Abstract
Objective
To assess the diagnostic accuracy of photodynamic diagnostic (PDD) ureterorenoscopy for detecting upper-urinary tract transitional cell carcinoma (UUT-TCC) in our initial 3 years, and compare the results with that of white light ureterorenoscopy (WLU).
Patients and methods
Between August 2007 and March 2010, 32 patients underwent PDD flexible ureterorenoscopy for UUT-TCC. Oral 5-aminolaevulinic acid (5-ALA) was used as the photosensitiser. The sensitivity, specificity and detection rate of PDD were calculated.
Results
The sensitivity, specificity, positive and negative predictive values of PDD for detecting abnormal tissue were 96%, 100%, 100% and 88%, compared to the results of WLU of 80%, 86%, 95% and 55%, respectively. PDD was able to detect 96% (24/25) of the abnormal tissue and 96% (21/22) of cancerous tissue, while WLU detected 80% (20/25) of abnormal tissue and 91% (20/22) of the tumour tissue. Three patients of the 32 (10%) developed side-effects related to 5-ALA; two patients developed a facial-skin photosensitive reaction and one developed hypotension. All were treated conservatively, with no long-term effects.
Conclusion
PDD can be used safely in the UUT, and with a higher sensitivity, specificity and detection rate than standard WLU for detecting UUT tumours.
Abbreviations: PDD, photodynamic diagnosis; P(N)PV, positive (negative) predictive value; WL(U)(C), white-light (ureterorenoscopy) (cystoscopy); TURBT, transurethral resection of bladder tumour; UUT, upper urinary tract; FURS, flexible ureterorenoscopy; 5-ALA, 5-aminolaevulinic acid; TP, true-positive; TN, true-negative; FP, false-positive; FN, false-negative; CIS, carcinoma in situ
Keywords: Transitional cell carcinoma, Photodynamic, Diagnosis, 5-Aminolaevulinic acid, Flexible ureterorenoscopy
Introduction
Of urinary tract malignancies, 7% arise in the ureter and pelvicalyceal system, with TCC being the most common type [1–4]. The standard treatment of upper-urinary tract TCC (UUT-TCC) is a radical nephroureterectomy. A more conservative approach with repeated flexible ureterorenoscopy (FURS) in patients who are unfit for radical surgery or have small, unifocal, low-grade disease, produces similar results to that of radical surgery [3,5,6]. However, invisible lesions can be missed, leading to a progression or worsening of the disease.
To aid the visualisation of tumours during FURS, photodynamic diagnosis (PDD) has been attempted, following its success in detecting invisible bladder tumours [3,6,7]. PDD can detect more bladder tumours than white light cystoscopy (WLC) alone, and can facilitate a more complete resection if used during transurethral resection of bladder tumour (TURBT) [6].
PDD has been confirmed as a viable tool to facilitate the investigation of bladder tumours by helping to distinguish them from benign tissue [6,7]. The basic principle of PDD is the interaction of a photosensitising agent, such as 5-aminolaevulinic acid (5-ALA) with light. This agent has a high uptake by tumour cells and emits light with an appropriate wavelength [6,7] when light is absorbed as high energy and re-emitted with a lower energy, producing a different wavelength [6,7].
We audited the diagnostic accuracy of PDD to detect UUT tumours in our initial 3-year cohort of patients, compared with the findings of WL ureterorenoscopy (WLU) in the same patients.
Patients and methods
Between August 2007 and September 2010, we audited 32 patients who were unfit for radical surgery, and who had a small, unifocal, low-grade UUT-TCC, previously treated endoscopically for their tumour and requiring follow-up, or who required ureterorenoscopy to exclude UUT-TCC. All patients provided informed consent for the procedure.
