Abstract
Introduction
Over the past 15 years, the discovery and development of oral medications that selectively inhibit the enzyme phosphodiesterase type 5 (PDE5) have revolutionised the treatment of erectile dysfunction (ED). Currently, three PDE5 inhibitors are widely available clinically, i.e., sildenafil, vardenafil and tadalafil. New PDE5 inhibitors, including avanafil and udenafil, are now in clinical use in a few countries, and other compounds are under development.
Methods
We describe the current use and future direction of PDE5 inhibitors in the treatment of ED.
Results and conclusion
Each PDE5 inhibitor has an excellent and comparable efficacy and tolerability. These drugs are highly effective for ED of various causes, and are effective in preventing ED after radical prostatectomy. However, whilst being at least 60% effective, PDE5 inhibitors are still ineffective in at least 30% of patients, prompting current research into other pharmacological targets for ED.
Abbreviations: ED, erectile dysfunction; PDE5(i), phosphodiesterase type 5 (inhibitors); IIEF, International Index of Erectile Function; SHIM, Sexual Health Inventory in Men; NO, nitric oxide; sGC, soluble guanylyl cyclase; cGMP, cyclic guanosine monophosphate; GTP, guanosine triphosphate; FDA, USA Food and Drug Administration; Cmax, maximum serum concentration; Tmax, time to Cmax; RCT, randomised controlled trial
Keywords: Erectile dysfunction, Phosphodiesterase type 5 inhibitors, Penile disorders
The epidemiology of erectile dysfunction (ED)
ED is defined as the recurrent inability to obtain and maintain an erection for sexual function [1]. Clinically, the diagnosis of ED is based mostly on the patient’s report, which can be quantified using well-validated questionnaires including the International Index of Erectile Function (IIEF) and the shorter Sexual Health Inventory in Men (SHIM) [2,3]. In addition, laboratory and physiological studies can supplement the patient’s history and physical examination, to aid the clinician in determining the cause and severity of ED. These include, but are not limited to, serum testosterone levels, penile Doppler ultrasonography, combined intracavernous injection and stimulation, and monitoring nocturnal penile tumescence. However, the use of these tests has declined significantly with the advent of medications that are effective for all causes of ED [4].
ED is a common problem worldwide, especially among ageing men. Using a meta-analysis of over 24 international studies, the prevalence of ED in men in their 40s was 2–9%. This increased to 20–40% in men in their 60s, and by the age of 80 years, 75% of men report ED [5]. In 1995 there were >152 million men worldwide who experienced ED, and this total is estimated to reach 322 million by 2025 [6]. In the USA the crude incidence rate of ED in white men is estimated at 26/1000 man-years. This rate increases with each decade (per 1000 man-years) to 12.4 for 40–49 years, 29.8 for 50–59 years and 46.4 for 60–69 years [7].
The age-adjusted risk (per 1000 man-years) of ED was higher for men with diabetes mellitus (50.7 cases), treated heart disease (58.3 cases), and treated hypertension (42.5 cases). Using these data and the known population of the USA, it was estimated that there are 617,715 new cases of ED per year in those aged 40–69 years [8]. Some authors predict that continuing public education about ED and phosphodiesterase type 5 inhibitors (PDE5i) will increase the patient-reported incidence of this disease [9].
In Middle Eastern countries there is comparatively little information about the overall disease burden of ED, and how it compares to western countries. However, one study using random questionnaires via the website Facebook™ showed that among younger Arab men (mean age 35 years) there is a high prevalence of mild ED, based on the SHIM score, and a low willingness to treat this with PDE5i due to a high distrust of these medications [10]. The authors of this study suggest that this distrust might be due to a mass media campaign focusing on the overestimated side-effects of these medications.
The physiology and pathophysiology of ED
Erectile function depends on a complex interplay of psychological sexual stimulation, sensory feedback, peripheral neurotransmitter release, smooth muscle cell relaxation, and vascular engorgement of the corporal penile tissue, resulting in erection. After sexual stimulation, postsynaptic neurones and endothelial cells in the penis release various erectogenic substances, the most important of which is nitric oxide (NO). Despite its very short half-life, this gaseous molecule can diffuse quickly across the smooth muscle cell membrane to activate a signalling cascade that ultimately results in arteriolar smooth muscle relaxation, vascular engorgement, and erection. NO activates soluble guanylyl cyclase (sGC) which produces cyclic guanosine monophosphate (cGMP) from guanosine triphosphate (GTP). Cyclic GMP is the second messenger that sets in motion vascular smooth muscle relaxation. The enzyme PDE5 enzymatically inactivates cGMP to GMP, resulting in decreased downstream erectogenic signalling. Thus, PDE5i promote erections by increasing the stability of cGMP and potentiating the NO/cGMP-dependent signalling cascade (Fig. 1). However, without sexual stimulation and the production of NO, there is no signal to potentiate, which explains why PDE5i do not cause erections in the absence of sexual stimulation [11].
Figure 1.
