Abstract
Objective
To evaluate the feasibility of replacing a relatively long segment of the canine urethra by a tube of cell-seeded acellular collagen bladder matrix.
Materials and methods
The study included 14 female mongrel dogs in which a 3-cm segment of the whole urethral circumference was excised and replaced by a tube of acellular matrix seeded with autologous urothelial cells. The acellular matrix was obtained from the excised bladder of female donor dogs that were not included in the study. Autologous cells were obtained from the study dogs by open bladder biopsy, with subsequent in vitro expansion and cultivation. Urethroplasty was performed over a 16 F urethral catheter that was kept for 4 weeks. The dogs were killed humanely (one every week for 4 weeks and then one monthly for 10 months). After stent removal, retrograde urethrography was used each month in the living dogs. If retention occurred a urethrogram was taken and then the dog was killed humanely. All grafts from dogs were harvested and sent for histopathological examination.
Results
Exploration at 1, 2, 3 and 4 weeks showed progressive shrinkage in length, together with relative narrowing of the lumen. Three dogs developed retention within a week after stent removal and the other seven developed retention within 4 months. Retrograde urethrograms showed evidence of stricture and/or fistula at the graft site in all dogs. On exploration, grafts showed marked shrinkage (0.6–1.2 cm in length) with complete obliteration of their lumens. Histopathological examination showed extensive fibrosis of the matrix with no evident urothelial architecture.
Conclusion
Cell-seeded acellular matrix tube is insufficient to replace a 3-cm circumferential urethral defect in dogs.
Abbreviations: AM, acellular matrix; H&E, haematoxylin and eosin
Keywords: Acellular matrix, Urethroplasty, Tissue engineering
Introduction
A long-segment urethral stricture, where end-to-end anastomosis is not applicable, is a challenging problem. Several autologous tissues have been proposed for urethroplasty as free grafts, but most of these grafts have significant complications and can require additional surgery for graft harvesting, with subsequent donor-site morbidity and prolonged hospitalization [1]. Hence, alternative materials have been tried for urethral repair.
Biological tissue substitutes have been introduced through the developing techniques of tissue engineering to replace diseased and lost human tissues, including the urethra [1]. The development of acellular matrix (AM) by tissue engineering represents a remarkable stage in the field of reconstructive surgery. Several studies reported successful results when AMs were used to replace a partial urethral defect in an onlay fashion [2,3]. Sievert et al. [4] used a tube of AM to replace a circumferentially excised segment of rabbit urethra 0.8–1.1 cm long, and reported satisfactory results. Our group reported the inapplicability of replacing a longer tubular defect of the urethra (3 cm) with such an AM [5].
In further attempts to improve urethral regeneration, some studies reported successful results with urethral defect replacement using cell-seeded tubular AMs [6,7]. The use of AM facilitates easy neovascularisation and adequate nourishment of the seeded cells, as well as easy ‘creeping’ and regeneration from the edges of the surrounding viable tissue [6,7]. In both studies the urethral defect was <1.5 cm. In clinical practice, such short defects could be managed by primary resection and re-anastomosis. The question is whether a cell-seeded tubular AM could be used to replace a long tubular urethral defect; the present study was designed to answer this question.
Materials and methods
In this study we used dogs as a source of the collagen AM and for the urethral replacement experiment; dogs used for obtaining the AMs were not included in the study group; the latter including 14 mongrel female dogs with a body weight of 25–30 kg. All were vaccinated for common infectious diseases and health status was verified by a veterinarian. Cefoxitin (11–22 mg/kg) was administered before surgery and daily for 3 subsequent days to guard against infection. A preoperative ascending urethrogram was taken. The dogs were sedated with acepromazin (0.05 mg/kg) intramuscularly, and then anaesthesia was induced with ketamine (5 mg/kg intravenously) and diazepam (0.25 mg/kg intravenously). The dogs were intubated and isofluran (1–2%) was used to maintain anaesthesia with spontaneous breathing.
