Abstract
Objectives
To give a comprehensive and focused overview on the current knowledge of the causal relations of metabolic syndrome and/or central obesity with kidney stone formation.
Methods
Previous reports were reviewed using PubMed, with a strict focus on the keywords (single or combinations thereof): urolithiasis, nephrolithiasis, kidney stones, obesity, metabolic syndrome, bariatric surgery, calcium oxalate stones, hyperoxaluria, insulin resistance, uric acid stones, acid–base metabolism.
Results
Obesity (a body mass index, BMI, of >30 kg/m2) affects 10–27% of men and up to 38% of women in European countries. Worldwide, >300 million people are estimated to be obese. Epidemiologically, a greater BMI, greater weight, larger waist circumference and major weight gain are independently associated with an increased risk of renal stone formation, both for calcium oxalate and uric acid stone disease.
Conclusions
There are two distinct metabolic conditions accounting for kidney stone formation in patients with metabolic syndrome/central obesity. (i) Abdominal obesity predisposes to insulin resistance, which at the renal level causes reduced urinary ammonium excretion and thus a low urinary pH; the consequence is a greater risk of uric acid stone formation. (ii) Bariatric surgery, the only intervention that facilitates significant weight loss in morbidly obese people, carries a greater risk of calcium oxalate nephrolithiasis. The underlying pathophysiological mechanisms are profound enteric hyperoxaluria due to intestinal binding of calcium by malabsorbed fatty acids, and severe hypocitraturia due to soft or watery stools, which lead to chronic bicarbonate losses and intracellular metabolic acidosis.
Abbreviations: BMI, body mass index; CaOx, calcium oxalate; UA, uric acid; HDL, high-density lipoprotein; RYGB, Roux-en-Y-gastric bypass
Keywords: Metabolic syndrome, Obesity, Uric acid stones, Bariatric surgery, Enteric hyperoxaluria, Hypocitraturia
Introduction
Obesity, i.e. a body mass index (BMI) of >30 kg/m2, has become an epidemic condition around the world, and affects 10–27% of men and up to 38% of women in European countries [1,2]. In the USA the percentage of obese adults increased from 15% in 1995 to 24% in 2005 [1]. Of the adult population of the USA >5% are considered morbidly obese, defined as having a BMI of ⩾40 kg/m2 [3]. Worldwide, over 300 million people are estimated to be obese [1].
Epidemiological studies suggest that a greater BMI, greater body weight, larger waist circumference and major weight gain are independently associated with a greater risk of renal stone formation [4]. Among nearly 6000 renal stone-formers those with a body weight of >120 kg had significantly higher urinary excretion rates of calcium, oxalate and uric acid (UA), all established risk factors for nephrolithiasis, than those of <100 kg [5]. However, in a study of >2000 male and female stone-formers and >1000 male and female healthy controls from the Health Professionals Follow-Up Study and the Nurses Health Studies I and II [6] the positive association between BMI and urinary calcium disappeared after adjusting for urinary sodium level (i.e. salt consumption) and for urinary phosphate (i.e. an index of protein consumption). Furthermore, urinary supersaturation of calcium oxalate (CaOx), the main driving force for the most prevalent CaOx stone formation, was not related to BMI [6].
However, the same large database showed that urinary pH was inversely related to BMI, i.e. more obese people have lower urinary pH values [6]. A very similar finding was obtained when plotting urinary pH against body weight in nearly 5000 kidney-stone formers from the two main stone centres in the USA [7]. Because urinary supersaturation of UA progressively increases with decreasing urinary pH [8], it is not surprising that a direct relationship between urinary UA supersaturation to BMI was found by Taylor and Curhan [6]. Thus the greater incidences of renal stones in more recent years in the USA [9] and Europe [10] might primarily be caused by a specific increase in UA nephrolithiasis in increasing numbers of obese people [6]. Indeed, Daudon et al. [11] showed that the prevalence of UA stone disease both in men and in women progressively increased with increasing BMI, which is not the case for the prevalence of CaOx and calcium phosphate stone formers (Fig. 1).
