Abstract
Objective
The most common urinary stones (calcium salts, uric acid) form due to genetic factors and lifestyle. This review describes why, if and how medication and lifestyle changes can reduce the risk of formation.
Methods
Previous reports were reviewed to obtain information on three aspects of urolithiasis, i.e. epidemiology, mechanisms linking lifestyle and urolithiasis and lifestyle intervention for preventing urolithiasis.
Results
Epidemiological evidence links the prevalence of urinary stone formation to general lifestyle factors. Detailed analysis has identified individual lifestyle elements that affect the risk of urinary stone formation. Currently there are several concepts that explain the mechanism of stone formation. Urinary markers like calcium, oxalate, phosphate, uric acid and urinary pH are involved in all these concepts. Many studies show that changing (combinations of) specific lifestyle elements has a favourable effect on these urinary markers. Based on this evidence, protocols have been developed that use a combination of these lifestyle changes and medication to prevent stone formation. In well-controlled studies where patients are optimally informed and continuously motivated, these protocols clearly reduce the stone formation rate. In general practice the result is less clear, because the time and tools are insufficient to maintain long-term patient compliance in the use of medication and lifestyle advice.
Conclusion
The risk of stone formation can be reduced in general practice when the patient’s compliance is optimised by providing individualised advice, continuous information, and feedback and incorporation of the advice into a regular lifestyle. The use of ‘e-tools’ might enable this without increasing the time required from the physician.
Abbreviations: BMI, body mass index; LUT, lower urinary tract; UUT, upper urinary tract; AA-SH, sulphur-containing amino acid; PTH, parathyroid hormone
Keywords: Urolithiasis, Lifestyle, Compliance, Diet, Alkali load, Acid load, Calcium, Serum
Introduction
Most urinary stones formed in patients worldwide contain calcium oxalates, calcium phosphates, uric acid or urates, and form due to genetic factors and lifestyle. This review describes why, if and how medication and lifestyle factors can reduce the risk of forming stones. Further sections provide epidemiological evidence of links between lifestyle and urinary stone formation, specific lifestyle elements that affect urinary stone formation and its urinary markers, the cascade of lifestyle elements, urinary composition, and stone formation, a protocol for the preventive treatment of stones that is based on the consented protocol used in Germany [1], and finally a discussion of why such protocols reduce stone formation much less in general practice than in research settings. The main reason for the latter is that patient compliance with long-term use of medication and lifestyle advice is low, and the key elements for success are continuous access to practical advice, efforts to maintain patient’s motivation, continuous feedback and incorporation of the advice into regular lifestyle. An in-depth discussion of compliance by stone formers with treatment is provided elsewhere in this journal.
Lifestyle and prevalence of urinary tract stone disease
In a review published 40 years ago Prien [3] discussed the prevalence of stone formation throughout the world at that time and the changes in prevalence over time in relation to lifestyle. In any population the first form is endemic bladder stone disease (lower urinary tract, LUT) affecting mainly children. Specific changes in the lifestyle of that population move the disease to upper urinary tract (UUT) stone formation, affecting mainly adults [3]. During this transformation the prevalence first declines and later increases again. Endemic LUT stones contain mainly uric acid, urates and struvite [4]. In Thailand, calcium oxalate is also a major component of LUT stones [5,6]; the background for this is low hygiene and malnutrition, and in Thailand the malnutrition can be accompanied by endemic [7] renal tubular acidosis. A monotonous diet where energy is derived mainly from cereals and vegetables provides high doses of purines that can be converted to uric acid and lead to hyperuricosuria [4]. UTI with urease-producing bacteria increases both urinary pH and ammonium concentration. This favours the precipitation of ammonium–magnesium-phosphate (struvite) and calcium phosphates. In combination with hyperuricosuria the product can be ammonium urate. When the standard of living increases in an area with endemic bladder stone disease the incidence of bladder stone disease decreases. This has been attributed to the more balanced diet, including more dairy products and animal protein [8,9].
Whereas the change from a ‘primitive’ lifestyle to an ‘intermediate economic’ lifestyle [10] reduces the incidence of overall stone formation, further changes in lifestyle, towards the so-called western lifestyle, increases the prevalence of urinary stone formation again, now as UUT stones [10]. Well-balanced might be the keyword here. Those vegetarians who succeed in maintaining a well-balanced diet have a low risk of LUT stone disease but also of UUT stone formation [11]. Periods of stone disease were apparent in European countries and Japan between the First and Second World Wars and after World War II [12,13]. In 1971, the situation was one where UUT stones were most prevalent in the economically most-developed countries [3]. The stones contained calcium salts and affected mainly men. In the decades thereafter endemic bladder stone formation has further diminished throughout the world, while the incidence of UUT stone formation increased even further in western countries [14–16]. Again, lifestyle changes appeared to cause this further increase. The highest prevalence of UUT (71% calcium oxalate and 15% uric acid) stone disease has been reported for Arab countries that combined a high economic standard with a hot and dry climate, and local diet components high in oxalate, e.g. 8–13% in Europe and USA vs. 18% in the United Arab Emirates and 20% in Saudi Arabia [17].
Overall it appears that the working hypothesis formulated by Andersen (Table 1) over half a century ago still holds true, and that stone formers are best helped by the advice to maintain a balanced diet and that can be accompanied with medication. But what is a balanced diet for stone formers?
Table 1.