The AM was obtained using the same modified technique described by Probst et al. [8]. The whole bladder from a female mongrel dog was excised and placed in a beaker containing 50 mL of 10 mmol/L PBS, pH 7.0, and 0.1% sodium azide. The tissue was stirred for 24 h to allow partial cell lysis. The tissue was then treated with 50 mL of 1 mol/L DNase (Sigma Chemical Co., St. Louis, MO, USA) and stirred for 24 h. With this step complete, cell lysis occurred and all intracellular components were released. The sample was then treated with 50 mL 4% sodium deoxycholate (Sigma Chemical Co.) containing 0.1% sodium azide, and stirred for 24 h to solubilise the lipid cell membrane and intracellular membrane lipids; this step was repeated once more. The resultant AM was washed three times with 50 mL PBS and stored in 10% neomycin sulphate at 4 °C until grafted.
To prepare the cell-seeded collagen AM we followed similar steps to those reported by Filippo et al. [6]. Bladder biopsies were obtained from the 14 dogs in the study group. A small laparotomy incision was made above the pubic symphysis, exposing the bladder. A 2 × 2 cm biopsy specimen was excised from the anterior bladder wall, and the defect was repaired in two layers with 2/0 polyglactin sutures. Dogs were kept on cefoxitin (11–22 mg/kg) intramuscularly for 3 days after surgery for prophylaxis against infection.
The seromuscular layer of the bladder tissue was removed carefully via microdissection under loupe magnification (×5). Primary cultures of smooth muscle cells were obtained by explanting several pieces of seromuscular tissue on 10-mL culture plates in Dulbecco’s modified Eagle’s medium plus 10% foetal bovine serum. Enzymatic dissection with collagenase and dispase was used to isolate epithelial cells in keratinocyte serum-free medium. The cells were expanded to a density of 1.0–3.0 × 107 cells/cm2 for seeding onto the urethral grafts.
The cells were incubated with trypsin EDTA solution and then centrifuged; the cell pellet was resuspended to a concentration of 1 × 107 cells/mL of fresh medium. A 3-cm segment of the prepared AM was reconfigured as a tube over a 16 F Nelaton tube, then sequentially seeded with epithelial cells within the lumen and smooth muscle cells on the outer surface. The seeded grafts were incubated for 7 days then examined microscopically to ensure that cells are well attached to the AM (Fig. 1).
Figure 1.

The cell-seeded AM before grafting shows an intact framework of the matrix with an homogenous distribution of the seeded cells (H&E, reduced from ×100).
Operative technique
The urethra was exposed intra-abdominally and a 3-cm segment of the mid-urethra was excised and replaced by a similar length of a tube of the cell-seeded AM. The anastomosis was made using interrupted watertight 7/0 polyglactin sutures. Identifying nonabsorbable sutures were placed at the distal and proximal anastomosis. A 16 F silicon stent was left inside the urethra; its upper end was fixed by a 4/0 catgut suture to the bladder mucosa, and its lower end was cut 1 cm distal to the urethral opening and sutured to the vaginal wall with a 2/0 silk suture via an episiotomy. The urethral stent was kept in place for 4 weeks and then removed by cutting the external silk suture.
Postoperative assessment
Abdominal and vaginal wounds were irrigated twice daily with povidone iodine, and garamycin cream was applied at the urethral meatus around the stent to avoid ascending infection. A retrograde urethrogram was taken after removing the urethral stent, and then monthly until death. The dogs were killed humanely at one per week for 4 weeks and then one per month for 10 months. If there was urinary retention an ascending urethrogram was taken and the dog was killed. The urethra was exposed, the graft was examined and the distance between the identification sutures was measured. The urethra was calibrated by antegrade and retrograde insertion of a Nelaton catheter. The whole specimen was then excised for histopathological examination.