Figure 1.
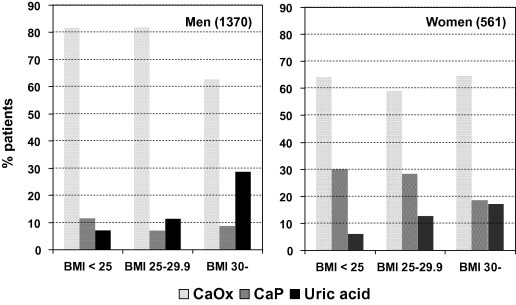
The prevalence of CaOx, calcium phosphate (CaP) and UA stone disease in French men and women in relation to BMI. Adapted from Daudon et al. [11].
Abdominal (central) obesity is the key feature of the so-called metabolic syndrome, as defined in Box 1 [12,13].
Box 1.
The definition of the metabolic syndrome according to the International Diabetes Federation, 2005. Adapted from Alberti et al. [12].
Central (abdominal) obesity that is ethnicity-specific (Europeans, waist circumference, men >94 cm, women >80 cm; USA, waist circumference, men >102 cm, women >88 cm, according to the Adult Treatment Panel III 2001 definition likely to be used [13]).
Plus two of the four following criteria:
-
1)
Arterial hypertension:
systolic >130 mmHg
diastolic >85 mmHg
or treatment for diagnosed hypertension
-
1)
Triglycerides >1.7 mmol/L or specific treatment
-
2)
Low HDL cholesterol
<1.30 mmol/L (women)
<1.00 mmol/L (men)
or specific treatment
-
1)
Plasma glucose >5.6 mmol/L or previously diagnosed type 2 diabetes.
This cluster of cardiovascular risk factors in obese people, increasingly common around the world, affected 27% of the American population in 2000 [14]. According to the epidemiological trial NHANES III, traits of the metabolic syndrome are significantly associated with a self-reported history of kidney stones; with no traits of the metabolic syndrome the prevalence of kidney stones was 3%, and increased to 7.5% with three traits and to 9.8% with five traits, respectively [14]. Lately, similar findings were obtained in a cross-sectional analysis from Korea, where kidney stones were diagnosed by ultrasonography or CT. Among 34,895 screened individuals the prevalence of stones progressively increased with the number of metabolic syndrome components [15]. The multivariate adjusted odds ratio for kidney stones was highest for individuals with hypertension (1.47, 95% CI 1.25–1.71), and higher than for those with the whole metabolic syndrome (1.25, 95% CI 1.03–1.50) [15]. Other significant risk factors for kidney stones were increases in age and waist circumference, as well as male gender, but not serum triglyceride or high-density lipoprotein (HDL) cholesterol levels [15].
The aim of the present review was to show that the global increase in the prevalence of obesity and metabolic syndrome might soon be followed by an epidemic of kidney stone disease, mainly through two pathophysiological mechanisms, i.e. (i) an increased prevalence of UA stone formation due to increasingly low urinary pH values (‘undue urine acidity’), and (ii) an increased prevalence of CaOx stones due to hyperoxaluria/hypocitraturia after bariatric surgery.
Metabolic syndrome, low urinary pH and UA stone formation
Pathophysiology
When the urinary pH is low, UA is primarily present as poorly soluble undissociated UA (solubility 0.54 mmol/L) [8]. Therefore, even at normal urinary UA excretion rates, abundant UA crystallisation with subsequent stone formation can occur. Indeed, clinical experience shows that UA stone formation is mainly caused by a low urinary pH (‘undue urine acidity’) but not by hyperuricosuria [8]. As such abnormally low urinary pH values are often associated with primary gout, it was initially thought that idiopathic UA nephrolithiasis might be a manifestation of primary gout [16]. Two independent studies subsequently showed that the reason for a low urinary pH in most normo-uricosuric UA stone formers was a reduced renal ammonium excretion [17,18]. Furthermore, the increase in urinary ammonium excretion after acid loading in UA stone formers was five to seven times lower than in ‘common’ calcium stone formers or healthy controls [18].