Adaptation of the original working hypothesis, from Andersen [10].
| Economy |
|||
|---|---|---|---|
| Primitive | Intermediate | Modern | |
| Diet | Unbalanced deficient | Balanced | Unbalanced overloaded |
| Stone risk site | High, bladder | Low | High, UUT |
Individual lifestyle elements and the risk of urinary tract stone formation
To appreciate which lifestyle elements these could be, it helps to understand the basic reasons for stone formation. Stones can only form when the urinary tract contains higher concentrations of the stone-forming material than can be kept in solution [18,19]. High concentrations of the mineral components in the urinary tract thus pose a risk of stone formation. For UUT stones the relevant components are calcium, oxalate, phosphate and urate. These can derive from intrinsic pools (calcium from bone or the non-filterable pool in blood), from intrinsic production (uric acid, oxalate) and from the diet (all components). Release from intrinsic pools and intrinsic production both can also be subject to dietary effects. When an attempt is made to prevent stone formation both by medication and lifestyle changes, the intrinsic production, the dietary content and the fraction of the dietary content that is actually taken up must be known. Furthermore, it must be known whether dietary load fluctuations during the day cause fluctuations in the risk of stone formation. This is not a linear relationship. Super-saturation will eventually lead to crystal formation but there is a time lag. The mineralisation drive increases exponentially with the super-saturation. Periods of the day where recent events cause an extreme deviation of urinary composition thus pose the highest risk. The body might be prepared for such mineralisation, as there is a mechanism for renal crystal removal [20,21]. Possibly, stone formation starts when this defence is overwhelmed, but undoubtedly a reduction of the super-saturation drive will be beneficial.
Finally it must be acknowledged that the mineralisation drive depends not on total concentrations of stone components but on their free concentrations plus the solubility of the mineral in that urine. Here other lifestyle elements come into play, those that determine urine volume (water intake and sweat loss), complexation of mineral components (magnesium and citrate), urinary ionic strength (water/salt intake and sweat loss), urinary pH (acid–base balance related to intake of protein and organic acids). The effects of different individual lifestyle elements are discussed first in relation to the formation of calcium stones and then in relation to the formation of uric acid/urate stones.
Medication and lifestyle advice for calcium-containing stones
Calcium stones form when the urinary concentrations of calcium, oxalate and phosphate are high enough and (for calcium phosphate stones) urinary pH dictates the correct ionic phosphate form.
Calcium, oxalate and phosphate content
Initially it would seem that a high dietary supply of calcium, oxalate and phosphate all pose a risk of calcium-salt stone formation. Hypercalciuria was the first urinary abnormality to be found in stone formers and at present, next to a low urine volume, remains the most common problem found in calcium-salt stone formers. Next to the advice to drink more, the first lifestyle advice therefore was to reduce the intake of calcium. Unfortunately a strict reduction of calcium intake caused bone loss and did not reduce stone formation; it might actually have increased it. There are several explanations for this. First, the glomerular filtrate and thus the free concentration of calcium in the serum dictate how much calcium enters the nephron. This free calcium concentration is kept in a narrow range by regulation of intestinal calcium uptake and renal calcium reabsorption. A high dietary supply of calcium does not dramatically change either the free serum concentration or the supply of calcium at the glomerular level. It will down-regulate the fractional intestinal uptake of calcium and reduce reabsorption of calcium in the distal nephron parts. Thus hypercalciuria based on dietary calcium intake starts and increases the mineralisation drive only during the short time that urine needs to pass these parts [22]. Hypercalciuria that is induced by a dietary acid load (see below) or NaCl load, or by a disease associated with hypercalcaemia, increases the mineralisation drive earlier in the nephron [22]. The latter state also increases that drive for the whole day, not only after meals.
For preventing hypercalciuria, the best advice to give to calcium-salt stone formers is to maintain a normal calcium intake, a low salt intake and to avoid a high dietary acid load. When hypercalciuria persists and intrinsic factors are suspected hydrochlorothiazide can be prescribed. This medication has been shown to reduce stone formation by correcting hypercalciuria at the cost of moderate to severe side-effects [23].
By contrast, the dietary supply of oxalate plays a more prominent role and oxalate restriction does reduce the risk of stone formation. Part of the reason for this is that the oxalate content of urine usually is about 10 times lower than the calcium content. An 0.1-mm change in the urinary oxalate concentration has a 10 times greater effect on the calcium oxalate concentration product (which drives crystal formation) than a 0.1-mm increase in calcium concentration. The property of calcium to bind oxalate drives crystal formation in the urine but also reduces the amount of oxalate available for uptake in the intestine. When dietary calcium is restricted, more oxalate is available for uptake. The body compensates for the lower calcium supply by increasing uptake efficiency. However, neither intestinal oxalate uptake nor renal re-absorption is regulated. The extra intestinal uptake causes an increase in urinary oxalate that outweighs the decrease in urinary calcium. Epidemiological studies have confirmed this adverse effect of a low dietary calcium intake [24]. The same mechanism explains the increased urinary oxalate excretion and stone risk after bariatric surgery, where the aim is to reduce the uptake of fatty acids [25]. The remaining fatty acids bind calcium, which increases the free intestinal oxalate concentration [26,27].
In general the advice is to reduce oxalate uptake. In a few stone formers a genetic defect causes increased production of oxalate, i.e. primary hyperoxaluria. In these patients the effect of dietary oxalate restriction can be insufficient and they might need drugs. Pyridoxine aims to correct the overproduction, but at the cost of side-effects. Correcting oxalate uptake can be achieved both by restricting the intake of oxalate and by maintaining a normal intake of calcium or by supplementation with magnesium, which will also bind intestinal oxalate. However, magnesium supplementation should not be given in the presence of renal insufficiency. There is a short list of food items that contain much oxalate, mainly vegetables and plant extracts like tea. In general it is not difficult to explain this list to the patient, and for the patient to avoid their intake. However, specific cultural or individual preferences can frustrate this. There is a second much longer list of foods that contain less oxalate but still contribute to the overall intake. It is very difficult for patients to monitor their total oxalate intake. Furthermore, it is a problem for patients to assess how much oxalate was bound by calcium and magnesium in the intestine. To obtain such insights, patients can rely on an Internet-based dietary assessment which has become available in recent years (e.g. www.niersteen.com).