All patients had CT urography routinely as a follow-up investigation. Urinary cytology is not used routinely, as the test is not sensitive enough to detect UUT-TCC. Each patient received 20 mg/kg body weight of oral 5-ALA (Medac, Scion House, Stirling University Innovation Park, Stirling, UK) dissolved in 50 mL of water. Direct exposure of patients to sunlight or strong room light was avoided for 24 h. All the procedures were performed by an experienced endourologist. Rigid cystoscopy and FURS were conducted using the D-light system (Karl Storz, Tuttlingen, Germany) to detect fluorescence, using a xenon arc lamp with blue light at 380–440 nm wavelength (PDD cystoscopy with 12° and 70° telescopes, and a 7.5 F Flex-X PDD system, Karl Storz).
All suspicious lesions were biopsied (using haematoxylin and eosin staining) and tumours ablated. Random biopsies from normal-looking mucosa (if reported abnormal on CT urography) were also taken, and from areas of previous ablations (first follow-up ureterorenoscopy). Ablation was done with curative intent, using a holmium laser (365 μm); the power levels for ablation were 1–1.2 J and the pulse frequencies 10–15 Hz. After laser ablation of UUT-TCC, biopsies were taken from the base, which was ablated with diathermy thereafter. Our technique of ureterorenoscopy, biopsy and ablation is based on the principles described by Tawfiek et al. [8] and Grasso et al. [9]. Eleven patients requiring ablation were stented afterwards. We recommend removing the stent 1 month before the first check ureterorenoscopy, to avoid falsely positive fluorescence. The follow-up comprised an initial ureterorenoscopy at 3 months, and if there is no recurrence, then follow-up ureterorenoscopy is done 6-monthly for the first 2 years and then annually. However, if there are recurrences then the follow-up is 3-monthly until no recurrence is detected.
Lesions visualised under WLU and PDD were correlated with the biopsy results to obtain true-positive (TP), true-negative (TN), false-positive (FP) or false-negative (FN) values. These were used to calculate the sensitivity, specificity, negative and positive predictive values (NPV and PPV), and accuracy for the correct detection of the lesion for WLU and PDD blue light. For all analyses we used the Meta-analysis of Diagnostic and Screening Tests 1.4 programme (Unidad de Bioestadistica Clinica, Hospital Ramon y Cajal, Madrid) and Review Manager 5.1.4 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011).
The Improvement and Quality Committee of NHS Tayside Board approved the implementation of PDD FURS in these patients.
Results
All patients were previously diagnosed with unifocal, low-grade UUT-TCC or were unfit for nephroureterectomy. The mean (SD) age of the patients was 73.34 (6.52) years, with a male–female ratio of 25:7. Seven of the patients had obvious tumours, which were ablated. In all there were 25 patients whose biopsy confirmed abnormal UUT, while 22 had UUT-TCC, two carcinoma in situ (CIS), 16 G2pTa, two G3pTa, one G2pT1, one G3pT1 and three had dysplasia. We considered the dysplastic biopsy tissue as abnormal due to the high risk of cancerous transformation.
The TP, TN, FP, and FN of WLU and PDD are shown in Table 1. PDD-guided FURS was able to detect 96% (24/25) of the abnormal tissue and 95.5% (21/22) of the tumour tissue. WLU detected 80% (20/25) of the abnormal tissue and 91% (20/22) of the tumour tissue. The sensitivity, specificity, PPV and NPV (with 95% CIs) of WLU and PDD to detect abnormal tissue were 0.8 (0.59–0.93) vs. 0.96 (0.80–1.0), 0.86 (0.42–1.0) vs. 1.0 (0.53–1.0), 0.95 vs. 1.0, and 0.55 vs. 0.88, respectively. Despite the better results with PDD, there was no statistically significant difference between them for sensitivity (P = 0.07) or specificity (P = 0.226).
Table 1.
