Instead, feedback regulation by LPA and S1P is mediated by inhibition of ATX expression resulting from phosphatidylinositol-3-kinase activation. Inhibiting ATX activity in mice with ONO-8430506 severely decreased plasma LPA concentrations and increased ATX mRNA in adipose tissue, which is a major site of ATX production. Consequently, the amount of inhibitor-bound ATX protein in the plasma increased. We, therefore, demonstrate the concept that accumulation of LPA in the circulation decreases ATX production. However, this feedback regulation can be overcome by the inflammatory cytokines, TNF-α or interleukin 1β. This enables high LPA and ATX levels to coexist in inflammatory conditions. The results are discussed in terms of ATX regulation in wound healing and cancer.
Keywords: autotaxin inhibition, fluorescent substrate-3 assay, lysophosphatidylcholine, inflammation, cytokines
Autotaxin (ATX) is a secreted enzyme, which converts extracellular lysophosphatidylcholine (LPC) into lysophosphatidate (LPA). This is an important reaction in cell signaling because LPA activates at least six G protein-coupled receptors to increase cell division, survival, and migration (1, 2). ATX serves an essential function in embryonic development because ATX knockout mice die in utero at day 9.5 with defects in vasculogenesis and the development of the neural crest (3–6). In the postnatal organism, the major function of ATX appears to be in wound healing. ATX is produced in response to inflammation to mediate wound repair. If the inflammation is not resolved, then high ATX concentrations persist in association with inflammatory diseases such as arthritis and inflammatory bowel disease (1, 7, 8). In addition, inflammatory bowel disease and hepatitis can progress to carcinogenesis in these organs (1). Significantly, increased ATX activity is associated with the growth of breast tumors (9, 10) and the ATX gene is among the top 40 most upregulated in metastatic cancer (11). Blocking LPA production by inhibiting ATX activity with ONO-8430506 decreases the first phase of breast tumor growth and subsequent metastasis by about 60% in mice (9, 12). Conversely, increasing the low lipid phosphate phosphatase (LPP)1 activity in cancer cells so as to increase LPA turnover in the tumor and attenuate LPA signaling decreased breast tumor growth and metastasis in mice by about 80% (13). These studies emphasize the importance of ATX in controlling cell signaling in several physiological and pathological conditions. Consequently, it is important to understand how ATX activity and signaling by LPA are regulated. The present work focuses on the role of ATX and how its expression is controlled.
LPA turns over with a half-life of about 3 min in the circulation (14, 15). Steady-state LPA concentrations are regulated partly by the balance of ATX activity versus LPA degradation by the ecto-activities of a family of three LPPs (16, 17). Consequently, knocking down LPP1 expression in mice increased the half-life on LPA (14). One hypothesis for the regulation of the concentrations of extracellular LPA is that ATX activity is controlled by a product feedback inhibition from LPA or its sphingolipid analog, sphingosine 1-phosphate (S1P). This work is based mainly on studies where ATX activity was measured with very low concentrations (1–5 μM) of fluorescent LPC-analog substrates (18, 19). This technique was also used to identify LPA analogs as possible ATX inhibitors (20–23).
We questioned whether the inhibition of ATX with LPA and S1P could have physiological relevance because these inhibitions are competitive with the substrate concentration (1). Circulating LPC concentrations in mice and human beings are in the range of 200–400 μM (2, 24). These concentrations are very high compared with those of fluorescent substrates used in the ATX assay (1, 25). Our work demonstrates that LPA and S1P produce very little inhibition of ATX activity in assays where the substrate for ATX is raised to the physiological concentrations. However, we did show that ATX activity is regulated by feedback from LPA and S1P, but that this depends on the production of ATX in cancer cells and adipose tissue. This feedback regulation controls the mRNA concentrations for ATX and ATX secretion rather than a direct effect on ATX activity. This level of feedback regulation is overcome by inflammatory cytokines, which explains why ATX activity and LPA concentrations can be increased in inflammation and cancer.
MATERIALS AND METHODS
Reagents
C18:1-LPA, C18:1-LPC, and VPC23019 were purchased from Avanti Polar Lipids (Alabaster, AL). Fluorescent substrate-3 (FS-3) fluorogenic ATX substrate (L-2000) was purchased from Echelon (Salt Lake City, UT). FBS was from Gibco/Life Technologies (Burlington, ON, Canada). FBS was charcoal-treated (FBSC) to remove lipids by adding activated charcoal washed twice with phosphate-buffered saline (2 g per 50 ml of FBS) and then rotated overnight at 4°C. The mixture was then centrifuged and the supernatant was filtered twice through 0.22 μm sterile filters. Ki16425, SEW2871, and human recombinant ATX were from Cayman Chemical (Ann Arbor, MI). LY294002, wortmannin, PD98059, and Gö6983 were purchased from Calbiochem/EMD Millipore (Etobicoke, ON, Canada). Wls-31 was a gift from Dr. W. Santos and Mr. N. Patwardhan (Department of Chemistry, Virginia Polytechnic Institute, VA) (26, 27). TNF-α and interleukin 1β (IL-1β) were from PeproTech (Rocky Hill, NJ). ONO-8430506 was obtained from Ono Pharmaceuticals (Osaka, Japan; patent number WO2012005227) (28, 29) under a materials transfer agreement. S32826 was from Institut de Recherches Servier (Croissy-sur-Seine, France) (30). Anti-ATX antibody was a gift from Dr. T. Clair (National Cancer Institute, Bethesda, MD) (31). Other reagents were from Sigma-Aldrich (Oakville, ON, Canada) unless otherwise indicated.
Cell culture
SW-579 (HTB-107™) thyroid cancer cells and MDA-MB-435S (HTB-129™) melanoma cells were purchased from ATCC (Manassas, VA), and cultured in RPMI 1640 medium supplemented with 10% FBS and 1% penicillin/streptomycin at 37°C, 5% CO2, and 95% humidity. Cells were used at low passage number. For all experiments, cells were seeded in 6-well plates and grown until 80% confluent. Cells were then washed with HEPES buffer saline, and 2 ml RMPI 1640 medium containing either 10% FBS or FBSC with reagents was added for 24 h for RNA experiments or 48 h for ATX ELISA experiments. In some experiments, cells were pretreated in 10% FBS with 1 μM wortmannin, 10 μM LY294002, 10 μM Gö6983, or 20 μM PD98059 for 6 h before addition of LPA or S1P to fresh medium with inhibitors. In experiments with 100 μM LPC, medium was supplemented with 1% BSA. TNF-α and IL-1β were used at a final concentration of 10 ng/ml.
ATX activity assays
For FS-3 assay measurements, FS-3 substrate was prepared at 1.25 times the final working concentration in FS-3 assay buffer [140 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 50 mM Tris-HCl (pH 8.0), and 1 mg/ml BSA]. LPA, S1P, and recombinant ATX were prepared at 10 times the final working concentration in FS-3 assay buffer. FS-3 substrate (80 μl) was added to 10 μl of ATX protein and 10 μl of either C18:1-LPA, S1P, C18:1-LPC, or FS-3 assay buffer for a final volume of 100 μl. Final ATX protein concentrations of 75, 100, and 125 ng/ml were used to demonstrate that the activity results were directly proportional to enzyme quantity. Measurements were taken at 5 min intervals for 4 h at 37°C and the kinetics analyzed as previously described (32). For ATX inhibition experiments, 0.25–10 μl of mouse plasma, FBS, or FBSC was added to 10 μl of 100 nM of ONO-8430506 (final concentration 10 nM) and 80 μl of 6.25 μM FS-3 substrate (final concentration 5 μM). To account for slight dilutions of FBSC during the delipidation process, initial rates were normalized to total serum protein as measured by the BCA protein assay (Thermo Fisher Scientific, Rockford, IL).
For the choline release method, 10 μl of ATX protein was incubated with 25 μl of 400 μM C18:1-LPC and 15 μl of C18:1-LPA or S1P or choline-assay buffer for a total volume of 50 μl for 4 h at 37°C. All assay conditions and buffers have been previously described (9). Final ATX concentrations of 750, 1,000, and 1,250 ng/ml were used to demonstrate that the activity results were directly proportional to protein. ATX activity in mouse plasma was also assessed using 3 mM C18:1-LPC in the choline-release assay as previously described (9).
