Abstract
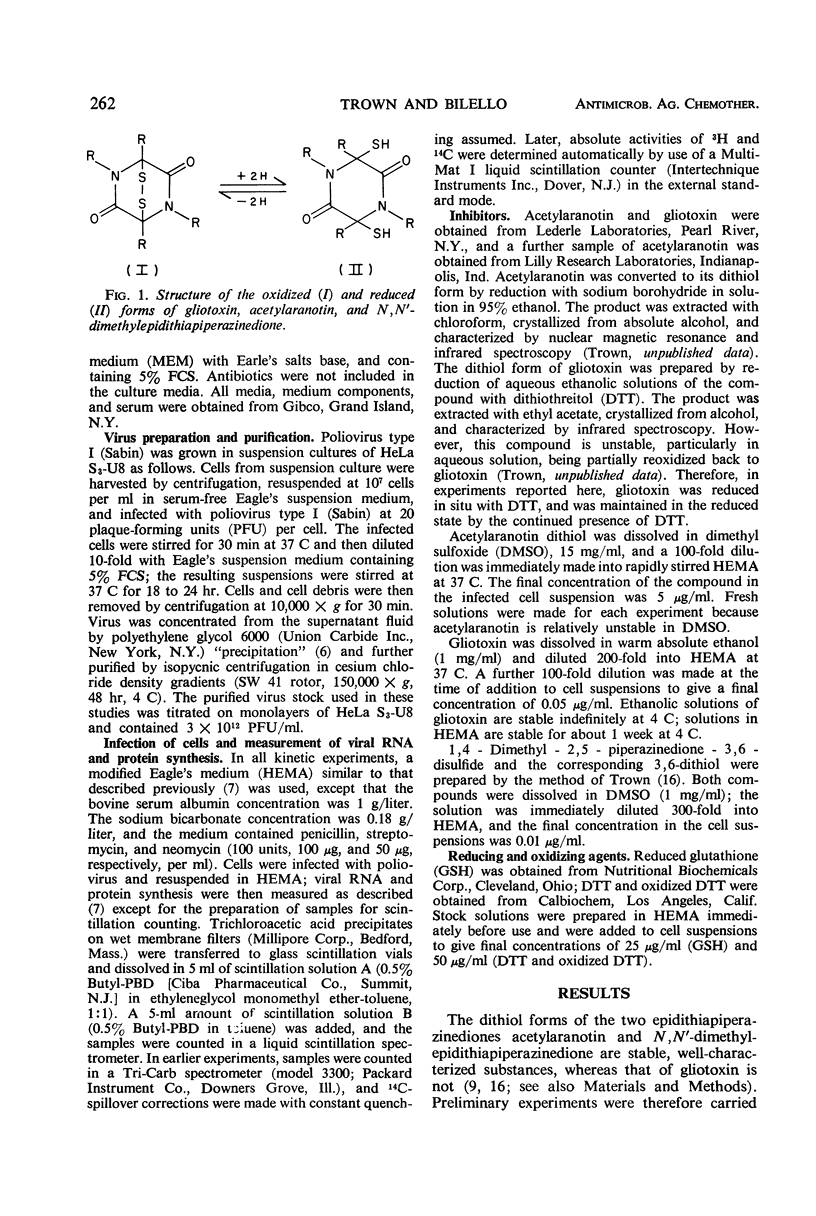
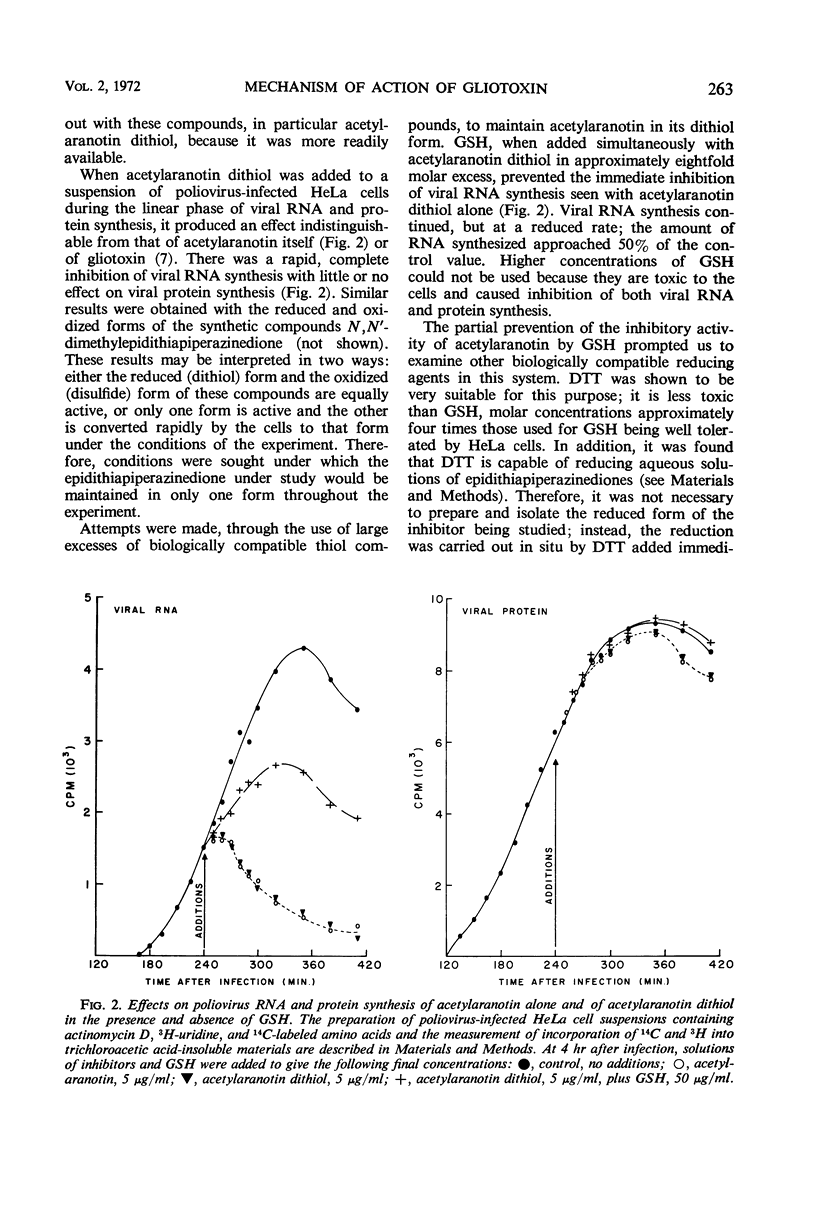
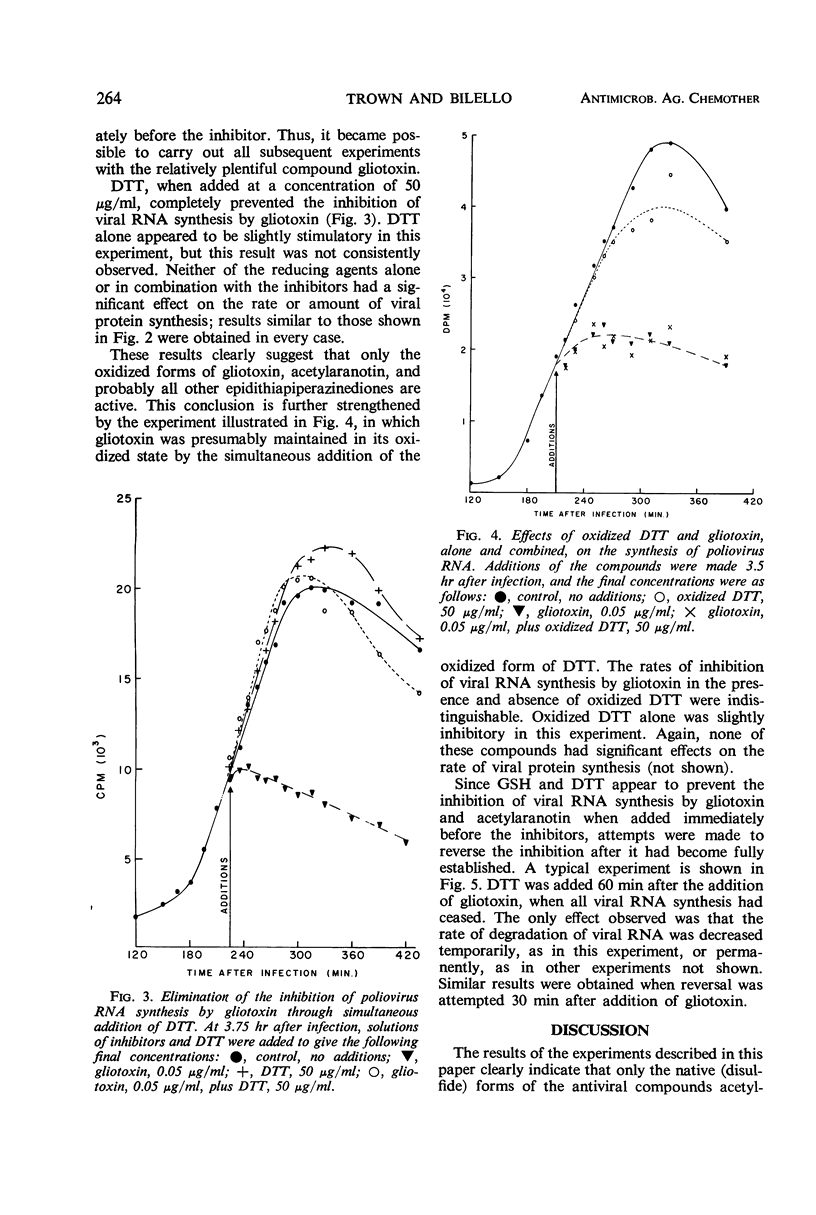
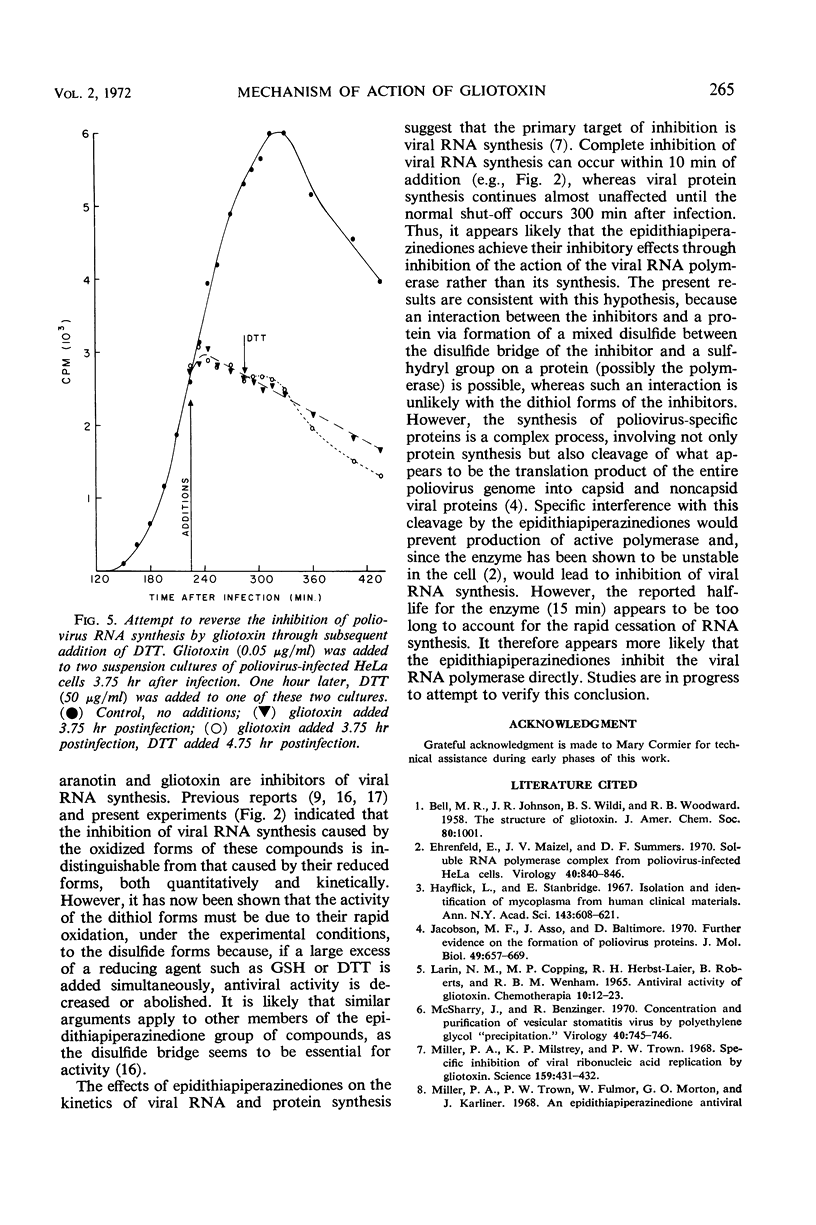
Gliotoxin and two other compounds, with antiviral activity against a number of ribonucleic acid (RNA) viruses and structurally related via the epidithiapiperazinedione moiety, appeared to be equally active in their oxidized and reduced forms. However, the ability of the reduced forms to inhibit viral RNA synthesis was abolished when these compounds were maintained in the reduced state by the simultaneous presence of a large molar excess of dithiothreitol or reduced glutathione. The active form therefore appeared to be that containing a disulfide bridge, and the apparent activity of the dithiol was due to cellular oxidation. Possible mechanisms by which the compounds could interact with viral proteins, e.g., viral RNA-dependent RNA polymerase, are proposed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ehrenfeld E., Maizel J. V., Summers D. F. Soluble RNA polymerase complex from poliovirus-infected HeLa cells. Virology. 