Abstract
Echium oil (EO), which is enriched in 18:4 n-3, the immediate product of fatty acid desaturase 2 (FADS2) desaturation of 18:3 n-3, is as atheroprotective as fish oil (FO). The objective of this study was to determine whether botanical oils enriched in the FADS2 products 18:3 n-6 versus 18:4 n-3 are equally atheroprotective. LDL receptor KO mice were fed one of four atherogenic diets containing 0.2% cholesterol and 10% calories as palm oil (PO) plus 10% calories as: 1) PO; 2) borage oil (BO; 18:3 n-6 enriched); 3) EO (18:4 n-3 enriched); or 4) FO for 16 weeks. Mice fed BO, EO, and FO versus PO had significantly lower plasma total and VLDL cholesterol concentrations; hepatic neutral lipid content and inflammation, aortic CE content, aortic root intimal area and macrophage content; and peritoneal macrophage inflammation, CE content, and ex vivo chemotaxis. Atheromas lacked oxidized CEs despite abundant generation of macrophage 12/15 lipooxygenase-derived metabolites. We conclude that botanical oils enriched in 18:3 n-6 and 18:4 n-3 PUFAs beyond the rate-limiting FADS2 enzyme are equally effective in preventing atherosclerosis and hepatosteatosis compared with saturated/monounsaturated fat due to cellular enrichment of ≥20 PUFAs, reduced plasma VLDL, and attenuated macrophage inflammation.
Keywords: fatty acid/metabolism, inflammation, lipoproteins/metabolism, macrophages/monocytes, mass spectrometry, very low density lipoprotein, fatty acid desaturase 2
Despite widespread use of lipid-lowering drugs, cardiovascular disease is still the leading cause of death in the United States (1). One of the first lines of cardiovascular disease treatment is reducing total fat intake and replacing saturated fatty acids with PUFAs (2). In particular, n-3 PUFAs from fatty fish or fish oil (FO) reduce the extent of cardiovascular and other chronic diseases in humans and experimental animals (3–6). The atheroprotective benefits of fatty fish and FO are attributed to two n-3 PUFAs, EPA (20:5 n-3) and DHA (22:6 n-3). However, despite the well-documented health benefits of n-3 PUFAs, consumption is low in the US (7). Furthermore, most botanical sources of n-3 fatty acids (i.e., flaxseed oil) contain α-linolenic acid (ALA, 18:3 n-3), which is inefficiently converted to EPA (4–15%) because of the rate-limiting nature of fatty acid desaturase 2 (FADS2; i.e., delta-6 desaturase) in the fatty acid elongation and desaturation pathway (8). Thus, botanical oils enriched in ALA lack the plasma lipid-lowering and atheroprotective properties observed with FO (9).
Dietary n-6 PUFAs are also atheroprotective in mouse and nonhuman primate models of atherosclerosis, and in human populations (10–13). Some of the atheroprotection is likely due to plasma lipid lowering, but there may also be an anti-inflammatory role for n-6 PUFAs through the generation of prostaglandin E (PGE)1 from dihomo-γ-linolenic acid (DGLA, 20:3 n-6), a potent inhibitor of thromboxane A2 (TXA2) formation that inhibits leukocyte adherence to endothelial cells (14, 15). However, most sources of botanical n-6 PUFAs are highly enriched in linoleic acid (LA; 18:2 n-6, ∼85–90% of North American dietary PUFA intake) (10, 12). LA also must be converted by FADS2 to longer-chain bioactive PUFAs, although some bioactive derivatives of LA are described as both pro- and anti-inflammatory (13).
One strategy to circumvent the poor conversion of dietary 18-carbon PUFAs to their ≥20 carbon bioactive products is to identify botanical oils enriched in PUFAs beyond the FADS2 rate-limiting step of desaturation and elongation. We previously showed that echium oil (EO), which is relatively enriched in stearidonic acid (SDA, 18:4 n-3), the immediate product of FADS2-mediated desaturation of ALA, effectively enriches plasma and tissue lipids in EPA (16). In LDL receptor KO (LDLrKO) mice, isocaloric replacement of palm oil (PO) with EO attenuated atherosclerosis severity, splenic monocytosis, monocyte influx into aortic intima, and aortic root intimal macrophage content to an equivalent extent as FO, lending proof of principle for this strategy (17). However, whether a similar strategy will be atheroprotective for the n-6 PUFA pathway is unknown.
To address this gap in knowledge, we identified borage oil (BO) as a potential botanical oil to test this strategy in the n-6 PUFA conversion pathway. BO is enriched (∼20%) in γ-linolenic acid (GLA, 18:3 n-6), the immediate product of FADS2-mediated desaturation of LA. GLA can be elongated to DGLA, a precursor of the anti-inflammatory PGE1, or arachidonic acid (AA, 20:4 n-6), a precursor of pro-inflammatory eicosanoid species. The atheroprotective effects of BO have not been explored, and little is known about its effects on plasma lipids and lipoproteins. The purpose of this study was to directly compare the atheroprotective potential of BO with EO and elucidate potential mechanisms for atheroprotection.
MATERIALS AND METHODS
Dietary oils
The seed oil of Borago officinalis L. (a member of the Boraginaceae family) and the seed oil of Echium plantagineum L. (a member of the Boraginaceae family) were generous gifts from Croda Europe Ltd. (Leek, Staffordshire, UK) and authenticated by the Wake Forest University Center for Botanical Lipids and Inflammatory Disease Prevention. The seed oil of palm, Elaeis guineensis Jacq (a member of the Arecaceae family) was purchased from Shay and Company (Portland, OR). For these oils, a certificate of analysis is on file and retention samples are deposited at the Wake Forest School of Medicine. The FO source, Brevoortia tyrannis Latrobe (a member of the Clupeidae family), was manufactured and generously provided by Omega Protein (Reidsville, VA) with a report of analysis on file for reference.
Animals and atherogenic diets
Female LDLrKO (C57BL/6 background) mice (5–6 weeks of age) were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were housed in a specific pathogen-free facility on a 12 h light/dark cycle. All protocols and procedures were approved by the Institutional Animal Care and Use Committee. Mice were allowed to acclimate for 1–2 weeks during which time they ate a chow diet. At 7–8 weeks of age, mice were randomly assigned to one of four atherogenic diet (AD) groups (n = 15 per diet group) containing 10% calories as PO and 0.2% cholesterol, supplemented with an additional 10% of calories as: 1) PO, 2) BO (18:3 n-6 enriched), 3) EO (18:4 n-3 enriched), or 4) FO (20:5 n-3 and 22:6 n-3 enriched) for 16 weeks. The synthetic ADs were prepared by the diet kitchen in the Department of Pathology at Wake Forest School of Medicine as previously described (18). Detailed composition and quality control data for similar ADs have been published (16).
Body and organ weights
Mice were weighed every 2–4 weeks. After 16 weeks of AD feeding, body weights were recorded after a 4 h fast. Blood was collected via the tail vein at baseline and after 2, 4, 8, and 16 weeks of AD feeding. Mice were then anesthetized using ketamine-xylazine and perfused via the left ventricle using cold PBS at the rate of ∼3 ml/min for 3–4 min before organs were harvested. After perfusion, liver wet weights were measured and normalized to terminal body weight.
Fatty acid analysis
Lipid extraction of lyophilized diets, red blood cells (RBCs), plasma, and liver was performed using the Bligh-Dyer method (19). The total lipid extracts from plasma and liver were separated into cholesteryl ester (CE), TG, and phospholipid (PL) fractions by TLC. Lipids were re-extracted from CE, TG, and PL fractions and then transmethylated using boron trifluoride (20), and percentage fatty acid composition was quantified by gas-LC (GLC) as described previously (18). To estimate recovery of fatty acids from TLC and the transmethylation procedure, a known amount of tripentadecanoin (C15:0) and cholesteryl nonadecanoate (C19:0) were used as internal standards prior to TLC and triheptadecanoin (C17:0) was used as an internal standard prior to transmethylation. All internal standards were purchased from Nu-Chek-Prep, Inc., (T-145, Ch-818, and T-155). Percentage loss during TLC and transmethylation was <10% and <15%, respectively. Diet and RBC lipid extracts were directly transmethylated for fatty acid analysis.
Plasma lipid and lipoprotein analysis
Plasma was isolated by low-speed centrifugation. Plasma total and free cholesterol (FC) (Wako), and TG (Roche) concentrations were determined using enzymatic assays as described earlier (21) at baseline and after 2, 4, 8, and 16 weeks of AD feeding. CE content was calculated as [total cholesterol (TC)−FC] × 1.67; this calculation corrects for loss of fatty acid during the cholesterol esterase step of the assay. Data were expressed as area under the curve (AUC), which integrates plasma lipid concentrations over the 16 weeks, representing a time average estimate of hypercholesterolemia during the 16 week experiment. Plasma lipoprotein cholesterol mass distribution was determined using fast protein LC (FPLC) fractionation on a Superose 6 10/300 column (GE Healthcare). Three equal volumes of plasma samples from each time point (5 mice per group) were pooled and subjected to FPLC fractionation and cholesterol quantification. VLDL, LDL, and HDL cholesterol concentrations were determined and then expressed as AUC.
In vivo quantification of VLDL TG secretion rate
VLDL TG secretion rate was determined using detergent inhibition of intravascular TG lipolysis (20, 22). Tyloxapol (500 mg/kg body weight) was intravenously injected into anesthetized mice (n = 5 per group) fed the AD for 16 weeks and fasted for 4 h. Plasma TG concentrations before (0 min), and 60, 120, and 180 min after injection were determined by enzymatic assay. TG secretion rate was calculated using linear regression analysis to determine the slope of the time versus TG concentration plot for each animal (20).
Liver lipid analysis
Livers were harvested at necropsy, flash-frozen in liquid N2, and stored at −80°C. Liver lipids were quantified using a detergent-based enzymatic assay (23).
Western blot analysis
Nuclear proteins were prepared from frozen livers using ultracentrifugation as described (24, 25). Equal aliquots (20 μg) of nuclear protein from individual livers were subjected to SDS-PAGE on 4–12% gels and transferred to a polyvinylidene difluoride membrane. Immunoblot analyses were performed using monoclonal anti-mouse SREBP-1 and rabbit polyclonal SREBP-2 antibodies as described (26). Anti-SREBP-1 and -2 antibodies were generously donated by Dr. Timothy Osborne (Sanford Burnham Medical Research Institute). Anti-YY1 antibody (Abcam #43058) was used as a nuclear loading control. Whole cell lysates were prepared using frozen livers (27) and equal aliquots (50 μg) of protein from individual livers were subjected to SDS-PAGE on 4–12% gels and transferred to polyvinylidene fluoride membranes. Immunoblot analyses were performed using rabbit monoclonal anti-FAS antibody (Cell Signaling #3180) and goat polyclonal anti stearoyl-CoA desaturase-1 (SCD-1) antibody (Santa Cruz Biotechnology #14719).