UA stones, metabolic syndrome and insulin resistance
As mentioned above, Maalouf et al. [7] studied almost 5000 stone patients from two main centres in the USA and found an inverse correlation between urinary pH and body weight. Most recently, Otsuki et al. [19] not only confirmed these findings but showed that lower fasting urinary pH values were significantly related to increases in waist circumference, fasting blood glucose level, glycosylated haemoglobin A1c and serum triglycerides, as well as decreases in HDL cholesterol. Thus there were significant associations of urinary pH with all traits of the metabolic syndrome except mean blood pressure [19].
Earlier Sakhaee et al. [18] showed that a third of pure UA stone-formers, but none of the calcium stone-formers or healthy controls, were diabetic. Abate et al. [20] later directly assessed insulin sensitivity in humans by measuring the glucose-disposal rate (a surrogate for insulin sensitivity) during a euglycemic hyperinsulinaemic clamp procedure, as originally described previously [21], in 55 healthy controls and 13 selected pure UA stone-formers. As shown in Fig. 2A the glucose-disposal rate was significantly higher in healthy controls than in UA stone-formers, thus UA stone-formers showed increased insulin resistance. A further important finding of this study was that, compared with healthy controls, lower urinary pH values in UA stone-formers were associated with a significantly lower ratio of urinary ammonium to net acid excretion (Fig. 2B) [20]. Moreover, urinary pH values were positively related to insulin sensitivity in the 55 healthy controls, i.e. a higher urinary pH was associated with higher insulin sensitivity. However, the lower urinary pH values in the 13 UA stone-formers tended to cluster in the area of higher insulin resistance [20].
Figure 2.
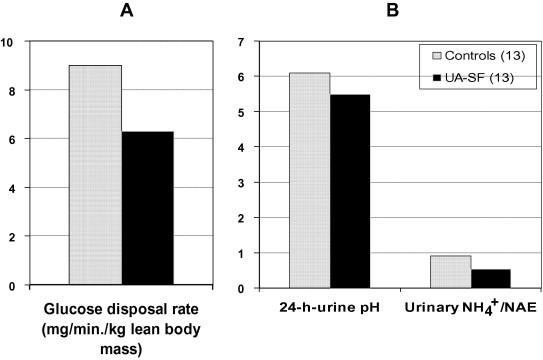
(A) Glucose disposal rate as a measure of insulin sensitivity in healthy controls and UA stone-formers (UA-SF). Values are means. (B) 24-h urinary pH and the ratio of urinary ammonium to net acid excretion (NAE) in healthy controls and UA stone formers (UA-SF). Values are means. Adapted from Abate et al. [20].
Similar findings were also obtained by Otsuki et al. [19] who showed that progressively lower fasting urinary pH values were not only correlated with greater waist circumferences, but also with increases in insulin resistance. Thus abdominal obesity appears to indicate insulin resistance [22] on the one hand and is directly linked to a low urinary pH (‘undue urine acidity’) on the other in patients with metabolic syndrome and UA stone disease. As noted by Otsuki et al. [19], a lower urinary pH was also associated with higher serum urea nitrogen levels, which might reflect a higher intake of protein. Indeed, in young adults, a diet-induced acid load has been shown to be the primary determinant of the potential renal acid load, whereas body surface area is a better estimate of urinary organic anion excretion [23].
Conclusion
Fig. 3 summarises the most likely pathophysiology of UA stone formation in patients with the metabolic syndrome, i.e. a low urinary pH, the key feature of UA stone formation, is due to reduced urinary ammonium excretion. The latter must be viewed as a novel renal manifestation of insulin resistance in the context of metabolic syndrome with central obesity as its primary key feature. Thus, UA stone formation might become a ‘renal bystander’ in some patients with the metabolic syndrome.