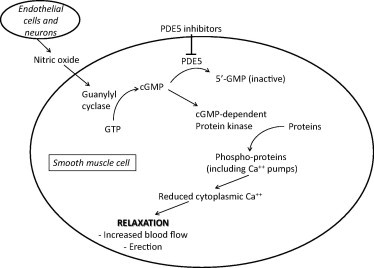
PDE5i promote penile smooth muscle cell relaxation and erection by preventing the degradation of cGMP.
It is now well accepted that, in most cases, ED is caused by several simultaneous factors, including psychogenic, vasculogenic, neurogenic, anatomical, endocrinological, and pharmacological causes (Fig. 2). Psychogenic causes, including performance anxiety, stress, depression, and relationship factors, are very important and are probably a significant component in most cases. In fact, ED from organic causes can lead to decreased sexual confidence, anxiety, and/or depression, with further worsening of ED (see Fig. 3).
Figure 2.
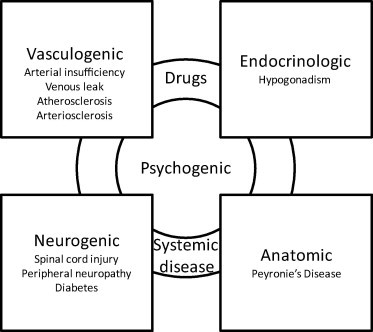
The multifactorial causes of ED.
Figure 3.
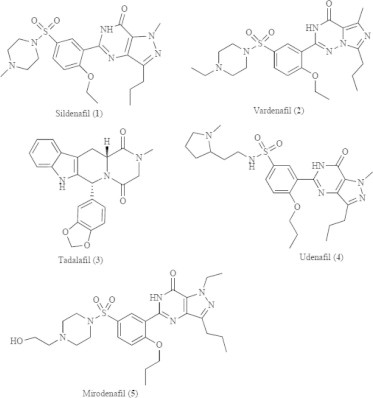
The molecular structures of clinically available PDE5i.
As erections are ultimately a neurovascular event, cardiovascular and neurological diseases are common causes of ED. Both hypertension and current smoking status increase the risk of ED by 60%, and diabetes mellitus carries an adjusted odds ratio of 2.9, an expected finding given its role in peripheral neuropathy, peripheral vascular disease, and endothelial dysfunction (Table 1). Furthermore, medications used in the treatment of hypertension and depression are well-recognised causes of ED.
Table 1.
Risk factors for ED.
| Condition | Multivariate adjusted odds ratio |
|---|---|
| Antidepressant use | 9.1 |
| Antihypertensive use | 4.0 |
| Diabetes mellitus | 2.9 |
| Obstructive urinary symptoms | 2.2 |
| Current cigarette smoking | 1.6 |
| Hypertension | 1.6 |
| Benign prostatic enlargement | 1.6 |
| Obesity | 1.5 |
| Physical inactivity | 1.5 |
| Cardiovascular disease | 1.1 |
Finally, the treatment of prostate cancer, either through damage of the neurovascular structures supplying the penis, or through ablation of the androgen axis, can result in profound ED that is particularly refractory to treatment.
Historical and alternative treatments for ED before PDE5i, and the discovery of PDE5i
Before the discovery of PDE5i the medical treatments for ED were prone to inefficacy and side-effects. Because of this, effective treatments were limited to relatively invasive therapies such as urethral suppositories, intracorporeal injectable medications, penile vacuum pumps, or penile prosthetic surgery. The ineffective medications included yohimbine (which is unlikely to be more effective than placebo), apomorphine (which has not been approved in the USA due to its side-effect profile), and trazadone (which, despite a well-known side-effect of priapism, has not been shown to reliably improve erectile function) [12–14].
Before the discovery of oral PDE5i, nonselective PDEi were used for treating ED. Papaverine is an injectable PDEi with a half-life of 1–2 h, that is 55% effective in achieving an erection when injected directly into the corpora. However, its side-effect profile, including a 35% risk of priapism, has led to a near abandonment of this medication as a monotherapy [15]. Non-selective PDEi, such as caffeine and pentoxifylline, have been shown to have minimal efficacy in vasculogenic ED [16].
In this context there was understandably great excitement in 1998 when Pfizer received the first USA Food and Drug Administration (FDA) approval for sildenafil (Viagra™), the first oral medication approved for treating ED. Given its activity as a vasodilator, sildenafil was actually developed as an antihypertensive and anti-anginal agent, but the lack of clinical efficacy for these endpoints, combined with a common side-effect of erections, led the company to quickly pursue ED as its primary indication. Five years later, in 2003, vardenafil and tadalafil received FDA approval for use in ED, providing three medications in the same class for treating ED.