A piece of unseeded AM was examined by light microscopy after staining with haematoxylin and eosin (H&E), and trichrome stains. The harvested urethral graft specimens were fixed by immersion overnight in 10% buffered formalin. After dehydration in graded ethanol solutions, clearing in Histo-Clear (National Diagnostics USA, Atlanta, GA, USA) and embedding in paraffin, 5-μm sections were cut and air-dried onto pre-coated glass slides. They were stained with trichrome for muscle and collagen, H&E for nuclei, and α-actin for smooth muscle. The graft length was expressed as the mean (SD).
The study was approved by the local ethical committee and by the local ‘Society of Animal Rights’. The dogs were treated in compliance with the Declaration of Helsinki.
Results
All dogs survived surgery and there were no remarkable complications. The urethral stents became displaced after 1 week in two dogs, and were re-introduced safely under complete aseptic precautions with adequate lubrication while the dogs were sedated, to complete the planned 4-week urethral stenting. Four dogs were killed at 1, 2, 3 and 4 weeks, respectively, for histopathological examination. At week 1, there was invasion of the graft by red blood cells and mononuclear cells in between the seeded epithelial cells. By the second week, there was an evidence of subepithelial fibrosis that became more apparent by the end of the fourth week, together with the appearance of some fibroblasts, but with no definite urethral urothelial architecture. Urethral stents were kept in place for 4 weeks in the remaining 10 dogs. After stent removal, three dogs developed urinary retention within a week. Retrograde urethrograms taken in these three dogs showed extensive stricture at the graft site in all, with evidence of anastomotic fistula in two of them. The three dogs were explored abdominally, and showed extensive shrinkage and fibrosis of the graft with severe periurethral adhesions. The graft lumens were completely obliterated. On histopathological examination, there was extensive fibrosis replacing most of the seeded cells.
In the remaining seven dogs, urinary retention developed by the end of the third month in two and the fourth month in five after stent removal. Retrograde urethrograms showed progressive narrowing at the graft site that increased each month until complete obliteration at the onset of urinary retention (Fig. 2). Those dogs were explored at the third month (two) and fourth month (five), when they developed retention. On exploration there was significant periurethral fibrosis with marked shrinkage of the graft. The mean (SD, range) graft length was 0.9 (0.1, 0.6–1.2) cm, with evidence of severe narrowing of the lumen (Fig. 3). Grafts were then removed en bloc and 5-μm sections of formalin-fixed, paraffin wax-embedded tissues were stained with H&E. On histopathological examination, there was no uniform urothelial architecture but the seeded epithelial and smooth muscle cells were invaded and progressively replaced by fibrosis (Figs. 4 and 5).
Figure 2.
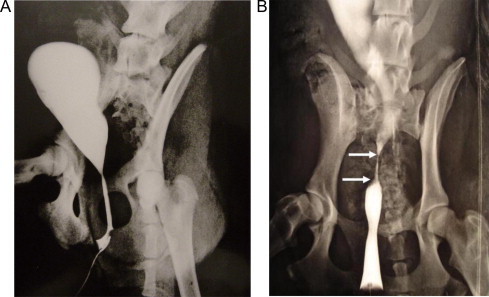
A retrograde urethrogram in a female dog. (A) normal findings before grafting. (B) a strictured segment (arrows) 6 weeks after surgery.
Figure 3.
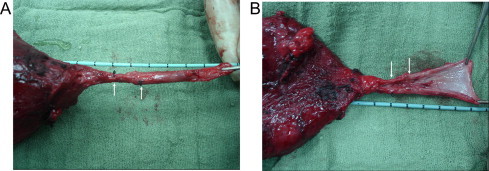
A postoperative specimen shows shrinkage and narrowing of the grafted matrix (between the arrows). (A) intact urethra. (B) opened urethra.
Figure 4.