Animal studies and measurement of LPA and S1P concentrations
Female Balb/c mice 8–10 weeks old (BALB/cAnNCrl, Strain Code 028) were from Charles River (Kingston, ON, Canada) and were housed and maintained as previously described (9). All procedures were performed in accordance with the Canadian Council of Animal Care as approved by the University of Alberta Animal Welfare Committee. ONO-8430506 was prepared at 10 mg/ml in 25 mM sodium hydroxide and heated to 60°C for 5 min to dissolve (9, 29). The solution was passed through a 0.22 μm filter prior to administration. Mice were then gavaged with water or 100 mg/kg ONO-8430506 (10 μl/g body weight) once a day for 4 days. Mice were euthanized at 6 h after the last treatment and tissues were processed as previously described (9). For tumor experiments, mice were either sham injected or injected with 20,000 4T1 Balb/c breast cancer cells into the fourth inguinal fat pad and were euthanized 21 days later as previously described (9). Plasma concentrations of LPA and S1P were measured by mass spectrometry as previously described (9).
Quantitative real-time PCR, Western blotting, and ELISA
Total RNA was extracted from cell lysates using the RNAquesous® kit (Ambion, Streetville, ON, Canada) or from mouse adipose tissue homogenized in Trizol® (Invitrogen Life Technologies, Carlsbad, CA) using the Direct-zol™ RNA MiniPrep kit (R2052; Zymo Research, Irvine, CA) according the manufacturer’s instructions. Extracted RNA was treated with DNAase and reverse transcription and quantitative RT-PCR was then performed (32). Gene expression was normalized to the housekeeping genes cyclophilin A (PPIA) or GAPDH. Relative changes in expression of ATX (ENPP2) mRNA were essentially the same with both reference mRNAs and results are routinely expressed relative to GAPDH mRNA. Primers purchased from Integrated DNA Technologies (Coralville, IA) that recognize both human and mouse transcripts were ATX (ENPP2): sense, 5′-CAT TTA TTG GTG GAA CGC AGA-3′ and antisense, 5′-CTA CAA AAA CAG TCT GCA TGC-3′ and GAPDH: sense, 5′-ACT TTG TCA AGC TCA TTT CC-3′ and antisense, 5′-TCT TAC TCC TTG GAG GCC AT-3′. ATX protein in mouse plasma was analyzed by Western blotting as previously described and quantified by ImageJ analysis (9). ATX protein concentration was quantified from 100 μl of centrifuged cell culture conditioned medium using the ATX sandwich ELISA kit (K-5600) from Echelon according to the manufacturer’s instructions. Secreted ATX protein was then normalized to both the incubation volume of conditioned medium and protein content from cell lysates by the BCA protein assay. Mouse plasma TNF-α concentrations were measured by Eve Technologies Corporation (Calgary, AB, Canada) using the Luminex™ 100 system (Luminex, Austin, TX).
Statistical analysis
Results are expressed as the mean ± SEM from at least three independent experiments. The two-tailed Student’s t-test for comparing two groups or the one-way ANOVA with a Bonferonni post hoc test for three or more groups were used to test for significance. P < 0.05 was used for significance. Statistics were calculated and results plotted using Origin Pro 9.1 (OriginLab Corporation, Northampton, MA).
RESULTS
LPA and S1P have little inhibitory effect on ATX activity at physiological concentrations of substrate concentrations
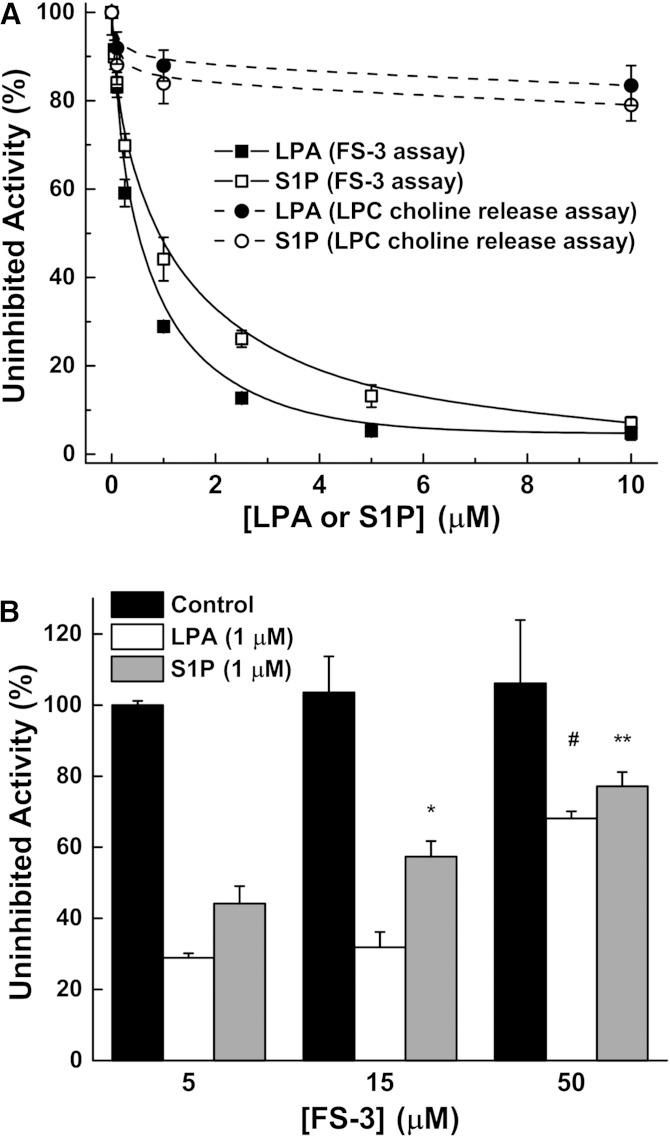
The hypothesis that LPA and S1P exert feedback regulation on ATX activity was developed mainly by using FS-3, which is an analog of LPC that yields a fluorescent product when cleaved by ATX (19, 33). This compound is relatively expensive and assays are normally performed at low micromolar concentrations of FS-3 (Km 4.5–6.3 μM) (19, 33). We used 5 μM FS-3 in the assay with recombinant ATX and demonstrated that the reaction was inhibited by increasing LPA and S1P concentrations (Fig. 1A). These inhibitions were progressively decreased when the concentration of FS-3 was increased to 15 and 50 μM (Fig. 1B) as expected from the mixed-type nature of the inhibition previously described (18, 19). C18:1-LPA and S1P had only marginal effects on ATX activity when this was measured using a choline release assay and a physiological concentration of 200 μM LPC as a substrate (Fig. 1A).
Fig. 1.
LPA and S1P are poor inhibitors of ATX activity at physiological concentrations of substrate. A: LPA and S1P potently inhibit ATX activity (ATX at 100 ng/ml concentration) when incubated with 5 μM FS-3 in the fluorogenic assay. However, this inhibition does not occur in the presence of 200 μM LPC in the choline-release assay (ATX at 1,000 ng/ml concentration). B: Increasing the FS-3 concentration decreases the inhibition effect of 1 μM LPA or S1P on ATX activity. Results are mean ± SEM from three independent experiments. *A significant increase (P < 0.05) in activity in the presence of S1P with 15 μM FS-3 compared with 5 μM FS-3. **A significant increase (P < 0.05) in activity in the presence of S1P at 50 μM FS-3 compared with 5 or 15 μM FS-3. #A significant increase (P < 0.05) in activity in the presence of LPA at 50 μM FS-3 compared with 5 or 10 μM FS-3.