Animal protein
While the addition of animal protein decreased the risk of LUT stone formation, it became clear that further addition to these levels in the so-called Western diet increased the risk of UUT stone formation [28]. In men with a normal body mass index (BMI) a high animal protein intake is even an independent risk factor for calcium-oxalate stone formation [29]. Conversely, when, in a randomised prospective trial with recurrent hypercalciuric calcium-oxalate stone formers, two characteristics of that diet (excess intake of animal protein and of salt) were reversed to normal values in addition to advice on drinking, the relative risk of UUT stone formation decreased to 0.49, compared to the traditional low-calcium diet [30]. This result might be exaggerated because the low-calcium diet might by itself pose a risk. What this effect would be compared to the Western-style diet cannot be concluded. What sets this study apart from the general situation is that the patients were examined during a 3-month period, received semi-personalised dietary advice, including intensive instruction on the effects of the diet, and had regular guidance during the follow-up. This high level of attention resulted in a withdrawal rate of only 5%. Considering the changes in urinary composition, compliance with all three aspects of advice (drinking, low animal protein and low salt) was good after 1 week. Adherence to the drinking advice stayed high for the whole 5-year period. Adherence to low salt and low protein intake decreased in the first year and the latter slightly deteriorated further over the following 4 years. By contrast, another study found no reduction in stone recurrence when stone formers who already had a normal intake of animal protein were advised to make a further reduction [31]. The best advice appears to be to avoid excess intake of animal protein and salt. A closer examination of the mechanism that links high animal protein intake to a high risk of stone formation explains this.
Acid–base balance
A high animal-protein intake has three negative effects; an increase in urinary calcium excretion, and decreases in urinary pH and urinary citrate excretion. All these increase the risk of forming large calcium-oxalate particles [32]. All three effects derive from the fact that animal protein constitutes an acid load for the body (Fig. 1) [33]. Sulphur-containing amino acids (AA-SH) from protein are converted in the liver, with protons and sulphate as by-products. Both plant- and animal-derived protein constitutes an acid load. Similar reductions in the risk of stone formation were found by preventing an excess intake of animal protein or preventing an excess intake of plant protein [34]. However, plant protein on average contains less AA-SH than animal protein, and plant material also contains high concentrations of organic acids. The latter constitute an alkali load for the body, as they can be converted into bicarbonate both in the liver and the kidney (Fig. 1). It is not the excess consumption of animal protein per se that poses a problem, but the net acid load from the total diet and the reaction of the body to that acid load.
Figure 1.
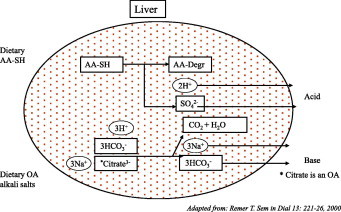
Why fruit juice (pH 2.8) is an alkali load and protein is an acid load. 1 L of orange juice (pH 2.8) contains 1.5 mmol H+. It also contains enough organic acids to produce ≈30 mmol HCO3 by conversion in both the liver and the kidney. 1 kg of meat causes the production of 80 mmol H+ from conversion of AA-SH. Adapted from [33].
A Western diet provides transient events of acid loading when a high animal-protein intake is not balanced by sources of organic acids. The acute effects of hypercalciuria, hypocitraturia and a low pH increase the risk of stone formation. In the long term these acute effects can accumulate and lead to bone loss. However, the body has mechanisms to compensate for the effect of renal calcium loss. When patients are accustomed to a constant high animal-protein diet they show hypercalciuria which is not directly related to the acid load but which can be reduced by a concomitant supply of alkali (potassium citrate) [35]. An (insignificant) trend for higher intestinal calcium can be sufficient to explain that the variables for bone turnover did not change. The question is what will happen in the long term with patients who vary their dietary acid load from day-to-day, a not uncommon situation. The same study showed that serum parathyroid hormone (PTH) levels were decreased by 10 pg/mL after 2 weeks of a high animal-protein diet, while they were increased by 5 pg/mL after 2 weeks of a high animal-protein diet that was balanced with potassium citrate. These changes were not significant, but were also found in another study [36], where serum and urine composition was followed on an hourly time scale after an acute load of animal protein. At the first time point, 1 h after the load, the serum PTH level was clearly decreased. Serum PTH is regulated in response to the serum free-calcium concentration. PTH production decreases in response to hypercalcaemia. As the half-life of PTH is ≈1 min, this leads to a decrease in serum PTH levels within minutes. Generally, when a low serum PTH level is found the interpretation is that there must have been a period of hypercalcaemia. Thus in both studies the patients appear to have experienced transient periods of hypercalcaemia in response to a net acid load. In Fig. 2, I propose a mechanism that might link acid-loading to hypercalciuria through transient hypercalcaemia. After consuming a protein load (animal or plant) the liver starts to produce protons. In the blood these protons are first bound by blood buffers. The level of blood buffers is, amongst other factors, determined by the production of bicarbonate in the liver and kidney. They produce bicarbonate from organic acids like citrate and tartrate. Thus the net balance between protein and organic acids determines whether the blood receives a net acid load, a neutral load or a net alkali load. When the net acid load exceeds the buffering capacity, blood pH decreases. The finding that reducing a high animal-protein intake decreases the stone formation rate, while further reducing normal animal protein intake does not [30,31] might be explained by this threshold for the dietary net-acid load. When the blood pH decreases unfilterable calcium that is bound to albumin in the blood, it is released to the free calcium pool, as that binding is pH-sensitive at around pH 7.4 [37]. This extra free calcium passes to the renal filtration system and this is signalled by the parathyroid glands, where it causes the unexplained decrease in PTH production reported previously [35,36]. This decreases intestinal calcium absorption and renal calcium reabsorption. The extra filtration at the glomerulus and reduced distal reabsorption increase the calcium concentration throughout the tubules. The decrease in blood pH also elicits bicarbonate production by the liver. If that is insufficient, the kidneys reabsorb organic acids like citrate from the proximal tubular fluid, to convert into bicarbonate. Reduced binding by citrate leaves more calcium available in the tubular fluid for combining with oxalate or phosphate. A low fluid intake and citrate also reduces the ability of the urine to inhibit the formation of large calcium oxalate particles [22,38,39]. After some time, addition of acid to the blood will decrease, blood pH will increase and this will be followed by a decrease in alkali production. Due to the higher blood pH, albumin will bind more calcium again, the serum free-calcium level will decrease and transient slight hypocalcaemia can occur, as the PTH level is still low and geared to reduce serum free-calcium levels. PTH production increases and the body starts to compensate for the calcium loss by increasing intestinal uptake. After the whole fluctuation has passed (the time scale is unknown) the end result is urine with a higher calcium content that seems to originate from the intestine, a lower excretion of citrate, and a lower pH.