Atherosclerotic lesion analysis
A subset of mice (n = 3 per group) was euthanized after 8 weeks of AD feeding to measure aortic CE content. In the remaining mice, aortic CE content, aortic root intimal area, and intimal macrophage content (CD68+) were measured after 16 weeks of AD feeding (17). Aortic root intimal area was measured with Oil Red O staining.
Aortic oxidized CE and aortic cholesterol content analysis
At necropsy, aortas were cleaned of visible adventitial fat and placed into a 15 ml etched glass tissue grinder containing 1 ml of 1:1 methanol:water (v/v) and a known amount of internal standard 5-α cholestane. Aortas were homogenized on ice and neutral lipids were extracted twice using 75:25 isooctane:ethyl acetate (v/v). The combined organic layers were brought to a volume of 2 ml with 75:25 isooctane:ethyl acetate. One milliliter of the extract was added to an argon-purged ampule, heat-sealed, and shipped on dry ice to the University of Colorado Denver for LC/MS/MS analysis. Before analysis, the organic layer was dried under N2. The residue was then weighed and diluted to 1 μg/μl with isooctane, and stored at −20°C until analysis of oxidized CE (ox-CE) using LC/MS (/MS) (28). The remaining 1 ml of the original aortic extract in 75:25 isooctane:ethyl acetate was used to quantify aortic total cholesterol (TC) and FC content by GLC (17). Results were normalized to aortic wet weight.
Peritoneal macrophage studies
Thioglycollate-elicited peritoneal macrophages were isolated and cholesterol content was measured as described previously (21). Macrophage gene expression was measured after 2 h of PBS or lipopolysaccharide (LPS) (200 ng/ml) stimulation (21) and eicosanoids were identified and quantified using LC/MS/MS in macrophage-conditioned media (29, 30). In vitro macrophage chemotaxis in response to MCP-1 and MIP-1α was performed in a 48-well microtaxis chamber (31).
Real-time PCR analysis
Total RNA was isolated from mouse macrophages and liver using TRIzol (Invitrogen) and quantitative real-time PCR was performed to determine gene expression (21). Primer sequences have been reported previously (20, 27, 32). GAPDH was used as the control for normalization of results.
Statistics
Data are reported as mean ± SEM. Statistical analyses were performed using one-way ANOVA and individual paired diet comparisons were made using Tukey’s post hoc analysis. All statistical analyses were performed using GraphPad Prism software.
RESULTS
Systemic response to ADs enriched in n-3 versus n-6 fatty acid products of FADS2
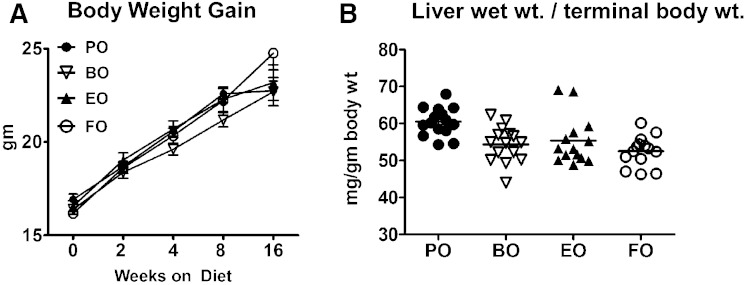
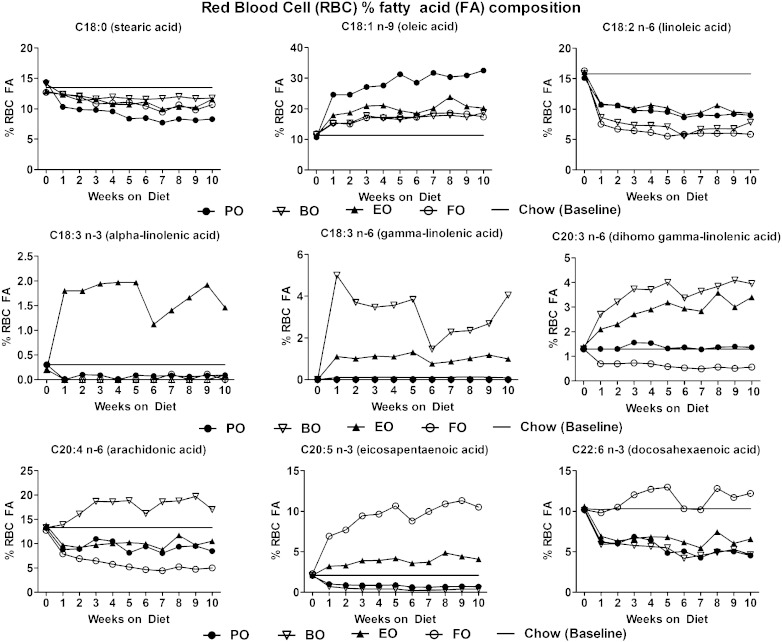
Dietary fatty acid compositions are given in Table 1 and show relative enrichment of 18-carbon fatty acids beyond FADS2 in the BO (19.7% 18:3 n-6) and EO (6% 18:4 n-3) diets. AD feeding over 16 weeks resulted in uniform food consumption (∼3–4 g/day/mouse), body weight gain, and terminal liver/body weight ratios among all groups (Fig. 1). Upon initiation of AD feeding, significant RBC fatty acid enrichment over baseline (chow diet) was evident within 1 week and appeared to reach equilibrium by 4 weeks (Fig. 2). RBC fatty acid compositions reflected efficient in vivo elongation-desaturation of dietary 18:3 n-6 derived from BO to its longer chain (20-carbon) counterparts, 20:3 n-6 and 20:4 n-6. Similarly, 18:4 n-3 derived from EO was elongated-desaturated to 20:5 n-3. These data indicate that dietary enrichment of 18-carbon fatty acid beyond FADS2 is sufficient to result in membrane enrichment of their respective 20-carbon chain counterparts. After 4 weeks of diet feeding, compared with chow (baseline), BO-fed mice had significant RBC fatty acid enrichment in GLA (∼5.0 vs. 0.1%), DGLA (∼4.0 vs. 1.1%), and AA (∼20.0 vs. 12.0%); whereas EO-fed mice had significant RBC fatty acid enrichment in ALA (∼2.0 vs. 0.4%) and EPA (∼3 vs. 2%). FO-fed mice had the greatest enrichment in EPA (∼10 vs. 2%) and DHA (∼12 vs. ∼10.0%), whereas PO-fed mice had the greatest enrichment in oleic acid (∼30 vs. 11%) and lowest enrichment in stearic acid (below 10 vs. ∼12–13%).
TABLE 1.
FA composition and total energy equivalence of individual FAs in each AD
| FA | PO | BO | EO | FO | ||||
| % FA | % EE | % FA | % EE | % FA | % EE | % FA | % EE | |
| Palmitic acid (C16:0) | 43.2 | 8.64 | 24.5 | 4.9 | 25.6 | 5.12 | 30.6 | 6.12 |
| Palmitoleic acid (C16:1) | 0.37 | 0.074 | 0.3 | 0.06 | 0.4 | 0.08 | 4.2 | 0.84 |
| Stearic acid (C18:0) | 4.5 | 0.90 | 4.4 | 0.88 | 4.1 | 0.82 | 4.5 | 0.9 |
| Oleic acid (C18:1 n-9) | 37.3 | 7.46 | 24.1 | 4.82 | 26.1 | 5.22 | 24.9 | 4.98 |
| LA (C18:2 n-6) | 11.1 | 2.22 | 17.4 | 3.48 | 15.4 | 3.08 | 8.2 | 1.64 |
| ALA (C18:3 n-3) | 0.37 | 0.074 | 0.5 | 0.11 | 13.8 | 2.76 | 1 | 0.2 |
| GLA (C18:3 n-6) | — | — | 19.7 | 3.94 | 5.0 | 1.0 | — | — |
| SDA (C18:4 n-3) | — | — | 0.2 | 0.04 | 6.0 | 1.2 | 1.5 | 0.3 |
| Euric acid (C22:1 n-9) | — | — | 2.6 | 0.52 | 0.2 | 0.04 | — | — |
| EPA (C20:5 n-3) | 0.1 | 0.02 | 0.3 | 0.06 | 0.3 | 0.06 | 6.9 | 1.38 |
| DHA (C22:6 n-3) | 0.2 | 0.04 | 0.3 | 0.06 | 0.3 | 0.06 | 7 | 1.40 |
Diets contained 0.2% cholesterol + 10% calories as PO + 10% calories as: PO, BO, EO, or FO. Percent FA composition (% FA) of PO, BO, EO and FO diets determined using GLC. Percent total energy equivalence (% EE) for individual FAs was calculated using total energy derived from FAs (i.e., 20%)/diet and percent FA composition of respective diet.
Fig. 1.
Body weight gain and terminal liver/body weight ratios. A: Body weight gain was monitored periodically from baseline (chow diet) to 16 weeks of feeding the indicated ADs. B: Mice were then euthanized and liver wet weights were measured and normalized to terminal body weight. Data are expressed as mean ± SEM; n = 15 per diet group. No significant differences were found by one-way ANOVA.
Fig. 2.
RBC FA composition. RBCs were harvested from mice consuming the indicated ADs from baseline (chow diet) to 10 weeks; percentage of FA distribution was measured as described in the Materials and Methods. Data for individual FAs are expressed as percentage composition of total FAs. An equal volume pool of RBCs from eight mice per diet group per week was used for analysis. Horizontal line denotes extrapolation of chow data (0 weeks) across the 10 week period.