The tests of diagnostic accuracy between PDD and WLU compared with final biopsy.
| Variable | Biopsy |
|
|---|---|---|
| Positive | Negative | |
| PDD | ||
| Positive | 24 | 0 |
| Negative | 1 | 7 |
| Sensitivity (95% CI) | 0.96 (0.80–1.0) | |
| Specificity (95% CI) | 1.0 (0.59–1.0 | |
| Overall accuracy, % | 96 | |
| PPV | 1.0 | |
| NPV | 0.88 | |
| WLU | ||
| Positive | 20 | 1 |
| Negative | 5 | 6 |
| Sensitivity (95% CI) | 0.8 (0.59–0.93) | |
| Specificity (95% CI) | 0.86 (0.42–1.0) | |
| Overall accuracy, % | 80 | |
| PPV | 0.95 | |
| NPV | 0.55 | |
Two patients developed a facial-skin photosensitive reaction and one developed hypotension. These were considered side-effects of the 5-ALA and resolved after a day. All patients were treated conservatively with no long-term (6-month) residual side-effects.
Of all patients, nine (28%) had concomitant bladder cancers, of whom four (13% of all patients) had their bladder lesions only detectable by PDD, with the remainder having clear lesions on WLU. Three of the four lesions detectable by PDD were CIS, while the fourth was dysplasia.
Discussion
This audit showed that PDD FURS can detect more UUT lesions than WLU alone (96%, 24/25, vs. 80%, 20/25, respectively). Furthermore, the use of oral 5-ALA as a photosensitising agent was safe, with minimal side-effects. In this cohort of patients the few side-effects experienced were treated conservatively, with no long-term complications.
PDD has been used in several specialities to aid in the diagnosis of abnormal tissue. These include the skin, brain, upper respiratory tract, biliary tract and the bladder. PDD is used specifically to improve the diagnosis of small, flat or invisible tumour tissue that might be missed by using FURS alone [3]. European guidelines have recommended the use of PDD-guided biopsy if bladder CIS is suspected; furthermore, they state that PDD-guided biopsy or resection is more sensitive in detecting tumours [10].
The physics underlying the success of PDD is the use of fluorochromes or photosensitising agents such as 5-ALA [11]. When these absorb photons of a specific wavelength (blue) they become electrically excited [3,7], absorb the high-energy light and emit a lower energy light of a different wavelength than that originally absorbed, and that can be readily detected [3,7]. As tumour cells have a high uptake of the fluorochromes they appear more red than the surrounding tissue seen under blue light [3,7]. However, one of the drawbacks of the method is that it is difficult to distinguish between inflamed tissue (e.g. after intravesical treatment with mitomycin or BCG) and cancerous tissue [7,11]. This has led to the low specificity of PDD in numerous studies [7,12]. Despite this, several studies report a higher sensitivity for detecting tumours with the use of PDD rather than WLC alone [7].
Figs. 1 and 2 show both WLC and PDD detections of UUT lesions in two obvious areas. However, as seen in Fig. 1, the three areas that are fluorescent are not visible with WLC.
Figure 1.
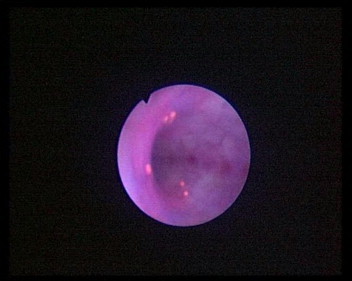
PDD using blue light, showing five areas of abnormal tissue.
Figure 2.
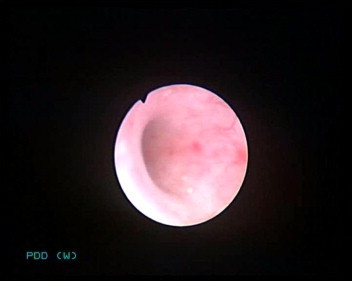
WLU of the same area as in Fig. 1, showing two lesions.