1970 Apr;40(4):840–846. doi: 10.1016/0042-6822(70)90129-7. [DOI] [PubMed] [Google Scholar]
- Hayflick L., Stanbridge E. Isolation and identification of mycoplasma from human clinical materials. Ann N Y Acad Sci. 1967 Jul 28;143(1):608–621. doi: 10.1111/j.1749-6632.1967.tb27705.x. [DOI] [PubMed] [Google Scholar]
- Jacobson M. F., Asso J., Baltimore D. Further evidence on the formation of poliovirus proteins. J Mol Biol. 1970 May 14;49(3):657–669. doi: 10.1016/0022-2836(70)90289-5. [DOI] [PubMed] [Google Scholar]
- Larin N. M., Copping M. P., Herbst-Laier R. H., Roberts B., Wenham R. B. Antiviral activity of gliotoxin. Chemotherapy. 1965;10(1):12–23. doi: 10.1159/000220389. [DOI] [PubMed] [Google Scholar]
- McSharry J., Benzinger R. Concentration and purification of vesicular stomatitis virus by polyethylene glycol "precipitation". Virology. 1970 Mar;40(3):745–746. doi: 10.1016/0042-6822(70)90219-9. [DOI] [PubMed] [Google Scholar]
- Miller P. A., Milstrey K. P., Trown P. W. Specific inhibition of viral ribonucleic acid replication by Gliotoxin. Science. 1968 Jan 26;159(3813):431–432. doi: 10.1126/science.159.3813.431. [DOI] [PubMed] [Google Scholar]
- Miller P. A., Trown P. W., Fulmor W., Morton G. O., Karliner J. An epidithiapiperazinedione antiviral agent from Aspergillus terreus. Biochem Biophys Res Commun. 1968 Oct 24;33(2):219–221. doi: 10.1016/0006-291x(68)90771-7. [DOI] [PubMed] [Google Scholar]
- Penman S., Summers D. Effects on host cell metabolism following synchronous infection with poliovirus. Virology. 1965 Dec;27(4):614–620. doi: 10.1016/0042-6822(65)90187-x. [DOI] [PubMed] [Google Scholar]
- RIGHTSEL W. A., SCHNEIDER H. G., SLOAN B. J., GRAF P. R., MILLER F. A., BARTZ O. R., EHRLICH J., DIXON G. J. ANTIVIRAL ACTIVITY OF GLIOTOXIN AND GLIOTOXIN ACETATE. Nature. 1964 Dec 26;204:1333–1334. doi: 10.1038/2041333b0. [DOI] [PubMed] [Google Scholar]
- Robb J. A. Microcloning and replica plating of mammalian cells. Science. 1970 Nov 20;170(3960):857–858. doi: 10.1126/science.170.3960.857. [DOI] [PubMed] [Google Scholar]
- Shedden W. I., Cole B. C. Rapid method for demonstrating intracellular pleuropneumonia-like organisms in a strain of hamster kidney cells (BHK 21 C13). Nature. 1966 May 21;210(5038):868–868. doi: 10.1038/210868a0. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Aaronson S. A., Rands E. Rapid detection of mycoplasma-infected cell cultures. Exp Cell Res. 1971 Mar;65(1):256–257. doi: 10.1016/s0014-4827(71)80077-0. [DOI] [PubMed] [Google Scholar]
- Trown P. W. Antiviral activity of N, N'-dimethyl-epidithiapiperazinedione, a synthetic compound related to the gliotoxins, LL-S88alpha and beta, chetomin and the sporidesmins. Biochem Biophys Res Commun. 1968 Nov 8;33(3):402–407. doi: 10.1016/0006-291x(68)90585-8. [DOI] [PubMed] [Google Scholar]
- Trown P. W., Lindh H. F., Milstrey K. P., Gallo V. M., Mayberry B. R., Lindsay H. L., Miller P. A. LL-S88-alpha, an antiviral substance produced by Aspergillus terreus. Antimicrob Agents Chemother (Bethesda) 1968;8:225–228. [PubMed] [Google Scholar]