The pharmacology of PDE5 inhibition and adverse events
Pharmacologically, the PDE5i are very similar, but slight differences in their chemical selectivity and bioavailability and/or metabolism can explain the slightly different side-effect profiles and the timing of the clinical response after dosing. There are 11 subtypes of PDEs that are expressed in a wide variety of tissues throughout the body. As a class, they function in the modulation of second messengers of signalling cascades, and their downstream cellular and physiological effects are broad. Nearly all PDEs are expressed in erectile tissue, but PDE5 is by far the most important for degrading cGMP in corporal smooth muscle cells. Conversely, PDE5 is expressed in vascular beds outside of the penis, including pulmonary arterioles, and in the smooth muscle cells of the bladder neck and prostate, which explains its clinical effectiveness in the treatment of primary pulmonary hypertension and BPH, respectively. Fortunately, the extreme selectivity of PDE5i results in few ‘off-target’ effects (Table 2). PDE6 is only expressed in photoreceptors, and sildenafil and vardenafil (and tadalafil to a lesser extent) inhibit PDE6. This results in a dose-related impairment of blue–green colour discrimination, and leads to the blue vision that <2% of patients report. Tadalafil inhibits PDE11 more strongly than the other drugs, although the clinical importance of this inhibition has not yet been shown.
Table 2.
The selectivity of PDE5i for PDE isoforms, compared to PDE5 (PDE5 = 1). Larger values denote a lower binding affinity.
| Isoform | PDE isoenzyme |
||
|---|---|---|---|
| Sildenafil | Tadalafil | Vardenafil | |
| PDE1 | 80 | >4000 | 690 |
| PDE2 | >19,000 | >4000 | 62,000 |
| PDE3 | 4628 | >4000 | 40,000 |
| PDE4 | 2057 | >4000 | 47,000 |
| PDE6 | 11 | 188 | 35 |
| PDE7 | 6100 | >14,000 | >300,000 |
| PDE8 | 8500 | >14,000 | >300,000 |
| PDE9 | 750 | >14,000 | 5800 |
| PDE10 | 2800 | >14,000 | 30,000 |
| PDE11 | 780 | 6 | 1620 |
Post-marketing studies have confirmed that PDE5i are extremely well tolerated, and the side-effects are minimal and similar between agents (Table 3). Most adverse events are the result of the vasoactivity of these agents, and the presence of PDE5 in vascular beds outside of the corpora cavernosa. These include headache, flushing, nasal congestion and dyspepsia. Perhaps the most clinically important side-effect is the additive vasodilation of PDE5i in patients taking nitrate medications, which can result in significant and potentially catastrophic hypotension. For this reason, in the management of patients with acute chest pain, it is recommended to delay nitrate administration for 24 h after sildenafil/vardenafil dosing, and 48 h after tadalafil dosing.
Table 3.
The common side-effects of PDE5i (>2% incidence).
| Adverse event (%) | Sildenafil | Tadalafil | Vardenafil |
|---|---|---|---|
| Headache | 16 | 15 | 15 |
| Flushing | 10 | 3 | 11 |
| Nasal congestion | 4 | 3 | 9 |
| Dyspepsia | 7 | 8 | 4 |
| Abnormal vision | 3 | – | – |
| Sinusitis | – | – | 3 |
| ‘Flu syndrome | – | – | 3 |
| Diarrhoea | 3 | – | – |
| Myalgia | – | 3 | – |
This issue has led in the past to the concern that PDE5i might be unsafe in patients with cardiac comorbidities. This has been shown not to be the case. The Princeton Consensus Guideline Conference II reported a careful review of the risks, adverse events, and safety of PDE5i in patients with cardiac comorbidities, and showed no increased risk of cardiac events [17]. In fact, several studies reviewed in this meta-analysis showed that patients taking PDE5i had fewer cardiac events than those not taking PDE5i, which could be expected when considering that sildenafil was initially developed as an anti-anginal agent.
Pharmacokinetics
Each of the three PDE5i have different pharmacokinetics, and their absorption is differentially affected by co-administration with fatty meals. An onset of action as early as 11 min with sildenafil and 14 min with vardenafil and tadalafil has been reported [18,19]. However, success at this early stage occurs in <40% of patients, and counselling patients to wait a full hour to allow for maximum serum accumulation, and to avoid early sexual activity after dose administration, can avoid performance anxiety, loss of confidence, and treatment failure. The most significant difference in the three medications is the serum half-life; whilst that of sildenafil and vardenafil are similar, at 4 h, that for tadalafil is ≈20 h. This has led to more frequent ‘on demand’ dosing with sildenafil and vardenafil, and more frequent daily dosing with tadalafil.
Co-administration of these medications with fatty meals can delay their absorption and result in failure. When taken with a fatty meal, sildenafil has a reduction in the maximum serum concentration (Cmax) of 29%, with a delay in the time to maximum serum concentration (Tmax) of up to 1 h [20]. Similarly, vardenafil taken with a fatty meal will reduce the Cmax by 18% and delay Tmax by 1 h. These effects are not seen with tadalafil, probably because of its long half-life, and can be overcome by instructing patients to delay sildenafil or vardenafil administration by 1–2 h after eating, or to take with a reduced-fat meal.