Figure 2.
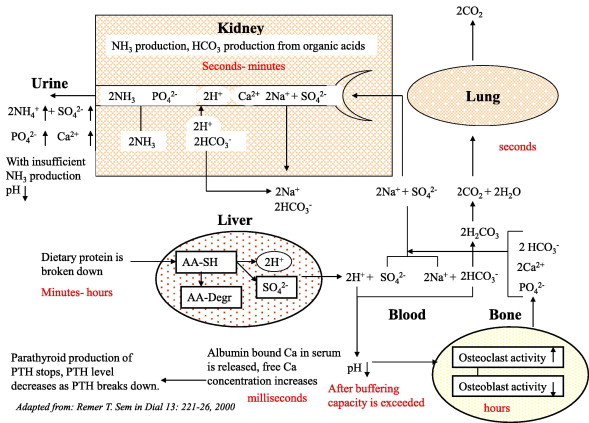
Cascade from acid-load to changes in urine composition. Adapted from [33].
This hypothesis has not been tested in all aspects and it does not take into account that stone-formers more often seem to have abnormalities in their renal acid handling, e.g. incomplete renal tubular acidosis [40]. A high prevalence of renal tubular acidosis might be involved, in Thailand, in the endemic formation of LUT stones also containing calcium oxalates in association with a high prevalence of hypocitraturia [4–7]. However, it explains why an especially acute excess of animal protein is a risk of urolithiasis and how this risk could best be reduced, i.e. by preventing acute events of high acid loading. In practical terms, stone formers should be asked not to eat too much meat, but when they do feel the urge to consume a large portion, compensate that load with enough fruits/vegetables or by alkali medication (potassium citrate).
Water intake
A simple and very effective advice is to drink enough. Drinking reduces the concentrations of the stone-forming components and improves the removal of particles from narrow parts of the urinary tract by increasing the flow rate [22]. Caveats can be that patients are not aware of how much water they lose by sweating, and that patients, upon the advice to drink more, should not drink more high oxalate fluids like tea. Again, if they do want to drink tea, they should know that adding a calcium source like milk lowers the risk posed by tea.
Medication and lifestyle advice for uric acid and urate stone formation
The risk of uric acid and urate stone formation depends both on urinary uric acid content and urinary pH. A daily uric acid excretion of > 4 mmol, hyperuricosuria, and a urinary pH continuously below 6.0 constitute increased risks for uric acid stone formation. High urinary uric acid levels can come from dietary over-consumption of purines, alcohol and fructose; intrinsic factors, as intrinsic overproduction in patients with specific enzyme defects; from excessive breakdown of body tissue; and from medication like probenecid. Uric acid excretion can be reduced by reducing the intake of uric acid precursors and by medication that attacks uric acid production, e.g. allopurinol. The lifestyle measures can be prescribed to all uric-acid and urate stone formers. Allopurinol is reserved for patients who have both hyperuricosuria and hyperuricaemia, with a blood uric acid level of > 380 μm.
Urinary pH determines how much urate is present in the protonated, uric acid, form (low urinary pH) or de-protonated, urate, form (high urinary pH). At ≈pH 5.5, 50% of uric acid is present in the undissociated uric acid form that forms uric acid stones. Above pH 6.5 urate will dominate and can precipitate together with ammonium if that is present in high quantities. In general, the risk of forming a uric acid stone starts when the urinary pH is constantly < 6.0 and it increases further with decreasing urinary pH [41].
Thus uric-acid stone formers are also sensitive to an acute dietary acid load. Furthermore, they are sensitive to the chronic increase in renal acid load that accompanies overweight [42], and the balance between renal ammonium production and excretion of titratable acids [43]. Each human cell produces some acid everyday. Consequently, intrinsic acid production increases with increasing body weight [42].
The contribution of intrinsic acid production is most apparent in the morning urine; the dietary component dominates for the rest of the day. Idiopathic uric-acid stone formers produce more acid directly after a given dietary load [44]. It is not clear if the total production is higher, or that other people take more time to clear the acid load to the urine. Irrespective of the mechanism, urinary pH will be lower in idiopathic uric-acid stone formers. Uric-acid stone formers should be advised to maintain a balance between acid (protein) and alkali load (organic acids from fruits and vegetables) or optionally to receive alkali medication. Again, patients will benefit from lifestyle-analysis tools.
With increasing pH the urate form will dominate and urate salts might precipitate. This will happen when the counter-ion is also present in large enough quantities. Thus patients who combine a high uric acid production with a high pH and high urinary ammonium concentration are at risk of forming ammonium-urate stones. Infection with urease-producing bacteria is the most likely reason for this, and antibiotics are the treatment of choice.
Role of obesity in calcium-salt and uric-acid stone formation
Obese people have a higher risk of urolithiasis [45]. As obesity affects over 300 million people worldwide [25] it contributes considerably to worldwide stone formation. In the USA obesity might have reached its maximum prevalence [46,47]. In countries that more recently have adopted a lifestyle leading to obesity, the prevalence could continue to rise in the future. The increased risk of stone formation in obese people is associated with the lifestyle, an over-rich diet and too little exercise, and the obesity itself (as explained above) [48]. Of all stone formers, those with severe overweight (>120 kg) have the highest daily urinary excretion rates of calcium, oxalate and uric acid [49,50]. As urinary pH is also inversely related to BMI [51], urinary super-saturation with uric acid increases with increasing BMI, and obesity is especially a risk for uric-acid stone formation [52]. For calcium-oxalate stone formation the link is less clear. Urinary super-saturation with calcium oxalate did not increase with BMI [52], and neither was the prevalence of calcium-oxalate stone formation significantly higher in obese people. Still, a reduction of overweight might be sensible advice to both types of stone formers, as this automatically means adapting to the desired balanced lifestyle and could provide extra motivation to maintain that new lifestyle in the long term.