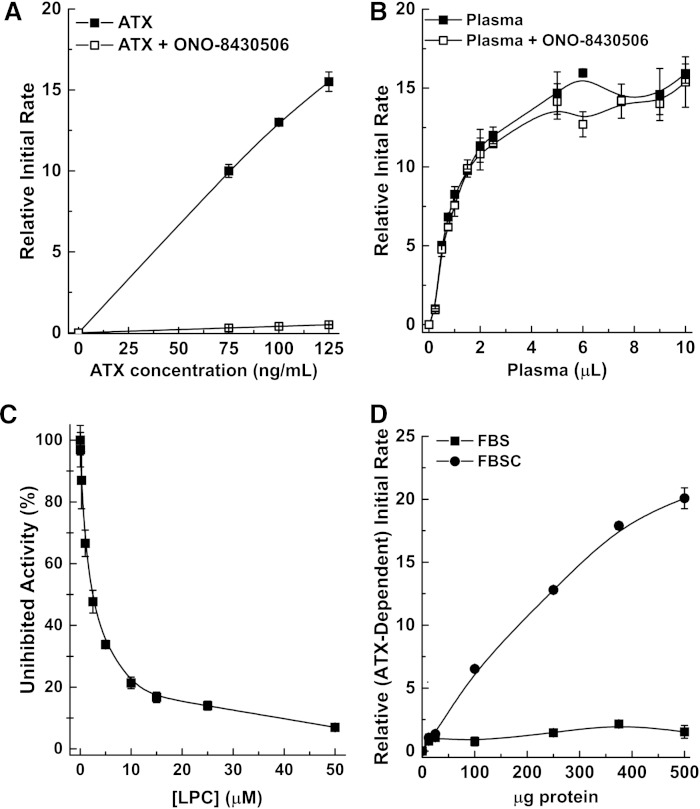
We also demonstrated that the FS-3 assay does not provide accurate measurements of ATX activity in biological samples if lysophospholipids are present. First, we defined ATX activity as the component of the FS-3 measurement that is suppressed by the ATX inhibitor ONO-8430506, as we did previously for the choline release assay (9). Using recombinant ATX, 10 nM ONO-8430506 suppressed >97% of the total measured activity (Fig. 2A). However, in plasma, the apparent activity measurements were not suppressed by ONO-8430506, and therefore cannot be attributable to ATX (Fig. 2B). Plasma and serum also contain >200 μM LPC in addition to LPA and S1P. LPC potently suppressed recombinant ATX activity measurements in the FS-3 assay (IC50 = 2.5 μM) (Fig. 2C). We, therefore, delipidated FBS to see whether this would eliminate the inhibition by lysophospholipids and this increased the ONO-8430506-inhibited activity by nearly 20-fold (Fig. 2D, supplementary Fig. 1).
Fig. 2.
ATX activity cannot be measured by the FS-3 assay in biological samples if lysophospholipids are present. A: Recombinant ATX (75, 100, and 125 ng/ml) was incubated with or without 10 nM of ONO-8430506 using 5 μM of FS-3. ONO-8430506 suppressed >97% of the total activity measured in the assay. B: The same experiment in (A) was conducted using 0.25–10 μl of plasma from three different mice (final assay volume of 100 μl). ONO-8430506 could not suppress the measured activity and is therefore not from ATX activity. C: LPC potently inhibits ATX activity (ATX at 100 ng/ml concentration) when incubated with FS-3. D: Removing lipids from FBS reveals a component of the measured total activity that can be suppressed by ONO-8430506. These results are obtained from the subtraction of the total measured activity from the activity remaining after adding 10 nM ONO-8430506 (see supplementary Fig. 1). All results are mean ± SEM from three independent experiments.
LPA and S1P cause feedback regulation on ATX expression in cultured thyroid cancer and melanoma cells
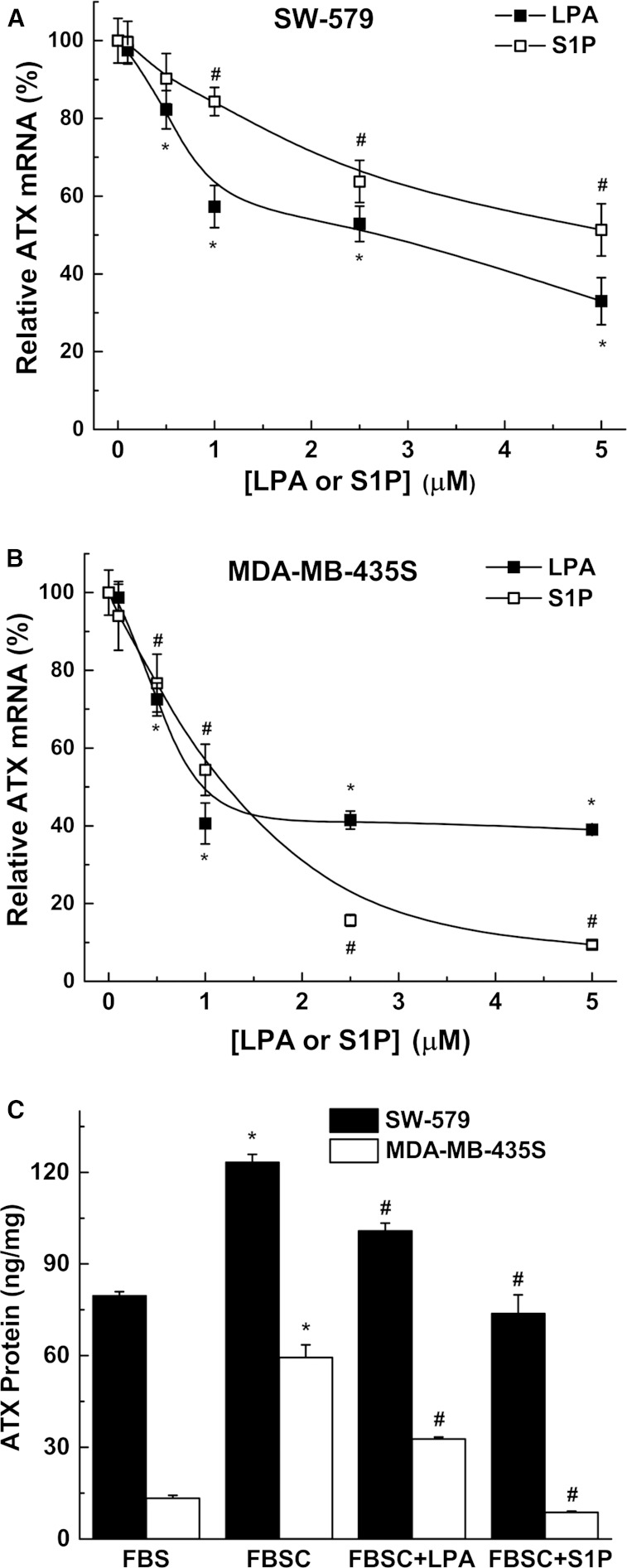
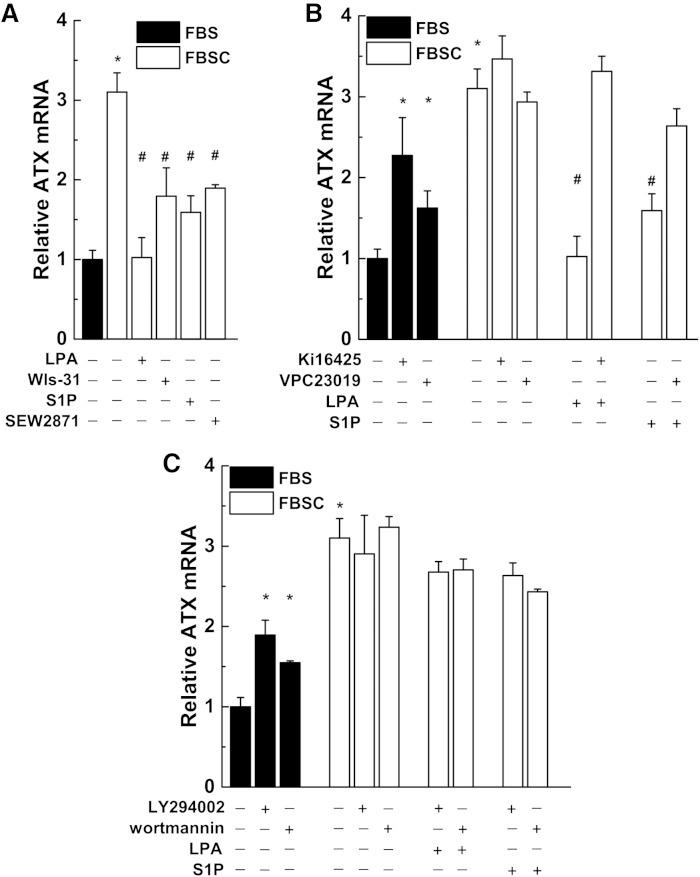
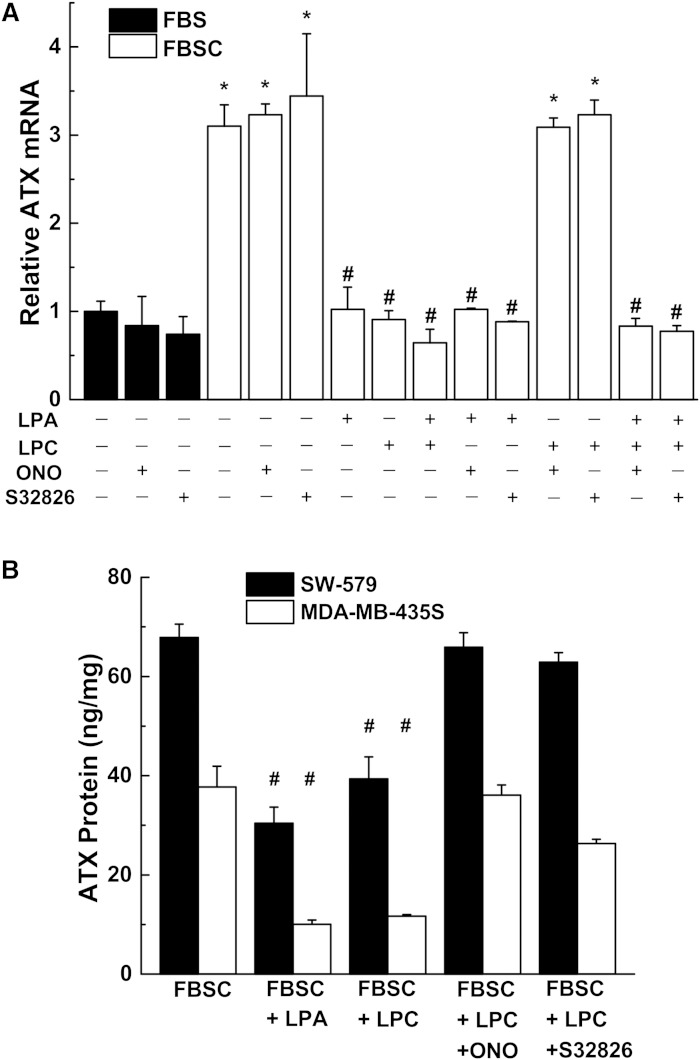
The concept that LPA exerts a feedback regulation on ATX is an attractive hypothesis and we tested whether this occurred at the level of ATX synthesis. We, therefore, used SW-579 thyroid cancer cells and MDA-MB-435S melanoma cells, which express very high amounts of ATX, to test this hypothesis. Addition of LPA or S1P to medium containing delipidated serum (FBSC) decreased ATX mRNA expression (Fig. 3A, B). We also verified this effect of LPA and S1P on ATX protein secretion by ELISA measurements (Fig. 3C). Incubation with medium containing delipidated serum increased mRNA concentrations for ATX compared with incubation in normal serum (Fig. 4A, supplementary Fig. 2). ATX mRNA expression was suppressed by adding 5 μM LPA or S1P to the delipidated medium and these effects were reversed by 1 μM Ki16425, a LPA receptor types 1/3 (LPA1/3) antagonist (34), and by 1 μM VPC23019, a S1P receptor types 1/3 (S1P1/3) antagonist (35), respectively (Fig. 4B, supplementary Fig. 2). Decreases in ATX mRNA expression were also obtained when the delipidated medium was supplemented with 1 μM wls-31 (26), a LPA1/2 receptor agonist, or SEW2871, a S1P1 receptor agonist (36) (Fig. 4A, supplementary Fig. 2). These results establish that LPA and S1P signaling negatively regulate the expression of ATX.
Fig. 3.