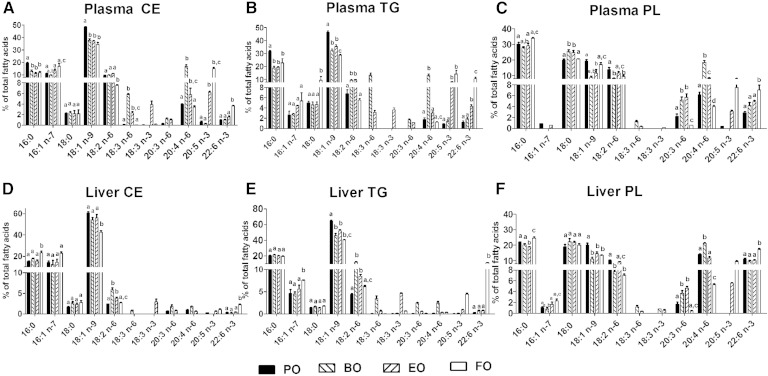
Fatty acid composition was also measured in plasma and liver CE, TG, and PL fractions (Fig. 3). In plasma from BO-fed mice, equivalent (∼20%) AA enrichment was observed in CE, TG, and PL fractions relative to plasma from PO-fed mice (Fig. 3 A–C). Plasma neutral lipids from mice fed BO were also relatively enriched in GLA [CE (∼6%) and TG (∼15%)], unlike plasma PL. In BO-fed livers, AA was modestly, but significantly, enriched in PL (∼20%), but not in neutral lipids (below 5%), relative to the other diet groups (Fig. 3 D–F). In general, PUFA enrichment in liver CE and TG was low for all diet groups relative to liver PL.
Fig. 3.
Percentage FA composition of plasma and liver lipids. A–F: LDLrKO mice were fed the indicated ADs for 16 weeks before harvesting plasma and liver for FA analysis. Data for individual FAs are expressed as percentage (mean ± SEM) composition of total FAs; n = 3 per diet group. Bars with different letters denote significant (P < 0.05) differences among diet groups by one-way ANOVA and Tukey’s post hoc analysis.
Plasma lipid and lipoprotein response to dietary BO versus EO
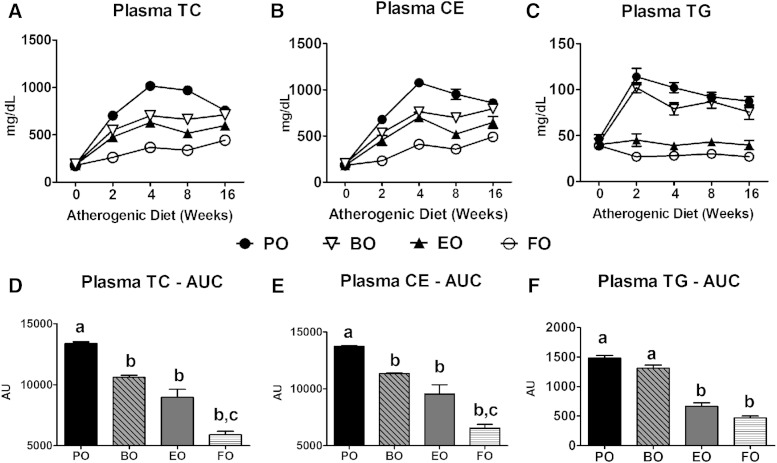
Chow-fed mice had similar baseline measurements, but after 2 weeks of AD feeding, plasma TC, CE, and TG concentrations increased significantly, peaked by week 4, and remained elevated over the 16 weeks of diet feeding for PO-fed mice (Fig. 4). This pattern was significantly attenuated in the other diet groups. BO and EO induced equivalent cholesterol lowering, whereas FO induced even further TC and CE lowering, compared with PO. Plasma TG concentrations increased equivalently in PO- and BO-fed mice after 2 weeks of AD feeding, but stayed at baseline levels in the EO- and FO-fed mice.
Fig. 4.
Plasma lipid concentrations. Fasting (4 h) plasma TC (A), CE (B), and TG (C) concentrations in LDLrKO mice during a 16 week AD feeding were measured by enzymatic assays (n = 15). CE = (TC − FC) × 1.67. Plasma TC, CE, and TG concentrations were integrated over the 16 week study and expressed as AUC (D–F). Data are expressed as mean ± SEM, n = 15 per diet. Groups with different letters are significantly different (P < 0.05) by one-way ANOVA and Tukey’s post hoc analysis.
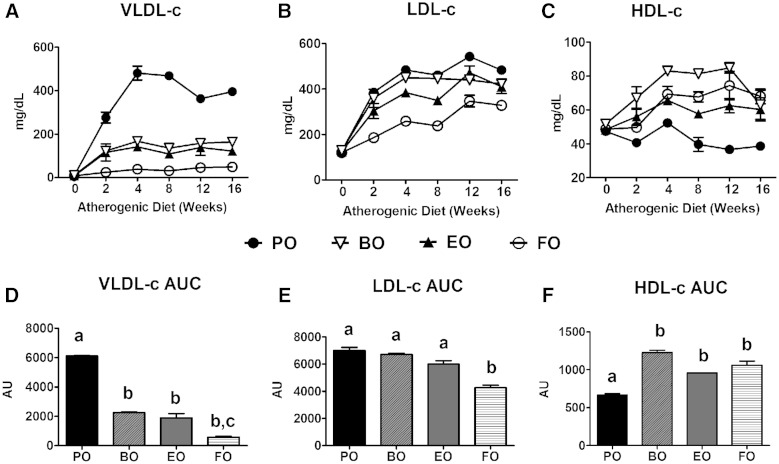
A sharp increase in VLDL cholesterol (VLDL-c) concentrations occurred within 2 weeks of AD feeding that peaked and equilibrated by 4 weeks for PO-fed mice (Fig. 5A). VLDL-c concentrations were significantly and equivalently attenuated in the other diet groups (Fig. 5 A, D). LDL cholesterol (LDL-c) levels showed a similar pattern; only FO-fed mice had significantly lower LDL-c concentrations versus PO-fed mice (Fig. 5B, E). HDL cholesterol (HDL-c) concentrations also increased sharply within 4 weeks among BO-, EO-, and FO-fed mice, and were relatively stable thereafter. HDL-c concentrations were significantly lower in PO-fed mice compared with the other groups (Fig. 5C, F); however, HDL-c was <10% of the total pool of plasma cholesterol. Hence, most of the TC lowering came from decreases in VLDL-c. BO-induced reduction in VLDL-c was equivalent to that of EO and FO, but BO did not reduce plasma TG concentrations.
Fig. 5.
Plasma lipoprotein cholesterol distribution. LDLrKO mice were fed the indicated ADs for 16 weeks. Fasted (4 h) plasma was harvested and fractionated by FPLC and cholesterol distributions among VLDL (A), LDL (B), and HDL (C) fractions were measured. Plasma VLDL-c (D), LDL-c (E), and HDL-c (F) concentrations were integrated over the 16 week study and expressed as AUC. Data are expressed as mean ± SEM, n = 3 equal volume pooled samples from 5 mice per group. Groups with different letters are significantly different (P < 0.05) by one-way ANOVA and Tukey’s post hoc analysis.
EO, but not BO, diet reduces hepatic VLDL TG secretion rate
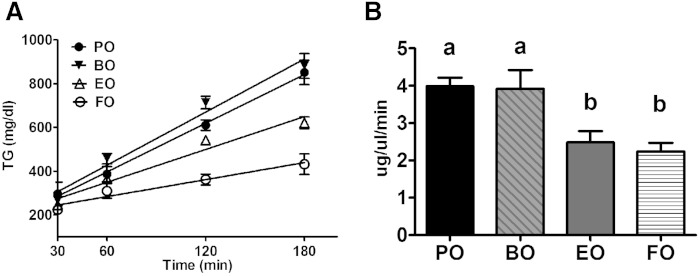
We investigated whether the difference in plasma TG concentrations between EO- versus BO-fed mice could be explained by hepatic TG secretion. Using detergent inhibition of plasma TG lipolysis, hepatic TG secretion rates were significantly lower for EO- and FO-fed groups compared with BO- and PO-fed mice (Fig. 6). These data suggest that the higher plasma TG concentrations in BO-versus EO-fed mice are likely due, in part, to a higher hepatic VLDL TG secretion rate.
Fig. 6.
Hepatic VLDL-TG secretion rate. A: Plasma TG levels were measured by enzymatic assay before (0 min) and after (60, 120, and 180 min) intravenous Triton® injection. B: Hepatic TG secretion rate during the 3 h experiment was calculated for each animal as the slope of the regression line. Data are plotted as mean ± SEM, n = 4–5. Groups with different letters are significantly different (P < 0.05) by one-way ANOVA and Tukey’s post hoc analysis.
BO- and EO-containing ADs are equally effective in reducing hepatosteatosis
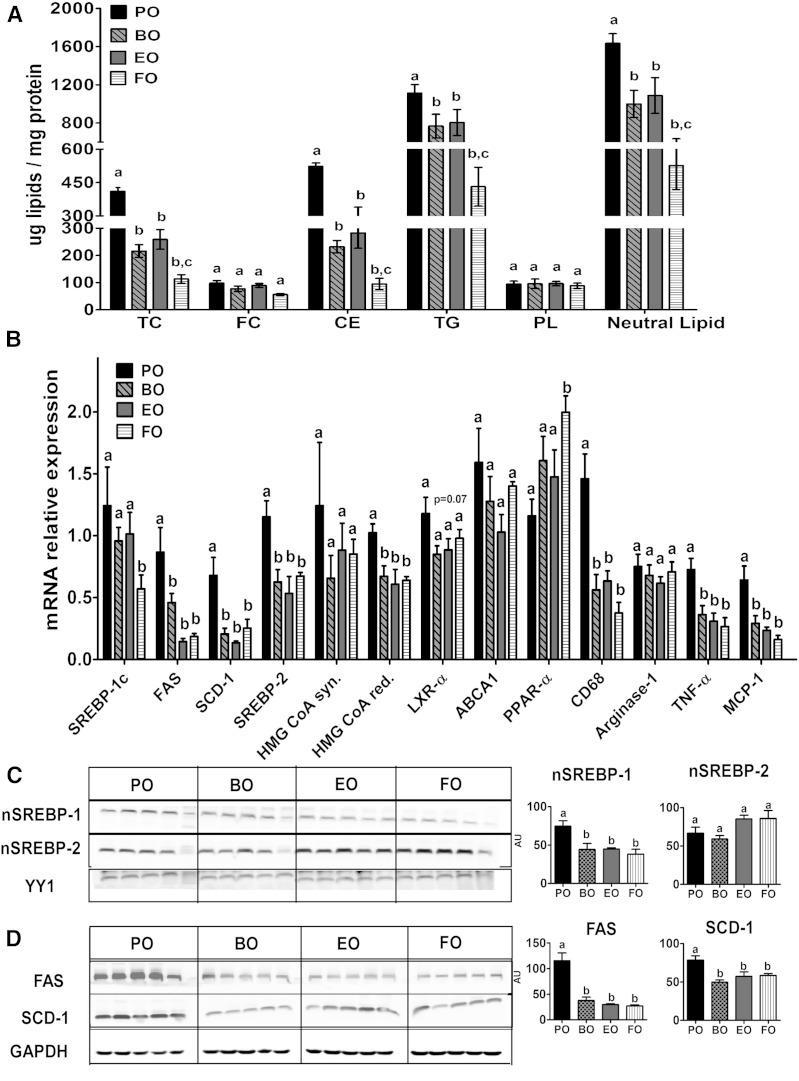
We and others have shown that ADs enriched in FO reduce hepatic lipid content (16, 33–35). In the present study, ADs containing BO, EO, or FO for 16 weeks reduced hepatic total neutral lipid content (i.e., TG and CE) relative to PO (Fig. 7A), but FC and PL contents were similar among all diet groups. Furthermore, BO and EO were equally effective in reducing hepatic neutral lipid content. To determine the reason for the decreased hepatic neutral lipid content with BO and EO feeding, we examined hepatic lipogenic gene expression using quantitative real-time PCR. Compared with PO, all three ADs significantly lowered SREBP-2 mRNA and its target gene HMG-CoA reductase, but not HMG-CoA synthase (Fig. 7B). Although SREBP-1c mRNA expression levels were similar among all diet groups, expression of its target genes, FAS and SCD-1, was significantly reduced in all three ADs relative to PO (Fig. 7B). There were no significant differences among diet groups in LXRα and ABCA1 expression, whereas PPARα expression was significantly induced in FO-fed mice (Fig. 7B). Because mice consuming the BO diet had reduced hepatosteatosis, similar to that of mice fed the EO or FO diets, we determined whether BO feeding attenuated hepatic inflammation as well. mRNA expression of CD68, TNF-α, and MCP-1 were significantly and equivalently reduced in BO-, EO-, and FO-fed mice compared with those fed PO (Fig. 7B).