The high urinary calcium excretion in obese people is largely due to the high intake of salt and animal protein. From the mechanism proposed in Fig. 2 it would follow that animal-protein intake will be a risk when it is not balanced by alkali intake. Obese people who eat both more animal protein and more vegetables and fruits might have an extra incentive to gain weight but no extra risk of calcium loss. Of course, their body weight-related higher intrinsic acid production reduces the tolerance for a dietary acid load. A more established explanation is that the (especially abdominal) obesity predisposes to insulin resistance [48]. This decreases the renal ability to excrete an acid load as ammonium, and induces renal consumption of organic acids like citrate for bicarbonate production.
As changing someone’s lifestyle is difficult, the surgical option to treat obesity, bariatric surgery, is becoming increasingly popular. This approach is effective in treating obesity and several of its complications, but it also increases the risk of especially calcium-oxalate stone formation [26,27]. An object of the surgery is to reduce the uptake of fatty acids. In the intestine these compete with oxalate for binding calcium. When more fatty acids remain behind, more oxalate becomes available for uptake. This explains the high urinary oxalate excretion after bariatric surgery. Furthermore, the stool will be soft or watery, inducing chronic bicarbonate loss, intracellular metabolic acidosis, renal consumption of organic acids and hypocitraturia. The hyperoxaluria and hypocitraturia are partly counteracted by a lower urinary calcium excretion, but overall the drive for calcium oxalate crystallisation is increased. Patients who have had bariatric surgery should thus receive extra calcium and extra alkali to compensate for these effects.
Consensus on medication and lifestyle advice to UUT stone formers
For all patients who form stones containing calcium salts and/or uric acid/urate, lifestyle advice is the primary approach to prevent recurrence. Medication should be reserved for cases where lifestyle advice proves insufficient. This can be when there is an intrinsic problem, but also when patient compliance with lifestyle advice is low and remains low despite efforts to optimise it. The drinking advice can be given to all stone formers. When the stone type is known, more specific lifestyle advice and medication can be added. Tables 2 and 3 show what type of lifestyle advice and medication can be prescribed for which patients. For optimisation of the lifestyle advice, both doctors and patients can use lifestyle-analysing tools.
Table 2.
Lifestyle advice for urolithiasis patients; adapted from [1].
| Stone type | Lifestyle advice/medication |
|---|---|
| Struvite | Antibiotics against urease producing bacteria Total stone removal To remove fragments transient acidification using l-methionine (200–500 mg, 3 times daily) to obtain a urine pH between 5.8 and 6.2 can be applied |
| Drinking advice during the period of stone/fragment removal | |
| Calcium oxalates/calcium phosphates Uric acid Ammonium urate |
Drinking advice, to reach a urine volume of > 2 litre a fluid intake of > 2.5 litre is needed. For children the advice is > 1.5 l/m2 body surface area. Drinking should be distributed over the day with some concentration around food intake |
| Extra fluid is needed to compensate sweat loss in a hot environment and during intensive physical activity | |
| Normal calcium intake, 1000–1200 mg/day except for patients with proven absorptive hypercalciuria (urine Ca > 8 mmol/day on average calcium intake and without ongoing bone loss) | |
| Limited NaCl intake, 4–5 g/day unless excessive sweat loss calls for extra NaCl intake | |
| Avoid excess animal protein (>1 g/kg/day). When a high animal protein intake does occur try to compensate this with extra fruit/vegetables | |
| Enough fruits and vegetables to maintain a neutral to slightly alkaline acid/base balance | |
| Try to obtain the recommended daily allowances for vitamins and minerals from the diet. Added vitamin/mineral can be applied but excess intake should be avoided | |
| Minimise intake of food items with a high oxalate content | |
| Strive for a BMI between 18 and 25 for adults. For children reduce overweight with respect to their age group. This advice is especially important for patients who form urate/uric acid containing stones |
Table 3.
When to use which medication.
| Medication/dose | Indication |
|---|---|
| Potassium citrate/ 9–12 g/day Sodium bicarbonate 1.5 g 3×/day |
When adjustment of the acid/base towards the alkaline region is needed and dietary measures are not sufficient. This can be the case for patients with high uric acid production, low dietary acid tolerance (overweight, renal acidification disorders), high intrinsic oxalate production. Stone types: uric acid, calcium-oxalates, ammonium urate |
| Hydrochlorothiazide 25–50 mg/day |
To correct hypercalciuria when that cannot be corrected by dietary advice (or by surgery in the case of primary hyperparathyroidism) Stone types: calcium-salts |
| Magnesium salts 200–400 mg/day |
For patients with oxalate overproduction (hyperoxaluria that cannot be corrected by dietary advice). Magnesium salts should not be given to patients with renal insufficiency Stone types: calcium oxalates |
| Pyridoxine 5–20 mg/kg/day |
Patients in whom hyperoxaluria remains present despite dietary restriction of oxalate and normalisation of calcium intake (primary hyperoxaluria) Goal: normo-oxaluria |
|
l-Methionine 200–500 mg 3× daily |
When acidification of the urine is needed. This can be to remove fragments of infection stones (struvite/calcium apatites) or patients with uric acid/ammonium urate stones Goal: urinary pH 5.8–6.2, where urine pH remains >6.2 despite advice to neutralise the dietary acid/base intake |
| Allopurinol 100–300 mg/day |
For patients with hyperuricosuria that is not corrected by dietary advice. These are patients who produce extra uric acid as a result of severe overweight or due to an enzymatic disorder. The high dose should be reserved for patients who have both hyperuricosuria and hyperuricosaemia |
Optimisation of patient compliance
Lifestyle advice is a cornerstone for preventing urinary stones and has confirmed its potential [1]. Low patient compliance greatly diminishes its effect. To obtain good compliance the patient must be well-informed about the pros and cons of the lifestyle changes that are requested, must be well motivated to start with the changes, and must maintain a high motivation or make the new lifestyle his or her own.