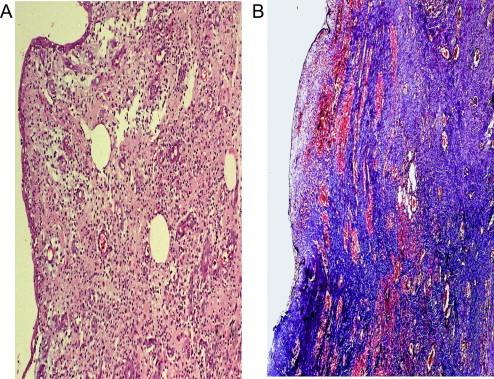
Histopathology of the specimen 4 weeks after surgery shows moderate subepithelial fibrosis. (A) H&E reduced from ×100. (B) trichrome stain, reduced from ×100.
Figure 5.
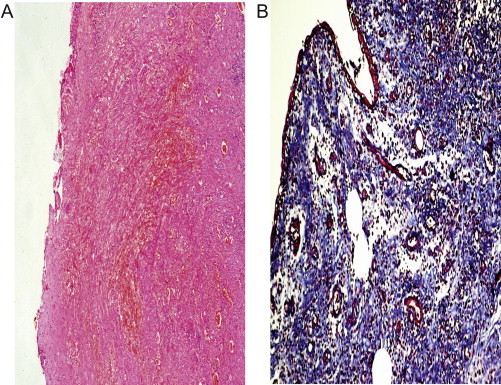
Histopathology of the specimen 6 weeks after surgery shows marked subepithelial fibrosis. (A) H&E reduced from ×50. (B) trichrome stain, reduced from ×100.
Discussion
This study included 14 female dogs for which AMs seeded with autologous urothelial cells were used as a tube to bridge a 3-cm circumferential urethral defect. Three dogs developed retention within a week after stent removal, while retention occurred within 4 months in the remaining dogs. Retrograde urethrograms showed evidence of stricture and/or fistula at the graft site in all dogs. Grafts showed marked shrinkage with complete obliteration of their lumens after harvesting. Histopathological examination showed extensive fibrosis of the matrix with no evident urothelial architecture.
Usually in cases of urethral strictures, urethral traumatic losses or genital defects there is paucity of urethral tissue for urethral reconstruction [9]. Autologous tissues from other sources have been tried for urethral reconstruction, e.g. genital and extragenital skin flaps or grafts, buccal mucosa, bladder mucosa grafts, tunica vaginalis and peritoneal flaps [10,11]. However, these procedures have a significant rate of complications, including stricture and fistula formation, graft shrinkage, and stone formation, in addition to donor-site morbidity [7]. The use of a variety of synthetic grafts composed of silicon, Teflon and Dacron has been proposed for urethral reconstruction. These materials have been associated with erosion, disruption, fistula, stenosis, extravasation or calcification [12,13].
In the last two decades the techniques of tissue engineering have developed dramatically. The structural and functional interaction and relationships of normal and abnormal tissue are better understood, and the repair or replacement of damaged tissue has become feasible [14]. When cells are used for tissue engineering, donor tissue is dissociated into individual cells that can be implanted into the host directly or expanded in culture attached to a support matrix, and then re-implanted after expansion. Harvesting autologous urothelium for cultivation in vitro usually requires a biopsy from the bladder or the meatus. Bladder washing is also a noninvasive but less productive way to obtain autologous urothelial cells for cultivation into stratified grafts [1,15]. Direct injection of cell suspensions (without matrices) has been used but controlling the location of the transplanted cells was difficult [16]. This was an opportunity for those involved in tissue engineering to search for a biomaterial to act as a scaffold for such autologous cells, to improve the pattern of healing and regeneration of the replaced damaged tissue until the AM was developed. AMs are collagen-rich matrices prepared by removing the cellular components from tissues. Matrices are often prepared by mechanical and chemical manipulation of particular tissues, like the bladder mucosa or the small intestinal submucosa [8].