Clinical outcomes
The evaluation of the clinical outcomes of the various PDE5i is aided by numerous high-quality, well-designed, blinded, randomised controlled trials (RCTs) comparing the various agents both to placebo and to the other agents. A recent meta-analysis that evaluated the outcomes of over 100 studies, including over 31,000 patients, showed each of the PDE5i to be highly effective over placebo, with tadalafil showing a very slight advantage against the other agents, followed by vardenafil [21]. The outcomes in this meta-analysis were based on questionnaire scores, including the IIEF, and the Global Assessment Questionnaire. These effects were seen in all questionnaires evaluated, and the overall end-study improvement of the IIEF questionnaire was 7.5 points for tadalafil vs. placebo, 7 points for vardenafil vs. placebo, and 6 points for sildenafil vs. placebo. Compared directly, tadalafil showed a 1.5 point advantage over sildenafil, and a 0.4 point advantage over vardenafil. Whether these small interclass differences in questionnaire outcomes represent true differences in clinically important outcomes is currently under debate.
Overall, in placebo-controlled RCTs, all three agents show a clinical response rate in ≈65% of patients, compared to ≈30% for placebo [18,22,23]. Importantly, all of these studies included diverse groups of patients, and it is clear that all three agents are effective in the treatment of ED, whatever the cause or severity. Moreover, even in refractory cases of ED, PDE5i were shown to be effective.
Diabetes
Because of its many deleterious effects on both the neuronal and vascular supply to the penis, diabetes can be an especially challenging cause of ED to treat. Sildenafil, tadalafil and vardenafil have all been shown to be effective treatments for diabetic ED. The Sildenafil Diabetes Study Group was a RCT of men with diabetic ED, and showed a 56% improvement of reported erections in the sildenafil group, compared to 10% in the placebo group, after 12 weeks of treatment. In addition, 61% of men randomised to sildenafil reported successful sexual intercourse, compared with 22% of controls (P < 0.001) [24]. A similar study comparing vardenafil to placebo for 12 weeks showed a significant increase in the erectile function domain score of the IIEF of 5.9 for men randomised to vardenafil at a dose of 10 mg, and 7.8 for a dose of 20 mg, compared to 1.4 for placebo (P < 0.001) [25]. Finally, tadalafil at a dose of 10 or 20 mg significantly improved the IIEF erectile function domain score by 6.4 and 7.3, respectively, compared with 0.1 for placebo (P < 0.001) [26].
ED after prostatectomy or radiotherapy
Prostatectomy and radiotherapy for prostate cancer both cause damage to the neurovascular structures supplying the penis, that can lead to severe and refractory ED. The bilateral nerve-sparing approach to prostatectomy, when oncologically appropriate, can help to reduce the rate of ED, but despite this surgical advance, significant numbers of patients still have ED after prostatectomy.
Fortunately, PDE5i have been shown to be effective in significant numbers of these patients. Whilst patient satisfaction rates with early postoperative sildenafil therapy have been shown to be low, i.e., 26% at 0–6 months after nerve-sparing prostatectomy, this increases to 60% satisfaction at 18–24 months [27]. Vardenafil treatment has shown more encouraging results. In 440 men with ED after nerve-sparing prostatectomy, treatment with vardenafil for 12 weeks significantly enhanced erectile function compared to placebo. Patients taking vardenafil reported improved erections (65% vs. 13% of controls, P < 0.001), successful vaginal penetration (48% vs. 22%, P < 0.001), and successful intercourse (34% vs. 10%, P < 0.001) [28].
Tadalafil has shown perhaps the most encouraging results in the treatment of ED after surgery. In a double-blind RCT of 303 men with ED 1–4 years after bilateral nerve-sparing prostatectomy, tadalafil at a dose of 20 mg for 12 weeks significantly enhanced erectile function, with patients on tadalafil reporting improved erectile function (62% vs. 23%, P < 0.001) and successful sexual intercourse (41% vs. 19%, P < 0.001) compared to controls [29]. The benefits of tadalafil therapy in this study were most pronounced in patients who had some detectable penile tumescence after surgery. In this group, 71% of patients on tadalafil reported improved erections, compared with 24% of controls, and 52% of men were able to have successful intercourse (P < 0.001).
PDE5i treatment failure
Despite initial success in 65–70% of patients, 30–40% do not respond to PDE5i alone, and alternative strategies must be considered to enhance the response rate. Most importantly, realistic expectations should be set, and patients should be encouraged to give the medications a chance to work. In some patients who have had prolonged ED, more than six doses of sildenafil might be required before there is a satisfactory response [30]. Patients should be counselled to avoid high fat meals, especially with sildenafil and vardenafil, and to delay sexual stimulation for at least 1 h after administration. If a particular PDE5i continues to be ineffective, a trial of another agent might result in positive response rates in 60% of cases [31].
If a patient continues to be poorly responsive to PDE5i in the absence of risk factors, he should be evaluated for hypogonadism. PDE5i require NO-dependent increases in cGMP to be effective, and a lack of libido associated with hypogonadism will result in low NO levels. Normalisation of testosterone levels with replacement therapy significantly improves the response rates to PDE5i. In a RCT comparing sildenafil and testosterone combined therapy vs. sildenafil and placebo, the combined therapy group showed an increase in the IIEF erectile function domain of 4.4 points, compared with 2.1 points in the placebo group. In addition, these patients showed an improvement in ejaculatory function [32]. Of course, if patients are on hormone ablation therapy for prostate cancer, testosterone replacement is not an option, and the ED is particularly refractory to PDE5i [33].