Figure 3.
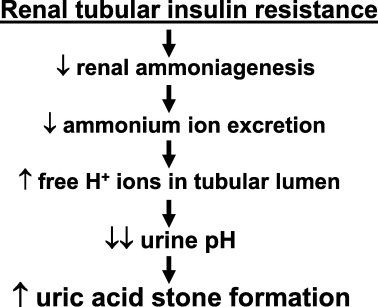
The most likely pathophysiology of UA stone formation in patients with metabolic syndrome.
Bariatric surgery and CaOx stone formation
Epidemiological background
Currently significant weight loss in the morbidly obese is increasingly attained using bariatric surgery. There are two main options: (i) Restrictive bariatric surgical procedures (gastric stapling, adjustable gastric banding, a combination of both, or vertical restrictive gastrectomy) create a small gastric reservoir that delays the emptying of the stomach and thereby limits calorie intake. (ii) malabsorptive procedures, such as the Roux-en-Y-gastric bypass (RYGB) and biliopancreatic diversion, bypass varying portions of the small intestine where nutrients are absorbed [1]. The RYGB has become the most common bariatric operation in the USA [24]. The RYGB results in sustained weight loss and improves abnormal glucose homeostasis, insulin resistance, sleep apnoea, hypertension and cardiovascular risk factors [23]. Although the consequences of malabsorption, i.e. osteoporosis and osteomalacia, have been recognised [25,26], RYGB is generally felt to be safe, effective and durable. Consequently there are increasingly many RYGB procedures in many areas of the world.
The renal complications of bariatric surgery
Previously the potential renal complications of bariatric surgery have had little attention. A first small study reported hyperoxaluria, nephrolithiasis and oxalate nephropathy as serious complications of the RYGB procedure [27], and subsequent independent reports emphasised that one major complication of modern bariatric surgery might be hyperoxaluric CaOx nephrolithiasis. In a retrospective study Asplin et al. [28] reported that compared with normal controls and ‘routine’ kidney-stone formers, 132 patients with nephrolithiasis after bariatric surgery excreted more than twice the amount of urinary oxalate. A smaller study from the Mayo Clinic [24] showed that increasingly many patients developed CaOx stones within a mean of 2.9 years after RYGB surgery. A small cross-sectional analysis of patients from the same cohort before and 12 months after a RYGB showed that hyperoxaluria and substantial increases in urinary CaOX supersaturation occurred in more than half of the previously normo-oxaluric patients [24].
The first prospective longitudinal study on 24 morbidly obese adults after the RYGB procedure [29] reported increases in urinary oxalate excretion rates and relative supersaturations of CaOx by 90 days after surgery. It was concluded that this early increase in urinary oxalate excretion could herald the onset of a clinically significant hyperoxaluric state [29]. To study the prevalence of hyperoxaluria after bariatric surgery, Patel et al. [30] screened 58 patients (52 with the RYGB procedure) and found hyperoxaluria (defined as a urinary oxalate excretion rate of >500 μmol/day) in three-quarters and profound hyperoxaluria (>1100 μmol/day) in a quarter of the patients [30].
The pathophysiology of hyperoxaluria after malabsorptive bariatric surgery
The pathophysiology of hyperoxaluria after RYGB is not completely understood. Two possibilities have been discussed [24,28]. First, RYGB might induce a functional short bowel syndrome with an enteric hyperoxaluric state secondary to increased fatty acid, bile salt and oxalate delivery to the intact colon, similar to what can be found in Crohn’s disease and after a jejuno-ileal bypass [31].
As ingested calcium binds to unabsorbed fatty acids (‘calcium soaps’), dietary oxalates reach the colon uncomplexed by calcium and are then absorbed. From this well-known mechanism, it could be hypothesised that a longer common channel after RYGB surgery might predispose to more significant fat malabsorption and thus hyperoxaluria. Previous studies in inflammatory bowel disease appear to confirm this hypothesis, as the degree of hyperoxaluria corresponds with the degree of steatorrhoea [31].