There are many reports about the use of the AM for urethral reconstruction. Angiogenesis and neovascularisation occur appropriately when a part of the urethral circumference is kept intact, leading to correct cell expansion and tissue formation [4]. By contrast, tubular grafts lack this rapid neovascularisation, cell expansion and healthy tissue formation with subsequent fibrosis [4,5]. There is growing evidence from many experimental and clinical studies that seeded or unseeded AM might be suitable for urethral repair when used to reconstruct an incomplete urethral defect in an onlay fashion [3,17,18]. However, the results are uncertain when those matrices are used to replace a complete urethral defect, especially a long one. Sievert et al. [4] reported satisfactory results when a tube of AM was used to replace a circumferential urethral defect 0.8–1.1 cm long. We tested the applicability of the AM tube to replace a longer complete urethral defect of 3 cm in a dog model. After 4 weeks of urethral stenting, all dogs developed fistula and/or stricture with urinary retention by the end of 3 months [5]. Another study reported that the maximum complete urethral defect that could be replaced by a tube of unseeded AM is 0.5 cm, which is in agreement with our report [19].
In an attempt to improve the outcome of AM when used to replace a tube from the urethra, some authors proposed seeding these matrices by host-harvested and in vitro cultivated autologous cells. The idea was that the presence of smooth muscle cells on the biodegradable scaffold stimulates the formation of both a urothelial layer and a muscle layer of normal thickness. These cells survive on scaffolds through nutrient diffusion (Table 1). Angiogenesis and neovascularisation start when the construct is implanted, and occur in concert with further cell expansion and tissue formation [20]. There are many growth factors suggested to promote this neovascularisation and tissue regeneration. Among these factors are TGF-α mRNA, TGF-β1 mRNA, IGF and heparin-binding epidermal growth factor, the last being the most important, and which was shown to play a major role in the process of graft neovascularisation and urothelial proliferation [4].
Table 1.
Experimental studies using an AM tube for a circumferential urethral defect.
| Reference | Animal model/n | Seeded cells | Graft length (cm) | Results |
|---|---|---|---|---|
| Sievert et al., [4] | Rabbits/30 | No | 1–1.5 | 30/30 Success |
| Fillippo et al., [6] | Rabbits/24 | 12 No | 1 | 12/12 Failure |
| 12 Urothelial cells | 12/12 Success | |||
| Shokeir et al., [5] | Dogs/14 | No | 3 | 14/14 Failure |
| Fu et al., [7] | Rabbits/18 | 9 No | 1–1.5 | 9/9 Failure |
| 9 Epidermal cells | 9/9 Success | |||
| Dorin et al., [19] | Rabbits/12 | No | 0.5–3 | ⩽0.5 cm Success |
| >0.5 cm Failure |
Filippo et al. [6] used urothelial-cell seeded tubularized collagen matrices to replace a 1-cm urethral defect in 12 male rabbits and used another 12 rabbits supplied with unseeded matrices as a control group. Serial urethrograms showed a wide urethral calibre with no strictures in rabbits implanted with the cell-seeded matrices, whereas those implanted with unseeded matrices showed graft collapse and strictures in all cases. Recently, a similar study was reported by Fu et al. [7], but they seeded the collagen matrices with autologous epidermal cells obtained from the rabbit foreskin, which was less invasive than obtaining the urothelial cells by open bladder biopsy, as used by Filippo et al. [6]. They also reported successful results with an epidermal-cell seeded matrix tube, replacing a 1.5 cm tubular urethral defect. At the same time, the control group with unseeded grafts showed stricture formation in all cases [7]. Notably, both studies showed failure when replacing a short urethral tubular defect of 1–1.5 cm with unseeded matrices. These results again are in contrast to what was reported previously by Sievert et al. [4], and are in agreement with our previous study, when we tried to replace a longer urethral tube using such unseeded AMs [5].