Indications other than ED
PDE5i have also been used in the prevention of long-term ED after prostatectomy. After neurovascular damage due to radical surgery, the incidence of nocturnal erections decreases, and the corpora undergo a relatively irreversible fibrosis that results in ED and penile shortening. Padma-Nathan et al. [34] showed that daily sildenafil after bilateral nerve-sparing prostatectomy improved the erectile function sevenfold compared to controls. Similar effects have been shown for tadalafil [35]. Unfortunately, the high ‘out-of-pocket’ cost to patients has prevented adequate adherence to these regimens, despite their proven benefit [36].
Interestingly, there is a high co-prevalence of ED with BPH and urinary obstruction, even after controlling for α-blocker therapy. This has led to clinical trials that have resulted in FDA approval for the use of PDE5i in the treatment of LUTS associated with BPH. Two studies showed that on-demand dosing with sildenafil can improve the IPSS and patient’s bother due to urinary symptoms [37,38]. Similar results with daily dosing of tadalafil have been reported, despite no significant change in urinary flow rate [39].
PDE5i have been evaluated and approved for various non-urological conditions. In 2005, sildenafil (Revatio™) was approved by the FDA for treating pulmonary arterial hypertension, followed by tadalafil (Adcirca™) in 2009. In RCTs, both increased the 6-min walking distance (the primary endpoint of the studies) significantly more than did placebo [40]. In a similar disease process, sildenafil and tadalafil have also been shown to improve the symptoms due to acute high-altitude pulmonary oedema and chronic high altitude pulmonary hypertension [41]. Whilst not yet approved by the FDA for the treatment of Raynaud’s disease, the results of clinical trials have been promising. Vardenafil has been shown to improve digital blood flow in 70% of patients and improve clinical symptoms in 68%, whilst sildenafil has been shown to significantly reduce the frequency of Raynaud’s attacks [42]. Finally, after encouraging preclinical studies, PDE5i are currently under investigation for the treatment of congestive heart failure [43].
Future studies and directions
The future of PDE5i in the treatment of ED is exciting, and researchers remain focused on providing a more rapid clinical onset with a more flexible clinical response, whilst improving the clinical efficacy. Avanafil was recently approved by the FDA in the USA for treating ED in early 2013, based on a RCT that showed that doses of 50, 100, and 200 mg had a rapid onset of full erection at 30 min after administration, with no restrictions on fatty food or alcohol consumption. The subjective efficacy was ≈70%, and the most commonly reported adverse events were headache, flushing, and nasal congestion. It is a fast-acting molecule with a Tmax of 35 min, but is also rapidly metabolised, with a half-life of 60–90 min [44]. This would make this compound particularly useful in cases where patients found it effective but wished to minimise the duration of side-effects.
Udenafil has recently undergone phase III trials and has been approved for use in Russia and Korea, with approval expected in the USA. In a single-centre placebo-controlled trial of Korean men, there was an average increase in IIEF erectile function domain score of 8.5 points, vs. 0.2 points in the placebo group [45]. Its pharmacokinetic profile mirrors that of tadalafil, with a combination of rapid onset and long duration of action, with a Tmax of 0.8–1.3 h, and a half-life of 7.3–12.1 h.
NCX-911, also referred to as sildenafil nitrate, is a recently developed compound that acts both as a potent inhibitor of PDE5 and an effective NO-dependent activator of sGC. It aims to improve erections in patients with difficult-to-treat ED due to conditions such as diabetes, atherosclerosis, and after prostatectomy, that result in decreased endogenous NO levels [46]. Pre-clinical studies have been encouraging and future clinical trials are eagerly awaited.
Conclusion
Oral PDE5i have revolutionised the treatment of ED due to their desirable combination of efficacy and tolerability. They currently represent the first line treatment for all causes of ED, according to the Sexual Medicine Society guidelines [47]. Results from randomised trials suggest that no one agent is more effective than the others, and certain pharmacological qualities of each medication are desirable to certain patients. However, PDE5i for ED are still ineffective in 30% of patients. As these medications depend on sexual stimulation and intact NO signalling, current research is underway to develop medications that provide erections through NO-independent mechanisms.
Conflict of interest
None.
Source of funding
None.
Footnotes
Peer review under responsibility of Arab Association of Urology.