LPA and S1P decrease ATX mRNA expression and secretion of ATX. SW-579 (A) and MDA-MB-435S (B) cells were incubated for 24 h in 10% FBSC medium with increasing concentrations of either LPA or S1P. Significant reduction (P < 0.05) in ATX mRNA expression with LPA (*) and with S1P (#) compared with no treatment. C: SW-579 and MDA-MB-435S cells were incubated for 48 h in medium containing FBS or FBSC with or without 5 μM LPA or S1P. Secreted ATX protein was quantified by ELISA and normalized to both volume of conditioned medium and cell lysate protein content. ATX protein from basal medium was subtracted from the total ATX protein measured after incubation. Results are mean ± SEM from three independent experiments. *A significant increase (P < 0.05) in ATX protein concentration in FBSC medium compared with FBS medium. #A significant decrease (P < 0.05) in ATX protein following LPA or S1P treatment in FBSC compared with FBSC alone.
Fig. 4.
LPA and S1P inhibit ATX mRNA expression through LPA and S1P receptor-mediated activation of phosphatidylinositol 3-kinase. A: SW-579 cells were incubated in 10% FBS or FBSC in the presence of 5 μM LPA or S1P, or 1 μM of the LPA receptor agonist wls-31 or S1P receptor agonist SEW2871. B: ATX mRNA expression increased in cells incubated in FBS when treated with 1 μM of the LPA receptor antagonist Ki16425 or SIP receptor antagonist VPC23019. These antagonists had no effect on ATX mRNA levels in cells incubated in FBSC alone. C: ATX mRNA expression increased in cells incubated in FBS when treated with phosphatidylinositol 3-kinase inhibitors (10 μM LY294402 or 1 μM wortmannin). These inhibitors had no effect on ATX mRNA levels in cells in FBSC alone. Results are mean ± SEM from three independent experiments. *A significant increase (P < 0.05) in ATX mRNA compared with FBS treatment. #A significant decrease (P < 0.05) in ATX mRNA compared with FBSC treatment.
The effects of LPA and S1P in decreasing mRNA for ATX were blocked by two inhibitors of phosphatidylinositol 3-kinase, 10 μM LY294002, and 1 μM wortmannin (Fig. 4C, supplementary Fig. 2). Inhibiting ERK activation or protein kinase C with 20 μM PD98059 or 10 μM Gö6983, respectively, had no significant effect on LPA- or S1P-induced decreases in ATX mRNA (results not shown).
We also determined whether the secretion of ATX by SW-579 thyroid cancer cells and MDA-MB-435S melanoma cells would establish a negative feedback regulatory loop on further ATX production. Cells were incubated in medium with delipidated serum to maximize the concentrations of mRNA for ATX and treated with 100 μM LPC. This decreased ATX mRNA concentrations, an effect that was reversed by inhibiting ATX activity with either ONO-8430506 or S32826 (Fig. 5A, supplementary Fig. 3). As controls, we demonstrated that these inhibitors had no significant effects on ATX mRNA when added in the absence of LPC, or in the presence of LPA (Fig. 5A, supplementary Fig. 3). The suppression of mRNA concentrations for ATX by LPA and LPC were paralleled by decreased secretion of ATX from the cells (Fig. 5B). Inhibiting ATX activity with ONO-8430506 or S32826 reversed these effects of LPC.
Fig. 5.
LPC-mediated inhibition of ATX mRNA expression is blocked by ATX inhibitors. A: SW-579 cells were incubated in 10% FBS or FBSC supplemented with 1% BSA in the presence of 5 μM LPA and/or 100 μM LPC with 10 μM of the ATX inhibitors ONO-8430506 (ONO) or S32826. B: SW-579 and MDA-MB-435S cells were incubated for 48 h in medium under the same conditions as (A). Secreted ATX protein was quantified by ELISA and normalized to both volume of conditioned medium and cell lysate protein content. ATX protein from basal medium was subtracted from the total ATX protein measured after incubation. Results are mean ± SEM from three independent experiments. *A significant increase (P < 0.05) compared with FBS treatment. #A significant decrease (P < 0.05) compared with FBSC treatment.