In the two studies [6,7] with cell-seeded collagen AMs the replaced urethral segments were 1–1.5 cm long. In clinical practice a stricture of such length is easily treated by primary resection and re-anastomosis. The actual challenge is whether or not these cell-seeded AMs can replace longer urethral defects. In the present study we tried to replace a complete 3-cm long urethral segment with a urothelial cell-seeded AM in the dog. We chose female dogs for urethral replacement as they provide easier surgical access and a less bloody field that allowed precise suturing. All dogs showed urethral strictures either early after stent removal or maximally after 4 months. At death, all grafts showed evident shrinkage and fibrosis. Histopathological examination confirmed the poor vascularity of those grafts, with replacement of the seeded AMs by fibrous tissue. It is well known that the immune response and the healing power of the ‘street mongrel’ dog is very powerful [21], which might be one of the causes of extensive fibrosis and graft failure in the present study. Our results are discouraging and differ from those of Filippo et al. [6] and Fu et al. [7], when they applied their cell-seeded AMs to replace short urethral segments of 1–1.5 cm. Although their studies were published as early as 2002, their results were neither reproduced by other experimental studies nor applied clinically to date.
By analysing our data in the current and previous studies [3,5], and those of others [4,6,7], it is evident that urethral regeneration could occur over a defective area when a strip of normal urethral circumference is intact. However, for circumferential urethral defects, the outcome of urethral replacement with AMs is doubtful even with short defects. The main additional finding of our study is that it tested the use of cell-seeded AM to bridge long circumferential defects, which are of more practical importance than short urethral defects not requiring grafting. One of the drawbacks of the present study was the few dogs included, and the use of dogs rather than rabbits as a model. Urine cultures were intended to be assessed routinely to elucidate the role of infection in graft failure. Further studies are needed to elucidate and circumvent the causes of failure of implanted engineered tissues. Every effort should be made to improve the nourishment, take, regeneration and growth of cell-seeded AMs when used as a tubular substitute for urethral replacement. Further research should not be precluded, but must be encouraged and other options tried to determine the exact humoral and cellular causes of failure of seeded and unseeded engineered scaffolds as tubular urethral substitutes, and how to circumvent and subordinate these factors.
In conclusion, the cell-seeded AM tube is insufficient to replace a 3-cm circumferential urethral defect in dogs, a situation of more practical importance in humans than bridging short defects.
Conflict of interest
No conflict of interest to declare.
References
- 1.Atala A. Experimental and clinical experience with tissue engineering techniques for urethral reconstruction. Urol Clin N Am. 2002;29:485–492. doi: 10.1016/s0094-0143(02)00033-2. [DOI] [PubMed] [Google Scholar]
- 2.Chen F., Yoo J.J., Atala A. A cellular collagen matrix as a possible ‘of the shelf’ biomaterial for urethral repairs. Urology. 1999;54:407–410. doi: 10.1016/s0090-4295(99)00179-x. [DOI] [PubMed] [Google Scholar]
- 3.Shokeir A.A., Osmen Y., El-Sherbiny M., Gabr M., Mohsen T., El-baz M. Comparison of partial urethral replacement with a cellular matrix versus spontaneous urethral regeneration in a canine model. Eur Urol. 1999;44:603–609. doi: 10.1016/j.eururo.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Sievert K., Bokircioglu M.E., Nunes L., Tu R., Dahiya R., Tanagho E.A. Homologous acellular matrix graft for urethral reconstruction in the rabbit Histological and functional evaluation. J Urol. 2000;163:1958–1965. [PubMed] [Google Scholar]