Second, alterations in the intestinal flora after RYGB could play a role. Oxalobacter formigenes, normally part of the human intestinal microflora, can metabolise oxalate as an energy source, and normal colonisation with O. formigenes protects against increases in oxalate absorption and excretion into urine [32]. Indeed, decreased intestinal colonisation with oxalate-degrading bacteria was reported in patients who had undergone jenuno-ileal bypass surgery [33]. Whether or not RYGB surgery also alters colonisation with O. formigenes is currently unknown.
In conclusion RYGB surgery can predispose to hyperoxaluric CaOx nephrolithiasis, primarily in the monohydrate form. The most likely cause of hyperoxaluria is gastrointestinal hyperabsorption of oxalate due to reduced intestinal precipitation by dietary calcium, i.e. enteric hyperoxaluria. Consequently, an increase in calcium intake to 4–6 g/day, ingested with meals, is expected to reduce oxalate absorption and hyperoxaluria, as shown by the teaching case (Box 2).
Box 2. Teaching case.
CaOx nephrolithiasis after RYBG surgery
In 1999, a 53-year-old female healthcare administrator received a adjustable gastric band to treat morbid obesity (body weight 113 kg, height 166 cm, BMI 41 kg/m2). Three years later she underwent a RYGB procedure due to intolerance of the gastric banding. In the following years she had several surgical procedures for postoperative internal abdominal hernias. By the end of 2009, 7 years after RYGB surgery, she had left-sided renal colic and ESWL was required for a left-sided kidney stone. At the beginning of 2010 a residual stone in the left kidney was again treated by ESWL. Stone analysis by X-ray diffraction showed 80% CaOx monohydrate and 20% CaOx dihydrate. She had a metabolic evaluation; the results of the 24-h urine measurements (Table 1) showed severe hyperoxaluria and profound hypocitraturia.
The patient was put on a calcium-rich diet (4–6 g/day, ingested with meals) to precipitate sufficient oxalate within the gastrointestinal tract and thereby avoid hyperabsorption of oxalate. In addition, she was treated with alkali in the form of potassium citrate (2 × 30 mmol/day). After repeated interventions to optimise the dietary and medical treatment, the 24-h urine collection 1.5 years later showed normal urinary citrate levels and only minimally elevated urinary oxalate excretion (Table 1). The patient remained stone-free.
The pathophysiology of hypocitraturia after malabsorptive bariatric surgery
As shown by the teaching case (Box 2), malabsorptive states such as the classic short-bowel syndrome, Crohn’s disease, colitis ulcerosa and a functionally short bowel such as after jejuno-ileal bypass or (more recently) the RYGB procedure, might induce severe hypocitraturia. Sufficient citrate in urine is important because citrate retards the crystallisation of stone-forming calcium salts, mainly by forming a pH-dependent calcium–citrate–phosphate complex [34], and mediates the inhibitory effects of macromolecular modulators of CaOx crystallisation [34]. The reason for severe hypocitraturia after malabsorptive bariatric procedures such as RYGB surgery is chronic diarrhoea with gastrointestinal losses of bicarbonate. Depending on the severity of the diarrhoea, this continuous, but often subtle, loss of alkali induces a more or less severe intracellular acidosis in proximal renal tubular cells [8,35].
It has been shown clearly that the degree of citraturia is mainly determined by changes in acid-base status [34]. At the apical membrane of the proximal tubule, a sodium-dependent dicarboxylic transporter is responsible for citrate reabsorption, whereas citrate uptake across the basolateral membrane appears to occur via a tricarboxylate transporter [34]. In metabolic acidosis with a lower luminal pH in the proximal tubule, the divalent species of citrate becomes much more prevalent, and thus citrate reabsorption from the tubular lumen into the proximal tubular cells increases. Furthermore, the activity of citrate lyase increases during intracellular acidosis, which lowers citrate concentrations within the proximal tubular cells and further favours reabsorption from the tubular fluid. Finally, there are more sodium-dependent citrate transporters in acidosis [34]. The combination of all these factors allows for a net increase in the use of citrate by renal cells and thus a decrease in the urinary excretion of citrate, even if the acidosis is not systemic but only subtle, i.e. intracellular.