Information on lifestyle advice
Good information on lifestyle advice is available to stone formers; in general practice this information is delivered during the doctor/patient contacts and provided on paper for use at home. After reading the first three sections of this review, the advice summarised in Table 2 might seem straightforward, but in practice the patient at home can still be confused and needs additional individual advice. ‘The doctor told me to drink more but also to be careful with tea. I only like tea. What must I do?’. ‘I wouldn’t mind eating less salt but how do I know how much salt I am eating?’. ‘I should reduce my intake of animal protein but I really like to eat meat’. ‘I have tried to be careful with eating oxalate-containing food and even tried to balance this with my calcium intake, but I am not sure if I am doing OK’. These are all the questions that can be answered when there is precise knowledge about the diet, about the composition of the items in the diet, and about the possible interactions (calcium oxalate, acid–base) as explained above. For these specialised answers the average patient needs assistance from a doctor/dietician or from interactive knowledge tools. In studies where teams of doctors and dieticians provide such intensive guidance, considerable reductions in stone formation rate are achieved with lifestyle advice, i.e. [30]. In general practice such guidance is not possible. To solve this problem there are two routes that must be taken together. First is the use of ‘e-tools’ that provide continuing information and motivation and have confirmed their worth in other diseases [53–55]. Patients with stone need automated Internet tools that analyse the individual diet and provide individual advice, just as the team doctor/dietician would do in the one-to-one situation. Second, the attention of the doctor/dietician should be concentrated into a ‘priming period’, after which the patient should have incorporated most of the advice into their lifestyle. The first steps in this direction are initiatives like the website www.niersteen.com that was developed for guiding patients in the Netherlands.
Continuing motivation
The starting motivation for patients who have just experienced colic pain is high. This motivation decreases as the memory of the pain dissipates, and the hurdle to visit the doctor increases. Providing enough attention during that initial high-motivation period is crucial to obtaining long-term high compliance. Especially important is that the patients want feedback at the moment that they have a question or a problem. In a large scale 2-year follow-up study on exercise advice for women, those women who received advice only at the start showed a significantly lower compliance than those where feedback was given during frequent visits [56]. As noted, such input from the doctor is not possible in general practice and e-tools that can provide the guidance on a (semi)automated basis will be of value. What needs to be done in the near future is to test them for user-friendliness, ensure simple and safe access, and involve the patients themselves in further developments to ensure optimal individualisation and thereby optimal long-term compliance.
Conflict of interest statement
We have no conflicts of interest to declare.
Footnotes
Peer review under responsibility of Arab Association of Urology.
References
- 1.Straub M., Strohmaier W.L., Berg W., Beck B., Hoppe B., Laube N. Diagnosis and metaphylaxis of stone disease. World J Urol. 2005;23:309–323. doi: 10.1007/s00345-005-0029-z. [DOI] [PubMed] [Google Scholar]
- 3.Prien E.L. The riddle of urinary stone disease. JAMA. 1971;216:503–507. [PubMed] [Google Scholar]
- 4.Joly J.S. International Congress of Urology; New York: 1939. The etiology and preventive treatment of urinary lithiasis, in reports. [Google Scholar]
- 5.Prasongwatana V., Sriboonlue P., Suntarapa S. Urinary stone composition in North-East Thailand. Br J Urol. 1983;55:353–355. doi: 10.1111/j.1464-410x.1983.tb03320.x. [DOI] [PubMed] [Google Scholar]
- 6.Tanthanuch M., Apiwatgaroon A., Pripatnanont C. Urinary tract calculi in southern Thailand. J Med Assoc Thai. 2005;88:80–85. [PubMed] [Google Scholar]
- 7.Nimmannit S., Malasit P., Susaengrat W., Ong-Aj-Yooth S., Vasuvattakul S., Pidetcha P. Prevalence of endemic distal renal tubular acidosis and renal stone in the northeast of Thailand. Nephron. 1996;72:604–610. doi: 10.1159/000188947. [DOI] [PubMed] [Google Scholar]
- 8.Aegukkatajit S. Reduction of urinary stone in children from north-eastern Thailand. J Med Assoc Thai. 1999;82:1230–1233. [PubMed] [Google Scholar]
- 9.Thomas J.M.R. Vesical calculus in Norfolk. Br J Urol. 1949;21:20–23. doi: 10.1111/j.1464-410x.1949.tb10746.x. [DOI] [PubMed] [Google Scholar]
- 10.Andersen D.A. Patterns of incidence of stones of the urinary tract (with special reference to bladder stones) Urologia. 1967;34:385–402. [Google Scholar]
- 11.Robertson W.G., Peacock M., Marshall D.H. Prevalence of urinary stone disease in vegetarians. Eur Urol. 1982;8:334–339. doi: 10.1159/000473551. [DOI] [PubMed] [Google Scholar]