Fig. 7.
Hepatic response to ADs. LDLrKO mice were fed the indicated ADs for 16 weeks before the livers were harvested to measure lipid content and gene expression. A: Hepatic lipid content. Neutral lipid = CE + TG. B: Hepatic mRNA expression of genes involved in cholesterol biosynthesis, lipogenesis, and inflammation. C: Nuclear accumulation of mature SREBP-1c and -2 isoforms in liver. D: Hepatic FAS and SCD-1 protein content. Data are expressed as mean ± SEM, n = 5. Groups with different letters are significantly different (P < 0.05) by one-way ANOVA and Tukey’s post hoc analysis.
Expression of other pro-inflammatory cytokines, such as IL-1β, IL-6, and IL-18, and the alternatively activated macrophage markers, arginase-1 and anti-inflammatory cytokine IL-10, were similar among all four diet groups (data not shown). To determine whether FADS2 products reduce hepatic lipogenic gene expression via attenuation of the SREBP pathway, we measured accumulation of proteolytically cleaved mature/nuclear SREBP-1 and -2 isoforms in liver nuclear preparations. Content of nuclear SREBP-1 (but not SREBP-2) was significantly reduced in BO-, EO-, and FO-fed mice relative to PO (Fig. 7C). Hepatic protein content of the SREBP-1c target genes, FAS and SCD-1, was also significantly lower in those groups relative to PO (Fig. 7D).
BO and EO are equally atheroprotective
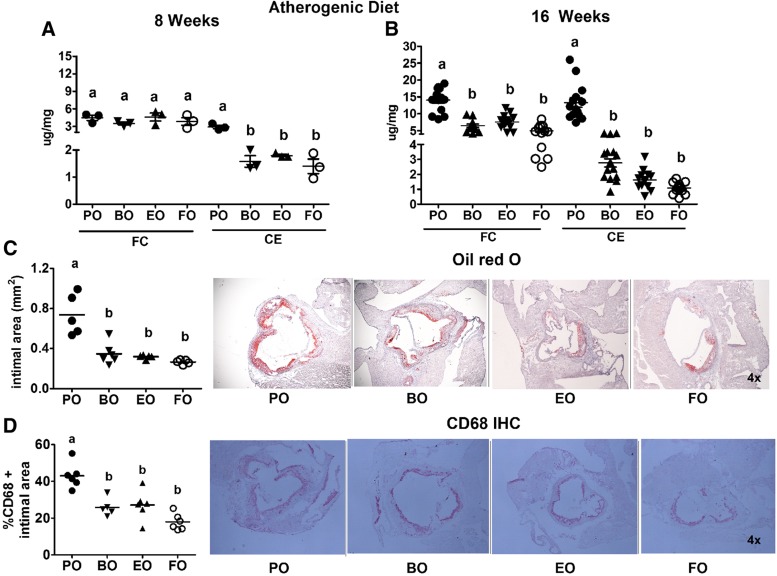
Aortic FC content was comparable among the groups after 8 weeks; however, CE content was significantly lower (∼2-fold lower) in all groups versus PO-fed mice (Fig. 8A). After 16 weeks of AD feeding, aortic FC and CE content was significantly lower in all groups (∼2-fold reduction in FC; ∼3- to 4-fold reduction in CE) versus PO-fed mice (Fig. 8B), indicating that BO, EO, and FO induced equivalent atheroprotection compared with PO. Significant aortic FC accumulation occurred between 8 and 16 weeks of AD feeding among all groups (∼3–4 μg/mg at 8 weeks vs. 6–14 μg/mg at 16 weeks). CE accumulation increased modestly among all groups (∼1–3 μg/mg at 8 weeks vs. ∼2–4 μg/mg at 16 weeks) relative to the PO group (∼4 μg/mg at 8 weeks to ∼16 μg/mg at 16 weeks), indicating a disproportionate increase of CE deposition in PO-fed mice.
Fig. 8.
Aortic atherosclerosis. LDLrKO mice were fed the indicated ADs for 16 weeks before aortas and hearts were harvested for atherosclerosis quantification. Aortas were lipid extracted for quantification of TC and FC content using GLC. CE content was calculated as (TC − FC) × 1.67. Aortic FC and CE content of mice fed PO, BO, EO, and FO for 8 weeks (A) or 16 weeks (B). Each data point represents an individual mouse aorta. C: Quantification of aortic root Oil Red O-positive intimal area (lesion area) and representative Oil Red O-stained aortic root sections. D: Quantification of percentage of aortic root lesional area occupied by CD68+ cells and representative CD68+ immunohistochemically stained aortic root sections. Each point represents the average lesion area of six to eight sections per mouse. Horizontal lines denote the mean for each diet group. Groups with different letters are significantly different (P < 0.05) by one-way ANOVA and Tukey’s post hoc analysis.
Aortic root intimal area was significantly lower (300–400 vs. 700 mm2) in the three PUFA-containing ADs compared with PO (Fig. 8C), as was aortic root intimal macrophage content (CD68+) (Fig. 8D). Collectively, these results show that BO, EO, and FO were equally effective in reducing aortic atherosclerosis and macrophage content.
Mouse aortas lack ox-CE species
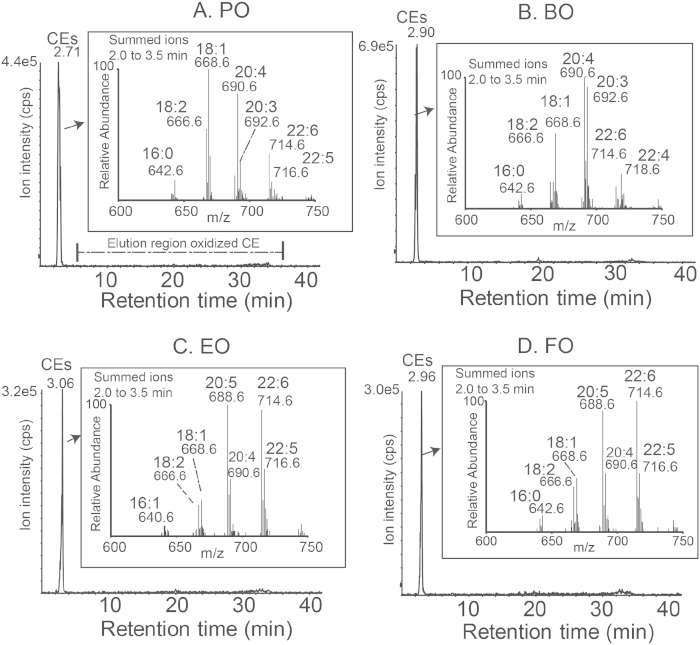
Human atheromas contain several distinct families of ox-CE species derived from PUFAs (e.g., 18:2 n-6, 20:4 n-6, and 22:6 n-3) that may play a role in atherogenesis (28). Because three of the four ADs showed substantial PUFA enrichment, we examined ox-CE content in mouse aortas after 16 weeks of AD feeding. Aortic CE fatty acyl molecular species reflected the fatty acid enrichment of the diet; however, aortas had undetectable levels of ox-CE in all diet groups (Fig. 9). The relative abundance of the nonoxidized CE molecular species presented in Fig. 9 insets do not precisely reflect absolute differences in molecular species due to different electrospray ionization response factors (mass spectrometric parameters) for the polyunsaturated CEs, as previously noted (29). Nonetheless, these raw data reveal dietary modification of PUFA-containing CE molecular species in aorta tissues reflecting the dietary fats.
Fig. 9.
Mouse atherosclerotic plaque ox-CE analysis. LDLrKO mice were fed the indicated ADs for 16 weeks before aortas were harvested for ox-CE analysis using normal phase-HPLC-MS/MS. Ion chromatograms of CE (2–4 min elution) and ox-CEs (6–36 min elution) for one aorta from each diet group. Inset presents the raw mass spectrometric data corresponding to CE molecular species labeled with m/z values and their acyl components, denoted by total acyl carbons and double bonds in this qualitative study. No ox-CE species were detectable for any of the four diet groups.