Inhibition of ATX in vivo increases adipose tissue ATX expression and plasma ATX concentrations
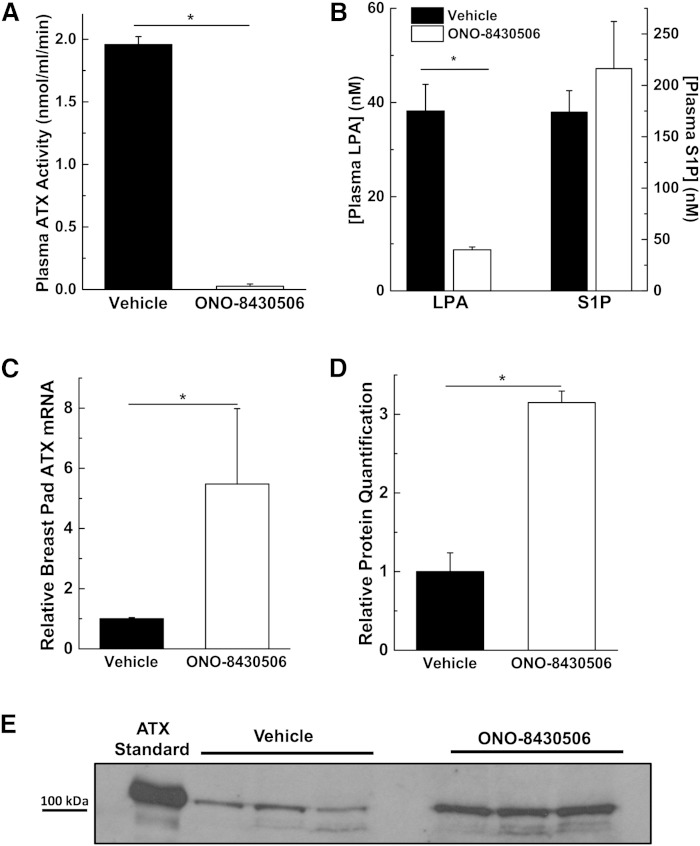
The role of ATX in regulating its own expression was also investigated in vivo by treating mice for 4 days with 100 mg/kg ONO-8430506. This inhibitor decreased the activity of ATX as measured in the plasma using 3 mM C18:1-LPC (9) by about 95% and this was accompanied by a decrease of about 80% in measured LPA molecular species concentrations (Fig. 6A, B). S1P concentrations were not affected by ONO-8430506 (Fig. 6B), as expected from previous work (9). Adipose tissue is a major source of ATX in the circulation (37–40), and therefore, we measured the expression of ATX mRNA in the mammary fat pads of the female mice. ATX mRNA was increased by about 5.5-fold in the ONO-8430506-treated mice and this was accompanied by 3-fold increase in the concentration of ATX protein in the plasma (Fig. 6C–E). We conclude from these results that the expression and secretion of ATX from adipose tissue is suppressed by plasma LPA concentrations. Conversely, decreasing plasma LPA concentrations increases ATX secretion by adipose tissue resulting in more ATX in the plasma.
Fig. 6.
Inhibition of ATX activity in mice increases ATX mRNA expression in adipose tissue and ATX protein concentration in plasma. Female mice were treated with 100 mg/kg ONO-8430506 once a day for 4 days and samples were taken at 6 h after the last treatment. Plasma ATX activity (A) and plasma concentrations (B) of LPA (sum of C16:0, C18:0, C18:1, and C20:4 molecular species) and S1P levels compared with vehicle-treated mice. ATX mRNA concentrations in breast adipose tissue (C) and plasma ATX protein (D, E) of mice treated with ONO-8430506 compared with vehicle-treated mice. ATX standard is from concentrated MDA-MB-435S conditioned medium. *A significant change in ONO-8430506-treated mice compared with vehicle-treated mice; n = 3–5 mice per group.
Both ATX and LPA levels increase in inflammatory conditions such as cancer
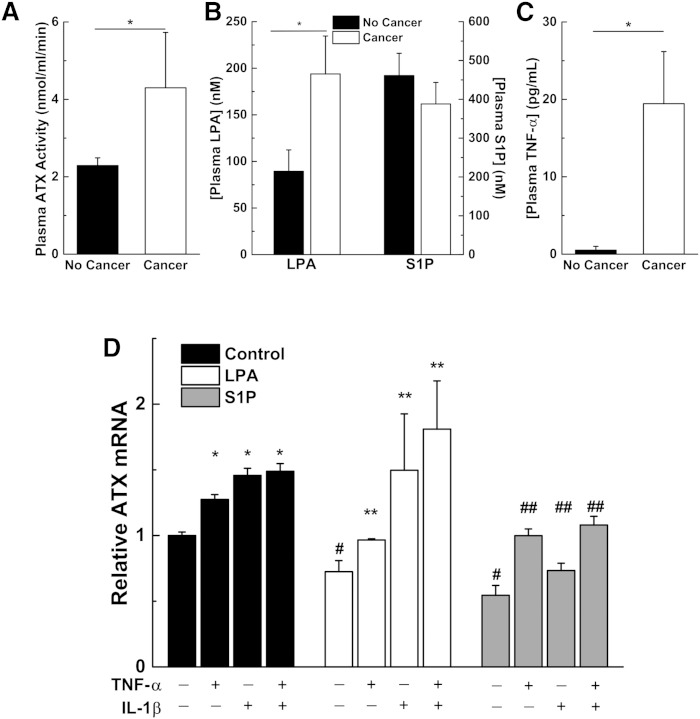
We also investigated the relationship between ATX and LPA levels in inflammatory conditions such as cancer. Consistent with previous results (9), Balb/c mice with 4T1 breast tumors at day 21 have significantly elevated plasma ATX levels (Fig. 7A). These mice also have nearly twice the plasma LPA concentrations of control mice without cancer (Fig. 7B). S1P concentrations were not affected (Fig. 7C). The mice also have nearly 40 times higher plasma levels of the pro-inflammatory cytokine, TNF-α (Fig. 7C). From these observations, we proposed that inflammatory cytokines counteract the inhibition of ATX mRNA expression by LPA and S1P. Stimulation of SW-579 cells with TNF-α or IL-1β alone or in combination increased ATX mRNA concentrations. TNF-α or IL-1β also overcame the inhibition on ATX mRNA expression by either 1 μM LPA or S1P (Fig. 7D).
Fig. 7.
Mice with cancer have higher plasma ATX activity, LPA concentrations, and TNF-α concentrations, and pro-inflammatory cytokines can overcome LPA- and S1P-mediated inhibition of ATX mRNA expression. Female Balb/c mice were either sham injected (No Cancer) or injected with 20,000 4T1 Balb/c breast cancer cells (Cancer) and plasma was collected 21 days later. Plasma ATX activity (A), plasma concentrations of LPA (sum of C16:0, C18:0, C18:1, and C20:4 molecular species) and S1P levels (B), and plasma TNF-α compared with mice with no cancer (C). *A significant increase in mice with cancer compared with mice without cancer (n = 5–8 mice per group). D: SW-579 cells were incubated in 10% FBSC for 24 h in the presence of 1 μM LPA or S1P and 10 ng/ml of the pro-inflammatory cytokines TNF-α and/or IL-β, as indicated. Results are mean ± SEM from three independent experiments. *A significant increase in ATX mRNA for cells treated with cytokines compared with no treatment; #LPA and S1P significantly suppress ATX mRNA expression compared with no treatment; **a significant increase in ATX mRNA for cells treated with both LPA and cytokines compared with LPA treatment alone; ##a significant increase in ATX mRNA for cells treated with both S1P and cytokines compared with S1P treatment alone.
DISCUSSION
We concluded from our results that the product inhibition of ATX by LPA and S1P is an artifact of the FS-3 assay and that it is unlikely to have physiological relevance. This conclusion also has implications for identifying ATX inhibitors because low concentrations of fluorescent probes are often used to screen libraries of compounds or test the efficacy of derivatives (41–43). While these probes may be efficient for screening potential compounds, positive hits for ATX inhibition or derivative analogs should be verified in the presence of physiological concentrations of LPC, as used in the choline release assay. Physiologically relevant LPC concentrations have been used to validate the in vitro efficacy of many such inhibitors (15, 20, 21, 29, 44).
