Abstract
Objectives
To describe our experience with ‘minipatch’ penile skin graft (PSG) urethroplasty, as at our institution we prefer excision and primary anastomosis (EPA) urethroplasty whenever feasible, as it gives better outcomes than substitution urethroplasty. However, despite careful preoperative planning, the unanticipated need for a small graft is occasionally recognised intra-operatively, and in such cases we have found that harvesting a minipatch is an efficient alternative to harvesting a buccal mucosal graft.
Patients and methods
Bulbar urethroplasty using a <3 cm PSG was performed via either a ventral onlay or augmented anastomotic technique. In each case the PSG was required to repair an unanticipated urethral defect recognised intra-operatively during various scenarios of challenging urethroplasty. We retrospectively reviewed our experience with this technique.
Results
Among a total of 425 urethral reconstructions over a 4-year period at our institution, four patients (1%) underwent minipatch PSG urethroplasty to repair either urethral strictures that were discovered intra-operatively to be too complex for EPA (two patients) or for intra-operatively identified, unanticipated synchronous strictures (two patients). The mean (range) stricture length was 2.4 (2–3) cm and the mean graft length was 2.1 (1.5–2.5) cm. At a mean follow-up of 18 months all repairs were patent with no need for further procedures or instrumentation.
Conclusion
Minipatch PSG urethroplasty is an efficient alternative to a buccal mucosal graft repair, especially when the unanticipated need for short-segment tissue transfer arises during complex urethral reconstruction.
Abbreviations: EPA, excision with primary anastomosis; PSG, penile skin graft; BMG, buccal mucosal graft
Keywords: Urethra, Urethral stricture, Urethral stenosis, Grafts
Introduction
Bulbar urethral stricture disease is a heterogeneous condition that often requires the creative application of various reconstructive techniques for a successful repair. At our institution we prefer complete stricture excision with primary anastomosis (EPA) whenever feasible, as it gives better success rates than substitution urethroplasty [1,2]. Despite careful preoperative planning and aggressive mobilisation intra-operatively, the unanticipated need for a small graft does occasionally arise. In such cases we have found that harvesting a small penile skin graft (PSG) ‘minipatch’ is an efficient alternative to harvesting a buccal mucosal graft (BMG). The objective of this study was to describe our technique, indications and initial results with minipatch PSG urethroplasty.
Patients and methods
We retrospectively reviewed all urethral reconstruction procedures between 2007 and 2011 by the senior author at our tertiary institution. Patients who underwent minipatch PSG urethroplasty were identified, and their demographics, stricture location and aetiology, operative technique, graft dimensions, outcomes and complications were reviewed.
Patients were evaluated with a comprehensive history, physical examination and uroflowmetry. Stricture anatomy was delineated with retrograde urethrography and/or flexible cystoscopy.
For surgery, the patient was placed in the lithotomy position, using adjustable stirrups, and under general anaesthesia. Patients with short anterior urethral strictures were treated whenever possible by complete stricture excision and re-anastomosis. If the length of the excised segment precluded a tension-free repair, the dorsal ends of the urethra were re-approximated with an interrupted 5–0 polydioxanone suture, with several initial sutures anchoring the anastomosis to the corpora, to eliminate tension. After roughly two-thirds of the anastomosis was completed primarily, the remaining short ventral urethral defect was then augmented with an appropriately tailored oval minipatch PSG, applied epithelial side towards the urethral lumen, with a running 5–0 polydioxanone suture over a 16-F silicone urethral catheter. For patients in whom a small urethral defect was initially recognised intra-operatively (e.g. an unanticipated synchronous stricture), a similar minipatch PSG was applied across the ventral stricturotomy, with the epithelial side towards the urethral lumen, with a running 5–0 polydioxanone suture over a 16-F silicone urethral catheter.
Each minipatch PSG was obtained by harvesting a small (<3 cm) elliptical graft from the lateral distal penile shaft in a transverse direction subcoronally (Fig. 1). The subepithelial tissue of graft was thinned to the level of the dermis, tailored as necessary, and applied over the urethral defect in the above manner. The graft was supported with a ventral spongioplasty and the wound was closed in several layers. The PSG harvest site was closed with an interrupted 4–0 chromic suture and dressed with a lightly compressive elastic bandage wrap.
Figure 1.