Thioglycollate-elicited peritoneal macrophage eicosanoid release is similar for BO- and EO-fed mice
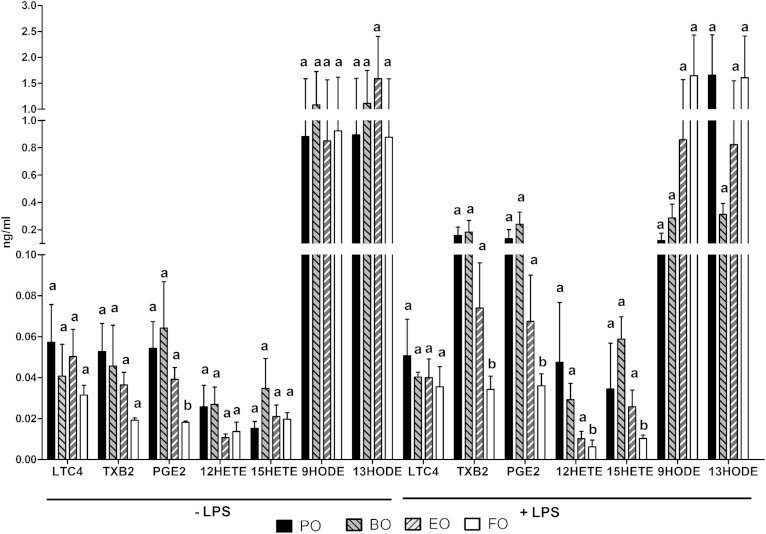
We studied the effects of AD feeding on thioglycollate-elicited peritoneal macrophage eicosanoid biosynthesis. After 16 weeks of AD feeding, thioglycollate-elicited peritoneal macrophages were incubated with or without LPS for 2 h before media eicosanoids were quantified. This model of stimulated eicosanoid biosynthesis has been recently reported, including details of gene expression related to PUFA metabolism stimulated by LPS (30). Among the eicosanoids measured, 12/15 lipoxygenase-derived LA metabolites 9- and 13-HODE were most abundant (Fig. 10). Furthermore, the type of dietary fat had little impact on production of thioglycollate-elicited peritoneal macrophage eicosanoid species in the basal state or after LPS stimulation. Only in the FO group were significant reductions observed for generation of TXB2, PGE2, and 12-hydroxyeicosatetraenoic acids after LPS stimulation (∼2- to 4-fold lowering vs. PO, EO, and BO). These data indicate that LPS stimulation revealed little difference in thioglycollate-elicited peritoneal macrophage eicosanoid biosynthesis among BO-, EO-, and PO-fed mice.
Fig. 10.
LPS-stimulated eicosanoid release from peritoneal macrophages. LDLrKO mice were fed the indicated ADs for 16 weeks. Mice were then injected with thioglycollate in the peritoneal cavity and macrophages were isolated 4 days later as described in the Materials and Methods. The macrophages were cultured for 3 h in serum-free medium and then treated with LPS (200 ng/ml) or PBS (−LPS) for 2 h before 1 ml medium was collected for eicosanoid quantification by LC/MS/MS analysis. Eicosanoids below the minimum detection level are not shown. Data are expressed as mean ± SEM, n = 5 per group. Groups with different letters are significantly different (P < 0.05) by one-way ANOVA and Tukey’s post hoc analysis.
BO and EO attenuate macrophage inflammatory response to LPS
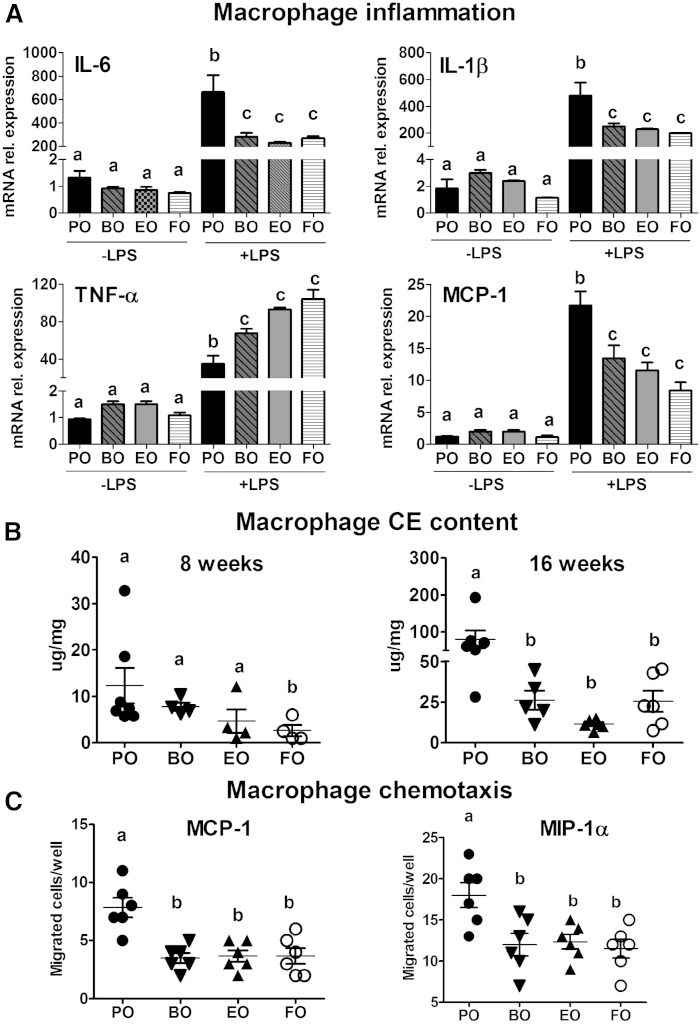
HODEs are natural ligands for macrophage PPARγ, which, when activated, can result in downregulation of the canonical NF-κB pathway (36, 37). Thus, we measured expression of NF-κB target genes after LPS-induced activation in thioglycollate-elicited peritoneal macrophages isolated from mice after 16 weeks of AD feeding. Basal (−LPS) gene expression of the pro-inflammatory cytokines IL-6, IL-1β, TNF-α, and the chemokine MCP-1 was comparable among all groups (Fig. 11A). LPS-treated macrophages from mice fed BO, EO, and FO versus PO had decreased mRNA expression of IL-6, IL-1β, and MCP-1, whereas TNF-α expression was significantly higher (Fig. 11A). However, 6 h after LPS stimulation, TNF-α mRNA expression was significantly lower in macrophages from BO-, EO-, and FO-fed mice versus PO-fed mice (data not shown), similar to the results for IL-6, IL-1β, and MCP-1 at 2 h. mRNA abundance for the alternatively activated macrophage markers, arginase 1 and CD206, was not different among groups (data not shown). Collectively, these data indicate that BO attenuates macrophage LPS-induced pro-inflammatory gene expression to the same extent as EO and FO, without affecting the gene expression of alternatively activated macrophage markers.
Fig. 11.
Macrophage inflammation, foam cell formation, and chemotaxis. LDLrKO mice were fed the indicated ADs for 8 or 16 weeks before thioglycollate-elicited macrophages were isolated. A: Inflammatory gene expression was measured using RT-PCR after 2 h treatment with LPS (200 ng/ml) or PBS (−LPS) in thioglycollate-elicited peritoneal macrophages. B: Peritoneal macrophage CE content was measured using GLC after 8 and 16 weeks of AD feeding. C: Ex vivo chemotaxis of thioglycollate-elicited peritoneal macrophages toward MCP-1 and MIP-1α was measured after 16 weeks of AD feeding. Data are expressed as mean ± SEM, n = 5. In (B) and (C), data points for individual mice are also shown. Groups with different letters are significantly different (P < 0.05) by one-way ANOVA and Tukey’s post hoc analysis.
BO and EO equally attenuate macrophage CE accumulation and chemotaxis
Given the equivalent effectiveness of BO in attenuating aortic CE and macrophage content, we sought to determine whether macrophages from BO-fed mice contributed to reduced aortic atherosclerosis via reduced macrophage foam cell formation. We used thioglycollate-elicited peritoneal macrophages (as a surrogate for aortic macrophages) from mice after 8 and 16 weeks of AD exposure to estimate CE content. At 8 weeks, macrophage CE content ranged from 5 to 20 μg/mg among the groups, but only the FO-fed mice had significantly lower macrophage CE content compared with PO-fed mice (Fig. 11B). After 16 weeks of AD feeding, macrophage CE content increased considerably (∼2- to 8-fold) compared with 8 weeks and was significantly attenuated in BO-, EO-, and FO-fed mice versus PO-fed mice (Fig. 11B). We next investigated whether reduced macrophage chemotaxis might partially explain the reduction in aortic root macrophage content in BO-, EO-, and FO-fed mice. All three PUFA-containing ADs were equally effective in reducing chemotaxis to MIP-1α and MCP-1 compared with PO (Fig. 11C).
DISCUSSION
In the current study, we tested the hypothesis that botanical oils enriched in 18:3 n-6 (BO) and 18:4 n-3 (EO) PUFAs beyond the rate-limiting FADS2 enzyme are equally atheroprotective compared with saturated/monounsaturated fat (PO) in LDLrKO mice. Although BO differs from EO and FO in its inability to lower plasma TGs, BO and EO were comparable in their ability to lower plasma cholesterol concentrations, especially VLDL-c. Additionally, BO was as effective as EO and FO in alleviating hepatic steatosis, and in regulating hepatic lipogenic and inflammatory gene expression compared with PO. As a result, BO and EO resulted in significant atheroprotection relative to PO at early (8 weeks) and advanced (16 weeks) atherosclerotic stages. We also report that ox-CEs were undetectable in early and advanced atherosclerotic arteries, suggesting that ox-CEs play a minimal role in murine atherosclerosis progression. Additionally, BO and EO significantly and equivalently attenuated macrophage inflammatory gene expression, CE content, and chemotaxis. BO had these atheroprotective outcomes despite significant enrichment of RBC membranes and plasma and liver lipids with 20:4 n-6, a precursor for several pro-inflammatory eicosanoids. Our results support the conclusion that botanical oils enriched in 18:3 n-6 and 18:4 n-3 PUFAs beyond the rate-limiting FADS2 enzyme are equally atheroprotective and hepatoprotective compared with saturated/monounsaturated fat.
Although previous studies in nonhuman primates and mice have demonstrated n-6 PUFA-mediated atheroprotection, these studies used dietary fats primarily enriched in LA (38–40). Our study focused on the hypothesis that dietary enrichment with n-6 PUFAs beyond FADS2 (i.e., GLA) would be as atheroprotective as EO, which we have previously demonstrated as equal to FO in preventing atherosclerosis progression (17, 41). On the other hand, flaxseed oil, which is enriched in ALA, a substrate for FADS2, was not as atheroprotective as FO; this outcome occurred despite significant enrichment of liver PLs with EPA and was likely due to the lower plasma LDL-c concentrations in the FO versus the flaxseed oil group (9). The combined results of the flaxseed oil and EO studies support our hypothesis that dietary enrichment in n-3 PUFAs beyond FADS2 is atheroprotective.