- 5.Shokeir A.A., Osman Y., Gabr M., Mohsen T., Dawaba M., El-Baz M. Acellular matrix tube for urethral replacement: Is it fact or fiction? J Urol. 2004;171:453–456. doi: 10.1097/01.ju.0000089776.52568.9e. [DOI] [PubMed] [Google Scholar]
- 6.Filippo R.E., Yoo J.J., Atala A. Urethral replacement using cell seeded tubularized collagen matrices. J Urol. 2002;168:1789–1793. doi: 10.1097/01.ju.0000027662.69103.72. [DOI] [PubMed] [Google Scholar]
- 7.Fu Q., Deng C.L., Liu W., Cao Y.L. Urethral replacement using cell-seeded tubular acellular bladder collagen matrix. Br J Urol. 2007;99:1162–1165. doi: 10.1111/j.1464-410X.2006.06691.x. [DOI] [PubMed] [Google Scholar]
- 8.Probst M., Dahiya R., Carrier S., Tanago E.A. Reproduction of functional smooth tissue and partial bladder replacement. Br J Urol. 1997;79:505–515. doi: 10.1046/j.1464-410x.1997.00103.x. [DOI] [PubMed] [Google Scholar]
- 9.Atala A., Guzman L., Retik A.B. A novel inert collagen matrix for hypospadias repair. J Urol. 1999;162:1148–1151. doi: 10.1016/S0022-5347(01)68105-9. [DOI] [PubMed] [Google Scholar]
- 10.Dessanti A., Rigamant W., Meroll V., Falchetti D., Caccia G. Autologous buccal mucosa graft for hypospadias repair: An initial report. J Urol. 1992;147:1081–1084. doi: 10.1016/s0022-5347(17)37478-5. [DOI] [PubMed] [Google Scholar]
- 11.Snow B., Cartwright P.C. Tunica vaginalis urethroplasty. Urology. 1992;40:442–445. doi: 10.1016/0090-4295(92)90460-e. [DOI] [PubMed] [Google Scholar]
- 12.Anwar H., Dave B., Seebode J.J. Replacement of partially resected canine urethra by polytetrafluoroethylene. Urology. 1984;24:583–586. doi: 10.1016/0090-4295(84)90107-9. [DOI] [PubMed] [Google Scholar]
- 13.Hakky S.I. The use of fine double siliconised Dacron in urethral replacement. Br J Urol. 1977;49:167–172. doi: 10.1111/j.1464-410x.1977.tb04093.x. [DOI] [PubMed] [Google Scholar]
- 14.Desgrandchamps F. Biomaterials in functional reconstruction. Curr Opin Urol. 2000;10:201–206. doi: 10.1097/00042307-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Gustafson G.J., Kratz G. Tissue engineering in urology. Curr Opin Urol. 2001;11:275–279. doi: 10.1097/00042307-200105000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Brittberg M., Lindahl A., Nilsson A., Ohlsson C., Isaksson O., Peterson L. Treatment of deep cartilage defect in the knee with autologous chondrocyte transplantation. N Eng J Med. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 17.El-Kassaby A.W., Retik A.B., Yoo J.J., Atala A.A. Urethral stricture repair with an off-the-shelf collagen matrix. J Urol. 2003;169:170–173. doi: 10.1016/S0022-5347(05)64060-8. [DOI] [PubMed] [Google Scholar]
- 18.Li C., Xu Y.M., Song L.J., Fu Q., Cui L., Yin S. Urethral reconstruction using oral keratinocyte seeded bladder acellular matrix grafts. J Urol. 2008;180:1538–1542. doi: 10.1016/j.juro.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 19.Dorin R.P., Pohl H.G., De Filippo R.E., Yoo J.J., Atala A. Tubularized urethral replacement with unseeded matrices: what is the maximum distance for normal tissue regeneration ? Worl J Urol. 2008;26:323–326. doi: 10.1007/s00345-008-0316-6. [DOI] [PubMed] [Google Scholar]
- 20.Kannan R.V., Salacinski H.J., Sales K., Butler P., Seifalian A.M. The role of tissue engineering and vascularisation in the development of microvascular networks. Biomaterials. 2005;26:1857–1875. doi: 10.1016/j.biomaterials.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 21.De Santis E., Lang N.P., Scala A., Viganò P., Salata L.A., Botticelli D. Healing outcomes at implants installed in grafted sites: an experimental study in dogs. Clin Implants Res. 2011;16:237–241. doi: 10.1111/j.1600-0501.2011.02326.x. [DOI] [PubMed] [Google Scholar]