- 12.Grossman W. The current urinary stone wave in central Europe. Br J Urol. 1938;10:46–54. [Google Scholar]
- 13.Inada T., Miyasaki S., Omori T., Nihira H., Hino T. Statistical study on urolithiasis in Japan. Urol Int. 1958;7:150–165. doi: 10.1159/000277382. [DOI] [PubMed] [Google Scholar]
- 14.Stamatelou K.K., Francis M.E., Jones C.A., Nyberg L.M., Curhan G.C. Time trends in reported prevalence of kidney stones in the United States 1976–94. Kidney Int. 2003;63:1817–1823. doi: 10.1046/j.1523-1755.2003.00917.x. [DOI] [PubMed] [Google Scholar]
- 15.Hesse A., Brändle E., Wilbert D., Köhrmann K.U., Alken P. Study on the prevalence and incidence of urolithiasis in Germany comparing the years 1979 vs 2000. Eur Urol. 2003;44:709–713. doi: 10.1016/s0302-2838(03)00415-9. [DOI] [PubMed] [Google Scholar]
- 16.Yoshida O., Okada Y. Epidemiology of urolithiasis in Japan: a chronological and geographical study. Urologia Intis. 1990;45:104–111. doi: 10.1159/000281680. [DOI] [PubMed] [Google Scholar]
- 17.Robertson W.G., Hughes H. Epidemiology of urinary stone disease in Saudi Arabia. In: Ryall R.L., Bais R., Marshall V.R., Rofe A.M., Smith L.H., Walker V.R., editors. Urolithiasis 2. Pelum Press; New York: 1994. pp. 453–455. [Google Scholar]
- 18.Kok D.J. Clinical implications of physicochemistry of stone formation. Endocr Metab Clin N Am. 2002;31:855–867. doi: 10.1016/s0889-8529(02)00037-3. [DOI] [PubMed] [Google Scholar]
- 19.Hess B., Kok D.J. Nucleation, growth and aggregation of stone forming crystals. In: Coe F.L., Favus M., Pak C.Y.C., Parks J., Preminger G., editors. Chapter 1: kidney stones: medical and surgical management. Raven Press; 1995. pp. 3–32. [Google Scholar]
- 20.Thurgood L.A., Sørensen E.S., Ryall R.L. The effect of intracrystalline and surface-bound osteopontin on the degradation and dissolution of calcium oxalate dehydrate crystals in MDCKII cells. Urol Res. 2012;40:1–5. doi: 10.1007/s00240-011-0423-5. [DOI] [PubMed] [Google Scholar]
- 21.de Water R., Leenen P.J.M., Noordermeer C., Nigg A.L., Houtsmuller A.B., Kok D.J. Macrophages in nephrolithiasis: cytokine production induced by binding and processing of calcium oxalate crystals. Am J Kidney Dis. 2001;38:331–338. doi: 10.1053/ajkd.2001.26098. [DOI] [PubMed] [Google Scholar]
- 22.Kok D.J., Khan S.R. Calcium oxalate nephrolithiasis, a free or fixed particle disease. Kidney Int. 1994;46:847–854. doi: 10.1038/ki.1994.341. [DOI] [PubMed] [Google Scholar]
- 23.Ettinger B., Citron J.T., Livermore B., Dolman L.I. Chlorthalidone reduced calcium oxalate calculus recurrence but magnesium hydroxide does not. J Urol. 1988;139:679–684. doi: 10.1016/s0022-5347(17)42599-7. [DOI] [PubMed] [Google Scholar]
- 24.Curhan G.C., Willet W.C., Rimm E.B., Stampfer M.J. A prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney stones. N Engl J Med. 1993;328:833–838. doi: 10.1056/NEJM199303253281203. [DOI] [PubMed] [Google Scholar]
- 25.DeMaria E.J. Bariatric surgery for morbid obesity. N Engl J Med. 2007;356:2176–2183. doi: 10.1056/NEJMct067019. [DOI] [PubMed] [Google Scholar]
- 26.Nelson W.K., Houghton S.G., Milliner D.S., Lieske J.C., Sarr M.G. Enteric hyperoxaluria, nephrolithiasis, and oxalate nephropathy: potentially serious and unappreciated complications of Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2005;1:481–485. doi: 10.1016/j.soard.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Asplin J.R., Coe F.L. Hyperoxaluria in kidney stone formers treated with modern bariatric surgery. J Urol. 2007;177:565–569. doi: 10.1016/j.juro.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 28.Robertson W.G., Heyburn P.J., Peacock M., Hanes F.A., Swaminathan R. The effect of high animal protein intake on the risk of calcium stone-formation in the urinary tract. Clin Sci (Lond) 1979;57:285–288. doi: 10.1042/cs0570285. [DOI] [PubMed] [Google Scholar]
- 29.Taylor E.N., Stampfer M.J., Curhan G.C. Dietary factors and the risk of incident kidney stones in men: new insights after 14 years of follow-up. J Am Soc Nephrol. 2004;15:3225–3232. doi: 10.1097/01.ASN.0000146012.44570.20. [DOI] [PubMed] [Google Scholar]
- 30.Borghi L., Schianchi T., Meschi T., Guerra A., Allegri F., Maggiore U. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med. 2002;346:77–84. doi: 10.1056/NEJMoa010369. [DOI] [PubMed] [Google Scholar]
- 31.Hiatt R.A., Ettinger B., Caan B., Quesenberry C.P., Duncan D., Citron J.T. Randomized controlled trial of a low animal protein, high fiber diet in the prevention of recurrent calcium oxalate kidney stones. Am J Epidemiol. 1996;44:25–33. doi: 10.1093/oxfordjournals.aje.a008851. [DOI] [PubMed] [Google Scholar]
- 32.Kok D.J., Iestra J., Doorenbos J.C., Papapoulos S.E. The effects of chronic dietary loads with salt and animal protein on the calcium oxalate crystallization kinetics in urines of healthy men. J Clin Endocr Metab. 1990;71:861–867. doi: 10.1210/jcem-71-4-861. [DOI] [PubMed] [Google Scholar]
- 33.Remer T. Influence of diet on acid-base balance. Semin Dial. 2000;13:221–226. doi: 10.1046/j.1525-139x.2000.00062.x. [DOI] [PubMed] [Google Scholar]