Our current results show that this is also true for the n-6 pathway of fatty acid elongation-desaturation. BO, which is enriched in GLA, was as atheroprotective as EO and FO despite its significant enrichment of RBCs and plasma and liver lipid fractions with AA, a fatty acid precursor to pro-inflammatory leukotrienes and prostaglandins (42). Concerns that elevated membrane AA may result in increased cellular inflammation that exacerbates atherosclerosis lack support in human studies (13). Moreover, LA-enriched diets have not enriched AA in plasma and tissue lipid fractions (40, 43, 44), likely due to inefficient FADS2 conversion of LA to AA (45, 46). In addition, a recent meta-analysis of 13 cohort studies (involving 310,602 individuals and 12,479 coronary heart disease events) revealed an inverse association between dietary LA intake and coronary heart disease risk, such that a 5% increase in energy intake from LA was associated with a 10% and 13% lower risk of coronary heart disease events and deaths, respectively (12). Collectively, these results suggest that increased consumption of dietary LA and GLA is not harmful and is potentially atheroprotective in the general population. Our results also suggest that assessing cardiovascular risk and inflammatory potential by dietary n-3/n-6 PUFA ratio may be an oversimplification that does not take into account differences between 18- versus ≥20-carbon PUFAs.
A recent study reported a single nucleotide polymorphism (rs174537) in the FADS1/2 gene cluster that affects plasma AA levels (47). The GG allele, which is nearly twice as frequent in African Americans as in European Americans, was associated with a small (∼3%), but statistically significant, enrichment in plasma AA. A subsequent study demonstrated that GG homozygotes for the rs174537 allele had increased plasma AA enrichment and production of LTB4 and 5-hydroxyeicosatetraenoic acids in zymosan-stimulated blood, suggesting that genetic polymorphisms may influence tissue AA content and inflammatory response to external pathogens (48). Thus, individuals harboring rs174537 homozygous GG alleles may be hyperresponsive to diets enriched in LA and GLA. Further studies are required to determine whether this potential hyperresponsiveness affects coronary heart disease risk.
Our results also suggest that BO and EO slow atherosclerosis progression in multiple ways, including reduced plasma VLDL-c concentrations, macrophage cholesterol content, inflammatory gene expression, and decreased macrophage migration toward a chemokine gradient. Plasma VLDL-c reduction likely had the greatest influence on atherosclerosis outcome in this study, because VLDL-c concentrations are the best lipoprotein/lipid predictor of aortic root atherosclerosis in LDLrKO mice (49), and we observed a strong positive association between plasma VLDL-c and aortic CE content (r2 = 0.88; P < 0.0001, data not shown). LDL-c concentrations were similar among PO-, BO-, and EO-fed mice, suggesting they had a minimal effect on atheroprotection in BO- and EO-fed mice. Plasma HDL-c was significantly elevated in all three diet groups, and may have contributed to atheroprotection relative to the PO group; however, only a small fraction (∼10%) of plasma cholesterol was distributed in HDL particles, making this a less likely possibility. In other studies investigating the influence of dietary n-3 and n-6 PUFAs on atherogenesis, LDL was the predominant plasma atherogenic lipoprotein (9, 38). Differences in plasma apoB lipoprotein response (VLDL vs. LDL) among these studies are likely due to genetic background (LDLrKO vs. apoB100 only-LDLrKO) and diet composition (0.02 vs. 0.2% cholesterol; 10 vs. 20% calories as fat).
A significant driver of atherogenesis in nonhuman primates and LDLrKO mice is hepatic production of saturated and monounsaturated CEs that are core constituents of secreted VLDL particles (11). Plasma VLDL particles undergo intravascular metabolism to become LDL particles, a major atherogenic lipoprotein particle in plasma. Deletion of the cholesterol esterification enzyme, steroyl O-acyltransferase 2 (SOAT2), strikingly reduces atherosclerosis regardless of dietary fat saturation (38), supporting a critical role for SOAT2 in the generation of atherogenic CEs in apoB-containing lipoproteins, such as VLDL and LDL. Hepatic CE content was significantly and equivalently reduced in mice fed BO and EO, suggesting the production of atherogenic CEs was blunted in these mice compared with those fed PO. This likely contributed to lower VLDL-c concentrations for the EO and BO groups, and reduced monounsaturated 18:1 n-9 CE species in plasma at the expense of FADS2-derived CEs.
One of the earliest events in atherogenesis is arterial retention of apoB lipoproteins by proteoglycans (50). A recent study showed that SOAT2 KO versus WT mice or mice fed FO versus monounsaturated fat had plasma LDL enriched in PUFA CE species and bound with less affinity to proteoglycans, suggesting a mechanism by which apoB lipoproteins from mice fed dietary PUFA may be less atherogenic (51). Although PUFA lipid species are more easily oxidized than their saturated and monounsaturated counterparts (52, 53) and ox-CE species have been identified in human tissue samples from endarterectomies (28), no arterial ox-CEs were detected in our study, despite significant enrichment of circulating and tissue lipids with AA in the BO group (Figs. 2, 3, 9). Thus, ox-CEs did not appear to contribute significantly to atherogenesis in our study.
CE-loaded macrophages are a hallmark of atherosclerosis, which result from chemokine-induced chemotaxis of monocytes to atherosclerotic lesions, differentiation of monocytes into macrophages expressing scavenger receptors, and unregulated uptake of modified apoB lipoproteins and apoB lipoprotein-proteoglycan complexes (54). In addition to reduced aortic cholesterol and aortic root intimal area in mice fed BO and EO versus PO, we also observed decreased aortic root macrophage (CD68+ cells) content and decreased macrophage chemotaxis in vitro. Thioglycollate-elicited peritoneal macrophages isolated from BO- and EO-fed mice also had reduced sterol content and inflammatory response to LPS compared with their PO-fed counterparts. Macrophage FC accumulation resulting from genetic deletion of macrophage efflux genes ABCA1 and ABCG1 results in hyper-responsiveness to pro-inflammatory stimuli and increased chemotaxis in vitro and in vivo (21, 55). However, in this study, the cholesterol elevation was due to CE, not FC, and there was no increase in macrophage ABCA1 and ABCG1 gene expression among diet groups (data not shown). These results suggest that decreased uptake of cholesterol from apoB lipoproteins likely explained the decreased macrophage atherogenic phenotype for mice fed EO and BO.
Diets enriched in n-3 PUFAs reduce hepatosteatosis (9, 16, 33, 56), unlike those containing n-6 PUFAs (27, 56). The reduced hepatic neutral lipid content in animals fed n-3 PUFAs is primarily mediated through decreased hepatic lipogenesis and is usually accompanied by reduced hepatic TG secretion (34, 57). In vitro, n-3 and n-6 PUFAs suppress SREBP-1c gene transcription (58, 59), decreasing hepatic lipogenesis, by competing with activators of LXR, a potent inducer of hepatic TG synthesis (58, 60). The n-3 and n-6 PUFAs also increase mRNA degradation (61), inhibit the proteolytic processing of SREBP-1c in vitro (59), and accelerate the degradation of nuclear SREBP-1c (62), reducing lipogenesis. Thus, we hypothesize that BO, but not the other n-6 PUFA-enriched diets (27, 56), reduces hepatosteatosis relative to diets containing saturated/monounsaturated fatty acids via its ability to enrich liver lipids in AA through elongation and desaturation of GLA. Many n-6 PUFA-enriched botanical oils contain LA as the predominant fatty acyl species, accounting for 85–90% of n-6 PUFA consumption in the US (10). However, as discussed above, diets enriched in LA do not result in plasma and tissue AA enrichment (44, 63). Genetic deletion of Elovl5, the gene encoding the enzyme that elongates 18:3 n-6 to 20:3 n-6 and 18:4 n-3 to 20:4 n-3, results in diminished hepatic lipid AA and DHA and increased neutral lipid storage (64). Feeding Elovl5 KO mice AA or DHA rescued the hepatosteatosis phenotype by decreasing nuclear SREBP-1c and de novo lipogenesis with no changes in mRNA or membrane-bound SREBP-1c, supporting a role for AA in blocking SREBP-1c cleavage and activation. In our study, liver membrane (i.e., PL) AA content was elevated, nuclear SREBP-1 content was reduced, and SREBP-1c-targeted genes (FAS, SCD-1) were reduced in BO- versus PO-fed mice, supporting a role for elevated AA in reducing hepatosteatosis in BO-fed mice. In another study, BO reduced ethanol-induced hepatosteatosis (65), suggesting that BO protects against hepatosteatosis regardless of the method of induction. How elevated liver AA inhibits the proteolytic processing of membrane SREBP-1c is unclear, but may be related to fluidity of the endoplasmic reticulum membrane. Regardless of the detailed mechanism for decreasing hepatic lipogenesis, our study reveals a distinct advantage of BO in reducing hepatosteatosis and atherosclerosis in contrast to other n-6 PUFA-enriched botanical oils that are atheroprotective, but do not prevent hepatosteatosis.
Although BO, EO, and FO all reduced hepatosteatosis comparably relative to PO, BO did not reduce hepatic TG secretion or plasma TG concentrations, whereas EO and FO did (Fig. 6). FO consistently reduces hepatic TG secretion, likely due to reduced hepatic lipogenesis (34, 41). This paradox is likely due to the fact that only a small fraction of hepatic TG is mobilized for secretion; therefore, a large decrease in hepatic TG content does not necessarily result in decreased TG secretion. For example, knockdown of SCD-1 with a targeting anti-sense oligonucleotide resulted in a >90% reduction in hepatic TG content, but did not affect hepatic TG secretion compared with mice treated with a nontargeting anti-sense oligonucleotide (66). We also observed a 50% decrease in newly synthesized TG secreted from livers of monkeys fed FO versus lard diets, although liver TG synthesis was similar between diet groups (34). These combined results suggest a unique secretory pool of TG that may be regulated differently than the bulk TG storage pool in hepatocytes.
Collectively, our results support the hypothesis that dietary enrichment with FADS2 fatty acid products, such as SDA and GLA, results in membrane and plasma lipid enrichment in EPA and AA, which in turn is associated with reduced plasma lipids, atherosclerosis, and hepatosteatosis. This hypothesis was based on data in humans and animal models showing that conversion of 18-carbon PUFAs to ≥20-carbon PUFAs is inefficient, and that bypassing the rate-limiting FADS2 step is possible by feeding botanical oils enriched in FADS2 products. Furthermore, allowing for body surface area differences between mice and humans (67), achieving this dose of botanical oils in the diet is feasible. For example, our ADs contained BO and EO as 10% energy, which corresponds to a human equivalent dose of 0.81% energy [10% × 3/37 (67)], well within the range of n-3 PUFA doses administered in many randomized clinical trials (68) and the American Heart Association’s recommended n-3 PUFA intake for individuals with documented coronary heart disease (69). While replacing dietary saturated fat with PUFAs is viewed as atheroprotective in general, our study suggests a more targeted approach of dietary PUFA replacement using known biochemical pathways may enhance the beneficial outcomes of increased dietary PUFA consumption.