FBS has been used as a source of ATX to test the efficacy of novel ATX inhibitors in the FS-3 assay (45, 46), but our work now shows this assay detects little true ATX activity unless lysophospholipids are removed from the serum. Similar problems are likely to occur if fluorescent probes are used to measured ATX activity in concentrated conditioned medium from cell culture (32, 47, 48). This can result from the production of lysophospholipids by the cells or the addition of FBS to the medium. We demonstrate that the presence of very low concentrations of serum can inhibit the assays because low micromolar concentrations of LPC, LPA, and S1P can significantly inhibit ATX activity as measured with fluorescent substrates. Therefore, if serum has to be used in these cultures to maintain cell viability, it should be delipidated.
We also conclude from our work that activation of phosphatidylinositol 3-kinase through LPA1 and S1P1 receptors decreases the expression of mRNA for ATX. LPA generated from LPC by ATX, therefore, acts as a feedback suppressor of ATX mRNA production. This feedback effect can be blocked in vivo by inhibiting ATX activity and lowering LPA concentrations. The latter work demonstrates the physiological importance of the secreted ATX producing LPA from LPC in this feedback regulation. To our knowledge, no other studies have looked directly at LPA and S1P effects on ATX expression. ATX mRNA expression levels were not significantly changed in other cell types treated with LPA or S1P, as determined by gene arrays on cancer cells, smooth muscle cells, mouse embryonic fibroblasts, and human embryonic stem cells (49–53). However, some of these studies look at cells cultured in 10–20% FBS and all these studies were performed after ≤6 h LPA or S1P treatment. Our results show that the amount of LPA and S1P present in 10% FBS is sufficient to significantly suppress ATX mRNA expression, and the addition of more LPA or S1P is unlikely to cause a further suppression. Also, we observed maximum effects of LPA or S1P on ATX mRNA expression at 24 h in cells cultured in delipidated serum. We recommend that future studies on the role of LPA or S1P in regulating ATX mRNA expression should be performed in delipidated serum using significantly longer time scales.
Inhibition of ATX activity in vivo, and the consequent decrease in LPA, increased ATX production in adipose tissue resulting in a higher concentration of ATX protein in the plasma. However, the ATX inhibitor, ONO-8430506, keeps this ATX catalytically inactive, as verified by the marked decrease in LPA concentrations. This highly potent and selective ATX (ENPP2) inhibitor does not inhibit the activity of other ENPP family members or affect ligand binding to sixty tested pharmacological drug targets (29). The mechanism by which ATX would normally regulate its own secretion by increasing LPA concentrations is typical of a physiological feedback loop. It is, therefore, fully expected that a potent ATX inhibitor would increase ATX expression. In fact, such an increase can serve as a surrogate measure of the effectiveness of the inhibitor. In this feedback model, S1P concentrations were not changed by ATX inhibition. This finding is not surprising as ATX (+/−) mice have half the levels of plasma LPA compared with normal mice, but S1P levels are not affected (3, 54). This means that ATX is not a major regulator of S1P concentrations in vivo. However, our animal studies do not exclude S1P as being a physiological regulator of ATX expression in vivo, and further studies are warranted. We also demonstrated that the effects of removing LPA from serum are readily reversible and that increasing LPA concentrations decreased the elevated ATX expression.
This work also provides novel evidence concerning the regulation of ATX expression during wound healing, inflammation, and cancer. We detected significant increases in both plasma ATX activity and LPA concentrations in Balb/c mice with advanced 4T1 breast tumors together with increased plasma TNF-α levels. We established that addition of the pro-inflammatory cytokines, TNF-α and/or IL-1β, overcome LPA-mediated suppression of ATX expression. We also demonstrate in unpublished observations that ATX expression can be increased to similar levels by 10 other inflammatory chemokines and cytokines. These results indicate that cytokine signaling is extremely redundant with respect to the regulation of ATX expression and that loss of an individual cytokine is unlikely to produce a significant effect.
Our results from cell culture are compatible with the conclusion that the production of inflammatory cytokines, such as TNF-α and IL-1β, in damaged and inflamed tissue is a signal for increased ATX expression and the need for LPA production to heal the wound (1, 55). If this process is successful and inflammation subsides, then LPA produced by ATX feeds back and blocks further ATX production. However, if inflammation is unresolved, then TNF-α and IL-1β stimulate further ATX production and the consequent formation of LPA stimulates further cytokine production in a vicious cycle (1). This situation particularly applies to cancer, which is often described “as a wound that does not heal” (56–58). Breaking the inflammatory cycle with an ATX inhibitor decreases the production of both LPA and inflammatory cytokines, which decreases tumor progression, metastasis and the production of chemo-resistance (1, 2, 9, 12). This occurs even though ATX production is increased because the ATX remains catalytically inactive.
Supplementary Material
Footnotes
Abbreviations:
- ATX
- autotaxin
- FBSC
- charcoal-treated FBS
- FS-3
- fluorescent substrate-3
- IL-1β
- interleukin 1β
- LPA
- lysophosphatidate
- LPA1–6
- lysophosphatidate receptor types 1–6
- LPC
- lysophosphatidylcholine
- LPP
- lipid phosphate phosphatase
- S1P
- sphingosine 1-phosphate
- S1P1–5
- sphingosine 1-phosphate receptor types 1–5
M.G.K.B. is the recipient of a Vanier Canada Graduate Scholarship from the Government of Canada, a Canadian Institutes of Health Research MD/PhD Studentship, a MD/PhD Studentship from Alberta Innovates-Health Solutions, and a Killam Trust Award. This work was also supported by grants from the Canadian Breast Cancer Foundation, Canadian Institutes of Health Research with the Women and Children’s Health Research Institute at the University of Alberta, and Ono Pharmaceuticals Ltd. The following conflicts of interest are disclosed: D.N.B. and T.P.W.M. have received consulting fees from Ono Pharmaceuticals Ltd. The other authors declare no conflicts of interest.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of three figures.