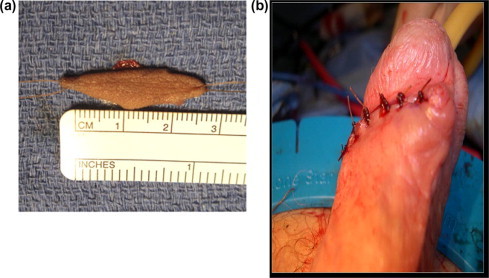
(A) A 2.5 cm × 1.0 cm minipatch PSG. (B) A wound after minipatch PSG harvesting adjacent to a previous circumcision scar.
Patients were discharged after overnight bed rest and observation. The subsequent follow-up was at 3–4 weeks, for urethral catheter removal and to obtain a voiding cysto-urethrogram to confirm the patency and integrity of the repair.
Results
Among a total of 425 urethral reconstructions performed over a 4-year period at our institution, four patients (1%, Table 1) underwent minipatch PSG urethroplasty to repair either urethral strictures that were discovered intra-operatively to be too complex for EPA (two patients) or for intra-operatively identified, unanticipated synchronous strictures (two patients). The mean (range) age of the patients was 62 (51–79) years, the mean stricture length was 2.4 (2.0–3.0) cm, and the mean graft length was 2.1 (1.5–2.5) cm; all minipatch grafts were 1.0 cm wide. The mean (range) operative blood loss was 263 (200–300) mL and the mean operative time was 227 (156–284) min. No complications were identified during or after surgery, and all four patients were discharged home after overnight observation.
Table 1.
Patient characteristics.
| Characteristic | Patient |
|||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Stricture | ||||
| Aetiology | Traumatic | Idiopathic | AdVance® urethral sling injury | Iatrogenic |
| Diagnosis | Recognised at time of synchronous posterior urethroplasty | Preoperative, RUG | Urethral defect after intraurethral mesh excision | Recognised during synchronous anterior urethroplasty |
| Location | Distal bulbar | Distal bulbar | Mid bulbar | Distal bulbar |
| Length (cm) | 2.0 | 3.0 | 2.2 | 2.5 |
| Synchronous | Yes, 3.0 cm | No | No | Yes, 6.0 cm |
| Stricture | posterior (EPA) | pendulous (circular flap) | ||
| Graft length (cm) | 2.0 | 1.5 | 2.2 | 2.5 |
| Repair technique | Ventral onlay | Augmented anastomotic | Ventral onlay | Augmented anastomotic |
RUG, retrograde urethrogram.
A voiding cysto-urethrogram after surgery showed patent repairs with no urinary extravasation in all patients. At a mean (range) follow-up of 18 (13–24) months all patients continued to have patent repairs, as determined by the lack of recurrent voiding symptoms (patients 2 and 4) or the ability to pass a 16-F urethral catheter at the time of a subsequent, unrelated surgery (patients 1 and 3). Also, patient 3 underwent successful transcorporal artificial urinary sphincter placement 5 months after minipatch PSG urethroplasty, and remains patent and dry at 24 months of total follow-up.
Discussion
Following Devine’s initial report in 1963, for over two decades PSGs became accepted worldwide as a standard, state-of-the-art method for reconstructing urethral strictures [3,4]. In the late 1990s, buccal mucosa began to replace penile skin as a preferred graft source for urethroplasty, due to its advantageous histological properties and superior handling characteristics. Over the past decade PSG urethroplasty has become virtually extinct, both clinically and in urological reports, due to the popularity of BMG.
We observed that many recently trained urologists are unfamiliar with the use of penile skin for urethral reconstruction. Although EPA is our preferred reconstructive technique for bulbar urethroplasty, because of its high success rate and efficiency [2], preoperative imaging might not always definitively ascertain which patients are appropriate candidates for EPA (Fig. 2). We sometimes encounter an unexpected need for a small graft, due to equivocal or outdated imaging, dense periurethral fibrosis, and/or those patients with synchronous strictures not recognised before surgery.
Figure 2.
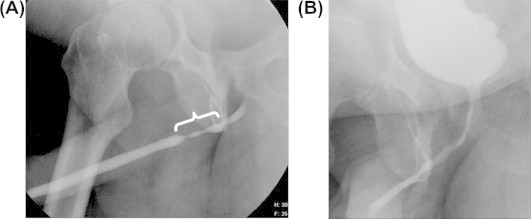
(A) A retrograde urethrogram (patient 2) showing a 3.0-cm distal bulbar stricture (bracket) which could not be completely reconstructed via excision and primary anastomosis alone. (B) A postoperative voiding cystourethrogram at 3 weeks of follow-up, showing a widely patent lumen after minipatch PSG augmented anastomotic urethroplasty.