References
- 1.Lue T.F. Erectile dysfunction. N Engl J Med. 2000;342:1802–1813. doi: 10.1056/NEJM200006153422407. [DOI] [PubMed] [Google Scholar]
- 2.Rosen R.C., Cappelleri J.C., Smith M.D., Lipsky J., Pena B.M. Development and evaluation of an abridged, 5-item version of the international index of erectile function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11:319–326. doi: 10.1038/sj.ijir.3900472. [DOI] [PubMed] [Google Scholar]
- 3.Rosen R.C., Riley A., Wagner G., Osterloh I.H., Kirkpatrick J., Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822–830. doi: 10.1016/s0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- 4.Meuleman E.J., Hatzichristou D., Rosen R.C., Sadovsky R. Diagnostic tests for male erectile dysfunction revisited. Committee consensus report of the international consultation in sexual medicine. J Sex Med. 2010;7:2375–2381. doi: 10.1111/j.1743-6109.2010.01841.x. [DOI] [PubMed] [Google Scholar]
- 5.Lewis R.W., Fugl-Meyer K.S., Bosch R., Fugl-Meyer A.R., Laumann E.O., Lizza E. Epidemiology/risk factors of sexual dysfunction. J Sex Med. 2004;1:35–39. doi: 10.1111/j.1743-6109.2004.10106.x. [DOI] [PubMed] [Google Scholar]
- 6.Ayta I.A., McKinlay J.B., Krane R.J. The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int. 1999;84:50–56. doi: 10.1046/j.1464-410x.1999.00142.x. [DOI] [PubMed] [Google Scholar]
- 7.Johannes C.B., Araujo A.B., Feldman H.A., Derby C.A., Kleinman K.P., McKinlay J.B. Incidence of erectile dysfunction in men 40–69 years old: longitudinal results from the Massachusetts male aging study. J Urol. 2000;163:460–463. [PubMed] [Google Scholar]
- 8.Lewis R.W., Hatzichristou D., Laumann E. Epidemiology and natural history of erectile dysfunction; risk factors including iatrogenic and aging. In: Jardin A., Wagner G., Khoury S., Giuliano F., editors. Proceedings of the first international consultation on erectile dysfunction. Health Publication; Plymbridge (UK): 2000. pp. 21–51. [Google Scholar]
- 9.Salonia A., Ferrari M., Saccà A., Pellucchi F., Castagna G., Clementi M.C. Delay in seeking medical help in patients with new-onset erectile dysfunction remained high over and despite the PDE5 era – an ecological study. J Sex Med. 2012;9:3239–3246. doi: 10.1111/j.1743-6109.2012.02953.x. [DOI] [PubMed] [Google Scholar]
- 10.Shaeer O., Shaeer K. The global online sexuality survey (GOSS): erectile dysfunction among arabic-speaking internet users in the middle east. J Sex Med. 2011;8:2152–2160. doi: 10.1111/j.1743-6109.2011.02297.x. [DOI] [PubMed] [Google Scholar]
- 11.Ghofrani H.A., Osterloh I.H., Grimminger F. Sildenafil. from angina to erectile dysfunction to pulmonary hypertension and beyond. Nat Rev Drug Discov. 2006;5:689–702. doi: 10.1038/nrd2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montague D.K., Barada J.H., Belker A.M., Levine L.A., Nadig P.W., Roehrborn C.G. Clinical guidelines panel on erectile dysfunction: summary report on the treatment of organic erectile dysfunction. The American Urological Association. J Urol. 1996;156:2007–2011. doi: 10.1016/s0022-5347(01)65419-3. [DOI] [PubMed] [Google Scholar]
- 13.Heaton J.P. Apomorphine. An update of clinical trial results. Int J Impot Res. 2000;12(Suppl. 4):S67–S73. doi: 10.1038/sj.ijir.3900581. [DOI] [PubMed] [Google Scholar]
- 14.Costabile R.A., Spevak M. Oral trazodone is not effective therapy for erectile dysfunction: a double-blind, placebo controlled trial. J Urol. 1999;161:1819–1822. [PubMed] [Google Scholar]
- 15.Moemen M.N., Hamed H.A., Kamel I.I., Shamloul R.M., Ghanem H.M. Clinical and sonographic assessment of the side effects of intracavernous injection of vasoactive substances. Int J Impot Res. 2004;16:143–145. doi: 10.1038/sj.ijir.3901194. [DOI] [PubMed] [Google Scholar]
- 16.Korenman S.G., Viosca S.P. Treatment of vasculogenic sexual dysfunction with pentoxifylline. J Am Geriatr Soc. 1993;41:363–366. doi: 10.1111/j.1532-5415.1993.tb06941.x. [DOI] [PubMed] [Google Scholar]
- 17.Kostis J.B., Jackson G., Rosen R., Barrett-Connor E., Billups K., Burnett A.L. Sexual dysfunction and cardiac risk (the second Princeton consensus conference) Am J Cardiol. 2005;96:313–321. doi: 10.1016/j.amjcard.2005.03.065. [DOI] [PubMed] [Google Scholar]