REFERENCES
- 1.Benesch M. G. K., Ko Y. M., McMullen T. P. W., Brindley D. N. 2014. Autotaxin in the crosshairs: Taking aim at cancer and other inflammatory conditions. FEBS Lett. 588: 2712–2727. [DOI] [PubMed] [Google Scholar]
- 2.Brindley D. N., Lin F. T., Tigyi G. J. 2013. Role of the autotaxin-lysophosphatidate axis in cancer resistance to chemotherapy and radiotherapy. Biochim. Biophys. Acta. 1831: 74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Meeteren L. A., Ruurs P., Stortelers C., Bouwman P., van Rooijen M. A., Pradere J. P., Pettit T. R., Wakelam M. J., Saulnier-Blache J. S., Mummery C. L., et al. 2006. Autotaxin, a secreted lysophospholipase D, is essential for blood vessel formation during development. Mol. Cell. Biol. 26: 5015–5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koike S., Keino-Masu K., Masu M. 2010. Deficiency of autotaxin/lysophospholipase D results in head cavity formation in mouse embryos through the LPA receptor-Rho-ROCK pathway. Biochem. Biophys. Res. Commun. 400: 66–71. [DOI] [PubMed] [Google Scholar]
- 5.Koike S., Keino-Masu K., Ohto T., Sugiyama F., Takahashi S., Masu M. 2009. Autotaxin/lysophospholipase D-mediated lysophosphatidic acid signaling is required to form distinctive large lysosomes in the visceral endoderm cells of the mouse yolk sac. J. Biol. Chem. 284: 33561–33570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koike S., Yutoh Y., Keino-Masu K., Noji S., Masu M., Ohuchi H. 2011. Autotaxin is required for the cranial neural tube closure and establishment of the midbrain-hindbrain boundary during mouse development. Dev. Dyn. 240: 413–421. [DOI] [PubMed] [Google Scholar]
- 7.Nikitopoulou I., Oikonomou N., Karouzakis E., Sevastou I., Nikolaidou-Katsaridou N., Zhao Z., Mersinias V., Armaka M., Xu Y., Masu M., et al. 2012. Autotaxin expression from synovial fibroblasts is essential for the pathogenesis of modeled arthritis. J. Exp. Med. 209: 925–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hozumi H., Hokari R., Kurihara C., Narimatsu K., Sato H., Sato S., Ueda T., Higashiyama M., Okada Y., Watanabe C., et al. 2013. Involvement of autotaxin/lysophospholipase D expression in intestinal vessels in aggravation of intestinal damage through lymphocyte migration. Lab. Invest. 93: 508–519. [DOI] [PubMed] [Google Scholar]
- 9.Benesch M. G. K., Tang X., Maeda T., Ohhata A., Zhao Y. Y., Kok B. P. C., Dewald J., Hitt M., Curtis J. M., McMullen T. P. W., et al. 2014. Inhibition of autotaxin delays breast tumor growth and lung metastasis in mice. FASEB J. 28: 2655–2666. [DOI] [PubMed] [Google Scholar]
- 10.Teo K., Brunton V. G. 2014. The role and therapeutic potential of the autotaxin-lysophosphatidate signalling axis in breast cancer. Biochem. J. 463: 157–165. [DOI] [PubMed] [Google Scholar]
- 11.Euer N., Schwirzke M., Evtimova V., Burtscher H., Jarsch M., Tarin D., Weidle U. H. 2002. Identification of genes associated with metastasis of mammary carcinoma in metastatic versus non-metastatic cell lines. Anticancer Res. 22: 733–740. [PubMed] [Google Scholar]
- 12.Venkatraman G., Benesch M. G., Tang X., Dewald J., McMullen T. P., Brindley D. N. 2015. Lysophosphatidate signaling stabilizes Nrf2 and increases the expression of genes involved in drug resistance and oxidative stress responses: implications for cancer treatment. FASEB J. 29: 772–785. [DOI] [PubMed] [Google Scholar]
- 13.Tang X., Benesch M. G., Dewald J., Zhao Y. Y., Patwardhan N., Santos W. L., Curtis J. M., McMullen T. P., Brindley D. N. 2014. Lipid phosphate phosphatase-1 expression in cancer cells attenuates tumor growth and metastasis in mice. J. Lipid Res. 55: 2389–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tomsig J. L., Snyder A. H., Berdyshev E. V., Skobeleva A., Mataya C., Natarajan V., Brindley D. N., Lynch K. R. 2009. Lipid phosphate phosphohydrolase type 1 (LPP1) degrades extracellular lysophosphatidic acid in vivo. Biochem. J. 419: 611–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albers H. M., Dong A., van Meeteren L. A., Egan D. A., Sunkara M., van Tilburg E. W., Schuurman K., van Tellingen O., Morris A. J., Smyth S. S., et al. 2010. Boronic acid-based inhibitor of autotaxin reveals rapid turnover of LPA in the circulation. Proc. Natl. Acad. Sci. USA. 107: 7257–7262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kok B. P., Venkatraman G., Capatos D., Brindley D. N. 2012. Unlike two peas in a pod: lipid phosphate phosphatases and phosphatidate phosphatases. Chem. Rev. 112: 5121–5146. [DOI] [PubMed] [Google Scholar]
- 17.Brindley D. N., Pilquil C. 2009. Lipid phosphate phosphatases and signaling. J. Lipid Res. 50(Suppl): S225–S230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Meeteren L. A., Ruurs P., Christodoulou E., Goding J. W., Takakusa H., Kikuchi K., Perrakis A., Nagano T., Moolenaar W. H. 2005. Inhibition of autotaxin by lysophosphatidic acid and sphingosine 1-phosphate. J. Biol. Chem. 280: 21155–21161. [DOI] [PubMed] [Google Scholar]
- 19.Saunders L. P., Cao W., Chang W. C., Albright R. A., Braddock D. T., De La Cruz E. M. 2011. Kinetic analysis of autotaxin reveals substrate-specific catalytic pathways and a mechanism for lysophosphatidic acid distribution. J. Biol. Chem. 286: 30130–30141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.East J. E., Kennedy A. J., Tomsig J. L., De Leon A. R., Lynch K. R., Macdonald T. L. 2010. Synthesis and structure-activity relationships of tyrosine-based inhibitors of autotaxin (ATX). Bioorg. Med. Chem. Lett. 20: 7132–7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cui P., Tomsig J. L., McCalmont W. F., Lee S., Becker C. J., Lynch K. R., Macdonald T. L. 2007. Synthesis and biological evaluation of phosphonate derivatives as autotaxin (ATX) inhibitors. Bioorg. Med. Chem. Lett. 17: 1634–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gendaszewska-Darmach E., Laska E., Rytczak P., Okruszek A. 2012. The chemical synthesis of metabolically stabilized 2-OMe-LPA analogues and preliminary studies of their inhibitory activity toward autotaxin. Bioorg. Med. Chem. Lett. 22: 2698–2700. [DOI] [PubMed] [Google Scholar]
- 23.Zhang H., Xu X., Gajewiak J., Tsukahara R., Fujiwara Y., Liu J., Fells J. I., Perygin D., Parrill A. L., Tigyi G., et al. 2009. Dual activity lysophosphatidic acid receptor pan-antagonist/autotaxin inhibitor reduces breast cancer cell migration in vitro and causes tumor regression in vivo. Cancer Res. 69: 5441–5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakanaga K., Hama K., Aoki J. 2010. Autotaxin–an LPA producing enzyme with diverse functions. J. Biochem. 148: 13–24. [DOI] [PubMed] [Google Scholar]
- 25.Moolenaar W. H., Perrakis A. 2011. Insights into autotaxin: how to produce and present a lipid mediator. Nat. Rev. Mol. Cell Biol. 12: 674–679. [DOI] [PubMed] [Google Scholar]
- 26.Hooks S. B., Santos W. L., Im D. S., Heise C. E., Macdonald T. L., Lynch K. R. 2001. Lysophosphatidic acid-induced mitogenesis is regulated by lipid phosphate phosphatases and is Edg-receptor independent. J. Biol. Chem. 276: 4611–4621. [DOI] [PubMed] [Google Scholar]
- 27.Pilquil C., Dewald J., Cherney A., Gorshkova I., Tigyi G., English D., Natarajan V., Brindley D. N. 2006. Lipid phosphate phosphatase-1 regulates lysophosphatidate-induced fibroblast migration by controlling phospholipase D2-dependent phosphatidate generation. J. Biol. Chem. 281: 38418–38429. [DOI] [PubMed] [Google Scholar]
- 28.Morimoto T., Nakatani S., Ohhata A., Sugiyama T., inventors; Ono Pharmaceuticals Co. Ltd., assignee. Tetrahydrocarboline derivative. World Patent WO2012005227 A1, Japanese. January 12, 2012. [Google Scholar]