We rediscovered PSG as a valuable time-saving alternative to BMG harvesting, especially during selected complex cases lasting for >3 h. Because the penis was already prepared into the surgical field in each of our patients, small PSGs were rapidly harvested and prepared, thus avoiding the time, expense and potential morbidity of oral graft harvest.
Although we harvest BMGs at our referral centre, a PSG might be helpful to consider for those not skilled in BMG harvesting, as it obviates the need to urgently mobilise additional specialised nursing and/or surgical personnel for oral graft harvest. Furthermore, the use of a small PSG avoids the need to prepare a separate operative field, eliminates the delay in obtaining additional oral surgery instrumentation, and in deploying a second surgical team and/or technician for BMG harvest.
Harvesting of penile skin is safe and technically simple for all urologists, as it requires no special experience with oral surgery or knowledge of oral anatomy. Cosmetic concerns are limited by harvesting the graft transversely, with suture-line integration into the circumcision scar. Bleeding and/or infection of these small penile incisions are rare and the formation of a urethral diverticulum is prevented by using grafts no wider than 1 cm. Penile shaft skin is ample for a small graft in most normal men, even when circumcised, although caution is advised in men with unhealthy or limited penile skin, such as those with lichen sclerosus or hypospadias.
While our study is limited by including few patients with a short follow-up, we believe that PSG urethroplasty remains an expedient and reliable tool in the options for contemporary urethral reconstruction. Although we prefer BMGs for most distal bulbar urethral defects of >3 cm, due to its superior handling characteristics, existing data suggest that a PSG is no less effective than the BMG [5–7]. Accordingly, we have encountered no cases of re-stenosis in the four patients who underwent minipatch PSG repair in this series of complex cases.
In conclusion, minipatch PSG urethroplasty is an efficient, effective alternative to BMGs for reconstructing bulbar strictures that are unexpectedly complex.
Conflict of interest
No conflict of interest to declare.
Acknowledgements
No extra-institutional funding was used.
This study was approved by the UT Southwestern Institutional Review Board.
Footnotes
Peer review under responsibility of Arab Association of Urology.
References
- 1.Barbagli G., Guazzoni G., Lazzeri M. One-stage bulbar urethroplasty. Retrospective analysis of the results in 375 patients. Eur Urol. 2008;53:828–833. doi: 10.1016/j.eururo.2008.01.041. [DOI] [PubMed] [Google Scholar]
- 2.Terlecki R.P., Steele M.C., Valadez C., Morey A.F. Grafts are unnecessary for proximal bulbar reconstruction. J Urol. 2010;184:2395–2399. doi: 10.1016/j.juro.2010.08.034. [DOI] [PubMed] [Google Scholar]
- 3.Devine P.C., Sakati I.A., Poutasse E.F., Devine C.J., Jr. One stage urethroplasty: repair of urethral strictures with a free full thickness patch of skin. J Urol. 1968;99:191–193. doi: 10.1016/S0022-5347(17)62670-3. [DOI] [PubMed] [Google Scholar]
- 4.Devine P.C., Horton C.E., Devine C.J., Sr., Devine C.J., Jr., Crawford H.H., Adamson J.E. Use of full thickness skin grafts in repair of urethral strictures. J Urol. 1963;90:67–71. doi: 10.1016/S0022-5347(17)64362-3. [DOI] [PubMed] [Google Scholar]
- 5.Barbagli G., Palminteri E., Lazzeri M., Turini D. Interim outcomes of dorsal skin graft bulbar urethroplasty. J Urol. 2004;172:1365–1367. doi: 10.1097/01.ju.0000139727.70523.30. [DOI] [PubMed] [Google Scholar]
- 6.Markiewicz M.R., Lukose M.A., Margarone J.E., 3rd, Barbagli G., Miller K.S., Chuang S.K. The oral mucosa graft: a systematic review. J Urol. 2007;178:387–394. doi: 10.1016/j.juro.2007.03.094. [DOI] [PubMed] [Google Scholar]
- 7.Raber M., Naspro R., Scapaticci E., Salonia A., Scattoni V., Mazzoccoli B. Dorsal onlay graft urethroplasty using penile skin or buccal mucosa for repair of bulbar urethral stricture: results of a prospective single center study. Eur Urol. 2005;48:1013–1017. doi: 10.1016/j.eururo.2005.05.003. [DOI] [PubMed] [Google Scholar]