In conclusion, the passage of many soft or even watery stools after malabsorptive procedures such as RYGB surgery is associated with chronic bicarbonate losses and the development of intracellular metabolic acidosis, the latter being a well-known cause of hypocitraturia.
Overall conclusion
Based on present knowledge, RYGB surgery predisposes to hyperoxaluric and hypocitraturic CaOx nephrolithiasis. This appears to occur by two simultaneous pathophysiological effects; (i) RYGB induces an enteric hyperoxaluric state due to fat malabsorption and binding of calcium to unabsorbed fatty acids which allows for hyperabsorption of oxalates unbound by the missing free calcium; and (ii) the continuous passage of soft or watery stools after malabsorptive procedures such as RYGB surgery is associated with chronic bicarbonate losses which induce intracellular metabolic acidosis, a well-known cause of hypocitraturia.
Conflict of interest
The author has no conflict of interest to declare.
Source of Funding
None.
Table 1.
The 24-h urine analyses of a 53-year-old woman after the first kidney stone episode at 7 years following RYGB surgery for morbid obesity (4/2010), and after the dietary intervention and medical treatment with potassium citrate (10/2011).
| Analysis | Normal levels | After RYGB | After treatment |
|---|---|---|---|
| Promoters | |||
| Volume (mL) | >1200 | 2500 | 3050 |
| 24-h urinary excretion rate (mmol): | |||
| Na | <200 | 130 | 180 |
| Ca | <8.00 | 2.33 | 4.82 |
| Oxalate | <0.450 | 1.075 | 0.458 |
| UA | <4.00 | 2.25 | 1.86 |
| Phosphate | Diet-dependent | 29.0 | 13.1 |
| Urea | Diet-dependent | 358 | 268 |
| Inhibitors | |||
| Citrate | >1.90 | 0.50 | 2.87 |
| Mg | >2.20 | 6.15 | 9.03 |
Footnotes
Peer review under responsibility of Arab Association of Urology.
References
- 1.DeMaria E.J. Bariatric surgery for morbid obesity. N Engl J Med. 2007;356:2176–2183. doi: 10.1056/NEJMct067019. [DOI] [PubMed] [Google Scholar]
- 2.James W.P.T., Van de Werf F. Obesity management: the cardiovascular benefits. Eur Heart J. 2005;7(Suppl. L):L3–L4. [Google Scholar]
- 3.Flum D.R., Khan T.V., Dellinger E.P. Toward the rational and equitable use of bariatric surgery. JAMA. 2007;298:1442. doi: 10.1001/jama.298.12.1442. [DOI] [PubMed] [Google Scholar]
- 4.Taylor E.N., Stampfer M.J., Curhan G.C. Obesity, weight gain, and the risk of kidney stones. JAMA. 2005;293:455–462. doi: 10.1001/jama.293.4.455. [DOI] [PubMed] [Google Scholar]
- 5.Powell C.R., Stoller M.L., Schwartz B.F., Kane C., Gentle D.L., Bruce J.E. Impact of body weight on urinary electrolytes in urinary stone formers. Urology. 2000;55:825–830. doi: 10.1016/s0090-4295(99)00617-2. [DOI] [PubMed] [Google Scholar]
- 6.Taylor E.N., Curhan G.C. Body size and 24-hour urine composition. Am J Kidney Dis. 2006;48:905–915. doi: 10.1053/j.ajkd.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Maalouf N.M., Sakhaee K., Parks J.H., Coe F.L., Adams-Huet B., Pak C.Y.C. Association of urinary pH with body weight in nephrolithiasis. Kidney Int. 2004;65:1422–1425. doi: 10.1111/j.1523-1755.2004.00522.x. [DOI] [PubMed] [Google Scholar]