Acknowledgments
Anti-SREBP-1 and -2 antibodies were generously donated by Dr. Timothy Osborne (Sanford Burnham Medical Research Institute). The authors gratefully acknowledge Karen Klein (Biomedical Research Services and Administration, Wake Forest School of Medicine) for editing the manuscript.
Footnotes
Abbreviations:
- AA
- arachidonic acid
- AD
- atherogenic diet
- ALA
- α-linolenic acid
- AUC
- area under the curve
- BO
- borage oil
- CE
- cholesteryl ester
- DGLA
- dihomo-γ-linolenic acid
- EO
- echium oil
- FADS2
- fatty acid desaturase 2 (delta six fatty acid desaturase enzyme)
- FC
- free cholesterol
- FO
- fish oil
- FPLC
- fast protein LC
- GLA
- γ-linolenic acid
- GLC
- gas-LC
- HDL-c
- HDL cholesterol
- LA
- linoleic acid
- LDL-c
- LDL cholesterol
- LDLrKO
- LDL receptor KO
- LPS
- lipopolysaccharide
- ox-CE
- oxidized cholesteryl ester (fatty acyl species)
- PGE
- prostaglandin E
- PL
- phospholipid
- PO
- palm oil
- RBC
- red blood cell
- SCD-1
- stearoyl-CoA desaturase-1
- SDA
- stearidonic acid
- SOAT2
- steroyl O-acyltransferase 2
- TC
- total cholesterol
- VLDL-c
- VLDL cholesterol
REFERENCES
- 1.Lloyd-Jones D., Adams R., Carnethon M., De Simone G., Ferguson T. B., Flegal K., Ford E., Furie K., Go A., Greenlund K., et al. 2009. Heart disease and stroke statistics–2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 119: 480–486. [DOI] [PubMed] [Google Scholar]
- 2.Mozaffarian D., Micha R., Wallace S. 2010. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta-analysis of randomized controlled trials. PLoS Med. 7: e1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bigger J. T., Jr, El-Sherif T. 2001. Polyunsaturated fatty acids and cardiovascular events: a fish tale. Circulation. 103: 623–625. [DOI] [PubMed] [Google Scholar]
- 4.Calder P. C., Grimble R. F. 2002. Polyunsaturated fatty acids, inflammation and immunity. Eur. J. Clin. Nutr. 56(Suppl 3): S14–S19. [DOI] [PubMed] [Google Scholar]
- 5.Kang J. X., Leaf A. 1996. Antiarrhythmic effects of polyunsaturated fatty acids. Recent studies. Circulation. 94: 1774–1780. [DOI] [PubMed] [Google Scholar]
- 6.Parks J. S., Kaduck-Sawyer J., Bullock B. C., Rudel L. L. 1990. Effect of dietary fish oil on coronary artery and aortic atherosclerosis in African green monkeys. Arteriosclerosis. 10: 1102–1112. [DOI] [PubMed] [Google Scholar]
- 7.Hu F. B., Bronner L., Willett W. C., Stampfer M. J., Rexrode K. M., Albert C. M., Hunter D., Manson J. E. 2002. Fish and omega-3 fatty acid intake and risk of coronary heart disease in women. JAMA. 287: 1815–1821. [DOI] [PubMed] [Google Scholar]
- 8.Horrobin D. F. 1993. Fatty acid metabolism in health and disease: the role of delta-6-desaturase. Am. J. Clin. Nutr. 57: 732S–736S; discussion 736S–737S. [DOI] [PubMed] [Google Scholar]
- 9.Degirolamo C., Kelley K. L., Wilson M. D., Rudel L. L. 2010. Dietary n-3 LCPUFA from fish oil but not alpha-linolenic acid-derived LCPUFA confers atheroprotection in mice. J. Lipid Res. 51: 1897–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris W. S., Mozaffarian D., Rimm E., Kris-Etherton P., Rudel L. L., Appel L. J., Engler M. M., Engler M. B., Sacks F. 2009. Omega-6 fatty acids and risk for cardiovascular disease: a science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation. 119: 902–907. [DOI] [PubMed] [Google Scholar]
- 11.Degirolamo C., Shelness G. S., Rudel L. L. 2009. LDL cholesteryl oleate as a predictor for atherosclerosis: evidence from human and animal studies on dietary fat. J. Lipid Res. 50(Suppl): S434–S439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farvid M. S., Ding M., Pan A., Sun Q., Chiuve S. E., Steffen L. M., Willett W. C., Hu F. B. 2014. Dietary linoleic acid and risk of coronary heart disease: a systematic review and meta-analysis of prospective cohort studies. Circulation. 130: 1568–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris W. S., Shearer G. C. 2014. Omega-6 fatty acids and cardiovascular disease: friend or foe? Circulation. 130: 1562–1564. [DOI] [PubMed] [Google Scholar]
- 14.Chopra J., Webster R. O. 1988. PGE1 inhibits neutrophil adherence and neutrophil-mediated injury to cultured endothelial cells. Am. Rev. Respir. Dis. 138: 915–920. [DOI] [PubMed] [Google Scholar]
- 15.Jones D. A., Fitzpatrick F. A. 1990. “Suicide” inactivation of thromboxane A2 synthase. Characteristics of mechanism-based inactivation with isolated enzyme and intact platelets. J. Biol. Chem. 265: 20166–20171. [PubMed] [Google Scholar]
- 16.Zhang P., Boudyguina E., Wilson M. D., Gebre A. K., Parks J. S. 2008. Echium oil reduces plasma lipids and hepatic lipogenic gene expression in apoB100-only LDL receptor knockout mice. J. Nutr. Biochem. 19: 655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown A. L., Zhu X., Rong S., Shewale S., Seo J., Boudyguina E., Gebre A. K., Alexander-Miller M. A., Parks J. S. 2012. Omega-3 fatty acids ameliorate atherosclerosis by favorably altering monocyte subsets and limiting monocyte recruitment to aortic lesions. Arterioscler. Thromb. Vasc. Biol. 32: 2122–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rudel L. L., Kelley K., Sawyer J. K., Shah R., Wilson M. D. 1998. Dietary monounsaturated fatty acids promote aortic atherosclerosis in LDL receptor-null, human ApoB100-overexpressing transgenic mice. Arterioscler. Thromb. Vasc. Biol. 18: 1818–1827. [DOI] [PubMed] [Google Scholar]
- 19.Bligh E. G., Dyer W. J. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37: 911–917. [DOI] [PubMed] [Google Scholar]
- 20.Bi X., Zhu X., Gao C., Shewale S., Cao Q., Liu M., Boudyguina E., Gebre A. K., Wilson M. D., Brown A. L., et al. 2014. Myeloid cell-specific ATP-binding cassette transporter A1 deletion has minimal impact on atherogenesis in atherogenic diet-fed low-density lipoprotein receptor knockout mice. Arterioscler. Thromb. Vasc. Biol. 34: 1888–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu X., Lee J. Y., Timmins J. M., Brown J. M., Boudyguina E., Mulya A., Gebre A. K., Willingham M. C., Hiltbold E. M., Mishra N., et al. 2008. Increased cellular free cholesterol in macrophage-specific Abca1 knock-out mice enhances pro-inflammatory response of macrophages. J. Biol. Chem. 283: 22930–22941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Millar J. S., Cromley D. A., McCoy M. G., Rader D. J., Billheimer J. T. 2005. Determining hepatic triglyceride production in mice: comparison of poloxamer 407 with Triton WR-1339. J. Lipid Res. 46: 2023–2028. [DOI] [PubMed] [Google Scholar]
- 23.Carr T. P., Andresen C. J., Rudel L. L. 1993. Enzymatic determination of triglyceride, free cholesterol, and total cholesterol in tissue lipid extracts. Clin. Biochem. 26: 39–42. [DOI] [PubMed] [Google Scholar]
- 24.Hattori M., Tugores A., Veloz L., Karin M., Brenner D. A. 1990. A simplified method for the preparation of transcriptionally active liver nuclear extracts. DNA Cell Biol. 9: 777–781. [DOI] [PubMed] [Google Scholar]
- 25.Lee J. H., Giannikopoulos P., Duncan S. A., Wang J., Johansen C. T., Brown J. D., Plutzky J., Hegele R. A., Glimcher L. H., Lee A. H. 2011. The transcription factor cyclic AMP-responsive element-binding protein H regulates triglyceride metabolism. Nat. Med. 17: 812–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeon T. I., Esquejo R. M., Roqueta-Rivera M., Phelan P. E., Moon Y. A., Govindarajan S. S., Esau C. C., Osborne T. F. 2013. An SREBP-responsive microRNA operon contributes to a regulatory loop for intracellular lipid homeostasis. Cell Metab. 18: 51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rong S., Cao Q., Liu M., Seo J., Jia L., Boudyguina E., Gebre A. K., Colvin P. L., Smith T. L., Murphy R. C., et al. 2012. Macrophage 12/15 lipoxygenase expression increases plasma and hepatic lipid levels and exacerbates atherosclerosis. J. Lipid Res. 53: 686–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hutchins P. M., Moore E. E., Murphy R. C. 2011. Electrospray MS/MS reveals extensive and nonspecific oxidation of cholesterol esters in human peripheral vascular lesions. J. Lipid Res. 52: 2070–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy R. C., Barkley R. M., Zemski Berry K., Hankin J., Harrison K., Johnson C., Krank J., McAnoy A., Uhlson C., Zarini S. 2005. Electrospray ionization and tandem mass spectrometry of eicosanoids. Anal. Biochem. 346: 1–42. [DOI] [PubMed] [Google Scholar]
- 30.Maurya M. R., Gupta S., Li X., Fahy E., Dinasarapu A. R., Sud M., Brown H. A., Glass C. K., Murphy R. C., Russell D. W., et al. 2013. Analysis of inflammatory and lipid metabolic networks across RAW264.7 and thioglycolate-elicited macrophages. J. Lipid Res. 54: 2525–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu X., Westcott M. M., Bi X., Liu M., Gowdy K. M., Seo J., Cao Q., Gebre A. K., Fessler M. B., Hiltbold E. M., et al. 2012. Myeloid cell-specific ABCA1 deletion protects mice from bacterial infection. Circ. Res. 111: 1398–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chung S., Timmins J. M., Duong M., Degirolamo C., Rong S., Sawyer J. K., Singaraja R. R., Hayden M. R., Maeda N., et al. 2010. Targeted deletion of hepatocyte ABCA1 leads to very low density lipoprotein triglyceride overproduction and low density lipoprotein hypercatabolism. J. Biol. Chem 285: 12197–12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown J. M., Chung S., Sawyer J. K., Degirolamo C., Alger H. M., Nguyen T. M., Zhu X., Duong M. N., Brown A. L., Lord C., et al. 2010. Combined therapy of dietary fish oil and stearoyl-CoA desaturase 1 inhibition prevents the metabolic syndrome and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 30: 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parks J. S., Johnson F. L., Wilson M. D., Rudel L. L. 1990. Effect of fish oil diet on hepatic lipid metabolism in nonhuman primates: lowering of secretion of hepatic triglyceride but not apoB. J. Lipid Res. 31: 455–466. [PubMed] [Google Scholar]