- 29.Saga H., Ohhata A., Hayashi A., Katoh M., Maeda T., Mizuno H., Takada Y., Komichi Y., Ota H., Matsumura N., et al. 2014. A novel highly potent autotaxin/ENPP2 inhibitor produces prolonged decreases in plasma lysophosphatidic acid formation in vivo and regulates urethral tension. PLoS ONE. 9: e93230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferry G., Moulharat N., Pradere J. P., Desos P., Try A., Genton A., Giganti A., Beucher-Gaudin M., Lonchampt M., Bertrand M., et al. 2008. S32826, a nanomolar inhibitor of autotaxin: discovery, synthesis and applications as a pharmacological tool. J. Pharmacol. Exp. Ther. 327: 809–819. [DOI] [PubMed] [Google Scholar]
- 31.Murata J., Lee H. Y., Clair T., Krutzsch H. C., Arestad A. A., Sobel M. E., Liotta L. A., Stracke M. L. 1994. cDNA cloning of the human tumor motility-stimulating protein, autotaxin, reveals a homology with phosphodiesterases. J. Biol. Chem. 269: 30479–30484. [PubMed] [Google Scholar]
- 32.Gaetano C. G., Samadi N., Tomsig J. L., Macdonald T. L., Lynch K. R., Brindley D. N. 2009. Inhibition of autotaxin production or activity blocks lysophosphatidylcholine-induced migration of human breast cancer and melanoma cells. Mol. Carcinog. 48: 801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferguson C. G., Bigman C. S., Richardson R. D., van Meeteren L. A., Moolenaar W. H., Prestwich G. D. 2006. Fluorogenic phospholipid substrate to detect lysophospholipase D/autotaxin activity. Org. Lett. 8: 2023–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohta H., Sato K., Murata N., Damirin A., Malchinkhuu E., Kon J., Kimura T., Tobo M., Yamazaki Y., Watanabe T., et al. 2003. Ki16425, a subtype-selective antagonist for EDG-family lysophosphatidic acid receptors. Mol. Pharmacol. 64: 994–1005. [DOI] [PubMed] [Google Scholar]
- 35.Davis M. D., Clemens J. J., Macdonald T. L., Lynch K. R. 2005. Sphingosine 1-phosphate analogs as receptor antagonists. J. Biol. Chem. 280: 9833–9841. [DOI] [PubMed] [Google Scholar]
- 36.Bolick D. T., Srinivasan S., Kim K. W., Hatley M. E., Clemens J. J., Whetzel A., Ferger N., Macdonald T. L., Davis M. D., Tsao P. S., et al. 2005. Sphingosine 1-phosphate prevents tumor necrosis factor-{alpha}-mediated monocyte adhesion to aortic endothelium in mice. Arterioscler. Thromb. Vasc. Biol. 25: 976–981. [DOI] [PubMed] [Google Scholar]
- 37.Boucher J., Quilliot D., Praderes J. P., Simon M. F., Gres S., Guigne C., Prevot D., Ferry G., Boutin J. A., Carpene C., et al. 2005. Potential involvement of adipocyte insulin resistance in obesity-associated up-regulation of adipocyte lysophospholipase D/autotaxin expression. Diabetologia. 48: 569–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dusaulcy R., Rancoule C., Gres S., Wanecq E., Colom A., Guigne C., van Meeteren L. A., Moolenaar W. H., Valet P., Saulnier-Blache J. S. 2011. Adipose-specific disruption of autotaxin enhances nutritional fattening and reduces plasma lysophosphatidic acid. J. Lipid Res. 52: 1247–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferry G., Tellier E., Try A., Gres S., Naime I., Simon M. F., Rodriguez M., Boucher J., Tack I., Gesta S., et al. 2003. Autotaxin is released from adipocytes, catalyzes lysophosphatidic acid synthesis, and activates preadipocyte proliferation. Up-regulated expression with adipocyte differentiation and obesity. J. Biol. Chem. 278: 18162–18169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nishimura S., Nagasaki M., Okudaira S., Aoki J., Ohmori T., Ohkawa R., Nakamura K., Igarashi K., Yamashita H., Eto K., et al. 2014. ENPP2 contributes to adipose tissue expansion and insulin resistance in diet-induced obesity. Diabetes. 63: 4154–4164. [DOI] [PubMed] [Google Scholar]
- 41.Cavalli S., Houben A. J., Albers H. M., van Tilburg E. W., de Ru A., Aoki J., van Veelen P., Moolenaar W. H., Ovaa H. 2010. Development of an activity-based probe for autotaxin. ChemBioChem. 11: 2311–2317. [DOI] [PubMed] [Google Scholar]
- 42.Kawaguchi M., Okabe T., Okudaira S., Nishimasu H., Ishitani R., Kojima H., Nureki O., Aoki J., Nagano T. 2013. Screening and X-ray crystal structure-based optimization of autotaxin (ENPP2) inhibitors, using a newly developed fluorescence probe. ACS Chem. Biol. 8: 1713–1721. [DOI] [PubMed] [Google Scholar]
- 43.Gierse J., Thorarensen A., Beltey K., Bradshaw-Pierce E., Cortes-Burgos L., Hall T., Johnston A., Murphy M., Nemirovskiy O., Ogawa S., et al. 2010. A novel autotaxin inhibitor reduces lysophosphatidic acid levels in plasma and the site of inflammation. J. Pharmacol. Exp. Ther. 334: 310–317. [DOI] [PubMed] [Google Scholar]
- 44.Nikitopoulou I., Kaffe E., Sevastou I., Sirioti I., Samiotaki M., Madan D., Prestwich G. D., Aidinis V. 2013. A metabolically-stabilized phosphonate analog of lysophosphatidic acid attenuates collagen-induced arthritis. PLoS ONE. 8: e70941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nozaki E., Gotoh M., Tanaka R., Kato M., Suzuki T., Nakazaki A., Hotta H., Kobayashi S., Murakami-Murofushi K. 2012. Pharmacological evaluation of a novel cyclic phosphatidic acid derivative 3-S-cyclic phosphatidic acid (3-S-cPA). Bioorg. Med. Chem. 20: 3196–3201. [DOI] [PubMed] [Google Scholar]
- 46.Nozaki E., Gotoh M., Hotta H., Hanazawa S., Kobayashi S., Murakami-Murofushi K. 2011. Synthesis of enantiopure 2-carba-cyclic phosphatidic acid and effects of its chirality on biological functions. Biochim. Biophys. Acta. 1811: 271–277. [DOI] [PubMed] [Google Scholar]
- 47.Lee S. C., Fujiwara Y., Liu J., Yue J., Shimizu Y., Norman D. D., Wang Y., Tsukahara R., Szabo E., Patil R., et al. 2015. Autotaxin and LPA1 and LPA5 receptors exert disparate functions in tumor cells versus the host tissue microenvironment in melanoma invasion and metastasis. Mol. Cancer Res. 13: 174–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xia Q., Deng A. M., Wu S. S., Zheng M. 2011. Cholera toxin inhibits human hepatocarcinoma cell proliferation in vitro via suppressing ATX/LPA axis. Acta Pharmacol. Sin. 32: 1055–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stortelers C., Kerkhoven R., Moolenaar W. H. 2008. Multiple actions of lysophosphatidic acid on fibroblasts revealed by transcriptional profiling. BMC Genomics. 9: 387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fuerst E., Foster H. R., Ward J. P., Corrigan C. J., Cousins D. J., Woszczek G. 2014. Sphingosine 1-phosphate induces pro-remodelling response in airway smooth muscle cells. Allergy. 69: 1531–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Avery K., Avery S., Shepherd J., Heath P. R., Moore H. 2008. Sphingosine 1-phosphate mediates transcriptional regulation of key targets associated with survival, proliferation, and pluripotency in human embryonic stem cells. Stem Cells Dev. 17: 1195–1205. [DOI] [PubMed] [Google Scholar]
- 52.Bennett G., Sadlier D., Doran P. P., Macmathuna P., Murray D. W. 2011. A functional and transcriptomic analysis of NET1 bioactivity in gastric cancer. BMC Cancer. 11: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murray D., Horgan G., Macmathuna P., Doran P. 2008. NET1-mediated RhoA activation facilitates lysophosphatidic acid-induced cell migration and invasion in gastric cancer. Br. J. Cancer. 99: 1322–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tanaka M., Okudaira S., Kishi Y., Ohkawa R., Iseki S., Ota M., Noji S., Yatomi Y., Aoki J., Arai H. 2006. Autotaxin stabilizes blood vessels and is required for embryonic vasculature by producing lysophosphatidic acid. J. Biol. Chem. 281: 25822–25830. [DOI] [PubMed] [Google Scholar]
- 55.Brindley D. N. 2004. Lipid phosphate phosphatases and related proteins: signaling functions in development, cell division, and cancer. J. Cell. Biochem. 92: 900–912. [DOI] [PubMed] [Google Scholar]
- 56.Dvorak H. F. 1986. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N. Engl. J. Med. 315: 1650–1659. [DOI] [PubMed] [Google Scholar]
- 57.Schäfer M., Werner S. 2008. Cancer as an overhealing wound: an old hypothesis revisited. Nat. Rev. Mol. Cell Biol. 9: 628–638. [DOI] [PubMed] [Google Scholar]
- 58.Hanahan D., Weinberg R. A. 2011. Hallmarks of cancer: the next generation. Cell. 144: 646–674. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.