- 8.Hess B. Acid-base metabolism: implications for kidney stone formation. Urol Res. 2006;34:134–138. doi: 10.1007/s00240-005-0026-0. [DOI] [PubMed] [Google Scholar]
- 9.Stamatelou K.K., Francis M.E., Jones C.A., Nyberg L.M., Curhan G.C. Time trends in reported prevalence of kidney stones in the United States in 1976–94. Kidney Int. 2003;63:1817–1823. doi: 10.1046/j.1523-1755.2003.00917.x. [DOI] [PubMed] [Google Scholar]
- 10.Hesse A., Brändle E., Wilbert D., Köhrmann K.U., Alken P. Study on the prevalence and incidence of urolithiasis in Germany comparing the years 1979 vs. 2000. Eur Urol. 2003;44:709–713. doi: 10.1016/s0302-2838(03)00415-9. [DOI] [PubMed] [Google Scholar]
- 11.Daudon M., Lacour B., Jungers P. Influence of body size on urinary stone composition in men and women. Urol Res. 2006;34:193–199. doi: 10.1007/s00240-006-0042-8. [DOI] [PubMed] [Google Scholar]
- 12.Alberti K.G., Zimmet P., Shaw J. For the IDF Epidemiology Task Force Consensus Group. The metabolic syndrome – a new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 13.Ford E.S., Giles W.H., Dietz W.H. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 14.West B., Luke A., Durazo-Arvizu R.A., Cao G., Shoham D., Kramer H. Metabolic syndrome and self-reported history of kidney stones: the National Health and Nutrition Examination Survey (NHANES III) 1988–94. Am J Kidney Dis. 2008;51:741–747. doi: 10.1053/j.ajkd.2007.12.030. [DOI] [PubMed] [Google Scholar]
- 15.Jeong I.G., Kang T., Bang J.K., Park J., Kim W., Hwang S.S. Association between metabolic syndrome and the presence of kidney stones in a screened population. Am J Kidney Dis. 2011;58:383–388. doi: 10.1053/j.ajkd.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 16.Pak C.Y.C., Sakhaee K., Peterson R.D., Poindexter J.R., Frawley W.H. Biochemical profile of idiopathic uric acid nephrolithiasis. Kidney Int. 2001;60:757–761. doi: 10.1046/j.1523-1755.2001.060002757.x. [DOI] [PubMed] [Google Scholar]
- 17.Kamel K.S., Cheema-Dhadli S., Halperin M.L. Studies on the pathophysiology of the low urine pH in patients with uric acid stones. Kidney Int. 2002;61:988–994. doi: 10.1046/j.1523-1755.2002.00197.x. [DOI] [PubMed] [Google Scholar]
- 18.Sakhaee S., Adams-Huet B., Moe O.W., Pak C.Y.C. Pathophysiologic basis for normouricosuric uric acid nephrolithiasis. Kidney Int. 2002;62:971–979. doi: 10.1046/j.1523-1755.2002.00508.x. [DOI] [PubMed] [Google Scholar]
- 19.Otsuki M., Kitamura T., Goya K., Saito H., Mukai M., Kasayama S. Association of urine acidification with visceral obesity and the metabolic syndrome. Endocr J. 2012;58:363–367. doi: 10.1507/endocrj.k10e-319. [DOI] [PubMed] [Google Scholar]
- 20.Abate N., Chandalia M., Cabo-Chan A.V., Moe O.W., Sakhaee K. The metabolic syndrome and uric acid nephrolithiasis: novel features of renal manifestation of insulin resistance. Kidney Int. 2004;65:386–392. doi: 10.1111/j.1523-1755.2004.00386.x. [DOI] [PubMed] [Google Scholar]
- 21.DeFronzo R.E., Tobin J.D., Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;233:E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 22.Carey D.G., Jenkins A.B., Campbell L.V., Freund J., Chisholm D.J. Abdominal fat and insulin resistance in normal and overweight women: direct measurements reveal a strong relationship in subjects at both low and high risk of NIDDM. Diabetes. 1996;45:633–638. doi: 10.2337/diab.45.5.633. [DOI] [PubMed] [Google Scholar]