- 18.Brock G.B., McMahon C.G., Chen K.K., Costigan T., Shen W., Watkins V. Efficacy and safety of tadalafil for the treatment of erectile dysfunction: results of integrated analyses. J Urol. 2002;168:1332–1336. doi: 10.1016/S0022-5347(05)64442-4. [DOI] [PubMed] [Google Scholar]
- 19.Porst H., Padma-Nathan H., Giuliano F., Anglin G., Varanese L., Rosen R. Efficacy of tadalafil for the treatment of erectile dysfunction at 24 and 36 hours after dosing: a randomized controlled trial. Urology. 2003;62:121–125. doi: 10.1016/s0090-4295(03)00359-5. [DOI] [PubMed] [Google Scholar]
- 20.Corbin J.D., Francis S.H. Pharmacology of phosphodiesterase-5 inhibitors. Int J Clin Pract. 2002;56:453–459. [PubMed] [Google Scholar]
- 21.Yuan J., Zhang R., Yang Z., Lee J., Liu Y., Tian J. Comparative effectiveness and safety of oral phosphodiesterase type 5 inhibitors for erectile dysfunction: a systematic review and network meta-analysis. Eur Urol. 2013;63:902–912. doi: 10.1016/j.eururo.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 22.Padma-Nathan H., Steers W.D., Wicker P.A. Efficacy and safety of oral sildenafil in the treatment of erectile dysfunction: a double-blind, placebo-controlled study of 329 patients. The Sildenafil study group. Int J Clin Pract. 1998;52:375–379. [PubMed] [Google Scholar]
- 23.Hellstrom W.J., Gittelman M., Karlin G., Segerson T., Thibonnier M., Taylor T. Vardenafil for treatment of men with erectile dysfunction: efficacy and safety in a randomized, double-blind, placebo-controlled trial. J Androl. 2002;23:763–771. [PubMed] [Google Scholar]
- 24.Rendell M.S., Rajfer J., Wicker P.A., Smith M.D. Sildenafil for treatment of erectile dysfunction in men with diabetes: a randomized controlled trial. The Sildenafil diabetes study group. JAMA. 1999;281:421–426. doi: 10.1001/jama.281.5.421. [DOI] [PubMed] [Google Scholar]
- 25.Goldstein I., Young J.M., Fischer J., Bangerter K., Segerson T., Taylor T. Vardenafil, a new phosphodiesterase type 5 inhibitor, in the treatment of erectile dysfunction in men with diabetes: a multicenter double-blind placebo-controlled fixed-dose study. Diabetes Care. 2003;26:777–783. doi: 10.2337/diacare.26.3.777. [DOI] [PubMed] [Google Scholar]
- 26.Saenz de Tejada I., Anglin G., Knight J.R., Emmick J.T. Effects of tadalafil on erectile dysfunction in men with diabetes. Diabetes Care. 2002;25:2159–2164. doi: 10.2337/diacare.25.12.2159. [DOI] [PubMed] [Google Scholar]
- 27.Hong E.K., Lepor H., McCullough A.R. Time dependent patient satisfaction with sildenafil for erectile dysfunction (ED) after nerve-sparing radical retropubic prostatectomy (RRP) Int J Impot Res. 1999;11(Suppl. 1):S15–S22. doi: 10.1038/sj.ijir.3900466. [DOI] [PubMed] [Google Scholar]
- 28.Brock G., Nehra A., Lipshultz L.I., Karlin G.S., Gleave M., Seger M. Safety and efficacy of vardenafil for the treatment of men with erectile dysfunction after radical retropubic prostatectomy. J Urol. 2003;170:1278–1283. doi: 10.1097/01.ju.0000086947.00547.49. [DOI] [PubMed] [Google Scholar]
- 29.Montorsi F., Nathan H.P., McCullough A., Brock G.B., Broderick G., Ahuja S. Tadalafil in the treatment of erectile dysfunction following bilateral nerve sparing radical retropubic prostatectomy: a randomized, double-blind, placebo controlled trial. J Urol. 2004;172:1036–1041. doi: 10.1097/01.ju.0000136448.71773.2b. [DOI] [PubMed] [Google Scholar]
- 30.McCullough A.R. An update on the PDE-5 inhibitors (PDE-5i) J Androl. 2003;24(Suppl. 6):S528. doi: 10.1002/j.1939-4640.2003.tb02747.x. [DOI] [PubMed] [Google Scholar]
- 31.Carson C.C., Hatzichristou D.G., Carrier S., Lording D., Lyngdorf P., Aliotta P. Erectile response with vardenafil in sildenafil nonresponders: a multicentre, double-blind, 12-week, flexible-dose, placebo-controlled erectile dysfunction clinical trial. BJU Int. 2004;94:1301–1309. doi: 10.1111/j.1464-410X.2004.05161.x. [DOI] [PubMed] [Google Scholar]
- 32.Shabsigh R., Kaufman J.M., Steidle C., Padma-Nathan H. Randomized study of testosterone gel as adjunctive therapy to sildenafil in hypogonadal men with erectile dysfunction who do not respond to sildenafil alone. J Urol. 2004;172:658–663. doi: 10.1097/01.ju.0000132389.97804.d7. [DOI] [PubMed] [Google Scholar]