Zaak et al. [13] conducted a study on 713 patients and reported a sensitivity of 97% for detecting tumour tissue by PDD. Hungerhuber et al. [12] reported a PDD study on 875 patients, in which they compared the PDD findings with WLC. They reported that PDD had a sensitivity of 92% compared to 76.3% for WLC [12]. Recent randomised controlled trials comparing PDD with WLC found that PDD is more effective than WLC for detecting malignant bladder lesions and CIS, in addition to having a more complete resection during TURBT [7]. Furthermore, two of these randomised controlled trials found that PDD-TURBT increases the recurrence-free survival of patients with non-muscle-invasive cancers [7].
However, these high sensitivities were all reported for bladder tissue, and a thorough search of previous reports failed to identify any studies reporting the use of PDD in the UUT, other than two case series of four patients each [4,14], one of which [4] was our initial experience. In this audit, although there was no statistically significant difference between WLU and PDD, PDD detected more lesions and tumours. WLU missed all the CIS and dysplastic lesions, but these were detected with PDD (Figs. 3 and 4). The FP result with WLU was a scar tissue, which was not fluorescent under PDD. While PDD did not detect a G3pTa lesion, WLU detected this, but we are unable to explain why there was no fluorescence.
Figure 3.
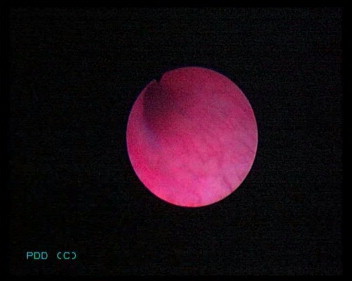
PDD showing a single area of CIS.
Figure 4.
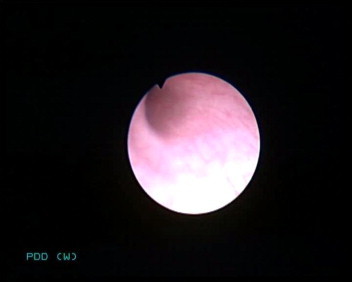
WLU of the same area as in Fig. 3, showing no obvious lesion.
One of the limitations of this audit is that there were few patients. However, the low incidence of the disease makes it difficult to include many patients. Furthermore, PDD is not readily used by most units for UUT lesions and therefore it is difficult to initiate a multi-institutional study. A further limitation of the study is that we did not report complications unrelated to 5-ALA administration; these were beyond the scope of this audit. We did not evaluate the site of each lesion in relation to anatomical orientation, and we classified the whole UUT as one renal unit.
Nonetheless, this paper is unique in presenting the results of using PDD for the UUT. Our method using oral 5-ALA was consistent with all patients and the ureteroscopy procedure itself was conducted by one endourologist, which eliminates inconsistency due to several surgeons operating differently or use of different agents.
This study showed that PDD can safely be administered to patients undergoing UUT endoscopy, and similar to bladder PDD, can increase the detection rate of lesions. However, to further evaluate the diagnostic accuracy of PDD, more patients are needed. Because UUT lesions are relatively rare, a multi-centred trial using a uniform agent and method of delivery is required.