- 23.Berkemeyer S., Remer T. Anthropometrics provide a better estimate of urinary organic acid excretion than a dietary mineral intake-based estimate in children, adolescents and young adults. J Nutr. 2006;136:1203–1208. doi: 10.1093/jn/136.5.1203. [DOI] [PubMed] [Google Scholar]
- 24.Sinha M.K., Collazo-Clavell M.L., Rule A., Milliner D.S., Nelson W., Sarr M.G. Hyperoxaluric nephrolithiasis is a complication of Roux-en-Y-gastric bypass surgery. Kidney Int. 2007;71:100–107. doi: 10.1038/sj.ki.5002194. [DOI] [PubMed] [Google Scholar]
- 25.DePrisco C., Levine S.N. Metabolic bone disease after gastric bypass surgery for obesity. Am J Med Sci. 2005;329:57–61. doi: 10.1097/00000441-200502000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Colazzo-Clavell M.L., Jiminez A., Hodgson S.F., Sarr M.G. Osteomalacia after Roux-en-Y-gastric bypass. Endocr Pract. 2004;10:195–198. doi: 10.4158/EP.10.3.195. [DOI] [PubMed] [Google Scholar]
- 27.Nelson W.K., Houghton S.G., Milliner D.S., Lieske J.C., Sarr M.G. Enteric hyperoxaluria, nephrolithiasis, and oxalate nephropathy: potentially serious and unappreciated complications of Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2005;1:481–485. doi: 10.1016/j.soard.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 28.Asplin J.R., Coe F.L. Hyperoxaluria in kidney stone formers treated with modern bariatric surgery. J Urol. 2007;177:565–569. doi: 10.1016/j.juro.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 29.Duffey B.G., Pedro R.N., Makhlouf A., Kriedberg C., Stessman M., Hinck B. Roux-en-Y gastric bypass is associated with early increased risk factors for development of calcium oxalate nephrolithiasis. J Am Coll Surg. 2008;206:1145–1153. doi: 10.1016/j.jamcollsurg.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 30.Patel B.N., Passman C.M., Fernandez A., Asplin J.R., Coe F.L., Kim S.C. Prevalence of hyperoxaluria after bariatric surgery. J Urol. 2009;181:161–166. doi: 10.1016/j.juro.2008.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McLeod R.S., Churchill D.N. Urolithiasis complicating inflammatory bowel disease. J Urol. 1992;148:974–978. doi: 10.1016/s0022-5347(17)36794-0. [DOI] [PubMed] [Google Scholar]
- 32.Siener R., Ebert D., Hesse A. Urinary oxalate excretion in female calcium oxalate stone formers with and without a history of recurrent urinary tract infections. Urol Res. 2001;29:245–248. doi: 10.1007/s002400100198. [DOI] [PubMed] [Google Scholar]
- 33.Allison M.J., Cook H.M., Milne D.B., Gallagher S., Clayman R.V. Oxalate degradation by gastrointestinal bacteria from humans. J Nutr. 1986;116:455–460. doi: 10.1093/jn/116.3.455. [DOI] [PubMed] [Google Scholar]
- 34.Hess B. Urinary citrate and citrate metabolism. In: Rao P.N., Preminger G.M., Kavanagh J.P., editors. Urinary Tract Stone Disease, Part II. London; Springer: 2011. pp. 181–184. Chapter 14. [Google Scholar]
- 35.Tasca A. Metabolic syndrome and bariatric surgery in stone disease etiology. Curr Opin Urol. 2011;21:129–133. doi: 10.1097/MOU.0b013e3283435cbc. [DOI] [PubMed] [Google Scholar]