- 34.Massey L.K., Kynast-Gales S.A. Diets with either beef or plant proteins reduce risk of calcium oxalate precipitation in patients with a history of calcium kidney stones. J Am Diet Assoc. 2001;101:326–331. doi: 10.1016/S0002-8223(01)00085-2. [DOI] [PubMed] [Google Scholar]
- 35.Maalouf N.M., Moe O.W., Adams-Huet B., Sakhaee K. Hypercalciuria associated with high dietary protein intake is not due to acid load. J Clin Endocr Metab. 2011;96:3733–3740. doi: 10.1210/jc.2011-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Houillier P., Normand M., Froissart M., Blanchard A., Jungers P., Paillard M. Calciuric response to an acute acid load in healthy subjects and hypercalciuric calcium stone formers. Kidney Int. 1996;50:987–997. doi: 10.1038/ki.1996.400. [DOI] [PubMed] [Google Scholar]
- 37.Kragh-Hansen Vorum H. Quantitative analysis of the interaction between calcium-ions and human serum albumin. Clin Chem. 1993;39:202–208. [PubMed] [Google Scholar]
- 38.Kok D.J., Papapoulos S.E., Bijvoet O.L.M. Excessive crystal agglomeration with low citrate excretion in recurrent stone formers. Lancet. 1986;i(1056):1058. doi: 10.1016/s0140-6736(86)91329-2. [DOI] [PubMed] [Google Scholar]
- 39.Kok D.J., Papapoulos S.E., Bijvoet O.L.M. Crystal agglomeration is a major element in calcium oxalate urinary stone formation. Kidney Int. 1990;37:51–56. doi: 10.1038/ki.1990.7. [DOI] [PubMed] [Google Scholar]
- 40.Arampatzis S., Röpke-Rieben B., Lippuner K., Hess B. Prevalence and densitometric characteristics of incomplete distal tubular acidosis in men with recurrent calcium nephrolithiasis. Urol Res. 2012;40:53–59. doi: 10.1007/s00240-011-0397-3. [DOI] [PubMed] [Google Scholar]
- 41.Grases F., Costa-Bauzá A., Gomila I., Ramis M., Garciá-Raja A., Prieto R.M. Urinary pH and renal lithiasis. Urol Res. 2012;40:41–46. doi: 10.1007/s00240-011-0389-3. [DOI] [PubMed] [Google Scholar]
- 42.Asplin J.R. Uric acid stones. Semin Nephrol. 1996;16:412–424. [PubMed] [Google Scholar]
- 43.Kok D.J., Poindexter J., Pak C.Y.C. Calculation of titratable acidity. Kidney Int. 1993;44:120–126. doi: 10.1038/ki.1993.221. [DOI] [PubMed] [Google Scholar]
- 44.Cameron M.A., Maalouf N.M., Adams-Huet B., Moe O.W., Sakhaee K. Urine composition in type 2 diabetes; predisposition to uric acid nephrolithiasis. J Am Soc Nephrol. 2006;17:1422–1428. doi: 10.1681/ASN.2005121246. [DOI] [PubMed] [Google Scholar]
- 45.Taylor E.N., Stampfer M.J., Curhan G.C. Obesity, weight gain, and the risk of kidney stones. JAMA. 2005;293:455–462. doi: 10.1001/jama.293.4.455. [DOI] [PubMed] [Google Scholar]
- 46.Ogden C.L., Carroll M.D., Kit B.K., Flegal K.M. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA. 2012;307:483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Flegal K.M., Carroll M.D., Kit B.K., Ogden C.L. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 48.Maalouf N.M., Sakhaee K., Parks J.H., Coe F.L., Adams-Huet B., Pak C.Y.C. Association of urinary pH with body weight in nephrolithiasis. Kidney Int. 2004;65:1422–1425. doi: 10.1111/j.1523-1755.2004.00522.x. [DOI] [PubMed] [Google Scholar]
- 49.Powell C.R., Stoller M.L., Schwartz B.F., Kane C., Gentle D.L., Bruce J.E. Impact of body weight on urinary electrolytes in urinary stone formers. Urology. 2000;55:825–830. doi: 10.1016/s0090-4295(99)00617-2. [DOI] [PubMed] [Google Scholar]
- 50.Del Valle E.E., Negri A.L., Spivacow F.R., Rosende G., Forrester M., Pinduli I. Metabolic diagnosis in Stone formers in relation to body mass index. Urol Res. 2012;40:47–52. doi: 10.1007/s00240-011-0392-8. [DOI] [PubMed] [Google Scholar]
- 51.Taylor E.N., Curhan G.C. Body size and 24-hour urine composition. Am J Kidney Dis. 2006;48:905–915. doi: 10.1053/j.ajkd.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 52.Daudon M., Lacour B., Jungers P. Influence of body size on urinary stone composition in men and women. Urol Res. 2006;34:193–199. doi: 10.1007/s00240-006-0042-8. [DOI] [PubMed] [Google Scholar]
- 53.Blanson-Henkemans O.A., van der Boog P.J.M., Lindenberg J., van der Mast C.A.P.G., Neerincx M.A., Zwetsloot-Schonk B.J.H.M. An online lifestyle diary with a persuasive computer assistant providing feedback on self-management. Technol Health Care. 2009;17:253–267. doi: 10.3233/THC-2009-0545. [DOI] [PubMed] [Google Scholar]
- 54.Wang X.H., Istepanian R.S.H., Geake T., Hayes J., Desco M., Kontaxakis G. Feasibility study of a personalized, internet-based compliance system for chronic disease management. Telemed E-Health. 2005;11:559–566. doi: 10.1089/tmj.2005.11.559. [DOI] [PubMed] [Google Scholar]
- 55.Haugen H.A., Tran Z.V., Wyatt H.R., Barry M.J., Hill J.O. Using telehealth to increase participation in weight maintenance programs. Obesity. 2007;15:3067–3077. doi: 10.1038/oby.2007.365. [DOI] [PubMed] [Google Scholar]
- 56.Lawton B.A., Rose S.B., Elley R., Dowell A.C., Fenton A., Moyes S.A. Exercise on prescription for women aged 40–74 recruited through primary care: two year randomized controlled trial. BMJ. 2008;337:a2509. doi: 10.1136/bmj.a2509. [DOI] [PMC free article] [PubMed] [Google Scholar]