In conclusion, our audit showed that PDD can be applied safely for the diagnosis of UUT lesions, and for surveillance and follow-up of UUT tumours in selective conservatively managed patients. This audit showed that PDD ureterorenoscopy increased the detection rates of abnormal lesions and tumour tissues compared to WLU. Similarly to recommendations for bladder PDD, we believe that PDD should be implemented for ureterorenoscopic inspection. However, a large multicentre trial is required for further validation of PDD in the UUT.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Browne R.F., Meehan C.P., Colville J., Power R., Torreggiani W.C. Transitional cell carcinoma of the upper urinary tract: spectrum of imaging findings. Radiographics. 2005;25:1609–1627. doi: 10.1148/rg.256045517. [DOI] [PubMed] [Google Scholar]
- 2.Anderson E.M., Murphy R., Rennie A.T., Cowan N.C. Multidetector computed tomography urography (MDCTU) for diagnosing urothelial malignancy. Clin Rad. 2007;62:324–332. doi: 10.1016/j.crad.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 3.Audenet F., Traxer O., Yates D.R., Cussenot O., Roupret M. Potential role of photodynamic techniques combined with new generation flexible ureterorenoscopes and molecular markers for the management of urothelial carcinoma of the upper urinary tract. BJU Int. 2011 doi: 10.1111/j.1464-410X.2011.10363.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Somani B.K., Moseley H., Eljamel M.S., Nabi G., Kata S.G. Photodynamic diagnosis (PDD) for upper urinary tract transitional cell carcinoma (UT-TCC). Evolution of a new technique. Photodiagnosis Photodyn Ther. 2010;7:39–43. doi: 10.1016/j.pdpdt.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Bader M.J., Sroka R., Gratzke C., Seitz M., Weidlich P., Staeheler M. Laser therapy for upper urinary tract transitional cell carcinoma. Indications and management. Eur Urol. 2009;56:65–71. doi: 10.1016/j.eururo.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Mowatt G., N’Dow J., Vale L., Nabi G., Boachie C., Cook J.A. Photodynamic diagnosis of bladder cancer compared with white light cystoscopy: systematic review and meta-analysis. Int J Technol Assess Healthc. 2011;27:3–10. doi: 10.1017/S0266462310001364. [DOI] [PubMed] [Google Scholar]
- 7.Jocham D., Stepp H., Waidelich R. Photodynamic diagnosis in urology: state-of-the-art. Eur Urol. 2008;53:1138–1148. doi: 10.1016/j.eururo.2007.11.048. [DOI] [PubMed] [Google Scholar]
- 8.Tawfiek E., Bibbo M., Bagley D.H. Ureteroscopic biopsy. Technique and specimen preparation. Urology. 1997;50:117–119. doi: 10.1016/S0090-4295(97)00216-1. [DOI] [PubMed] [Google Scholar]
- 9.Grasso M., Fraiman M., Levine M. Ureteropyeloscopic diagnosis and treatment of upper urinary tract urothelial malignancies. Urology. 1999;54(2):240–246. doi: 10.1016/s0090-4295(99)00121-1. [DOI] [PubMed] [Google Scholar]
- 10.Babjuk M., Oosterlinck W., Sylvester R., Kaasinen E., Bohle A., Palou-Redorta J. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder. Eur Urol. 2008;54:303–314. doi: 10.1016/j.eururo.2008.04.051. [DOI] [PubMed] [Google Scholar]
- 11.Berg K., Selbo P.K., Weyergang A., Dietze A., Prasmickaite L., Bonsted A. Porphyrin-related photosensitizers for cancer imaging and therapeutic applications. J Microscopy. 2005;218:133–147. doi: 10.1111/j.1365-2818.2005.01471.x. [DOI] [PubMed] [Google Scholar]
- 12.Hungerhuber E., Stepp H., Kriegmair M., Stief C., Hofstetter A., Hartmann A. Seven years’ experience with 5-aminolevulinic acid in detection of transitional cell carcinoma of the bladder. Urology. 2007;69:260–264. doi: 10.1016/j.urology.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 13.Zaak D., Hungerhuber E., Schneede P., Stepp H., Frimberger D., Corvin S. Role of 5-aminolevulinic acid in the detection of urothelial premalignant lesions. Cancer. 2002;95:1234–1238. doi: 10.1002/cncr.10821. [DOI] [PubMed] [Google Scholar]
- 14.Waidelich R., Hofstetter A., Stepp H., Baumgartner R., Weninger E., Kriegmair M. Early clinical experience with 5-aminolevulinic acid for the photodynamic therapy of upper tract urothelial tumors. J Urol. 1998;159(2):401–404. doi: 10.1016/s0022-5347(01)63932-6. [DOI] [PubMed] [Google Scholar]