- 33.Corona G., Maggi M. The role of testosterone in erectile dysfunction. Nat Rev Urol. 2010;7:46–56. doi: 10.1038/nrurol.2009.235. [DOI] [PubMed] [Google Scholar]
- 34.Padma-Nathan H., McCullough A., Forest C. Erectile dysfunction secondary to nerve-sparing radical retropubic prostatectomy. Comparative phosphodiesterase-5 inhibitor efficacy for therapy and novel prevention strategies. Curr Urol Rep. 2004;5:467–471. doi: 10.1007/s11934-004-0072-0. [DOI] [PubMed] [Google Scholar]
- 35.Carson C., Shabsigh R., Segal S., Murphy A., Fredlund P., Kuepfer C. Efficacy, safety, and treatment satisfaction of tadalafil versus placebo in patients with erectile dysfunction evaluated at tertiary-care academic centers. Urology. 2005;65:353–359. doi: 10.1016/j.urology.2004.09.056. [DOI] [PubMed] [Google Scholar]
- 36.Lee D.J., Cheetham P., Badani K.K. Penile rehabilitation protocol after robot-assisted radical prostatectomy: assessment of compliance with phosphodiesterase type 5 inhibitor therapy and effect on early potency. BJU Int. 2010;105:382–388. doi: 10.1111/j.1464-410X.2009.08820.x. [DOI] [PubMed] [Google Scholar]
- 37.Sairam K., Kulinskaya E., Boustead G.B., Hanbury D.C., McNicholas T.A. Prevalence of undiagnosed prostate cancer in men with erectile dysfunction. BJU Int. 2002;89:261–263. doi: 10.1046/j.1464-4096.2001.01271.x. [DOI] [PubMed] [Google Scholar]
- 38.McVary K.T. Sildenafil citrate improves erectile function and urinary symptoms in men with erectile dysfunction and lower urinary tract symptoms associated with benign prostatic hyperplasia: a randomized, double-blind trial. J Urol. 2007;177:1071–1077. doi: 10.1016/j.juro.2006.10.055. [DOI] [PubMed] [Google Scholar]
- 39.McVary K.T., Monnig W., Camps J.L., Jr., Young J.M., Tseng L.J., van den Ende G. Tadalafil relieves lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol. 2007;177:1401–1407. doi: 10.1016/j.juro.2006.11.037. [DOI] [PubMed] [Google Scholar]
- 40.Archer S.L., Michelakis E.D. Phosphodiesterase type 5 inhibitors for pulmonary arterial hypertension. N Engl J Med. 2009;361:1864–1871. doi: 10.1056/NEJMct0904473. [DOI] [PubMed] [Google Scholar]
- 41.Aldashev A.A., Kojonazarov B.K., Amatov T.A., Sooronbaev T.M., Mirrakhimov M.M., Morrell N.W. Phosphodiesterase type 5 and high altitude pulmonary hypertension. Thorax. 2005;60:683–687. doi: 10.1136/thx.2005.041954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwartz B.G., Levine L.A., Comstock G., Stecher V.J., Kloner R.A. Cardiac uses of phosphodiesterase-5 inhibitors. J Am Coll Cardiol. 2012;59:9–15. doi: 10.1016/j.jacc.2011.07.051. [DOI] [PubMed] [Google Scholar]
- 43.Cooper T.J., Guazzi M., Al-Mohammad A., Amir O., Bengal T., Cleland J.G. Sildenafil in Heart failure (SilHF). An investigator-initiated multinational randomized controlled clinical trial. Rationale and design. Eur J Heart Fail. 2013;15:119–122. doi: 10.1093/eurjhf/hfs152. [DOI] [PubMed] [Google Scholar]
- 44.Goldstein I., McCullough A.R., Jones L.A., Hellstrom W.J., Bowden C.H., Didonato K. A randomized, double-blind, placebo-controlled evaluation of the safety and efficacy of avanafil in subjects with erectile dysfunction. J Sex Med. 2012;9:1122–1133. doi: 10.1111/j.1743-6109.2011.02629.x. [DOI] [PubMed] [Google Scholar]
- 45.Kim B.H., Lim H.S., Chung J.Y., Kim J.R., Lim K.S., Sohn D.R. Safety, tolerability and pharmacokinetics of udenafil, a novel PDE-5 inhibitor, in healthy young Korean subjects. Br J Clin Pharmacol. 2008;65:848–854. doi: 10.1111/j.1365-2125.2008.03107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kalsi J.S., Kell P.D., Cellek S., Ralph D.J. NCX-911, a novel nitric oxide-releasing PDE5 inhibitor relaxes rabbit corpus cavernosum in the absence of endogenous nitric oxide. Int J Impot Res. 2004;16:195–200. doi: 10.1038/sj.ijir.3901157. [DOI] [PubMed] [Google Scholar]
- 47.Hackett G., Kell P., Ralph D., Dean J., Price D., Speakman M. British society for sexual medicine guidelines on the management of erectile dysfunction. J Sex Med. 2008;5:1841–1865. doi: 10.1111/j.1743-6109.2008.00773.x. [DOI] [PubMed] [Google Scholar]