- 35.Parks J. S., Wilson M. D., Johnson F. L., Rudel L. L. 1989. Fish oil decreases hepatic cholesteryl ester secretion but not apoB secretion in African green monkeys. J. Lipid Res. 30: 1535–1544. [PubMed] [Google Scholar]
- 36.Nagy L., Tontonoz P., Alvarez J. G., Chen H., Evans R. M. 1998. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell. 93: 229–240. [DOI] [PubMed] [Google Scholar]
- 37.Ricote M., Li A. C., Willson T. M., Kelly C. J., Glass C. K. 1998. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 391: 79–82. [DOI] [PubMed] [Google Scholar]
- 38.Bell T. A., 3rd, Kelley K., Wilson M. D., Sawyer J. K., Rudel L. L. 2007. Dietary fat-induced alterations in atherosclerosis are abolished by ACAT2-deficiency in ApoB100 only, LDLr-/- mice. Arterioscler. Thromb. Vasc. Biol. 27: 1396–1402. [DOI] [PubMed] [Google Scholar]
- 39.Rudel L. L., Johnson F. L., Sawyer J. K., Wilson M. S., Parks J. S. 1995. Dietary polyunsaturated fat modifies low-density lipoproteins and reduces atherosclerosis of nonhuman primates with high and low diet responsiveness. Am. J. Clin. Nutr. 62: 463S–470S. [DOI] [PubMed] [Google Scholar]
- 40.Rudel L. L., Parks J. S., Sawyer J. K. 1995. Compared with dietary monounsaturated and saturated fat, polyunsaturated fat protects African green monkeys from coronary artery atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 15: 2101–2110. [DOI] [PubMed] [Google Scholar]
- 41.Forrest L. M., Boudyguina E., Wilson M. D., Parks J. S. 2012. Echium oil reduces atherosclerosis in apoB100-only LDLrKO mice. Atherosclerosis. 220: 118–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sala A., Folco G., Murphy R. C. 2010. Transcellular biosynthesis of eicosanoids. Pharmacol. Rep 62: 503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thornburg J. T., Parks J. S., Rudel L. L. 1995. Dietary fatty acid modification of HDL phospholipid molecular species alters lecithin: cholesterol acyltransferase reactivity in cynomolgus monkeys. J. Lipid Res. 36: 277–289. [PubMed] [Google Scholar]
- 44.Rett B. S., Whelan J. 2011. Increasing dietary linoleic acid does not increase tissue arachidonic acid content in adults consuming Western-type diets: a systematic review. Nutr. Metab. (Lond). 8: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Demmelmair H., Iser B., Rauh-Pfeiffer A., Koletzko B. 1999. Comparison of bolus versus fractionated oral applications of [13C]-linoleic acid in humans. Eur. J. Clin. Invest. 29: 603–609. [DOI] [PubMed] [Google Scholar]
- 46.Hussein N., Ah-Sing E., Wilkinson P., Leach C., Griffin B. A., Millward D. J. 2005. Long-chain conversion of [13C]linoleic acid and alpha-linolenic acid in response to marked changes in their dietary intake in men. J. Lipid Res. 46: 269–280. [DOI] [PubMed] [Google Scholar]
- 47.Mathias R. A., Sergeant S., Ruczinski I., Torgerson D. G., Hugenschmidt C. E., Kubala M., Vaidya D., Suktitipat B., Ziegler J. T., Ivester P., et al. 2011. The impact of FADS genetic variants on omega6 polyunsaturated fatty acid metabolism in African Americans. BMC Genet. 12: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hester A. G., Murphy R. C., Uhlson C. J., Ivester P., Lee T. C., Sergeant S., Miller L. R., Howard T. D., Mathias R. A., Chilton F. H. 2014. Relationship between a common variant in the fatty acid desaturase (FADS) cluster and eicosanoid generation in humans. J. Biol. Chem. 289: 22482–22489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.VanderLaan P. A., Reardon C. A., Thisted R. A., Getz G. S. 2009. VLDL best predicts aortic root atherosclerosis in LDL receptor deficient mice. J. Lipid Res. 50: 376–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Skålén K., Gustafsson M., Rydberg E. K., Hultén L. M., Wiklund O., Innerarity T. L., Borén J. 2002. Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature. 417: 750–754. [DOI] [PubMed] [Google Scholar]
- 51.Melchior J. T., Sawyer J. K., Kelley K. L., Shah R., Wilson M. D., Hantgan R. R., Rudel L. L. 2013. LDL particle core enrichment in cholesteryl oleate increases proteoglycan binding and promotes atherosclerosis. J. Lipid Res. 54: 2495–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thomas M. J., Thornburg T., Manning J., Hooper K., Rudel L. L. 1994. Fatty acid composition of low-density lipoprotein influences its susceptibility to autoxidation. Biochemistry. 33: 1828–1834. [DOI] [PubMed] [Google Scholar]
- 53.Whitman S. C., Fish J. R., Rand M. L., Rogers K. A. 1994. n-3 fatty acid incorporation into LDL particles renders them more susceptible to oxidation in vitro but not necessarily more atherogenic in vivo. Arterioscler. Thromb. 14: 1170–1176. [DOI] [PubMed] [Google Scholar]
- 54.Moore K. J., Tabas I. 2011. Macrophages in the pathogenesis of atherosclerosis. Cell. 145: 341–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yvan-Charvet L., Welch C., Pagler T. A., Ranalletta M., Lamkanfi M., Han S., Ishibashi M., Li R., Wang N., Tall A. R. 2008. Increased inflammatory gene expression in ABC transporter-deficient macrophages: free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation. 118: 1837–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bell T. A., 3rd, Wilson M. D., Kelley K., Sawyer J. K., Rudel L. L. 2007. Monounsaturated fatty acyl-coenzyme A is predictive of atherosclerosis in human apoB-100 transgenic, LDLr-/- mice. J. Lipid Res. 48: 1122–1131. [DOI] [PubMed] [Google Scholar]
- 57.Jump D. B., Clarke S. D. 1999. Regulation of gene expression by dietary fat. Annu. Rev. Nutr. 19: 63–90. [DOI] [PubMed] [Google Scholar]
- 58.Ou J., Tu H., Shan B., Luk A., DeBose-Boyd R. A., Bashmakov Y., Goldstein J. L., Brown M. S. 2001. Unsaturated fatty acids inhibit transcription of the sterol regulatory element-binding protein-1c (SREBP-1c) gene by antagonizing ligand-dependent activation of the LXR. Proc. Natl. Acad. Sci. USA. 98: 6027–6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hannah V. C., Ou J., Luong A., Goldstein J. L., Brown M. S. 2001. Unsaturated fatty acids down-regulate srebp isoforms 1a and 1c by two mechanisms in HEK-293 cells. J. Biol. Chem. 276: 4365–4372. [DOI] [PubMed] [Google Scholar]
- 60.Chen G., Liang G., Ou J., Goldstein J. L., Brown M. S. 2004. Central role for liver X receptor in insulin-mediated activation of Srebp-1c transcription and stimulation of fatty acid synthesis in liver. Proc. Natl. Acad. Sci. USA. 101: 11245–11250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu J., Teran-Garcia M., Park J. H., Nakamura M. T., Clarke S. D. 2001. Polyunsaturated fatty acids suppress hepatic sterol regulatory element-binding protein-1 expression by accelerating transcript decay. J. Biol. Chem. 276: 9800–9807. [DOI] [PubMed] [Google Scholar]
- 62.Botolin D., Wang Y., Christian B., Jump D. B. 2006. Docosahexaneoic acid (22:6,n-3) regulates rat hepatocyte SREBP-1 nuclear abundance by Erk- and 26S proteasome-dependent pathways. J. Lipid Res. 47: 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rioux F. M., Innis S. M. 1992. Arachidonic acid concentrations in plasma and liver phospholipid and cholesterol esters of piglets raised on formulas with different linoleic and linolenic acid contents. Am. J. Clin. Nutr. 56: 106–112. [DOI] [PubMed] [Google Scholar]
- 64.Moon Y. A., Hammer R. E., Horton J. D. 2009. Deletion of ELOVL5 leads to fatty liver through activation of SREBP-1c in mice. J. Lipid Res. 50: 412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lukivskaya O. Y., Naruta E., Sadovnichy V., Kirko S., Buko V. U. 2012. Reversal of experimental ethanol-induced liver steatosis by borage oil. Phytother. Res. 26: 1626–1631. [DOI] [PubMed] [Google Scholar]
- 66.Brown J. M., Chung S., Sawyer J. K., Degirolamo C., Alger H. M., Nguyen T., Zhu X., Duong M. N., Wibley A. L., Shah R., et al. 2008. Inhibition of stearoyl-coenzyme A desaturase 1 dissociates insulin resistance and obesity from atherosclerosis. Circulation. 118: 1467–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reagan-Shaw S., Nihal M., Ahmad N. 2008. Dose translation from animal to human studies revisited. FASEB J. 22: 659–661. [DOI] [PubMed] [Google Scholar]
- 68.Rizos E. C., Ntzani E. E., Bika E., Kostapanos M. S., Elisaf M. S. 2012. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA. 308: 1024–1033. [DOI] [PubMed] [Google Scholar]
- 69.Kris-Etherton P. M., Harris W. S., Appel L. J., American Heart Association Nutrition Committee 2002. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 106: 2747–2757. [DOI] [PubMed] [Google Scholar]